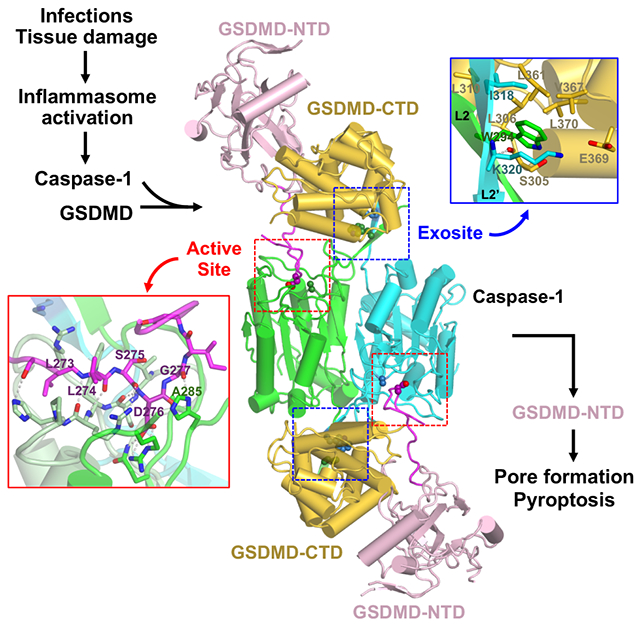
Summary
The recognition and cleavage of gasdermin D (GSDMD) by inflammatory caspases-1, 4, 5, and 11 is an essential step in initiating pyroptosis following inflammasome activation. Previous work has identified cleavage site signatures in substrates such as GSDMD, but it is unclear if these are the sole determinants for caspase engagement. Here we report the crystal structure of a complex between human caspase-1 and the full-length murine GSDMD. In addition to engagement of the GSDMD N- and C-domain linker by the caspase-1 active site, an anti-parallel β sheet at the caspase-1 L2 and L2’ loops bound a hydrophobic pocket within the GSDMD C-terminal domain distal to its N-terminal domain. This “exosite” interface endows an additional function for the GSDMD C-terminal domain as a caspase-recruitment module besides its role in autoinhibition. Our study thus reveals dual interface engagement of GSDMD by caspase-1, which may be applicable to other physiological substrates of caspases.
Keywords: Gasdermin D, inflammatory caspases, pyroptosis, inflammasome, crystal structure, active site, exosite, dual site engagement
Graphical Abstract
INTRODUCTION
The recognition and cleavage of gasdermin D (GSDMD) by inflammatory caspases-1, 4, 5, and 11 is an essential step in initiating an inflammatory form of cell death called pyroptosis downstream of the canonical and noncanonical inflammasome signaling pathways (Broz and Dixit, 2016; Cookson and Brennan, 2001; He et al., 2015; Kayagaki et al., 2015; Latz et al., 2013; Shi et al., 2015). GSDMD contains an N-terminal domain (NTD) and a C-terminal domain (CTD) connected by a linker that harbors the caspase cleavage site 272FLTD|G276 in human GSDMD (hGSDMD) and 273LLSD|G277 in murine GSDMD (mGSDMD) (Figure 1A). Binding of membrane lipids by the NTD leads to conformational changes and oligomerization that assemble membrane pores to induce cytolysis, whereas the CTD functions as an autoinhibition domain through intramolecular domain association with the NTD (Aglietti et al., 2016; Chen et al., 2016; Ding et al., 2016; Liu et al., 2016, 2018, 2019; Rogers et al., 2017; Sborgi et al., 2016; Wang et al., 2017). It is not clear if the GSDMD-CTD harbors any function other than as the autoinhibition domain.
Figure 1. CASP1 and GSDMD form stable protein complexes.
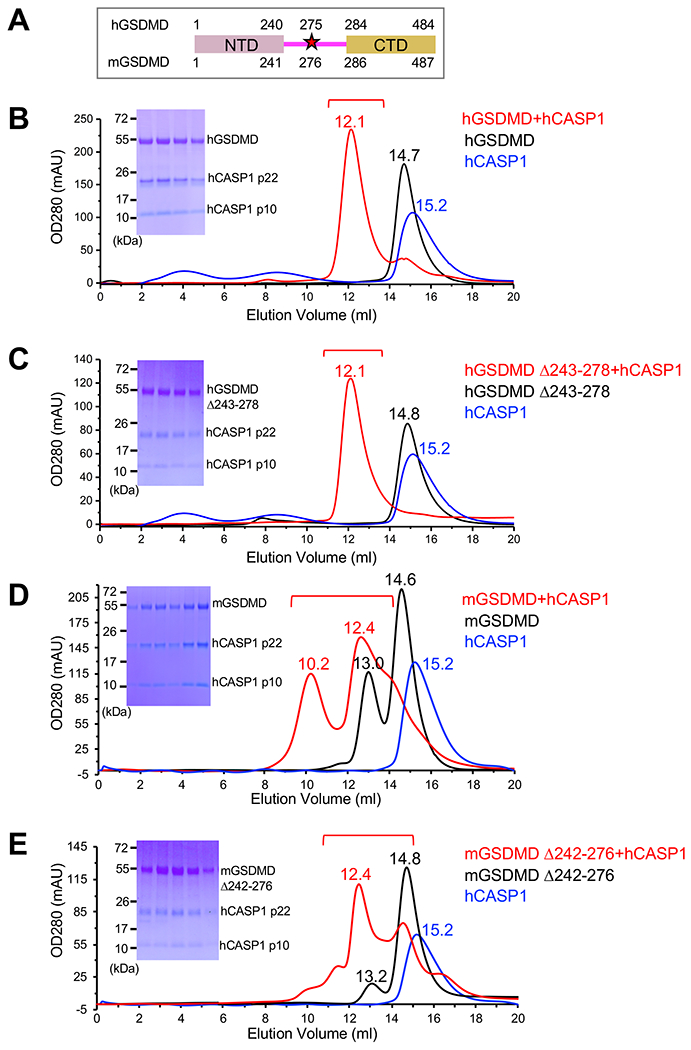
(A) The domain structures for human gasdermin D (hGSDMD) and murine gasdermin D (mGSDMD) are shown with residue numbers for the N-terminal domains (NTDs) (pink) and C-terminal domains (CTDs) (gold) marked. The inflammatory caspase cleavage site Asp residue is represented with a star marked with residue numbers in hGSDMD and mGSDMD.
(B-C) Formation of complexes between purified human caspase-1 (hCASP1) p22/p10 Cysteine 285 to Alanine (C285A) mutant and the full-length hGSDMD with (B) or without (C) linker residues 243-278 was analyzed through size-exclusion chromatography. Chromatograms for hCASP1, hGSDMD, or their complex are colored blue, black, or red, respectively, marked with elution volumes for the main peaks. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels for the peak fractions marked in the red chromatograms are shown in the inserts. Data shown are representative of three independent experiments.
(D-E) Formation of complexes between purified hCASP1 p22/p10 C285A and the full-length mGSDMD with (D) or without (E) linker residues 242-276 was analyzed through size-exclusion chromatography. Chromatograms for hCASP1, mGSDMD, or their complex are colored blue, black, or red, respectively, marked with elution volumes for the main peaks. SDS-PAGE gel for the peak fractions marked in the red chromatograms are shown in the inserts. Data shown are representative of three independent experiments.
Identical chromatograms were obtained using either the p20/p10 or p22/p10 catalytic domains of hCASP1 (data not shown).
See also Figure S1.
Caspase is a family of cysteine proteases that have a preference for Asp residues at the P1 sites, and play crucial roles in cell death, tissue remodeling and differentiation (Alnemri et al., 1996; Fuentes-Prior and Salvesen, 2004; Julien and Wells, 2017; Li and Yuan, 2008; Shi, 2002). Caspase-1 (CASP1) is a prototypical member of the inflammatory caspases implicated in innate immune responses to various infections and tissue damage (Jiménez Fernández and Lamkanfi, 2015; Julien and Wells, 2017). It cleaves several substrates such as GSDMD, pro-IL1β and pro-IL18. By contrast, the only known physiological substrate for caspases-4, 5, 11 is GSDMD (Jiménez Fernández and Lamkanfi, 2015; Kayagaki et al., 2011; Shi et al., 2015). The molecular basis for such substrate selectivity is poorly understood. Previous studies have reported the preference for hydrophobic/aromatic residues at the P4 site for inflammatory caspases, in contrast to negatively charged P4 site residues for apoptotic caspase-3 (Julien and Wells, 2017; Poreba et al., 2013; Stennicke et al., 2000; Wang et al., 2017). The roles for residues adjacent to the P1-P4 sites in substrate recognition remains unclear.
In addition to enzyme active sites that recognize cleavage sequences in their substrates, exosites have been identified as structural elements in enzymes that associate with partner proteins such as substrates or inhibitors, but are distinct from their active sites (Bar-Shavit et al., 1983; Grütter et al., 1990; Rydel et al., 1990). Such an exosite has been identified for caspase-7 that employs a positively-charged motif outside its catalytic domain to facilitate binding and cleavage of poly(ADP ribose) polymerase 1 (PARP), though no structural characterization of the caspase-7-PARP complex has been reported (Boucher et al., 2012). Recently Wang and colleagues reported the structures of caspases-1, 4, and 11 in complex with the GSDMD-CTDs, which revealed within the GSDMD-CTDs that mediate their recognition by a small β sheet in the inflammatory caspases referred to as an exosite (Wang et al., 2020). This exosite is critical for GSDMD cleavage and pyroptosis, and provides direct evidence that the tertiary structures of GSDMDs play essential roles in their recognition by inflammatory caspases (Wang et al., 2020). On the other hand, it is not clear whether and how inflammatory caspases engage the full-length GSDMDs through the exosites and active sites simultaneously, and how the GSDMD N-terminal domains are positioned within the caspase-GSDMD complexes.
To investigate the molecular mechanisms of inflammatory caspase recognition of their full-length substrates, we initiated studies of human caspase-1 (hCASP1) in complex with GSDMD. We crystallized hCASP1 in complex with the full-length murine GSDMD, which revealed that the mGSDMD-NTD did not interact with hCASP1, whereas a loop structure of the mGSDMD linker, only observed upon association with hCASP1, engaged the hCASP1 active site. In addition, the L2 and L2’ loops from the hCASP1 dimer formed an anti-parallel β sheet that bound a hydrophobic pocket within the mGSDMD-CTD. This exosite in hCASP1 was located adjacent to the caspase active site and distal to the mGSDMD-NTD. The exosite interface endows an additional function for the GSDMD-CTD as a caspase-recruitment module besides its role as an autoinhibition domain that regulates the cytolysis function of the GSDMD-NTD. Our study thus reveals dual interface engagement of GSDMD by CASP1, which may be essential for substrate selectivity that restricts the number of physiological substrates, and may be applicable to other caspase substrates or inhibitors.
RESULTS
Caspase-1 catalytic domain binds GSDMD in the absence of its linker cleavage site
To investigate the association of CASP1 and GSDMD, size-exclusion chromatography was used to analyze mixture of the hCASP1 catalytic domain (p22/p10) and the full-length hGSDMD or mGSDMD. Incubation of human hCASP1 (C285A mutant) and hGSDMD led to the formation of a higher molecular weight species as observed in the chromatogram, which contained both hCASP1 and hGSDMD (Figure 1B). Similarly, mixing hCASP1 and mGSDMD led to their co-elution as higher molecular weight species that contained both proteins (Figure 1D). Furthermore, such complexes were similarly observed when the caspase cleavage site in either hGSDMD or mGSDMD was removed: truncation of the hGSDMD linker residues 243-278 containing 272FLTD|G276, or the mGSDMD linker residues 242-276 containing 272FLTD|G276 did not substantially impact the formation of the CASP1-GSDMD complexes (Figures 1C and 1E). In agreement, hCASP1 catalytic domain co-precipitated with GST-hGSDMD or GST-mGSDMD with or without the linker segments that harbor the caspase cleavage sites (Figure S1). This suggests that the cleavage site residues in hGSDMD or mGSDMD are not essential for the formation of the CASP1-GSDMD complexes. As a result, structural elements outside of the GSDMD NTD-CTD linker likely make major contribution to engagement of GSDMD by CASP1.
The hCASP1-mGSDMD complex reveals extensive enzyme-substrate interface
Among the CASP1-GSDMD complexes that were purified (Figure 1), the hCASP1-mGSDMD complex yielded crystals that diffracted X-ray to 3.4 Å resolution (Table S1). The crystal structure was determined using molecular replacement method with the structures of the full-length mGSDMD (6N9N) (Liu et al., 2019) and the hCASP1 catalytic domain (6BZ9) (Yang et al., 2018) as search models (Figures S2). In the crystal lattice, two hCASP1 molecules within a dimer were related by a crystallographic two-fold rotation axis, with each of the hCASP1 dimer engaging an mGSDMD molecule (Figures 2A and S2C). About 2300 Å2 of solvent accessible surface area was buried at the hCASP1-mGSDMD interface, as calculated by program AREAIMOL (Lee and Richards, 1971). Half of the interface was contributed by the caspase active site binding the GSDMD linker, and the other half by the caspase exosite binding the GSDMD-CTD (see below). By contrast, the GSDMD-NTD did not contact CASP1 (Figure 2A). Comparison of the structures of mGSDMD from the current study and that determined previously in the absence of CASP1 (Liu et al., 2019) revealed no gross conformational changes, though the GSDMD linker encompassing the cleavage site adopted a long loop structure that was only observed in complex with CASP1, with continuous electron density connected to the GSDMD-NTD (Figures 2B and S2A). Similarly, no major structural changes in hCASP1 was observed upon superposition of its structures in complex with GSDMD, the N-acetyl-Phe-Leu-Thr-Asp-chloromethylketone (Ac-FLTD-CMK) peptide inhibitor (Yang et al., 2018), or the pan-caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp(O-Me)-fluoromethylketone (z-VAD-FMK) (Scheer et al., 2006)(Figure 2C). Such lack of gross structural changes suggests that CASP1 engages GSDMD through pre-formed structural elements or localized conformational changes such as within the GSDMD linker.
Figure 2. The hCASP1-mGSDMD complex structure reveals extensive interface.
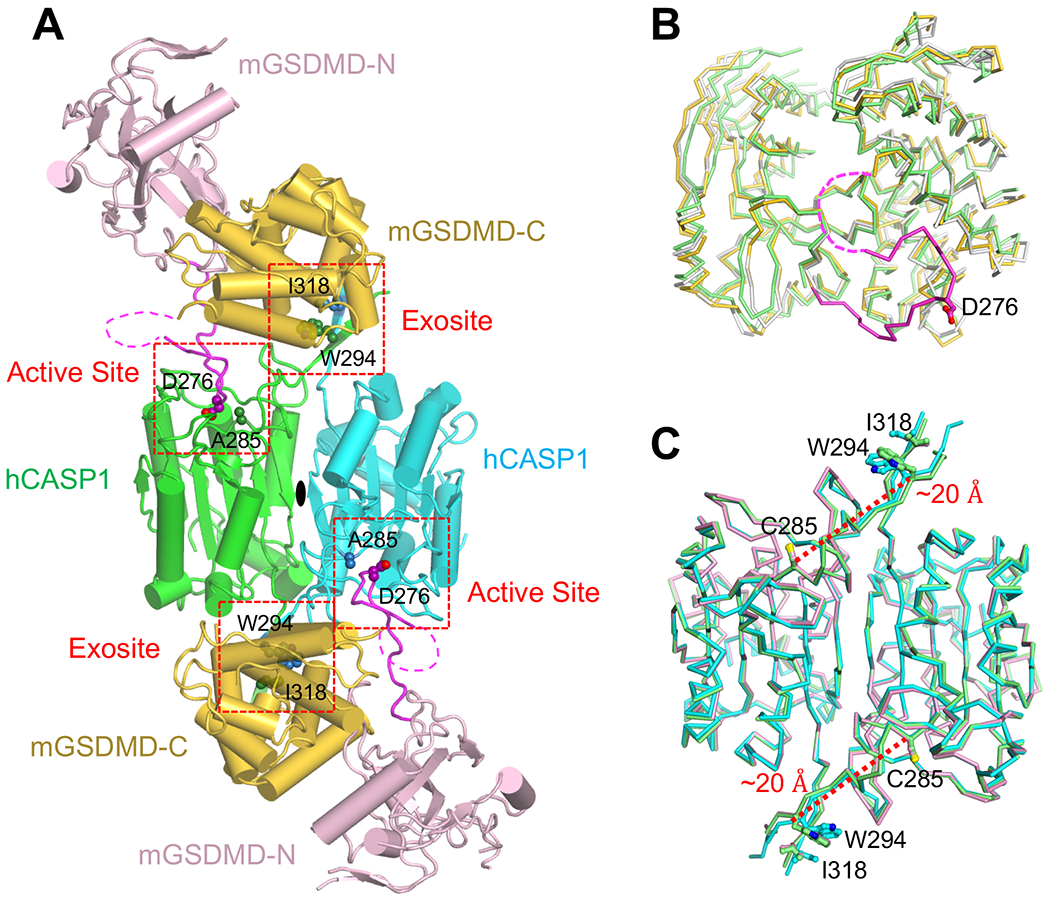
(A) Overall structure of the human CASP1 p22/p10 (C285A) in complex with the full-length murine GSDMD. The NTDs and CTDs from the mGSDMD molecules are colored light pink and gold, respectively. The linker regions are colored magenta with the caspase cleavage site residue D276 shown as spheres. The dimeric hCASP1 molecules are colored green and cyan, respectively, with residue A285 at the active site and residues W340 and K320 at the exosite shown as spheres. The magenta dotted lines indicate the portion of the GSDMD linkers that are disordered. The active site and exosite are marked with red dotted boxes.
(B) The mGSDMD structures determined previously in the absence of CASP1 (6N9N), determined here and bound to CASP1 (gold-colored molecules in figure S2C), or determined here and not in contact with CASP1 (gray-colored molecules in figure S2C) are superimposed and colored green, gold and gray, respectively. The linker region for the gold molecule is colored magenta, and the disordered portion is marked with a magenta dotted line.
(C) The hCASP1 structures in complex with GSDMD, the N-acetyl-Phe-Leu-Thr-Asp-chloromethylketone (Ac-FLTD-CMK) inhibitor (6BZ9), or N-benzyloxycarbonyl-Val-Ala-Asp(O-Me)-fluoromethylketone (z-VAD-FMK) (2HBQ) are superimposed and colored cyan, green, and pink, respectively. Residues C285 at the active site plus W294 and I318 at the exosite are shown as sticks. The distance between residues C285 and W294 are shown with red-dotted lines.
See also Figure S2.
Two interfaces mediate hCASP1 engagement of mGSDMD
The crystal structure of the hCASP1-mGSDMD complex revealed two distinct but adjacent interfaces mediating their association at the GSDMD linker and CTD, respectively, without contacts between the GSDMD-NTD and CASP1 (Figures 2A and 3A). At the GSDMD linker, the catalytic groove of CASP1 engaged GSDMD in a manner similar to its association with the active-site inhibitors such as z-VAD-FMK (2HBQ) (Scheer et al., 2006) or the GSDMD-derived peptide inhibitor (6BZ9) (Yang et al., 2018) (Figure 3B, left panel) (Yang et al., 2018). Specifically, the mGSDMD P1 site residue D276, which is conserved within most caspase cleavage sites (Clark, 2016; Julien and Wells, 2017; Poreba et al., 2013; Stennicke et al., 2000), was buried deep in the hCASP1 catalytic groove in close proximity to the catalytic residues C285 (mutated to Ala in the structure) and H237. D276 formed a mainchain hydrogen bond with S339 and three sidechain hydrogen bonds with residues R179 and Q283 from hCASP1. The adjacent mGSDMD residues L274 and S275 formed mainchain-mainchain and mainchain-sidechain hydrogen bonds with the hCASP1 residue R341 and hydrophobic contacts with residues W340 and P343. Such primary contribution through mainchain hydrogen bonds is consistent with the less stringent sequence conservation at the P2 and P3 sites (Julien and Wells, 2017; Poreba et al., 2013). At the N-terminus of the cleavage site, the P4 site residue L273 formed hydrophobic and van der Waals contacts with the hCASP1 residues R383, W340, and H342, which was in turn hydrogen bonded with the mGSDMD P5 site residue S272. The predominant hydrophobic contacts are in agreement with the preference for hydrophobic/aromatic residues at the substrate P4 site by the inflammatory caspases, in contrast to negatively charged residues by apoptotic caspase-3 (Julien and Wells, 2017; Poreba et al., 2013; Stennicke et al., 2000; Wang et al., 2017). At the C-terminus of the cleavage site, the P1 ‘ site residue G277 both anchored the linker through a mainchain-to-sidechain hydrogen bond with the hCASP1 catalytic residue H237, and provided conformational flexibility for a ~90° turn in the linker to exit the hCASP1 catalytic groove. Analysis of the known inflammatory caspase cleavage sites in GSDMD, GSDME, as well as caspases-1, 4, 5, and 11 zymogen revealed that in addition to the P1 Asp residue and P4 hydrophobic/aromatic residues, small residues such as Gly/Ala/Ser at the P1’ position were also conserved (Figures 3C and S3A). This is consistent with previous studies using peptide substrates for caspases (Stennicke et al., 2000), and the role of these C-terminal small residues in facilitating the sharp turn of the cleavage site loop observed here. The lack of the C-terminal small residues may explain why some of the other Asp residues adjacent to the substrate P1 site Asp are not cleaved by caspases. The current structure thus illustrates how a caspase active site engages its substrate beyond the P1-P4 residues that much of the previous studies have focused on.
Figure 3. Two interfaces mediate hCASP1 engagement of mGSDMD.
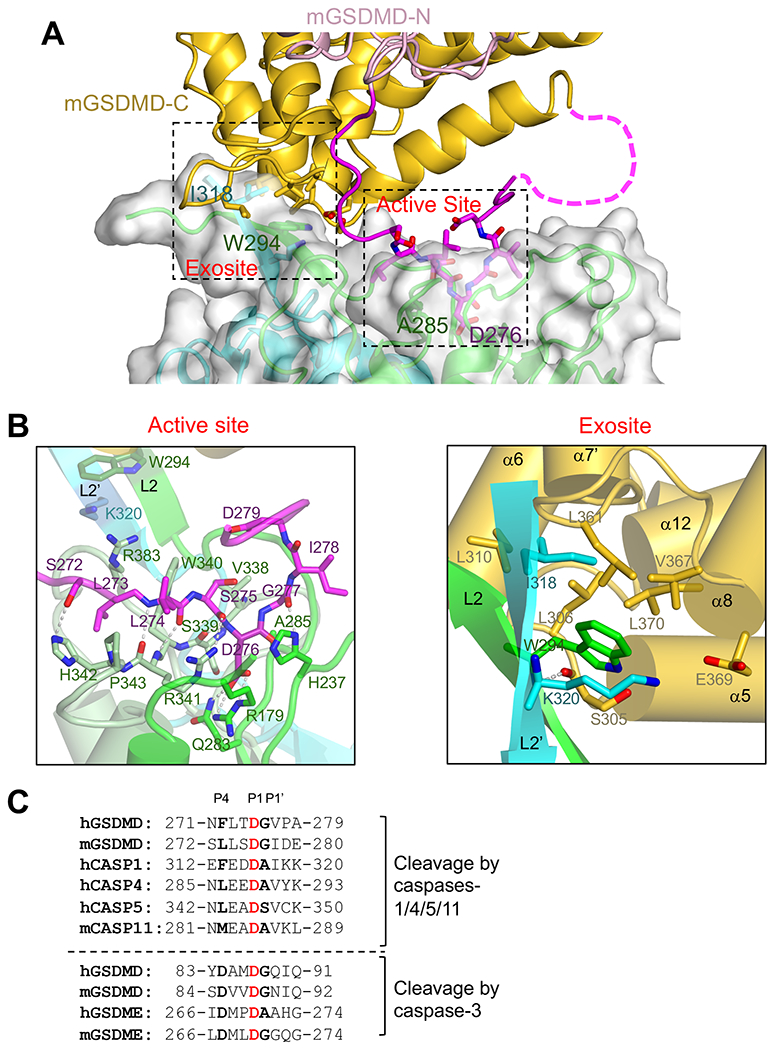
(A) A view of the dual interface between hCASP1 and mGSDMD, with the same color scheme as figure 2A. The CASP1 dimer is shown as both ribbons and transparent surface. The view is approximately 180 degrees from that in figure 2A.
(B)Left panel is a close-up view of the hCASP1-mGSDMD interface at the active site with the GSDMD linker colored magenta, and CASP1 colored green for p22, pale-green and cyan for p10 subunits, respectively. Residues participating in this interface, as well as residues W294 and K320 at the exosite, are shown in ball-and-sticks. Hydrogen bonds are shown as gray dotted lines.
Right panel is a close-up view of the hCASP1-mGSDMD interface at the exosite. The GSDMD-CTD is colored gold. The L2 and L2’ strands are colored green and cyan, respectively. The mainchain hydrogen bond between W294 in hCASP1 and S305 in mGSDMD is shown as a gray dotted line.
(C) The inflammatory caspase cleavage sites in GSDMD and caspases-1,4, 5, and 11 are shown above the dotted line. The caspase-3 cleavage sites in GSDMD and GSDME are shown below the dotted line for comparison. Identical residues at the P1 sites are in red, and conserved residues at the P4 and P1’ sites are in bold.
See also Figure S3.
In addition to the CASP1-GSDMD interface at the caspase active site, an adjacent but distinct interface, the “exosite”, mediated the association between the mGSDMD-CTD and an anti-parallel β sheet formed by the L2 and L2’ “loops” from the hCASP1 dimer (Figure 3B, right panel). This interface was centered on the hCASP1 residues W294 from L2 and I318 from L2’, which docked onto a hydrophobic pocket formed by residues L306, L310, L361, V367 and L370 from the α5-α6 and α7’-α8 structural elements in mGSDMD. At the peripheral of the hydrophobic interface, the hCASP1 residue W294 formed a mainchain hydrogen bond with the mGSDMD residue S305, and the hCASP1 residue K320 faced the mGSDMD residue E369 for a potential salt bridge. Of note, the five hydrophobic residues in mGSDMD that bind the hCASP1 exosite are conserved between the human and murine GSDMD, but are not conserved in GSDME (Figure S3A), suggesting that the exosite interface may contribute to the specific recognition of GSDMD but not GSDME by inflammatory caspases. The presence of this hydrophobic pocket at the GSDMD-CTD that bound the CASP1 exosite thus confers a previously unrecognized function for the CTD to recruit inflammatory caspases, in addition to its role as an autoinhibition domain (Ding et al., 2016; Liu et al., 2019). While this manuscript was in preparation, Wang and colleagues reported the structures of caspases-1,4, and 11 catalytic domains in complex with the GSDMD-CTDs, which revealed the critical importance of the same hydrophobic pocket within the GSDMD-CTD in mediating its recognition by inflammatory caspases (Wang et al., 2020). Comparison of our hCASP1-mGSDMD full-length structure with the hCASP1-hGSDMD-CTD structure indicates that even though the hGSDMD-CTD and mGSDMD-CTD superimpose with a root mean square deviation (rmsd) of 1.3 Å suggesting similar overall structures, a shift of the two CTDs relative to hCASP1 was observed (Figure S3B). Nonetheless, the same W294 and I318 residues from hCASP1 engaged the equivalent hydrophobic pockets at the GSDMD-CTDs (Figures S3 and 3B), in agreement with the conserved mode of exosite engagement in both hGSDMD and mGSDMD. Of note, in the murine caspase-11-mGSDMD-CTDlong complex structure reported by Wang and colleagues, the mGSDMD-CTD|ong contains the linker cleavage site (Wang et al., 2020). A continuous stretch of unmodeled electron density is observed and superimposes well with the mGSDMD P1-P4 residues observed in our study (Figure S3D), whereas the C-terminus of the caspase-11 p22 subunit is distal to the caspase active site therefore may not account for this density (6KMV).
The two CASP1-GSDMD interfaces contributes to their association
To probe the contribution of the observed CASP1-GSDMD interface to their association, we used immobilized GST-mGSDMD to pull-down the hCASP1 catalytic domain p22/p10. While the wild type GST-mGSDMD co-precipitated with hCASP1, mutation of the exosite-binding residues at the mGSDMD-CTD progressively reduced the pull-down of hCASP1, as more hydrophobic residues among L306, L310, L361, V367, and L370 are mutated to Ala (“5A” mutant), or potential salt bridge by E369 is mutated (Figure 4A). Similarly, mutation of the exosite W294, I318, or K320 residues in hCASP1 reduced pull-down by wild type mGSDMD (Figure 4B). Such reduced CASP1-GSDMD association was correlated with diminished cleavage by hCASP1, as mutation of the hydrophobic residues or charged residue E369 at the exosite-binding pocket led to less efficient cleavage of mGSDMD (Figure S4), even though the exosite is ~20 Å away from the caspase active site (Figure 2C). This suggests that mutations at the exosite-binding pocket reduced the CASP1-GSDMD engagement, which indirectly decreased substrate cleavage at the caspase active site. In addition, mutation at the P4 site residue L273 led to comparable reduction of mGSDMD cleavage as some of the exosite-pocket mutants (Figure S4). This is consistent with the preference of hydrophobic residues at the substrate P4 position for inflammatory caspases (Julien and Wells, 2017; Poreba et al., 2013; Stennicke et al., 2000).
Figure 4. The hCASP1-mGSDMD interface plays an important role in their association.
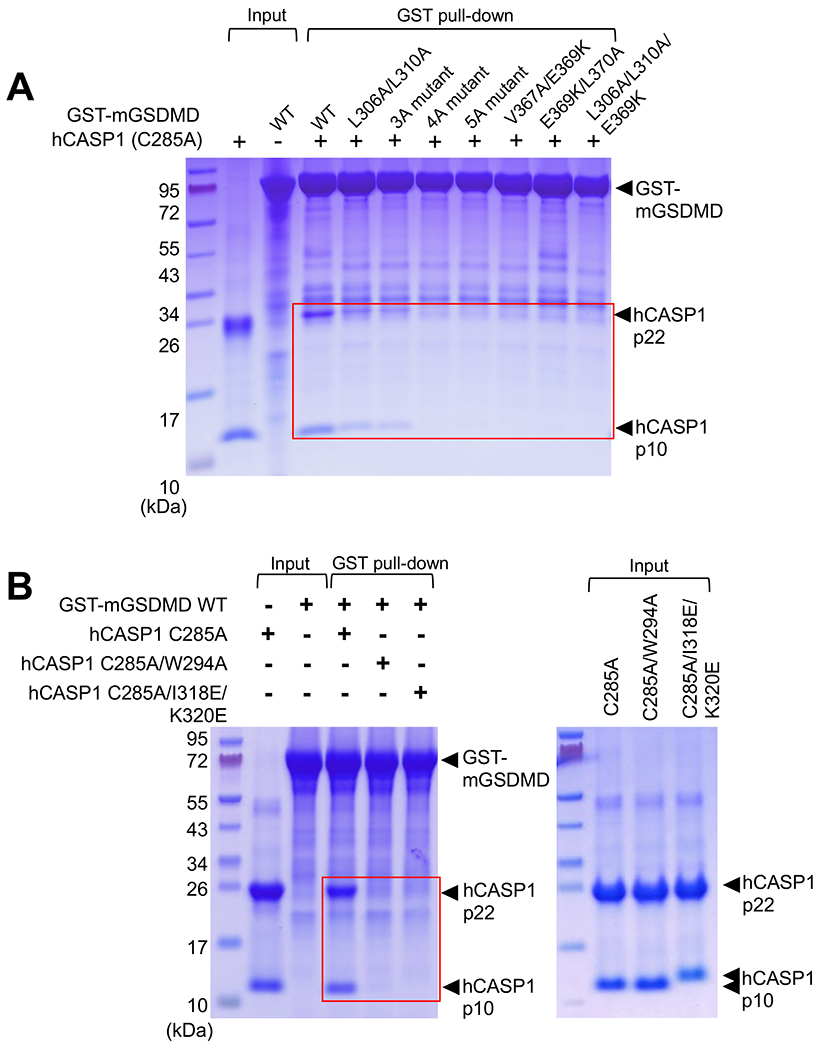
(A) Wild type or mutant GST-mGSDMD was used to pull down the hCASP1 (C285A) p22/p10 catalytic domain. The mutants harbor mutations of the exosite-binding residues in mGSDMD. Data shown are representative of three independent experiments.
(B) Wild type GST-mGSDMD was used to pull down hCASP1 catalytic domains harboring mutations such as C285A, W294A, I318E or K320E. Data shown are representative of three independent experiments.
See also Figure S4.
Disruption of the CASP1-GSDMD interface compromises pyroptosis
As the recognition and cleavage of GSDMD by inflammatory caspases leads to assembly of GSDMD pores and pyroptotic cell death in macrophages, we investigated the contribution of the observed exosite and active site interfaces to pyroptosis. Expression of the hCASP1 p22/p10 catalytic domain and wild type mGSDMD in HEK293T cells led to extracellular release of lactate dehydrogenase (LDH) in a time-dependent manner, a hallmark for pyroptosis (Figures 5A and S5A). By contrast, LDH release was either delayed or the magnitude of the release reduced upon mutation of either the exosite-binding residues or the cleavage site P4 residue L273. At different time points, the most severe phenotype was observed when the exosite-binding hydrophobic pocket was mutated (“5A” mutant). Similarly, expression of wild type mGSDMD in GSDMD deficient THP-1 cells led to time-dependent LDH release upon stimulation of the NLRP3 inflammasome using nigericin (Figures 5B and S5B). Such LDH release was delayed upon expression of mGSDMD harboring mutations at its interface with CASP1, particularly the hydrophobic pocket (“5A” mutant) that engaged the CASP1 exosite. By contrast, mutation at the peripheral of the exosite interface (E369K/L370A) marginally reduced LDH release at the early time points of nigericin stimulation. The LDH release data were corroborated by the decreased uptake of propidium iodide (PI) by THP-1 cells expressing mutant versus wild type mGSDMD post nigericin stimulation (Figure 5B lower panel). In agreement with the above mutations in mGSDMD, mutation of hCASP1 residues W294, I318 or K320 that engaged GSDMD led to diminished LDH release compared with the wild type hCASP1 (Figures 5C and S5C). Collectively, our data suggest that both the exosite and the active site interfaces contribute to pyroptosis following recognition and cleavage of GSDMD by CASP1.
Figure 5. Disruption of the hCASP1-mGSDMD interface compromises pyroptosis.
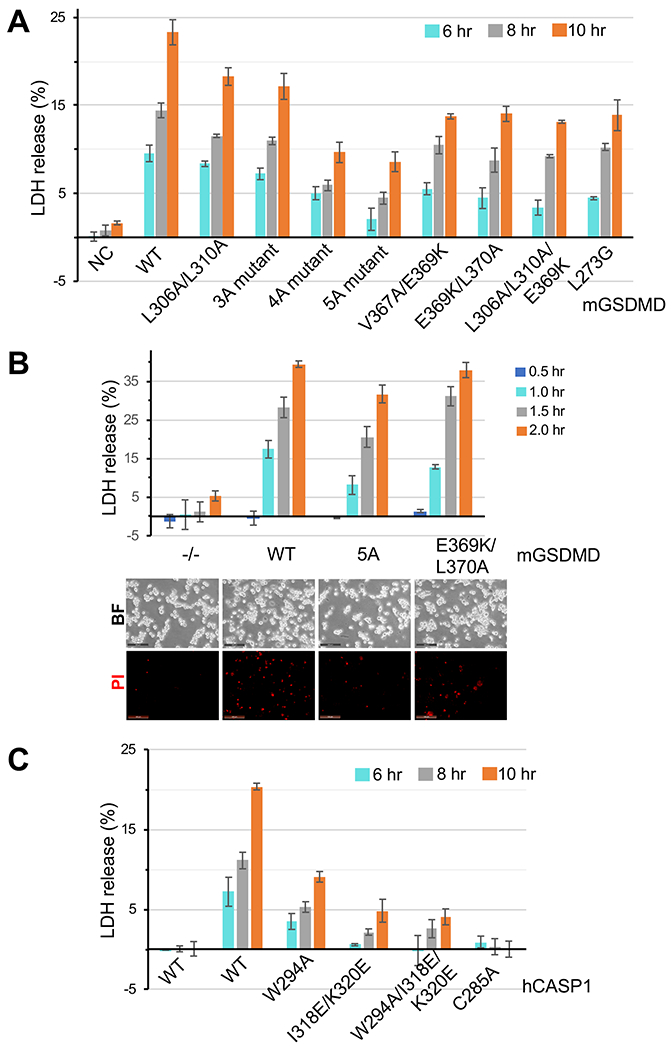
(A) LDH release upon expression of wild type or mutant mGSDMD along with wild type hCASP1 catalytic domain (p22/p10) in HEK293T cells at 6, 8 or 10 hours post-transfection is plotted as a percentage of total LDH content upon 1% Triton X-100 treatment of the cells. Data shown are mean ± SD of three independent experiments.
(B) Upper panel: LDH release upon expression of wild type or mutant mGSDMD in GSDMD deficient THP-1 cells upon stimulation with nigericin for 0.5, 1.0, 1.5 and 2.0 hr. Data shown are mean ± standard deviation (SD) of three independent experiments. Lower panel: Propidium iodide uptake by the same cells as the upper panel 1.0 hr post nigericin treatment. Data shown are representative of three independent experiments.
(C) LDH release upon expression of wild type or mutant hCASP1 catalytic domain (p22/p10) along with wild type mGSDMD in HEK293T cells at 6, 8 or 10 hours post-transfection is plotted as a percentage of total LDH content upon 1% Triton X-100 treatment of the cells. Data shown are mean ± SD of three independent experiments.
See also Figure S5.
DISCUSSION
Activation of GSDMD through its cleavage by inflammatory caspases underlies inflammatory responses downstream of both canonical and noncanonical inflammasome signaling pathways. The recognition and cleavage of GSDMD by inflammatory caspases is therefore a critical molecular event that initiates pyroptosis. The studies of substrate recognition by caspases have been mostly focused on the P1-P4 sites in their substrates, which have contributed to the identification of substrate sequence signatures for different groups of caspases (Julien and Wells, 2017; Poreba et al., 2013). By contrast, much less is known about the structural features of caspase substrates that are adjacent to the P1-P4 residues. Our current work presents the crystal structure of hCASP1 in complex with the full-length mGSDMD, which reveals that the GSDMD-NTD does not bind CASP1, whereas the cleavage site-containing linker in GSDMD adopts a long loop structure that engages the CASP1 active site. This active site interface resembles previously reported structures of CASP1 in complex with a pan-caspase inhibitor z-VAD-FMK (Scheer et al., 2006) or a GSDMD-derived peptide inhibitor (Yang et al., 2018). Here the P1 site residue D276 was buried deep in the CASP1 catalytic groove and adjacent to the catalytic dyad C285 and H237 (Clark, 2016), and the P4 site residue formed hydrophobic and van der Waals contacts with three CASP1 residues, in agreement with the preference for hydrophobic/aromatic residues at the substrate P4 site for the inflammatory caspases (Julien and Wells, 2017). Our structure further reveals how CASP1 engages other GSDMD linker residues, such as the P5 site residue S272 that forms a hydrogen bond with the CASP1 residue H342. The P1’ site residue G277 forms a mainchain to sidechain hydrogen bond with the CASP1 catalytic residue H237, as well as facilitates a sharp turn of the GSDMD linker out of the CASP1 catalytic groove. This explains the conservation of small residues at the P1’ site for most caspase substrates. Of note, the GSDMD residue D279 three residues from the P1 site D276 does not fit into the caspase catalytic groove, perhaps because the following residue E280 can not accommodate the sharp turn and does not fit the observed electron density. The lack of the C-terminal small residues may explain why some of the other Asp residues adjacent to the substrate P1 site Asp are not cleaved by caspases. The current work thus furnishes insights into how a caspase active site engages a protein substrate at and adjacent to the tetrapeptide sequence.
The identification of a CASP1 exosite in this study demonstrates a mode of engagement between a caspase and its substrate that is in agreement with the recent publication by Wang and colleagues reporting the structures of caspases-1,4, and 11 in complex with the GSDMD-CTDs (Wang et al., 2020). The fact that inflammatory caspases and GSDMDs can form stable protein complexes in the absence of the caspase cleavage sites, as observed by Wang and colleagues and by us, suggests that the exosites are the dominant structural determinants for the enzyme-substrate engagement. Exosites have been defined as structural elements in enzymes that participate in binding of partner proteins such as substrates or inhibitors, but are distinct from the enzyme active sites. Such exosites were observed in different enzymes such as alpha-thrombin (Bar-Shavit et al., 1983; Grütter et al., 1990; Rydel et al., 1990), matrix metalloproteinases (McQuibban et al., 2000; Overall, 2002; Steffensen et al., 1995), and bacterial nucleases (Kleanthous and Walker, 2001). Exosites were reported to offer enhanced substrate specificity and hydrolysis efficiency for a number of proteases (Fulcher and Van Doren, 2011; Jabaiah et al., 2012). The exosites in inflammatory caspases that bind a hydrophobic pocket formed by α5-α6 and α7’-α8 in GSDMD-CTDs, as observed by the Wang paper and by us, endow a previously unrecognized function for the CTD to recruit inflammatory caspases, in addition to its role as an autoinhibition domain. Such dual functionality of the CTD may allow stringent substrate selectivity in the resting state while facilitating cleavage and pyroptosis upon inflammasome activation. The residues forming the hydrophobic pocket are conserved between human and murine GSDMD, but not in GSDME, suggesting that the exosite interface may underlie the specific recognition of GSDMD but not GSDME by inflammatory caspases. Superposition of the hCASP1 structures in complex with the full-length mGSDMD reported here and with the hGSDMD-CTD (6KN0) reveals a shift of the hGSDMD-CTD relative to the mGSDMD-CTD, suggesting some plasticity in the enzyme-substrate interface. Nevertheless, the same exosite in hCASP1 engages the equivalent hydrophobic pockets at the GSDMD-CTDs. The absence of contacts between GSDMD-NTD and CASP1 is consistent with the observation that the GSDMD-NTD dissociates from its CTD to assemble membrane pores after linker cleavage (Aglietti et al., 2016; Chen et al., 2016; Ding et al., 2016; Liu et al., 2016; Sborgi et al., 2016). Dual-site engagement of caspase partner proteins has been reported previously. Studies of caspase-8 in complex with the baculovirus p35 protein, a broad spectrum caspase inhibitor, revealed that an interface distinct from the caspase active site mediates their association (Lu et al., 2006; Xu et al., 2001). This involves two anti-parallel β strands and the connecting loop from the p35 inhibitor binds caspase-8 at a surface adjacent to its active site. It is observed that the p35 loop also harbor a conserved W254 residue. In addition to caspase-8, caspase-7 was reported to employ a positively-charged motif outside its catalytic domain to bind and facilitate the cleavage of poly(ADP ribose) polymerase 1 (PARP) (Boucher et al., 2012). Clearly the recognition of caspase substrates or inhibitors may be influenced by their tertiary structures that either provide additional surface for caspase engagement as in exosites, or perhaps impact the accessibility of the signature motifs that caspases cleave.
Our mutagenesis studies of mGSDMD and hCASP1 suggest that both the exosite and the active site interfaces contribute to GSDMD binding, cleavage, and pyroptosis. Even though our data demonstrate that mutations of the exosite interface led to more severe phenotypes, disruption of the active site interface at the P4 position did result in diminished GSDMD cleavage and pyroptosis. In agreement, mutation of exosites from caspases-1,4, and 11, which resulted in no complex formation with the GSDMD-CTD, still led to reduced but not abrogated cell death upon inflammasome stimulation (Wang et al., 2020). These data are generally consistent with previous studies that identified substrate signature motifs for different caspases, particularly the hydrophobic/aromatic P4 residue for inflammatory caspases and acidic residues for apoptotic caspase-3 (Fuentes-Prior and Salvesen, 2004; Julien and Wells, 2017; Wang et al., 2017). The caspase-GSDMD-CTD complexes utilizing only the exosite interface may represent the initial binding of GSDMD by caspases through the exosites, referred to as exosite-mediated “priming interaction” (Wang et al., 2020). The initial enzyme-substrate contact may be followed by the dual interface engagement between caspases and the full-length GSDMD as illustrated in this work, which leads to close proximity of the caspase catalytic residues and the substrate Asp residue. This is conducive to the eventual cleavage of the GSDMD linker. Contribution of the dual interface may thus underlie the more stringent substrate selectivity for inflammatory caspases. Conversely, the exosite contribution may be less prominent for promiscuous apoptotic caspases such as caspase-3, which may engage substrates such as GSDME primarily through the essential cleavage site sequences. This was clearly demonstrated by the lack of caspase cleavage and pyroptosis activities when either the P1 site or the P4 site residue was mutated in GSDME (Wang et al., 2017). The modes of dual site engagement between inflammatory caspases and their substrates may not only offer more substrate selectivity that restricts the number of physiological substrates, but also enhance the overall efficiency of proteolysis through increased substrate binding affinities at two interfaces, as well as stabilization of the caspase dimers representative of the active enzyme conformation (Shi, 2002; Wang et al., 2020).
In summary, our study illustrates dual site engagement of GSDMD by CASP1. Crystal structure of hCASP1 in complex with the full-length mGSDMD reveals no binding between the GSDMD-NTD and CASP1, a loop structure of the GSDMD linker that binds the caspase active site, and a hydrophobic pocket at the GSDMD-CTD that engages the caspase exosite. This endows a previously unrecognized function for the GSDMD-CTD to recruit inflammatory caspases, which in turn contributes to specific substrate recognition by caspases and perhaps more efficient substrate cleavage. Our study provides a framework for understanding how caspases recognize full-length GSDMD, which suggests additional avenues of targeting gasdermins and pyroptosis. It remains to be determined if CASP1 binds other protein substrates such as proIL-1 b or proIL-18 using similar mode of dual interface engagement.
Limitations of study
While we have demonstrated the contribution of both the caspase-1 exosite and the active site interfaces to its engagement of GSDMD, quantitative analysis of the contribution from each interface would further establish that the exosite interface plays a more dominant role and may engage in the initial enzyme-substrate association. This may be followed by binding at the active site interface to facilitate proteolytic cleavage. Another limitation is that even though we have analyzed the exosite and active site mutants using HEK293T cells, further work is needed to analyze these mutants in relevant immune cells such as human and murine macrophages. Due to the COVID-19 pandemic, we were only able to reconstitute some of the exosite mutants into the GSDMD-deficient THP-1 cells, which demonstrated reduced LDH release consistent with data from HEK293T cells.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Tsan Sam Xiao, Ph.D. (tsx@case.edu).
Materials Availability
Plasmids generated in this study will be available from the authors under an MTA with the Case Western Reserve University.
Data and Code Availability
Coordinates and structural factors have been deposited with the Protein Data Bank with accession code 6VIE.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial strains
BL21 (DE3) Codon Plus RIPL cells were purchased from Agilent Technologies (Santa Clara, CA). NEB® 5-alpha Competent E. coli was purchased from New England Biolabs (Ipswich, MA). Both bacterial strains were cultured at 37 °C in Luria Broth Miller containing 10g/L sodium chloride, 10g/L tryptone and 5g/L yeast extract.
Mammalian cell lines
HEK-293T cell line derived from a female fetus kidney was purchased from American Type Culture Collection (Manassas, VA) (Cat# CRL-3216) and cultured in Dulbecco’s Modified Eagle medium (GE HyClone, Logan, UT) (Cat# SH30022) supplemented with 10% fetal bovine serum (Denville, Holliston, MA) (Cat# C788U22), non-essential amino acids (Cat# 11140050) and L-glutamine (Cat# 25030081) from Thermo Fisher Scientific (Waltham, MA). THP-1 cell line with GSDMD deficiency was reported previously (Rathkey et al., 2020) and cultured in RPMI medium from Corning (Corning, NY) (Cat# 15-040-CV) supplemented with 10% fetal bovine serum (Denville, Holliston, MA) (Cat# C788U22), non-essential amino acids (Cat# 11140050) and L-glutamine (Cat# 25030081) from Thermo Fisher Scientific (Waltham, MA). The original THP-1 cell line from ATCC (Cat# THB-202) was derived from acute monocytic leukemia cells in a male infant.
METHOD DETAILS
Protein Expression and Purification.
Recombinant murine (Q9D8T2) and human (P57764) GSDMD were expressed and purified using a bacterial expression vector encoding a His6-SUMO tag (Mossessova and Lima, 2000), as described previously (Liu et al., 2019). Site-directed mutagenesis was performed based on the QuikChange site-directed mutagenesis (Thermo Fisher Scientific, Waltham, MA) method and confirmed by sequencing. Protein expression constructs were transformed in BL21 (DE3) Codon Plus RIPL cells (Agilent Technologies, Santa Clara, CA), and the cells were grown at 37 °C until OD600 reached 0.6. Protein expression was induced at 18 °C overnight with 0.2 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). Cells were harvested and lysed by sonication in a lysis buffer containing 25 mM Tris-HCl (pH 8.0), 300 mM NaCl. The recombinant protein in the cleared cell lysate was purified using Ni-NTA (Thermo Fisher Scientific, Waltham, MA) affinity chromatography. Elution fractions containing the target protein were pooled, and the fusion protein was incubated overnight with the Ulp1 protease (Mossessova and Lima, 2000) at 4 °C, followed by a second Ni-NTA chromatography to remove the SUMO tag and uncleaved fusion protein. The GSDMD protein samples were further purified through size-exclusion chromatography using a Superdex 200 10/300 GL column (GE Healthcare Life Sciences, Pittsburgh, PA) in a buffer containing the lysis buffer plus 5 mM DTT at 4 °C. The purified protein was concentrated to ~20 mg/ml before frozen in aliquots at −80 °C.
The human caspase-1 (P29466) catalytic domain p20/p10 or p22/p10 was expressed and purified as described (Roschitzki-Voser et al., 2012; Yang et al., 2018). The p20 subunit contains residues 120-297, the p22 subunit contains residues 120-316, and the p10 subunit contains residues 317-404.
To obtain complexes of GSDMD and CASP1, 1:1.1 molar ratio of human or murine GSDMD and human caspase-1 catalytic domain C285A mutant were mixed and incubated on ice, followed by size-exclusion chromatography using a Superdex 200 10/300 GL column (GE Healthcare Life Sciences, Pittsburgh, PA) in a buffer containing 25 mM Tris-HCl, pH 8.0, 100 mM NaCl. Identical chromatograms for the CASP1-GSDMD complexes were obtained using either the p20/p10 or p22/p10 catalytic domains of hCASP1. The fractions containing CASP1-GSDMD complexes were pooled and concentrated to ~10 mg/ml and used for crystallization screening.
Crystallization, Data Collection and Structure Determination.
Crystallization screenings were performed using commercial screening kits from Hampton Research (Aliso Viejo, CA) and Molecular Dimensions (Maumee, OH) with sitting drop vapor diffusion method at 4°C. Among the GSDMD-CASP1 complexes that we tested, the hCASP1-mGSDMD complex yielded crystals that diffracted X-ray to 3.4 Å resolution. In this complex, mGSDMD harbors truncations at residues 182-187, 197-199 and 248-271 to facilitate crystallization; the hCASP1 catalytic domain contains p22/p10, whereas the p20/p10 form of hCASP1 did not yield crystals with GSDMD.
The hCASP1-mGSDMD complex was crystallized at 10 mg/ml using a reservoir solution containing 20% PEG3350, 0.2 M ammonium citrate tribasic, pH 7.0, and 5 mM DTT. The crystals were frozen in the reservoir solution plus 20%-25% glycerol using liquid nitrogen. X-ray diffraction data were collected at beamlines NE-CAT (24-ID) and GM/CAT (23-ID) at the Advanced Photon Source, Argonne National Laboratory, and processed with program XDS (Kabsch, 2010). The structure was determined using the murine GSDMD (PDB: 6N9N) and human CASP1 (PDB: 6BZ9) structures as search models. Manual model building and molecular replacement/refinement were performed with Coot (Emsley and Cowtan, 2004) and Phenix (Adams et al., 2010), respectively. The crystal structures were validated by the MolProbity server (Chen et al., 2010), which demonstrated MolProbity scores >2.0 at 100th percentile (best) among structures of similar resolutions. Figures were prepared using PyMol (The PyMOL Molecular Graphics System, Version 1.8.2.3 Schrödinger, LLC.).
Cell Culture and Cytotoxicity Assay
HEK-293T and THP-1 cells were purchased from ATCC (Monassas, VA). HEK-293T cells were grown in DMEM medium (GE HyClone, Logan, UT) supplemented with 10% fetal bovine serum (Denville, Holliston, MA), non-essential amino acids and L-glutamine (Thermo Fisher Scientific, Waltham, MA). THP-1 cells were grown in RPMI medium (Corning, Corning, NY) supplemented with 10% fetal bovine serum, non-essential amino acids and L-glutamine.
To express mGSDMD and hCASP1 catalytic domain in HEK293T cells, cells grown in DMEM plus 10% serum were co-transfected with pcDNA4/TO-mGSDMD WT or mutant plasmids, pCMV-3Tag-1-hCASP1 p22 and pcDNA3.1(+)-hCASP1 p10 plasmids at 3:1:1 molar ratio by using 5 μg of plasmids mixed with calcium phosphate for each 60 mm tissue culture dish. The pCDNA4/TO expression vector was from the T-REx expression system (Thermo Fisher Scientific, Waltham, MA). Culture supernatants were collected at different time points and were centrifuged to remove detached cells before assaying LDH release.
To express mGSDMD in THP-1 cells using lentiviral vectors, first HEK293T cells were transfected with plasmids for pLN-mGSDMD, pMD2 and pSPAX at a ratio of 5:3.5:1.5 (Rathkey et al., 2020). The culture supernatants were collected 72 hr after transfection and the viruses were concentrated through sucrose gradient ultracentrifugation. The titers of viruses were measured by flow cytometry after infecting HEK293T cells for 48 hr with different amounts of viral suspension in the presence of polybrene (MilliporeSigma, St Louis, MO). GSDMD deficient THP-1 cells (Rathkey et al., 2020) were infected with lentiviruses at an MOI of 1 for 36 hr, then treated overnight with 100 ng/ml phorbol 12-myristate 13-acetate (PMA) (MilliporeSigma, St. Louis, MO). The culture supernatants were collected between 0-2 hr after treatment with 20 μM nigericin (MilliporeSigma, St. Louis, MO) to assay for pyroptosis activities.
Lactate dehydrogenase (LDH) activities in the cell culture supernatants were measured using a cytotoxicity detection kit from Roche (Indianapolis, IN) or Thermo Fisher Scientific (Waltham, MA) following the manufacturers’ instructions. The LDH release was expressed as a percentage of total LDH content upon 1% Triton X-100 treatment of the cells. To examine cell morphology and propidium iodide (PI) uptake, bright field and fluorescent images of cells in 24-well plates were captured using a Leica DFC400 fluorescent microscope (Wetzlar, Germany) upon adding 1 μM PI to the culture media. For western blots, cells were lysed in 8 M urea plus 5% SDS, and cell extracts were used for SDS-PAGE.
Antibodies
Monoclonal anti-mouse GSDMD antibody (A-7, sc-393656) and monoclonal anti-β-actin antibody (sc-47778) were from Santa Cruz Biotechnology (Dallas, TX), anti-Flag antibody (F7425) was from MilliporeSigma (St Louis, MO), anti-GSDMD antibody (L60, #93709S) was from Cell Signaling Technology (Danvers, MA), and anti-human CASP1 p10 antibody (ab179515) was from Abcam (Cambridge, MA).
GST Pull-Down
Immobilized GST-tagged GSDMD was incubated with human caspase-1 (C285A) at a molar ratio of 1:3 in a buffer containing 25 mM Tris-HCl pH 8.0, 100 mM NaCl and 1 mM DTT for one hour at 4 °C. The GST resin was washed four times with the same buffer, and the bound protein was eluted using 20 mM reduced L-Glutathione (MilliporeSigma, St Louis, MO). The input and eluted samples from pull-down were analyzed with SDS-PAGE.
Caspase-1 Cleavage Assay
Purified GST-tagged wild type or mutant mGSDMD (3.5 pg) was incubated with 2 U human caspase-1 (Enzo Life Sciences, Ann Arbor, MI) in a 10 μl reaction mixture at 37 °C for one hour. The cleavage mixtures were analyzed with SDS-PAGE.
Quantification and Statistical Analysis
Statistical analyses were performed with program Excel (Microsoft Corporation, Redmond, WA). All data shown with error bars are mean values with standard deviation of three independent experiments.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-mouse GSDMDC1 (A-7) | Santa Cruz Biotech | Cat# sc-393656; RRID: AB_2728694 |
| Rabbit polyclonal anti-GSDMDC1 (L60) | Cell Signaling Technology (CST) | Cat# 93709S; RRID: AB_846135 |
| Mouse monoclonal anti-human GAPDH (0411) | Santa Cruz Biotech | Cat# sc-47724; RRID: AB_627678 |
| Rabbit polyclonal anti-FLAG | Sigma-Aldrich | Cat# F7425; RRID: AB_439687 |
| Rabbit Monoclonal anti-pro Caspase-1+p10+p12 | Abcam | Cat# ab179515 |
| Goat polyclonal anti-Rabbit IgG, HRP conjugated | Jackson ImmunoResearch Inc. | Cat# 111-035-144; RRID: AB_2307391 |
| Goat polyclonal anti-Mouse IgG, HRP conjugated | Jackson ImmunoResearch Inc. | Cat# 115-035-003; RRID: AB_10015289 |
| Mouse monoclonal anti-human β-actin | Santa Cruz Biotech | Cat# sc-47778; RRID:AB_626632 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Nigericin | MilliporeSigma | Cat# N7143 |
| Phorbol 12-myristate 13-acetate (PMA) | MilliporeSigma | Cat# P8139 |
| Propidium iodide | Thermo Fisher | Cat# P1304MP |
| Polybrene | MilliporeSigma | Cat# TR-1003 |
| Penicillin and streptomycin | Thermo Fisher Scientific | Cat# 15140122 |
| Non-Essential Amino Acids | Thermo Fisher Scientific | Cat# 11140050 |
| L-Glutamine | Thermo Fisher Scientific | Cat# 25030081 |
| Dulbecco’s Modified Eagle Medium (DMEM) | HyClone | Cat# SH30022 |
| RPMI 1640 | Corning | Cat# 15-040-CV |
| Fetal bovine serum (FBS) | Denville | Cat# C788U22 |
| NDSB-201 (Non-detergent sulfobetaine) | GOLDBIO | Cat# N-201 |
| hCaspase-1 (active) | Enzo | Cat# ALX-201-056-U025 |
| Mouse full-length wild type GSDMD | This paper | N/A |
| Human full-length wild type GSDMD | This paper | N/A |
| Mouse GSDMD harboring truncation of residues 243-276 | This paper | N/A |
| Human GSDMD harboring truncation of residues 243-278 | This paper | N/A |
| Mouse GSDMD harboring truncations of residues 182-187, 197-199 and 248-271 | This paper | N/A |
| GST-Mouse full-length wild type GSDMD | This paper | N/A |
| GST-Human full-length wild type GSDMD | This paper | N/A |
| GST-Mouse GSDMD harboring truncation of residues 243-276 | This paper | N/A |
| GST-Human GSDMD harboring truncation of residues 243-278 | This paper | N/A |
| GST-Mouse full-length GSDMD harboring L306A/L310A mutations (2A mutant) | This paper | N/A |
| GST-Mouse full-length GSDMD harboring L361A/V367A/L370A mutations (3A mutant) | This paper | N/A |
| GST-Mouse full-length GSDMD harboring L306A/L310A/V367A/L370A mutations (4A mutant) | This paper | N/A |
| GST-Mouse full-length GSDMD harboring L306A/L310A/L361A/V367A/L370A mutations (5A mutant) | This paper | N/A |
| GST-Mouse full-length GSDMD harboring V367A/E369K mutations | This paper | N/A |
| GST-Mouse full-length GSDMD harboring E369K/L370A mutations | This paper | N/A |
| GST-Mouse full-length GSDMD harboring L360A/L310A/E369K mutations | This paper | N/A |
| GST-Mouse full-length GSDMD harboring L273G mutation | This paper | N/A |
| hCaspase-1 (p22: residues 120-316, p10: residues 317-404) (C285A) | This paper | N/A |
| hCaspase-1 (p22: residues 120-316, p10: residues 317-404) (C285A) harboring W294A mutation | This paper | N/A |
| hCaspase-1 (p22: residues 120-316, p10: residues 317-404) (C285A) harboring I318E/K320E mutations | This paper | N/A |
| Critical Commercial Assays | ||
| Cytotoxicity Detection Kit (LDH) | Roche | Cat# 04 744 934 001 |
| LDH cytotoxicity assay kit | Thermo Fisher Scientific | Cat# 88953 |
| Quick Blunting Kit | New England Biolabs | Cat# E1201S |
| T4 DNA Ligase | New England Biolabs | Cat# M0202S |
| Phusion High-Fidelity DNA Polymerase | New England Biolabs | Cat# M0530L |
| Gibson Assembly Cloning Kit | New England Biolabs | Cat# E5510S |
| Deposited Data | ||
| Crystal structure of the mGSDMD-hCaspase-1 complex | RCSB PDB | PDB ID: 6VIE |
| Experimental Models: Cell Lines | ||
| HEK293T | ATCC | Cat# CRL-3216 |
| THP-1 cells with GSDMD knockout | Rathkey et al., 2020 | N/A |
| Experimental Models: Organisms/Strains | ||
| BL21 (DE3) Codon Plus RIPL | Agilent | Cat# 230280 |
| NEB® 5-alpha Competent E. coli | New England Biolabs | Cat# C2987I |
| Oligonucleotides: Please see Supplemental Table S2 for oligo/primer sequences. | ||
| Recombinant DNA | ||
| pSMT3-full length wild type hGSDMD | This paper | N/A |
| pSMT3-full length wild type mGSDMD | This paper | N/A |
| pSMT3-Mouse GSDMD harboring truncation of residues 243-276 | This paper | N/A |
| pSMT3-Human GSDMD harboring truncation of residues 243-278 | This paper | N/A |
| pSMT3-Mouse GSDMD harboring truncations of residues 182-187, 197-199 and 248-271 | This paper | N/A |
| pGEx-4T-1-Mouse full-length wild type GSDMD | This paper | N/A |
| pGEx-4T-1-Human full-length wild type GSDMD | This paper | N/A |
| pGEx-4T-1-Mouse GSDMD harboring truncation of residues 243-276 | This paper | N/A |
| pGEx-4T-1-Human GSDMD harboring truncation of residues 243-278 | This paper | N/A |
| pGEx-4T-1-Mouse full-length GSDMD harboring L306A/L310A mutations (2A mutant) | This paper | N/A |
| pGEx-4T-1-Mouse full-length GSDMD harboring L361A/V367A/L370A mutations (3A mutant) | This paper | N/A |
| pGEx-4T-1-Mouse full-length GSDMD harboring L306A/L310A/V367A/L370A mutations (4A mutant) | This paper | N/A |
| pGEx-4T-1-Mouse full-length GSDMD harboring L306A/L310A/L361A/V367A/L370A mutations (5A mutant) | This paper | N/A |
| pGEx-4T-1-Mouse full-length GSDMD harboring V367A/E369K mutations | This paper | N/A |
| pGEx-4T-1-Mouse full-length GSDMD harboring E369K/L370A mutations | This paper | N/A |
| pGEx-4T-1-Mouse full-length GSDMD harboring L360A/L310A/E369K mutations | This paper | N/A |
| pGEx-4T-1-Mouse full-length GSDMD harboring L273G mutation | This paper | N/A |
| pRSFDuet-hCaspase-1 (C285A) (ORF1: 120-316; ORF2: 317-404) | This paper | N/A |
| pRSFDuet-hCaspase-1 (C285A) harboring W294A mutation (ORF1: 120-316; ORF2: 317-404) | This paper | N/A |
| pRSFDuet-hCaspase-1 (C285A) harboring I318E/K320E mutations (ORF1: 120-316; ORF2: 317-404) | This paper | N/A |
| pCDNA4/TO-Mouse full-length GSDMD wild type | This paper | N/A |
| pCDNA4/TO-Mouse full-length GSDMD harboring L306A/L310A mutations (2A mutant) | This paper | N/A |
| pCDNA4/TO-Mouse full-length GSDMD harboring L361A/V367A/L370A mutations (3A mutant) | This paper | N/A |
| pCDNA4/TO-Mouse full-length GSDMD harboring L306A/L310A/V367A/L370A mutations (4A mutant) | This paper | N/A |
| pCDNA4/TO-Mouse full-length GSDMD harboring L306A/L310A/L361A/V367A/L370A mutations (5A mutant) | This paper | N/A |
| pCDNA4/TO-Mouse full-length GSDMD harboring V367A/E369K mutations | This paper | N/A |
| pCDNA4/TO-Mouse full-length GSDMD harboring E369K/L370A mutations | This paper | N/A |
| pCDNA4/TO-Mouse full-length GSDMD harboring L360A/L310A/E369K mutations | This paper | N/A |
| pCDNA4/TO-Mouse full-length GSDMD harboring L273G mutation | This paper | N/A |
| pCMV-3Tag-1-hCaspase-1 p22 (residues 120-316) | This paper | N/A |
| pCDNA3.1-hCaspase-1 p10 (residues 317-404) | This paper | N/A |
| pCMV-3Tag-1-hCaspase-1 (C285A) p22 (residues 120-316) | This paper | N/A |
| pCMV-3Tag-1-hCaspase-1 p22 (residues 120-316) W294A | This paper | N/A |
| pCDNA3.1-hCaspase-1 p10 (residues 317-404) I318E/K320E | This paper | N/A |
| psPAX2 | Addgene | Cat# 12260 |
| pMD2.G | Addgene | Cat# 12259 |
| LentiCRISPRv2 | Addgene | Cat# 52961 |
| LentiCRISPRv2-P2A-Mouse GSDMD full-length-WT-mNeonGreen | This paper | N/A |
| LentiCRISPRv2-P2A-Mouse GSDMD full-length-E369K/L370A-mNeonGreen | This paper | N/A |
| LentiCRISPRv2-P2A-Mouse GSDMD full-length-L306A/L310A/L361A/V367A/L370A-mNeonGreen | This paper | N/A |
| Software and Algorithms | ||
| Microsoft Excel | Microsoft Corporation | Version 16.16.8 |
| XDS | Kabsch, W. XDS. Acta Cryst. (2010). 66 (2): 125-132. | http://xds.mpimf-heidelberg.mpg.de/ |
| Phenix | Adams, P.V. et al., Acta Cryst. (2010). D66, 213-221 | https://www.phenix-online.org/ |
| Coot | Emsley P., Cowtan K. Acta Crystallogr. (2004). D60, 2126-2132. | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| PyMol | The PyMOL Molecular Graphics System, Schrödinger, LLC. | https://pymol.org/ |
| Other | ||
Acknowledgements
Z.L. is supported by a Careers in Immunology postdoctoral fellowship from the American Association of Immunologists. B.Z. is supported by NIH grant T32AI089474, D.W.A. is supported by NIH grants P01DK091222 and R01GM086550, and T.S.X. is supported by NIH grant R01GM127609. We would like to thank the Xiao, Abbott, Dubyak, Ramakrishnan, and Adoro laboratories for insightful discussions. X-ray diffraction data were collected at the Advanced Photon Source (APS) NE-CAT (24-ID) beamline funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165), and GM/CA-CAT (23-ID) beamline funded in whole or in part by the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006). This research used resources of the APS, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: T.S.X. is a scientific advisor for Quench Bio (Cambridge, MA), a biotechnology company developing therapeutics targeting the gasdermin proteins.
REFERENCES
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, and Dueber EC (2016). GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proceedings of the National Academy of Sciences of the United States of America 113, 7858–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, and Yuan J (1996). Human ICE/CED-3 protease nomenclature. Cell 87, 171. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit R, Kahn A, Wilner GD, and Fenton JW (1983). Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science 220, 728–731. [DOI] [PubMed] [Google Scholar]
- Boucher D, Blais V, and Denault J-B (2012). Caspase-7 uses an exosite to promote poly(ADP ribose) polymerase 1 proteolysis. Proceedings of the National Academy of Sciences of the United States of America 109, 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, and Dixit VM (2016). Inflammasomes: mechanism of assembly, regulation and signalling. Nature Reviews Immunology 16, 407–420. [DOI] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, and Richardson DC (2010). MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallographica Section D Biological Crystallography 66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, He W-T, Hu L, Li J, Fang Y, Wang X, Xu X, Wang Z, Huang K, and Han J (2016). Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Research 26, 1007–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AC (2016). Caspase Allostery and Conformational Selection. Chem. Rev 116, 6666–6706. [DOI] [PubMed] [Google Scholar]
- Cookson BT, and Brennan MA (2001). Pro-inflammatory programmed cell death. Trends in Microbiology 9, 113–114. [DOI] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang D-C, and Shao F (2016). Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116. [DOI] [PubMed] [Google Scholar]
- Emsley P, and Cowtan K (2004). Coot: model-building tools for molecular graphics. Acta Crystallographica Section D Biological Crystallography 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Fuentes-Prior P, and Salvesen GS (2004). The protein structures that shape caspase activity, specificity, activation and inhibition. Biochemical Journal 384, 201–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher YG, and Van Doren SR (2011). Remote Exosites of the Catalytic Domain of Matrix Metalloproteinase-12 Enhance Elastin Degradation. Biochemistry 50, 9488–9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grütter MG, Priestle JP, Rahuel J, Grossenbacher H, Bode W, Hofsteenge J, and Stone SR (1990). Crystal structure of the thrombin-hirudin complex: a novel mode of serine protease inhibition. EMBO J 9, 2361–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W-T, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang Z-H, Zhong C-Q, and Han J (2015). Gasdermin D is an executor of pyroptosis and required for interleukin-1 β secretion. Cell Research 25, 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaiah AM, Getz JA, Witkowski WA, Hardy JA, and Daugherty PS (2012). Identification of protease exosite-interacting peptides that enhance substrate cleavage kinetics. Biological Chemistry 393, 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez Fernández D, and Lamkanfi M (2015). Inflammatory caspases: key regulators of inflammation and cell death. Biological Chemistry 396, 193–203. [DOI] [PubMed] [Google Scholar]
- Julien O, and Wells JA (2017). Caspases and their substrates. Cell Death and Differentiation 24, 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W (2010). XDS. Acta Crystallographica Section D Biological Crystallography 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus PA, and Diederichs K (2012). Linking crystallographic model and data quality. Science 336, 1030–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. (2011). Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, and O’Rourke K (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671. [DOI] [PubMed] [Google Scholar]
- Kleanthous C, and Walker D (2001). Immunity proteins: enzyme inhibitors that avoid the active site. Trends in Biochemical Sciences 26, 624–631. [DOI] [PubMed] [Google Scholar]
- Latz E, Xiao TS, and Stutz A (2013). Activation and regulation of the inflammasomes. Nature Reviews Immunology 13, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, and Richards FM (1971). The interpretation of protein structures: estimation of static accessibility. Journal of Molecular Biology 55, 379–400. [DOI] [PubMed] [Google Scholar]
- Li J, and Yuan J (2008). Caspases in apoptosis and beyond. Oncogene 27, 6194–6206. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, and Lieberman J (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Rathkey JK, Yang J, Dubyak GR, Abbott DW, and Xiao TS (2018). Structures of the Gasdermin D C-Terminal Domains Reveal Mechanisms of Autoinhibition. Structure 26, 778–784.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Yang J, Zhou B, Yang R, Ramachandran R, Abbott DW, and Xiao TS (2019). Crystal Structures of the Full-Length Murine and Human Gasdermin D Reveal Mechanisms of Autoinhibition, Lipid Binding, and Oligomerization. Immunity 51, 43–49.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Min T, Eliezer D, and Wu H (2006). Native Chemical Ligation in Covalent Caspase Inhibition by p35. Chemistry & Biology 13, 117–122. [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Gong J-H, Tam EM, McCulloch CAG, Clark-Lewis I, and Overall CM (2000). Inflammation Dampened by Gelatinase A Cleavage of Monocyte Chemoattractant Protein-3. Science 289, 1202–1206. [DOI] [PubMed] [Google Scholar]
- Mossessova E, and Lima CD (2000). Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Molecular Cell 5, 865–876. [DOI] [PubMed] [Google Scholar]
- Overall CM (2002). Molecular Determinants of Metalloproteinase Substrate Specificity: Matrix Metalloproteinase Substrate Binding Domains, Modules, and Exosites. MB 22, 051–086. [DOI] [PubMed] [Google Scholar]
- Poreba M, Strózyk A, Salvesen GS, and Drag M (2013). Caspase substrates and inhibitors. Cold Spring Harbor Perspectives in Biology 5, a008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathkey JK, Xiao TS, and Abbott DW (2020). Human polymorphisms in GSDMD alter the inflammatory response. J. Biol. Chem 295, 3228–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, and Alnemri ES (2017). Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nature Communications 8, 14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschitzki-Voser H, Schroeder T, Lenherr ED, Frolich F, Schweizer A, Donepudi M, Ganesan R, Mittl PRE, Baici A, and Grutter MG (2012). Human caspases in vitro: expression, purification and kinetic characterization. Protein Expression and Purification 84, 236–246. [DOI] [PubMed] [Google Scholar]
- Rydel TJ, Ravichandran KG, Tulinsky A, Bode W, Huber R, Roitsch C, and Fenton JW (1990). The structure of a complex of recombinant hirudin and human alpha-thrombin. Science 249, 277–280. [DOI] [PubMed] [Google Scholar]
- Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, and Hiller S (2016). GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. The EMBO Journal 35, 1766–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer JM, Romanowski MJ, and Wells JA (2006). A common allosteric site and mechanism in caspases. Proceedings of the National Academy of Sciences of the United States of America 103, 7595–7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y (2002). Mechanisms of caspase activation and inhibition during apoptosis. Molecular Cell 9, 459–470. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, and Shao F (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. [DOI] [PubMed] [Google Scholar]
- Steffensen B, Wallon UM, and Overall CM (1995). Extracellular matrix binding properties of recombinant fibronectin type Il-like modules of human 72-kDa gelatinase/type IV collagenase. High affinity binding to native type I collagen but not native type IV collagen. J. Biol. Chem 270, 11555–11566. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Renatus M, Meldal M, and Salvesen GS (2000). Internally quenched fluorescent peptide substrates disclose the subsite preferences of human caspases 1, 3, 6, 7 and 8. Biochem J 350, 563–568. [PMC free article] [PubMed] [Google Scholar]
- Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, Li Z, Wang Y, Zhao Q, Shao F, et al. (2020). Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell 180, 941–955. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, and Shao F (2017). Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a Gasdermin. Nature 547, 99–103. [DOI] [PubMed] [Google Scholar]
- Xu G, Cirilli M, Huang Y, Rich RL, Myszka DG, and Wu H (2001). Covalent inhibition revealed by the crystal structure of the caspase-8/p35 complex. Nature 410, 494–497. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu Z, Wang C, Yang R, Rathkey JK, Pinkard OW, Shi W, Chen Y, Dubyak GR, Abbott DW, et al. (2018). Mechanism of gasdermin D recognition by inflammatory caspases and their inhibition by a gasdermin D-derived peptide inhibitor. Proceedings of the National Academy of Sciences of the United States of America 115, 6792–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Coordinates and structural factors have been deposited with the Protein Data Bank with accession code 6VIE.


