Significance
We report the identification of a cubane-modified aptamer (cubamer) against the malaria biomarker Plasmodium vivax lactate dehydrogenase (PvLDH). The cubamer contains the benzene isostere cubane, which is entirely alien to biology. The crystal structure of the cubamer–protein complex reveals a binding mechanism involving the formation of an unprecedented cubane pocket and an unusual C–H⋅⋅⋅O hydrogen bond. Importantly, the cubamer is capable of distinguishing the PvLDH from the Plasmodium falciparum LDH despite a very high sequence homology, which is impossible for unmodified aptamers. Finally, we have used the cubamer to detect PvLDH in a mimetic clinical situation. This approach blending medicinal chemistry and Darwinian evolution can easily be extended to other nonnatural, exotic functional groups.
Keywords: modified aptamer, SELEX, X-ray crystallography, cubane, malaria diagnosis
Abstract
Nucleic acid aptamers selected through systematic evolution of ligands by exponential enrichment (SELEX) fold into exquisite globular structures in complex with protein targets with diverse translational applications. Varying the chemistry of nucleotides allows evolution of nonnatural nucleic acids, but the extent to which exotic chemistries can be integrated into a SELEX selection to evolve nonnatural macromolecular binding interfaces is unclear. Here, we report the identification of a cubane-modified aptamer (cubamer) against the malaria biomarker Plasmodium vivax lactate dehydrogenase (PvLDH). The crystal structure of the complex reveals an unprecedented binding mechanism involving a multicubane cluster within a hydrophobic pocket. The binding interaction is further stabilized through hydrogen bonding via cubyl hydrogens, previously unobserved in macromolecular binding interfaces. This binding mechanism allows discriminatory recognition of P. vivax over Plasmodium falciparum lactate dehydrogenase, thereby distinguishing these highly conserved malaria biomarkers for diagnostic applications. Together, our data demonstrate that SELEX can be used to evolve exotic nucleic acids bearing chemical functional groups which enable remarkable binding mechanisms which have never been observed in biology. Extending to other exotic chemistries will open a myriad of possibilities for functional nucleic acids.
Directed evolution of nucleic acids through the iterative process of SELEX (1, 2) (selective evolution of ligands by exponential enrichment) provides routes to aptamers with exquisite structures that cannot be otherwise imagined or designed (3). Even using the simple natural nucleotides of DNA and RNA one can select and evolve aptamers with nanomolar and even picomolar binding affinities and surprising discriminatory specificity (4). Such aptamers are finding applications in therapeutics (5), diagnostics (6), biosensing (7), and nanotechnology (8) and can display numerous advantages relative to antibodies (9).
The chemistries of canonical DNA and RNA can be limiting when one compares them to antibodies (10). Postselection chemical modification can aid in vivo stability and residence time (11, 12) and can facilitate binding to problematic targets such as glycoproteins or proteins with low isoelectric points (13–15). However, these post-SELEX approaches often come at the expense of a loss of binding affinity and specificity. In contrast, preselection chemical modification—where modified nucleotides are directly included in the SELEX process—truly opens up directed evolution to extending the repertoire of aptamer-mediated molecular recognition (16–19). Many chemical modifications have been directed at the level of the sugar-phosphate backbone in order to improve the nuclease resistance of aptamers (18, 20–23). Other approaches include aptamers with artificially expanded genetic information systems going beyond the simple four building blocks of natural nucleic acids (24), even most recently to the eight-building-block Hachimoji system (25). Nucleobases have also been modified but have often been restricted to mimics of amino acid side chains (13, 26, 27) or small hydrophobic aromatic moieties (28, 29). Perhaps the most impressive demonstration of translational cross-proteome aptamer specificity are the SOMAmers (slow off-rate modified aptamers) (30), which enabled quantification of over 3,000 plasma proteins to create a genomic atlas of the human plasma proteome (31). Such SOMAmers include a simple modification where instead of dT they bear dU functionalized at the 5-position of the nucleobase with protein-like benzyl/2-napthyl/3-indolyl-carboxamide that confer some hydrophobic and/or base stacking character to the nucleic acid (10). New, engineered polymerases are not required to cope with unnatural chemistry introduced at position 5 of pyrimidines (32). Base modified nucleotides have also been proposed as elements in an RNA world to promote the catalytic activity of RNA-based catalysts (33). Alternatively, click chemistry with alkyne-modified nucleotides can be used to select for nucleobase-modified aptamers (34). Through such approaches, nucleobase-modified aptamers with nonnatural chemistries become accessible. One can then take advantage of the exponential enrichment and mutation enabled by the PCR to allow Darwinian molecular evolution, yet inclusion of more exotic unnatural functional groups allows evolutionary experiments well beyond the confined chemistries familiar of biology.
Here, we present an effort to stretch the idea of nonnatural chemistry within an evolutionary nucleic acid aptamer selection by applying the in vitro evolutionary technique of SELEX to macromolecular chemistries entirely alien to biology. We perform an aptamer selection incorporating unusual cubane-modified nucleotides, presenting structural data, mechanistic determination, and diagnostic application of cubane-modified nucleic acid aptamers, hereafter called cubamers. The extraordinary platonic solid cubanes have intrigued chemists for decades (35–37) and are now important functional groups in a wide range of pharmaceuticals and agrochemicals (36, 38). Cubanes are benzene bioisosteres with unique properties: 1) unlike benzene, cubane is biologically stable and inherently nontoxic; 2) enhanced water solubility compared to benzene due to the disruption of π stacking; and 3) formation of unusual C–H⋅⋅⋅O hydrogen bonds caused by the strong s-character in the C–H bonds itself induced by the p-character of the C–C bonds (39, 40). Synthetic and medicinal chemistry approaches for cubane function discovery can be challenging, so we considered the possibility of developing a Darwinian approach to the evolution of macromolecules functionalized by cubane bioisosteres.
A goal of this study was to evolve cubamers selective for binding to the malaria diagnostic biomarker Plasmodium vivax lactate dehydrogenase (PvLDH). We had previously selected canonical DNA aptamers binding to Plasmodium falciparum lactate dehydrogenase (PfLDH) (4) then applied these in various diagnostic approaches (41–44). P. falciparum is the parasite which causes the most severe disease with the highest mortality while P. vivax is more widely distributed and causes disease which is complex and recurring. As management and treatment of patients infected with the two different species differs, an ideal point-of-care diagnostic should be able to discriminate the two infections. However, canonical DNA aptamers with the ability to discriminate PfLDH vs. PvLDH had eluded us. We asked whether cubamers might provide a solution.
Results
Synthesis of Cubane-Modified Building Blocks for Selection and Synthesis of Cubane-Modified Aptamers.
We developed an approach to select for cubane-modified aptamers through initially synthesizing the cubane-modified deoxyuridine triphosphate dUCTP (SI Appendix, Scheme S2, compound 11). To incorporate the cubane on the nucleobase we started from the known cubane derivative 1 (SI Appendix, Scheme S1) to prepare the corresponding azide (compound 5) through previously published procedures (45). The 5-position alkyne-modified deoxyuridine derivative (compound 6) which had been prepared according to previously published procedures (46), was then reacted with the azide 5 through copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) to form nucleoside compound 10 which was then converted to the corresponding triphosphate (compound 11, Fig. 1B). The cubane-modified deoxyuridine phosphoramidite (compound 8, Fig. 1B) was synthesized through similar click chemistry approaches to yield compound 9.
Fig. 1.
Structure of PvLDH in complex with cubamer. (A) Crystal structure of tetrameric PvLDH binds to two cubamers. The four subunits of PvLDH are colored in yellow, green, cyan, and pink. The two 1501s cubamers are colored in gray and the cubane modification is colored in blue. (B) Protected cubane-modified deoxyuridine phosphoramidite (Left) and cubane-modified deoxyuridine triphosphate (Right). (C) Details of interactions between 1501s and PvLDH. Light blue indicates hydrogen bonding interactions between cubamer and protein. Blue indicates hydrophobic interactions between cubamer and protein. Orange indicates residues within interaction range of 3 Å. Connected nucleotides are within 3.8 Å. Below, the sequence of the 1501s cubamer is shown with X representing cubane-modified deoxyuridine. (D) Buried surface of PvLDH-1501s complex. We pinpointed by different colors the regions of interactions between the monomers A and B of PvLDH and a 1501 cubamer.
Investigating Compatibility of dUCTP with Primer Extension and PCR.
We next asked whether dUCTP 11 (Fig. 1B) would be compatible with enzymatic DNA synthesis under primer extension (PEX) reaction and PCR conditions. We used the 15-nucleotide-long, 5′ 6-FAM (fluorescein)-labeled primer P1 annealed to template T1 (see SI Appendix for sequence composition) then investigated the ability of various polymerases to perform a PEX reaction with a mixture of either four natural triphosphates or three natural triphosphates together with dUCTP replacing thymidine 5′-triphosphate (dTTP) (SI Appendix, Fig. S20). This analysis revealed that most polymerases accepted dUCTP as a substrate and the primer was extended by 16 nucleotides (accounting for the incorporation of four modified nucleotides). When a longer template was used (the 79-mer template T2) which would require extension of primer P2 by 60 nucleotides a clear higher-molecular-weight product is formed in the reaction with dUCTP when compared to that with natural triphosphates (SI Appendix, Figs. S21 and S22). Several polymerases including Therminator, Vent (exo−), Deep Vent, Taq, Bst, Klenow, Q5 DNA polymerase, and phi29 were all capable of forming the expected cubane-modified product. However, Vent (exo−) appeared to most consistently produce highest-yield product (SI Appendix, Fig. S22) so was carried forward to investigation of efficacy in PCR.
A PCR was performed using two simple 19/20 nucleotide DNA primers (P3 and P4) on the same 79 nucleotide template T2. Vent (exo−) was capable of forming the expected amplicon in high yields. Here also, the bands corresponding to cubane-modified DNA presented a lower electrophoretic mobility compared to the amplicon obtained with the natural dNTPs (SI Appendix, Fig. S23). Taq was not able to produce any product where Hemo KlenTaq, Q5 DNA polymerase, and Phusion did produce the expected product albeit with lower yields compared to Vent (exo−). We therefore concluded that Vent (exo−) was the most appropriate DNA polymerase to use with the dUCTP for subsequent SELEX procedures.
SELEX for Cubamers with High Affinity and Specificity for PvLDH.
PvLDH and PfLDH proteins were expressed and purified as we described previously (4, 43). For both proteins, we do not cleave the histidine tag during the purification procedure to facilitate immobilization onto nickel magnetic beads for aptamer selection. A single-stranded DNA library containing a 35-mer random region was used as previously described (4). This was amplified by PCR and transcribed to the cubane-modified pool using Vent (exo−). The cubane-modified pool was incubated with target PvLDH protein conjugated onto Ni-NTA beads and unbound species removed. Eluted cubamers were reamplified from a forward primer and reverse primer with a biotinylated 5′ end. Cubamers were purified through streptavidin magnetic beads followed by alkaline elution to result in the cubamer pool for next round of selection. Four classes of counterselection steps were incorporated: with Ni-NTA magnetic agarose beads after rounds 3 and 9, with PfLDH-immobilized Ni-NTA magnetic agarose beads after rounds 4 and 10, with hLDHB-immobilized Ni-NTA magnetic agarose beads after rounds 6 and 12, and with hLDHA1-immobilized Ni-NTA magnetic agarose beads after rounds 5 and 11. One can observe enrichment of PCR products up to round 15 with the expected depletion of PCR product after round 8 due to counterselection (SI Appendix, Fig. S26A). Amplification of selected pools by either dTTP or by dUCTP showed the enrichment and expected molecular weight size difference caused by the cubane modification (SI Appendix, Fig. S26B). An enzyme-linked oligonucleotide assay was performed as we previously described (43) to measure binding of the pool to target PvLDH (SI Appendix, Fig. S26C). One can see clear binding of the dUCTP-modified pool to PvLDH particularly after round 10 which is not present for the natural nucleotide pool or for other controls. These data indicate that SELEX was successful for evolving a pool of modified oligonucleotides binding to PvLDH, and that the binding is dependent on the cubane modification.
Next-generation sequencing was performed after round 15 (SI Appendix, Table S2). Two sequences dominated the pool: 1501s with five dUC (23.1% of pool sequences) and 1506s with six dUC (22.4% of pool sequences), then to a lesser extent 1516s (6.0%) and 1526s (4.9%) (SI Appendix, Table S4). These cubane-modified sequences were then synthesized by automated solid-phase DNA synthesis using the cubane-modified phosphoramidite 8 (SI Appendix, Fig. S13) and molecular weights were confirmed by mass spectrometry (SI Appendix, Table S1 and Figs. S14–S19). Interestingly, all attempts at synthesizing these oligonucleotides by click reaction with azide 5 and EdU-modified sequences failed due to the presence of a distribution of products regardless of the conditions.
We decided to focus our subsequent studies on the most abundant cubamer in our selection, 1501s. We determined the affinity for cubamers binding to PvLDH through surface plasmon resonance (SPR) analysis. SPR was performed through immobilization of PvLDH onto Ni-NTA with the cubamer in the mobile phase. The equilibrium dissociation constant (KD) was determined to be 670 ± 9 nM by SPR (Fig. 2A). The cubamer was also observed to be specific for PvLDH relative to PfLDH; PfLDH binding was over 30 times weaker at 26 µM (Fig. 2B).
Fig. 2.
Determination of binding affinity and specificity of cubamer toward PvLDH. (A) SPR (Biacore) analysis of the interaction between 1501s and PvLDH. PvLDH was immobilized to a Ni-NTA chip, then the response was observed at multiple concentrations of 1501s as shown in the figure. Raw data are shown background with the best fit overlain with a thick solid line. For 1501s binding to PvLDH, the association rate (kon), dissociation rate (koff), and the equilibrium dissociation constant (KD) were 2.2 × 104 M−1⋅s−1, 1.4 × 10−2 s−1, and 670 nM, respectively. (B) Specificity of 1501s binding to PvLDH relative to PfLDH. For 1501s binding to PfLDH, kon, koff, and KD were 6.8 × 103 M−1⋅s−1, 0.18 s−1, and 26 µM, respectively.
Crystal Structure of Cubamers in Complex with PvLDH.
The crystal structure of PvLDH in complex with the 1501s cubamer was solved at 2.5 Å resolution (Protein Data Bank [PDB] ID code 6TXR; Fig. 1 and SI Appendix, Table S3). As expected, the overall structure of PvLDH is similar to that previously reported (PDB ID code 3ZH2), indicating that binding of cubamers does not affect the three-dimensional structure of this protein. Difference Fourier electron density maps indicate the presence of two cubamers which bind to the target protein at each end of the tetrameric PvLDH (Fig. 1A), in some ways reminiscent of the canonical DNA aptamers we had previously selected binding to PfLDH (4). The 5′ end of the cubamer showed two base-paired loops in close interaction with the surface of PvLDH, while the 3′ end of the cubamer extended away from the protein surface and formed a duplex with an aptamer bound to a second protein molecule in the crystal packing (SI Appendix, Fig. S34). We mapped all of the interactions between PvLDH and cubamer in Fig. 1C. All of the dUC units were involved in some hydrogen bonding within the cubamer. Two of these dUC (T3-A21 and T17-A8) base-pairing interactions are conventional Watson–Crick base pairs to adenosine (SI Appendix, Fig. S27). Others are single hydrogen bond interactions either between themselves (T5 and T20) or with other noncanonical bases (T13–C11). Four of the cubanes were observed to cluster together through interactions both among themselves but then also through interactions with hydrophobic amino acid side chains in the PvLDH. A fifth cubane was somewhat separate from the other four and involved in interactions on another surface. The buried surface between the cubamer and PvLDH (Fig. 1D) was 1130 Å2 as calculated using the PISA server (https://www.ebi.ac.uk/pdbe/pisa/).
Mechanism of Discrimination of Binding Recognition by Cubamer for PvLDH vs. PfLDH.
The crystal structure of the complex revealed the binding site of the cubamer 1501s, which is in close proximity to an alpha helix where there are some amino acid differences between PvLDH and PfLDH (Fig. 3A). This alpha helix is remarkable with regard to its nonconserved nature, as overall PvLDH and PfLDH are highly conserved with 90% amino acid identity (47). Electrophoretic mobility shift assays demonstrate that our previous selected aptamers 2008s (selected as a canonical DNA aptamer binding to PfLDH) bound more strongly to PfLDH than PvLDH (Fig. 3B) (4). A previously selected pan-specific DNA aptamer, 1202s, bound to both PfLDH and PvLDH with similar affinity (Fig. 3B). The 1501s cubamer showed preference for PvLDH, with some weak binding to PfLDH (Fig. 3B), consistent with the SPR data (Fig. 2B). When the dUC nucleotides in 1501s were mutated to simple canonical dT units then binding was abolished, indicating the necessity of the cubane modification for the specific binding (Fig. 3B).
Fig. 3.
The cubamer discriminates PvLDH from PfLDH through binding to a variable region. (A) Superimposition of PvLDH and PfLDH shows they are highly conserved with some difference on the variable region. The variable region is colored in red, whereas the conserved region is in light green. (Inset) The cubanes on the modified nucleotides interact with the variable region. (B) Comparing the affinity of 1501s to PvLDH and PfLDH with the control of the PfLDH-specific aptamer, 2008s, and pan-LDH-specific aptamer, 1202s. EMSA analysis showed 1501s is highly specific to PvLDH. (C) EMSA analysis showed cubanes on modified nucleotides involved in the binding between 1501s and PvLDH. With the replaced cubane-modified triphosphate by dUCTP, dU1501s and dTTP, dT1501s, the affinity of the cubamers is significantly decreased.
It was noted that leucine 232 and alanine 233 of PvLDH played important roles in the binding, directly interacting through hydrophobicity with two of the cubane moieties (Fig. 1C) and being a critical part of the network of the cubanes in the binding pocket (Fig. 4A). We expressed PvLDH with leucine 232 mutated to glycine, and also with alanine 233 mutated to glycine, then both together. The L232G mutation decreased binding, the A233G mutation decreased binding slightly, and the PvLDH with the double mutation resulted in significant reduction in binding as shown by electrophoretic mobility shift assay (EMSA) (Fig. 4B) and by SPR (Fig. 4C). These experiments provided evidence that cubane interactions were dependent on hydrophobicity of amino acid side chains.
Fig. 4.
The cubamer interacts with PvLDH by hydrophobic interactions. (A) The structure of the PvLDH–1501s complex shows the cubane-modified nucleotides, T1, T5, T20, T13, and T17 formed a hydrophobic cluster. The hydrophobic surface of PvLDH is illustrated with a color code according to the polarity of the respective amino acids. Hydrophobic amino acids are colored red whereas hydrophilic residues are white. (B) EMSA reveals the binding of PvLDH and 1501s along with amino acid side chains. The affinities of 1501s to three mutants of PvLDH, PvLDHL232G, PvLDHA233G, PvLDHL232G/A233G, and the wide-type PvLDH were estimated. (C) SPR comparison of 1501s cubamer binding to PvLDH and the three mutants PvLDHL232G, PvLDHA233G, and PvLDHL232G/A233G. Results from B and C indicate the affinities among all of the mutants are significantly decreased by mutating the hydrophobic amino acids to glycine.
A Unique Hydrogen Bond within the Cubane–Protein Complex Structure.
Unlike phenyl or other simple arene moieties, cubane can also engage in the formation of stabilizing C−Hcubane⋅⋅⋅O bonds due to the rather high acidity of the cubyl H atoms (40). Such nonclassical hydrogen bonds also seem to occur between a dUC unit of the aptamer and a carbonyl unit of amino acids of PvLDH. Indeed, one C–H(2) atom lies in close proximity (2.4 to 2.6 Å) of the oxygen atom of the carbonyl group of Leu232 and forms a near-linear (hydrogen bond angle θ = 147.3 to 158.7°) angle (Fig. 5). Both values fall within the ranges that have been reported for the formation of syn‐anti catemers of small cubane derivatives (d of 2.2 to 3.0 Å and θ between 120 and 180°) (39, 40, 48, 49) and that of weak, nonclassical hydrogen bonds (cutoff d value of 2.8 Å and θ range between 150° and 180°) (50).
Fig. 5.
A unique hydrogen bond of the cubamer with PvLDH. Close up views of the interaction between a dUC unit (T5) of the two cubamers 1501s with PvLDH (Leu232) in monomer A and monomer B showing the respective angles and bond lengths of the unique C-H(2)⋅⋅⋅O hydrogen bond. The gray mesh corresponds to a Sigma-A weighted mFo-DFc difference map (contoured at 3 σ), in which Leu232 and the cubane moiety of T5 were omitted from the model before map calculation.
Using the Cubamer in Diagnostic Assays to Mimic a Clinical Situation.
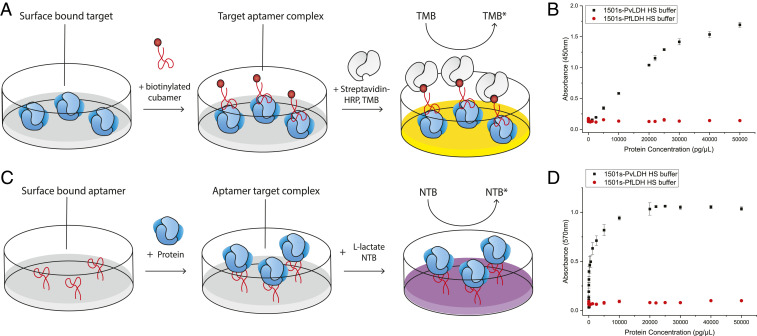
We have previously developed both enzyme-linked oligonucleotide (ELONA) assays (43) and an aptamer-tethered enzyme capture assay (41, 42, 44) to use our aptamers which bind to LDH as diagnostic approaches for malaria. In the ELONA assay proteins are adhered to a surface, then a biotinylated aptamer/cubamer is bound to the target proteins, then streptavidin/horseradish peroxidase (HRP) is added to reveal the binding and at the same time the presence or absence of the target protein (Fig. 6A). When using this assay it could be observed that the test was highly specific for PvLDH over PfLDH (Fig. 6B). Furthermore, when dUCTP was changed to dTTP during enzymatic synthesis of the aptamer, the assay was no longer effective, again proving the requirement for the cubane modifications here in the translational application (SI Appendix, Fig. S29A). The limit of detection for the ELONA assay in human serum buffer was determined as 1.05 ± 0.20 ng/µL (SI Appendix, Fig. S29A). The ELONA assay is not clinically relevant in the present format as the attachment is of His-tagged proteins to nickel-coated plates but is relevant for our comparison to previous data (43) and could be implemented through direct adherence of the native protein as per direct enzyme-linked immunosorbent assay (ELISA) methodologies. Furthermore, the mean levels of PvLDH in P. vivax isolates has been reported as 3.9 ± 6.1 ng/µL (51); this ELONA assay would occasionally be outside the clinical range as PvLDH in some low parasitemia conditions (51).
Fig. 6.
Determination of affinity and specificity of the 1501s cubamer against pLDHs in human serum (HS) buffer by ELONA and APTEC assay. (A) Diagram illustrating the ELONA. Target pLDHs are immobilized onto a microtiter plate, then biotinylated cubamers are added to recognize pLDHs. Streptavidin-HRP is added to recognize the biotin end of the cubamer, and TMB solution is used to allow for color development. (B) The response of the ELONA against the clinical range of PvLDH and PfLDH in human serum (HS) buffer. The limit of detection (LOD) of 1501s–PvLDH in the ELONA assay was determined to be 1.05 ± 0.20 ng·µL−1 (SI Appendix, Fig. S29). (C) Schematic diagram illustrating the APTEC assay. Biotinylated cubamers are immobilized onto streptavidin-coated wells, then pLDHs are added and captured by the cubamer. l-lactate/NTB solution is then added for color development. (D) The response of the APTEC assay against the clinical range of PvLDH and PfLDH in HS buffer. The LOD of 1501s-PvLDH in the APTEC assay was determined to be 4.33 ± 1.66 pg·µL−1 (SI Appendix, Fig. S29). Measurements were done in triplicate, reported in standard errors (SE).
Therefore, we switched to the aptamer-tethered enzyme capture (APTEC) assay. In the APTEC assay, the cubamer/aptamer is biotinylated and adhered to a streptavidin surface, then the clinical sample containing the target is applied, then washed, then color-developed through the intrinsic catalytic activity of the lactate dehydrogenase (41) (Fig. 6C). Observations were made for the APTEC assay similar to those made for the ELONA assay, confirming that the assay was specific for PvLDH (Fig. 6D) and required the cubane moieties (SI Appendix, Fig. S29B). The limit of detection of the APTEC assay in human serum buffer was determined as 4.33 ± 1.66 pg/µL (SI Appendix, Fig. S29B), which is much below the reported values of the clinical range for PvLDH (51). While further experiments in clinical samples would be important to ascertain diagnostic sensitivity, particularly at low parasitemia, we have evidence in these mimetic samples that the APTEC cubamer assay can potentially be used for clinical applications.
Discussion
We have used in vitro selection to evolve aptamers that are functionalized with the benzene isostere cubane. The so-called cubamers were selected against PvLDH and selectively recognized this protein target from PfLDH despite high sequence homology and bound with an appreciable affinity (KD of 670 nM). The binding affinity of the cubamer is ∼10-fold lower than a previously reported, unmodified aptamer (4), but this increase in KD is largely compensated by the capacity of discriminating PvLDH from PfLDH. The high sequence homology between both LDHs might have reduced the number of epitopes available for binding and thus limited the binding capacity. Importantly, we resolved the crystal structure of the binary aptamer–protein complex, which revealed the formation of a cubane cluster that interacts with hydrophobic residues of PvLDH. These findings mirror the functional interaction interface observed in crystal structures of SOMAmers in complex with their targets where hydrophobic interactions between the small aromatic moieties of the aptamer and of amino acid side chains were largely responsible for the binding mechanism (3, 29). As for SOMAmers and sequence-defined highly functionalized nucleic acid polymers (17, 52, 53), the specific location and sequence context of cubane moieties is crucial for binding to the protein target since it enables the formation of critical hydrophobic interactions accompanied by a C−Hcubane⋅⋅⋅O hydrogen bond.
In addition, cubamer 1501s was applied for the detection of the malaria biomarker PvLDH with an excellent limit of detection in the APTEC assay, underscoring the usefulness of cubane-modified aptamers in practical applications. Therefore, by merging medicinal chemistry approaches with traditional SELEX, we could isolate a cubamer that outperforms functional nucleic acids selected with canonical nucleotides that are unable to distinguish between both LDHs.
Molecular evolution approaches provide routes to molecules which can have translational application but which could otherwise never be imagined or designed. SELEX has provided an experimental approach toward evolving nucleic acid ligands by the blind watchmaker of evolution entirely without the need for a living organism through the replicative process of the PCR. There have been many extensions to SELEX in recent years through subtle differences of chemistry, but here we have demonstrated how entirely unnatural artificial functional group chemistries never observed in life can be subject to evolution under SELEX selection pressures.
This work demonstrates that the inclusion of nucleotides bearing the benzene isostere cubane on their nucleobases in a SELEX experiment has profound implications on the resulting functional nucleic acids. Besides opening the possibility of forming new binding modes with proteins via the formation of cubane-based pockets combined with C−Hcubane⋅⋅⋅O hydrogen bonding interactions, the modifications massively improve the specificity of the cubamers for binding a PvLDH target, enabling detection of this malaria biomarker in a mimetic clinical situation.
One can imagine steps beyond this work for completely new chemistries which could significantly extend such selection experiments where the selection pressure is not just binding but could be selection pressure for catalysis or other function. This is particularly the case when one considers expanded genetic codes of six-letter DNA alphabets (54–56), or even of eight-letter DNA (25, 57). In addition, the increase in diversity through unusual chemistries coupled to the replicative evolutionary power enabled by the PCR would provide a route to entirely new horizons for nucleic acid chemistry and give rise to hitherto unknown binding mechanisms. The introduction of exotic functional groups during SELEX may confer additional functional properties not accessible to canonical nucleic acids. This may, for example, be important for portable biosensors to detect biomarkers for pathogens in low-resource settings. Evolution of macromolecules incorporating chemistries entirely unknown in biology will have important repercussions across a plethora of applications.
Materials and Methods
Chemical Syntheses.
Synthetic procedures of the cubane-modified building blocks are given in SI Appendix.
PvLDH and PfLDH Expression and Purification.
Protein expression and purification was carried out at the platform for production and purification of recombinant proteins of Institut Pasteur. The PvLDH gene sequence was inserted into the pET28a (Novagen) vector and then recombinant PvLDH was transformed into Escherichia coli strain BL21 (DE3) pLysS. Bacteria were incubated at 37 °C in Luria–Bertani broth until optical density at 600 nm (OD600) reached 0.5. After cooling down to room temperature, protein expression was induced by adding 0.25 mM IPTG (isopropyl β-d-1-thiogalactopyranoside). Cells were grown at 25 °C for 4 h with agitation (220 rpm). Cells were then harvested by centrifuging the culture at 4,000 rpm for 45 min. The supernatant was discarded and the cell pellet was resuspended by using 1× phosphate-buffered saline (PBS). The cell suspension was transferred to 50-mL tubes and centrifuged at 4,000 rpm for 30 min. After discarding the supernatant, the cell pellet was resuspended in lysis buffer (100 mM Tris⋅HCl, 10 mM imidazole, and 0.3 M NaCl, pH 8) in a ratio of 1:50 lysis buffer to the original culture volume. After sonication, cell debris was removed by centrifugation at 19,000 rpm for 1 h at 4 °C. Supernatants were loaded onto a 5-mL Protino Ni-NTA column (Macherey Nagel) on an AKTA pure system (GE Healthcare) and eluted with 50 mM Tris⋅HCl, 0.3 M NaCl, and 500 mM imidazole, pH 8. The eluted fractions were then purified on a Hiload 16/60 (120 mL) Superdex 75 column using 50 mM Tris⋅HCl and 0.3 M NaCl, pH 8. After dialysis, the purified protein was stored in 25 mM Tris⋅HCl, 0.1 M NaCl, and 40% glycerol, pH 8; 120 mg of purified protein were obtained at a concentration of 20 mg/mL. PfLDH was expressed and purified in a similar manner.
Cloning, Expression, and Purification of PvLDH Mutants.
The mutants of PvLDH were amplified by using overlap extension PCR. For PvLDH-M1 that consists of a mutation at L232 to become G232, two fragments that contained the mutations corresponding to PvLDH-M1 were first amplified by PvLDH-S, ATTATTGCTAGCATGACGCCGAAACCCAAAATTGTGCTC, and PvLDH-L232G-AS, TGGGGCAACATAAGGAGAGGCACCGAGGTTCACAATCTCCAA, to become M1-a, and PvLDH-L232G-S, TTGGAGATTGTGAACCTCGGTGCCTCTCCTTATGTTGCCCCA, and PvLDH-AS, ATTATTCTCGAGTTAAATGAGCGCCTTCATCCTTTTAGTCTC, to become M1-b. The open reading frame (ORF) corresponded to PvLDH-M1 was further amplified by PvLDH-S, ATTATTGCTAGCATGACGCCGAAACCCAAAATTGTGCTC, and PvLDH-AS, ATTATTCTCGAGTTAAATGAGCGCCTTCATCCTTTTAGTCTC, using M1-a and M1-b as templates. The PvLDH-M1 ORF then was ligated into the NheI/XhoI-digested pET28a-PvLDH plasmid that was constructed in our previous study to become pET28a-PvLDH-M1. For PvLDH-M2 that consists of a mutation at A233 to become G233, two fragments that contained the mutations corresponding to PvLDH-M1 were first amplified by PvLDH-S, ATTATTGCTAGCATGACGCCGAAACCCAAAATTGTGCTC, and PvLDH-L232G-AS, TGGGGCAACATAAGGAGAACCACCGAGGTTCACAATCTCCAA, to become M2-a, and PvLDH-A233G-S, TTGGAGATTGTGAACCTCCTTGGTTCTCCTTATGTTGCCCCA, and PvLDH-AS, ATTATTCTCGAGTTAAATGAGCGCCTTCATCCTTTTAGTCTC, to become M2-b. The ORF of PvLDH-M2 was further amplified by PvLDH-S, ATTATTGCTAGCATGACGCCGAAACCCAAAATTGTGCTC, and PvLDH-AS, ATTATTCTCGAGTTAAATGAGCGCCTTCATCCTTTTAGTCTC, using M2-a and M2-b as templates. The PvLDH-M2 ORF then was ligated into the NheI/XhoI-digested pET28a-PvLDH plasmid that was constructed in our previous study to become pET28a-PvLDH-M2. For PvLDH-M3 that consists of mutations at L233 and A233 that became G233 and G233, respectively, two fragments that contained the mutations corresponding to PvLDH-M3 were first amplified by PvLDH-S, ATTATTGCTAGCATGACGCCGAAACCCAAAATTGTGCTC, and PvLDH-L232A233G-AS, TGGGGCAACATAAGGAGAACCACCGAGGTTCACAATCTCCAA, to become M3-a, and PvLDH-L232A233G-S, TTGGAGATTGTGAACCTCGGTGGTTCTCCTTATGTTGCCCCA, and PvLDH-AS, ATTATTCTCGAGTTAAATGAGCGCCTTCATCCTTTTAGTCTC, to become M3-b. The ORF of PvLDH-M3 was further amplified by PvLDH-S, ATTATTGCTAGCATGACGCCGAAACCCAAAATTGTGCTC, and PvLDH-AS, ATTATTCTCGAGTTAAATGAGCGCCTTCATCCTTTTAGTCTC, using M3-a and M3-b as templates. The PvLDH-M3 ORF then was ligated into the NheI/XhoI-digested pET28a-PvLDH plasmid that was constructed in our previous study to become pET28a-PvLDH-M3. The plasmids of the mutants were transformed into E. coli BL21 (DE3) pLysS for IPTG-induced expression. PvLDH mutants were expressed and purified according to our previous study (4). Bacterial culture was incubated at 37 °C until OD600 = 0.5, then 0.25 mM IPTG was added followed by 4-h expression at 25 °C. Pellets were collected and sonicated and expressed proteins were purified by using HisTrap chromatography (GE Healthcare).
Cubamer Selection.
A single-stranded DNA (ssDNA) library containing a 35-mer random region flanked by two 18-mer priming regions (5′–CGTACGGTCGACGCTAGC-[N35]-CACGTGGAGCTCGGATCC–3′) was used as starting material for in vitro selection. The ssDNA library was amplified by forward primer (5′–CGTACGGTCGACGCTAGC–3′) and reverse primer with biotinylated 5′-end (5′–biotin–GGATCCGAGCTCCACGTG–3′) using Vent (exo-) DNA Polymerase (NEB), dUcTP, dATP, dGTP, and dCTP. PCR conditions were as follows: denaturation at 95 °C for 15 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 15 s for 10 cycles. The amplified library was purified by streptavidin magnetic beads followed by a wash of 100 mM to elute the nonbiotinylated complementary strand as the cubane-modified ssDNA library. Two namomoles of cubane-modified ssDNA library were incubated with 1 nmol of PvLDH that was conjugated with Ni-NTA magnetic beads. The unbound species were removed, and the ssDNA–protein–magnetic bead complexes were suspended in 10 μL of water for PCR amplification of PfLDH-bound species by using forward primer (5′–CGTACGGTCGACGCTAGC–3′) and reverse primer with biotinylated 5′-end (5′–biotin–GGATCCGAGCTCCACGTG–3′) using Vent (exo-) DNA polymerase. PCR conditions were as follows: denaturation at 95 °C for 15 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 15 s for 10 cycles. Enriched DNA aptamer pool was purified by streptavidin magnetic beads followed by a wash of 100 mM NaOH to remove the nonbiotinylated complementary strand. The resultant ssDNA pool was used for the next round of selection. Counterselections by using Ni-NTA magnetic agarose beads (after rounds 3 and 9), PfLDH-immobilized Ni-NTA magnetic agarose beads (after rounds 4 and 10), hLDHB-immobilized Ni-NTA magnetic agarose beads (after rounds 6 and 12), and hLDHA1-immobilized Ni-NTA magnetic agarose beads (after rounds 5 and 11) were incorporated in between the SELEX cycles.
Cubamer Biophysical Characterization by SPR.
SPR measurements were collected using a Biacore X100 instrument (GE Healthcare). PvLDH was captured on the surface of an NTA sensor chip (GE Healthcare). A running buffer containing 25 mM Tris⋅HCl, pH 7.5, 100 mM NaCl, 20 mM imidazole, 0.005% (vol/vol) Tween 20, and 0.5 mg/mL bovine serum albumin was used for ligand capturing. The surface of the NTA chip was primed with running buffer and the PvLDH cubamer, 1501s, was injected in at a flow rate of 30 μL⋅min−1 and at 25 °C. All data were referenced for surface without captured cubamer and blank injections of buffer. Sensorgrams were analyzed with Biacore X100 Plus Package evaluation software (GE Healthcare).
Crystallization and Data Collection and Structure Refinement.
Crystallization screening trials of the protein–aptamer complex (14 mg/mL) were carried out by the sitting drop vapor-diffusion method with a Mosquito automated nanoliter dispensing system (TTP Labtech) at 291 K. Sitting drops of 400 nL were set up in Greiner plates for 672 commercially available screening solutions using a 1:1 mixture of protein sample and reservoir well solution (150 µL). The plates were stored in a RockImager (Formulatrix) automated imaging system to monitor crystal growth. The crystals appeared after 3 mo in crystallization condition 52 of Index HT kit (Hampton Research) containing 45% (vol/vol) 2-methyl-2,4-pentanediol, 0.2 M ammonium acetate, and 0.1 M Hepes, pH 7.5. The fished crystals were flash-cooled in liquid nitrogen directly, as the condition of crystallization served also as cryoprotectant. Diffraction data were collected at beamline PROXIMA-1 (SOLEIL synchrotron, St. Auban, France) and processed with XDS (58) and Aimless (59).
The structure of the PvLDH–cubamer complex was solved by the molecular replacement technique using a PvLDH tetramer (PDB ID code 3ZH2) as a search model with Phaser (60). A complete model of the complex was obtained through interactive cycles of manual model building with Coot (61) and reciprocal space refinement with Buster (62) and phenix.refine (63). The complex’s DNA moiety was gradually traced into difference electron density maps during this process. X-ray data collection and model refinement statistics are summarized in SI Appendix, Table S23. Figures showing the crystallographic model were generated and rendered with Pymol (Schrodinger, LLC) and/or Chimera (64). The atomic coordinates and structure factors for the PvLDH–cubamer complex have been deposited in the Protein Data Bank under ID code 6TXR.
EMSA.
EMSAs were carried out according to our previous study (4, 43). Protein was twofold serially diluted in binding buffer (25 mM Tris·HCl at pH 7.5 containing 0.1 M NaCl and 20 mM imidazole). The nucleotides, including 1501s, 2008s, 1202s, or corresponding cubamer variants, were mixed with the serially diluted proteins to a final concentration of 25 mM, followed by incubating at room temperature. The reactions were loaded on 12% native polyacrylamide gels and visualized by SYBR gold nucleic acid gel staining (Invitrogen).
Cubamer-Integrated ELONA.
A biotinylated complementary strand (3′–CTCCCGTGCCGTTTT–5′) was annealed to 1501s cubamer by incubating equal molar volumes in buffer (10 mM Tris, pH 7.5, 50 mM NaCl, and 1 mM EDTA), heated at 95 °C for 5 min, then allowed to cool at room temperature for 60 min before use. Annealing effectiveness was assessed by 12% TAE gel. Protein samples were prepared by diluting in PBS with 0.05% Tween 20 to concentrations of 10, 5, 2.5, 1.25, 0.63, 0.31, 0.16, and 0 µg/mL, which is 10,000, 5,000, 2,500, 1,250, 630, 310, 160, and 0 pg/µL, respectively. One hundred microliters of each protein were incubated in Ni-NTA HisSorb Plate (QIAGEN) wells for 1 h at room temperature. Wells were then washed three times with 200 µL PBST (0.05% Tween 20), and 100 µL of 50 nM biotinylated cubamer was added and incubated for 1 h at room temperature. PBST (0.05% Tween 20) was used as the cubamer binding buffer, while a 1:1 ratio of human AB plasma (Sigma-Aldrich) to PBS (0.05% Triton X-100) was used for the human serum binding buffer. Subsequently, wells were washed three times with PBST, then 100 µL of 1:10,000 streptavidin-HRP (Abcam) diluted in PBST were added and incubated for 30 min at room temperature. Following three washes with 200 µL of PBST, 100 µL of Pierce 1-Step Ultra TMB ELISA Substrate (Thermo Fisher Scientific) was added and incubated for 15 min at room temperature. The reaction was stopped by adding 2 M H2SO4 and absorbance was read at 450 nm.
Cubamer-Integrated APTEC Assay.
One hundred microliters of biotinylated cubamer was added to prewashed (PBST 0.1% Tween 20) Pierce streptavidin-coated plates (Thermo Fisher Scientific) for 2 h. Following three washes with PBST, 100 µL of sample was added to each cubamer-decorated well for 1 h, then washed five times with PBST. For color development, 100 µL of preprepared l-lactate/NTB solution: 12 mL l-lactate buffer (0.2 M sodium l-lactate, 100 mM Tris HCl, and 0.2% Triton X-100, pH 9.1), 158 µL NAD+ solution (50 mg/mL in water), 48 µL NBT solution (25 mg/mL in water), and 25 µL PES solution (5 mg/mL in water) was added and left to incubate for 45 min with mild shaking in the dark. Acetic acid (5%) was added to stop the reaction and the absorbance was read at 570 nm. For recombinant pLDH samples, 2× PBS was used as the binding buffer. For spiked human serum samples, 50 µL of human AB plasma (Sigma-Aldrich) was mixed with 50 µL PBS (0.5% Triton X-100) prior to adding protein.
Data Availability.
All data used in the study are included in the paper and in SI Appendix. The crystal structure of the cubamer–target complex has been deposited in the PDB (ID code 6TXR) (65).
Supplementary Material
Acknowledgments
We thank the Shirley Boyde Trust for their generous support through a Shirley Boyde Foundation Grant to J.A.T. and acknowledge Institut Pasteur funding to M.H., A.H., P.R., and F.L.-A.. J.A.T. acknowledges The University of Hong Kong Outstanding Young Researcher Award 2015-16, The University of Hong Kong Outstanding Research Student Supervisor Award 2016-2017, and The University of Hong Kong Seed Fund for Basic Research grants 201711159185 and 201611159269. We thank the staff of the platform for production and purification of recombinant proteins of Institut Pasteur for technical assistance during the expression and purification of the proteins steps and the staff of the crystallography platform at Institut Pasteur for carrying out robot-driven crystallization screening. We acknowledge synchrotron SOLEIL (Saint-Aubin, France) for granting access to their facility and the staff of Proxima1 for helpful assistance during the data collection.
Footnotes
Competing interest statement: J.A.T. is a scientific advisor for Zio Health, and J.A.T. and A.B.K. are founders of Jushan Bio.
This article is a PNAS Direct Submission.
Data deposition: The crystal structure of the cubamer–target complex has been deposited in the Protein Data Bank, http://www.wwpdb.org/ (PDB ID code 6TXR).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003267117/-/DCSupplemental.
References
- 1.Ellington A. D., Szostak J. W., In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990). [DOI] [PubMed] [Google Scholar]
- 2.Tuerk C., Gold L., Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Gelinas A. D., Davies D. R., Janjic N., Embracing proteins: Structural themes in aptamer-protein complexes. Curr. Opin. Struct. Biol. 36, 122–132 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Cheung Y. W. et al., Structural basis for discriminatory recognition of Plasmodium lactate dehydrogenase by a DNA aptamer. Proc. Natl. Acad. Sci. U.S.A. 110, 15967–15972 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur H., Bruno J. G., Kumar A., Sharma T. K., Aptamers in the therapeutics and diagnostics pipelines. Theranostics 8, 4016–4032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang S. et al., Measuring luteinising hormone pulsatility with a robotic aptamer-enabled electrochemical reader. Nat. Commun. 10, 852 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen V. T., Kwon Y. S., Gu M. B., Aptamer-based environmental biosensors for small molecule contaminants. Curr. Opin. Biotechnol. 45, 15–23 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Sakai Y. et al., DNA aptamers for the functionalisation of DNA origami nanostructures. Genes (Basel) 9, 571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold L., SELEX: How it happened and where it will go. J. Mol. Evol. 81, 140–143 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohloff J. C. et al., Nucleic acid ligands with protein-like side chains: Modified aptamers and their use as diagnostic and therapeutic agents. Mol. Ther. Nucleic Acids 3, e201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn M. R., Jimenez R. M., Chaput J. C., Analysis of aptamer discovery and technology. Nat. Rev. Chem. 1, 76 (2017). [Google Scholar]
- 12.Röthlisberger P., Hollenstein M., Aptamer chemistry. Adv. Drug Deliv. Rev. 134, 3–21 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Gawande B. N. et al., Selection of DNA aptamers with two modified bases. Proc. Natl. Acad. Sci. U.S.A. 114, 2898–2903 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimoto M., Yamashige R., Matsunaga K., Yokoyama S., Hirao I., Generation of high-affinity DNA aptamers using an expanded genetic alphabet. Nat. Biotechnol. 31, 453–457 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Pinheiro V. B. et al., Synthetic genetic polymers capable of heredity and evolution. Science 336, 341–344 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor A. I., Houlihan G., Holliger P., Beyond DNA and RNA: The expanding toolbox of synthetic genetics. Cold Spring Harb. Perspect. Biol. 11, a032490 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtor P. A., Chen Z., Elowe N. H., Chen J. C., Liu D. R., Side chain determinants of biopolymer function during selection and replication. Nat. Chem. Biol. 15, 419–426 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arangundy-Franklin S. et al., A synthetic genetic polymer with an uncharged backbone chemistry based on alkyl phosphonate nucleic acids. Nat. Chem. 11, 533–542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H., Zhang S., Chaput J. C., Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor. Nat. Chem. 4, 183–187 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Liu Z., Chen T., Romesberg F. E., Evolved polymerases facilitate selection of fully 2′-OMe-modified aptamers. Chem. Sci. (Camb.) 8, 8179–8182 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eremeeva E. et al., Highly stable hexitol based XNA aptamers targeting the vascular endothelial growth factor. Nucleic Acids Res. 47, 4927–4939 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei H. et al., Synthesis and evolution of a threose nucleic acid aptamer bearing 7-Deaza-7-substituted guanosine residues. J. Am. Chem. Soc. 140, 5706–5713 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Rose K. M. et al., Selection of 2′-deoxy-2′-fluoroarabino nucleic acid (FANA) aptamers that bind HIV-1 integrase with picomolar affinity. ACS Chem. Biol. 14, 2166–2175 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biondi E., Benner S. A., Artificially expanded genetic information systems for new aptamer technologies. Biomedicines 6, 53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshika S. et al., Hachimoji DNA and RNA: A genetic system with eight building blocks. Science 363, 884–887 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renders M., Miller E., Lam C. H., Perrin D. M., Whole cell-SELEX of aptamers with a tyrosine-like side chain against live bacteria. Org. Biomol. Chem. 15, 1980–1989 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Battersby T. R. et al., Quantitative analysis of receptors for adenosine nucleotides obtained via in vitro selection from a library incorporating a cationic nucleotide analog. J. Am. Chem. Soc. 121, 9781–9789 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Gupta S. et al., Chemically modified DNA aptamers bind interleukin-6 with high affinity and inhibit signaling by blocking its interaction with interleukin-6 receptor. J. Biol. Chem. 289, 8706–8719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren X. M., Gelinas A. D., von Carlowitz I., Janjic N., Pyle A. M., Structural basis for IL-1 alpha recognition by a modified DNA aptamer that specifically inhibits IL-1 alpha signaling. Nat. Commun. 8, 810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gold L., Walker J. J., Wilcox S. K., Williams S., Advances in human proteomics at high scale with the SOMAscan proteomics platform. N. Biotechnol. 29, 543–549 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Sun B. B. et al., Genomic atlas of the human plasma proteome. Nature 558, 73–79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaught J. D. et al., Expanding the chemistry of DNA for in vitro selection. J. Am. Chem. Soc. 132, 4141–4151 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Wolk S. K. et al., Modified nucleotides may have enhanced early RNA catalysis. Proc. Natl. Acad. Sci. U.S.A. 117, 8236–8242 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeiffer F. et al., Identification and characterization of nucleobase-modified aptamers by click-SELEX. Nat. Protoc. 13, 1153–1180 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Biegasiewicz K. F., Griffiths J. R., Savage G. P., Tsanaktsidis J., Priefer R., Cubane: 50 years later. Chem. Rev. 115, 6719–6745 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Reekie T. A., Williams C. M., Rendina L. M., Kassiou M., Cubanes in medicinal chemistry. J. Med. Chem. 62, 1078–1095 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Auberson Y. P. et al., Improving nonspecific binding and solubility: Bicycloalkyl groups and cubanes as para-phenyl bioisosteres. ChemMedChem 12, 590–598 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Houston S. D. et al., The cubane paradigm in bioactive molecule discovery: Further scope, limitations and the cyclooctatetraene complement. Org. Biomol. Chem. 17, 6790–6798 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Kuduva S. S., Craig D. C., Nangia A., Desiraju G. R., Cubanecarboxylic acids. Crystal engineering considerations and the role of C−H···O hydrogen bonds in determining O−H···O networks. J. Am. Chem. Soc. 121, 1936–1944 (1999). [Google Scholar]
- 40.Flanagan K. J., Bernhard S. S. R., Plunkett S., Senge M. O., Not your usual bioisostere: Solid state study of 3D interactions in cubanes. Chemistry 25, 6941–6954 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Dirkzwager R. M., Kinghorn A. B., Richards J. S., Tanner J. A., APTEC: Aptamer-tethered enzyme capture as a novel rapid diagnostic test for malaria. Chem. Commun. (Camb.) 51, 4697–4700 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Dirkzwager R. M., Liang S., Tanner J. A., Development of aptamer-based point-of-care diagnostic device for malaria using 3D printing rapid prototyping. ACS Sens. 1, 420–426 (2016). [Google Scholar]
- 43.Cheung Y. W. et al., Aptamer-mediated Plasmodium-specific diagnosis of malaria. Biochimie 145, 131–136 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Fraser L. A. et al., A portable microfluidic Aptamer-Tethered Enzyme Capture (APTEC) biosensor for malaria diagnosis. Biosens. Bioelectron. 100, 591–596 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Chalmers B. A. et al., Validating Eaton’s hypothesis: Cubane as a benzene bioisostere. Angew. Chem. Int. Ed. Engl. 55, 3580–3585 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Röthlisberger P., Levi-Acobas F., Hollenstein M., New synthetic route to ethynyl-dUTP: A means to avoid formation of acetyl and chloro vinyl base-modified triphosphates that could poison SELEX experiments. Bioorg. Med. Chem. Lett. 27, 897–900 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Turgut-Balik D. et al., Cloning, sequence and expression of the lactate dehydrogenase gene from the human malaria parasite, Plasmodium vivax. Biotechnol. Lett. 26, 1051–1055 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Kuduva S. S., Bläser D., Boese R., Desiraju G. R., Crystal engineering of primary cubanecarboxamides: Repetitive formation of an unexpected N-H...O hydrogen-bonded network. J. Org. Chem. 66, 1621–1626 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Das D., Jetti R. K. R., Boese R., Desiraju G. R., Stereoelectronic effects of substituent groups in the solid state. Crystal chemistry of some cubanecarboxylic and phenylpropiolic acids. Cryst. Growth Des. 3, 675–681 (2003). [Google Scholar]
- 50.Desiraju G. R., The C-H···O hydrogen bond: Structural implications and supramolecular design. Acc. Chem. Res. 29, 441–449 (1996). [DOI] [PubMed] [Google Scholar]
- 51.Jang J. W., Cho C. H., Han E. T., An S. S., Lim C. S., pLDH level of clinically isolated Plasmodium vivax and detection limit of pLDH based malaria rapid diagnostic test. Malar. J. 12, 181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Z., Lichtor P. A., Berliner A. P., Chen J. C., Liu D. R., Evolution of sequence-defined highly functionalized nucleic acid polymers. Nat. Chem. 10, 420–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong D., Yeung W., Hili R., In vitro selection of diversely functionalized aptamers. J. Am. Chem. Soc. 139, 13977–13980 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Kimoto M., Matsunaga K., Hirao I., DNA aptamer generation by genetic alphabet expansion SELEX (ExSELEX) using an unnatural base pair system. Methods Mol. Biol. 1380, 47–60 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Malyshev D. A. et al., A semi-synthetic organism with an expanded genetic alphabet. Nature 509, 385–388 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L. et al., Evolution of functional six-nucleotide DNA. J. Am. Chem. Soc. 137, 6734–6737 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dien V. T., Holcomb M., Romesberg F. E., Eight-letter DNA. Biochemistry 58, 2581–2583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabsch W., Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 66, 133–144 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans P. R., Murshudov G. N., How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCoy A. J. et al., Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Bricogne G., et al. , Buster (Version 2.11.1, Global Phasing Ltd, Cambridge, UK, 2011).
- 63.Afonine P. V. et al., Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pettersen E. F. et al., UCSF Chimera–A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Cheung Y., et al. , Structural insights into cubane-modified aptamer recognition of a malaria biomarker. Protein Data Bank. https://www.rcsb.org/structure/unreleased/6TXR. Deposited 15 January 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the study are included in the paper and in SI Appendix. The crystal structure of the cubamer–target complex has been deposited in the PDB (ID code 6TXR) (65).