Abstract
The cholinergic anti-inflammatory pathway (CAP) links the nervous and immune systems and modulates innate and adaptive immunity. Activation of the CAP by vagus nerve stimulation exerts protective effects in a wide variety of clinical disorders including rheumatoid arthritis and Crohn’s disease, and in murine models of acute kidney injury including ischemia/reperfusion injury (IRI). The canonical CAP pathway involves activation of splenic alpha7-nicotinic acetylcholine receptor (α7nAChR)-positive macrophages by splenic β2-adrenergic receptor-positive CD4+ T cells. Here we demonstrate that ultrasound or vagus nerve stimulation also activated α7nAChR-positive peritoneal macrophages, and that adoptive transfer of these activated peritoneal macrophages reduced IRI in recipient mice. The protective effect required α7nAChR, and did not occur in splenectomized mice or in mice lacking T and B cells, suggesting a bidirectional interaction between α7nAChR-positive peritoneal macrophages and other immune cells including β2-adrenergic receptor-positive CD4+ T cells. We also found that expression of hairy and enhancer of split-1 (Hes1), a basic helix-loop-helix DNA-binding protein, is induced in peritoneal macrophages by ultrasound or vagus nerve stimulation. Adoptive transfer of Hes1-overexpressing peritoneal macrophages reduced kidney IRI. Our data suggest that Hes1 is downstream of α7nAChR and is important to fully activate the CAP. Taken together, these results suggest that peritoneal macrophages play a previously unrecognized role in mediating the protective effect of CAP activation in kidney injury, and that Hes1 is a new candidate pharmacological target to activate the CAP.
Keywords: acute kidney injury, alpha7 nicotinic acetylcholine receptor, neuroimmune, ultrasound, vagus nerve
Neural pathways regulate immunity and inflammation.1 One of the well-studied neural pathways is the cholinergic anti-inflammatory pathway (CAP), the efferent arm of the inflammatory reflex mediated through the vagus nerve.2,3 Clinical studies showed effectiveness of implanted vagus nerve stimulators in rheumatoid arthritis4 and Crohn’s disease.5 Activation of the CAP by vagus nerve, a main part of the inflammatory reflex pathway, suppresses systemic and local inflammation, leading to an improvement of a wide variety of inflammation-related disorders,6–8 including acute kidney injury (AKI).9–12 Although AKI is a common and major concern especially in hospitalized patients because of its high morbidity and mortality, therapeutic or protective modalities for AKI are very limited.13,14 Previously we showed that ultrasound (US),9,10 optogenetic brainstem C1 neuron photostimulation,12 and vagus nerve stimulation (VNS)11 attenuate kidney ischemia-reperfusion injury (IRI) by activating the CAP (a summary of our previous findings is shown in Supplementary Figure S1). Implanted vagus nerve stimulators and noninvasive pulsed US treatment are promising therapeutic modalities; however, the mechanisms underlying the therapeutic efficacy of VNS or US need to be elucidated.
In vitro studies demonstrated that alpha7 nicotinic acetylcholine receptor (α7nAChR)-positive macrophages were identified as a key cellular component in the CAP.3 Using cells isolated from α7nAChR+/+ mice and α7nAChR−/− mice, Wang et al. revealed that α7nAChR on peritoneal macrophages regulated inflammation in vitro.3 Experiments using global α7nAChR−/− mice9–12 confirmed the requirement for α7nAChR in vivo in cholinergic-mediated anti-inflammatory effects.3,15,16 However, the role of α7nAChR expressed by peritoneal macrophages in the anti-inflammatory response in vivo is unclear. Considering the vagus afferent innervation of the peritoneum17 and vagus efferent innervation of myenteric plexus in the intestinal wall,18 peritoneal macrophages might be directly activated by VNS or US.
In the current studies, we explored the importance of α7nAChR on peritoneal macrophages in vivo, β2AR on CD4 T cells, and the interaction between these cells in the CAP in vivo. In addition, we found that hairy and enhancer of split-1 (Hes1), which is induced by either US or VNS, is downstream of α7nAChR and is necessary to fully activate the CAP. We believe that the current concept of CAP needs to be modified and consider the important in vivo contributory role of peritoneal macrophages in the CAP. These results should provide new therapeutic options for various diseases including AKI.
RESULTS
α7 nicotinic receptors on peritoneal macrophages are critical for renal protection induced by VNS or US
The requirement for α7nAChR on macrophages in regulating inflammation was demonstrated by in vitro experiments using peritoneal macrophages from α7nAChR−/− mice.3 The importance of α7nAChR in the CAP was also shown using global α7nAChR−/− mice in various disease models15,16 including our AKI studies.9–12 However, the role of α7nAChR expressed by peritoneal macrophages in the anti-inflammatory response in vivo is still unclear. Considering the vagus afferent innervation of the peritoneum17 and vagus efferent innervation of myenteric plexus in the intestinal wall,18 peritoneal macrophages might be directly activated by VNS or US through a pathway not yet identified. To test whether α7nAChR-positive peritoneal macrophages activated by VNS or US are important in an in vivo model, we performed adoptive transfer of α7nAChR+/+ or α7nAChR−/− peritoneal macrophages. Surprisingly, transfer of peritoneal macrophages from VNS-treated wild-type (WT) donor mice protected the kidneys of recipient mice from IRI to nearly the same extent as VNS treatment alone in the recipient (Figure 1a–d). Recipient mice that received peritoneal macrophages from US-treated WT mice were also protected against renal IRI (Figure 1e). Acetylcholine (ACh) is released from nerve terminals of vagus nerve upon stimulation, but the source of ACh for peritoneal macrophage activation is not yet known. We hypothesized that cholinergic activation of peritoneal macrophages, similar to activation of splenic macrophages by ACh released from T cells in the spleen (see below), would mimic VNS and US and have a protective effect in vivo. Peritoneal macrophages were treated with nicotine, a well-established agonist for nicotinic acetylcholine receptors. When recipient mice received nicotine-treated peritoneal macrophages from α7nAChR+/+ mice, the kidney was protected from IRI. On the other hand, when the recipient mice received nicotine-treated peritoneal macrophages from α7nAChR−/− mice, the protective effect was abolished (Figure 1f). These data clearly show the protective effect of α7nAChR-expressing peritoneal macrophages in the CAP in vivo, though pathways responsible for the activation of peritoneal macrophages by US or VNS or for their downstream protective effects are not yet known.
Figure 1 |. α7 nicotinic receptors on peritoneal macrophages are important for renal protection induced by vagus nerve stimulation (VNS) or ultrasound (US).
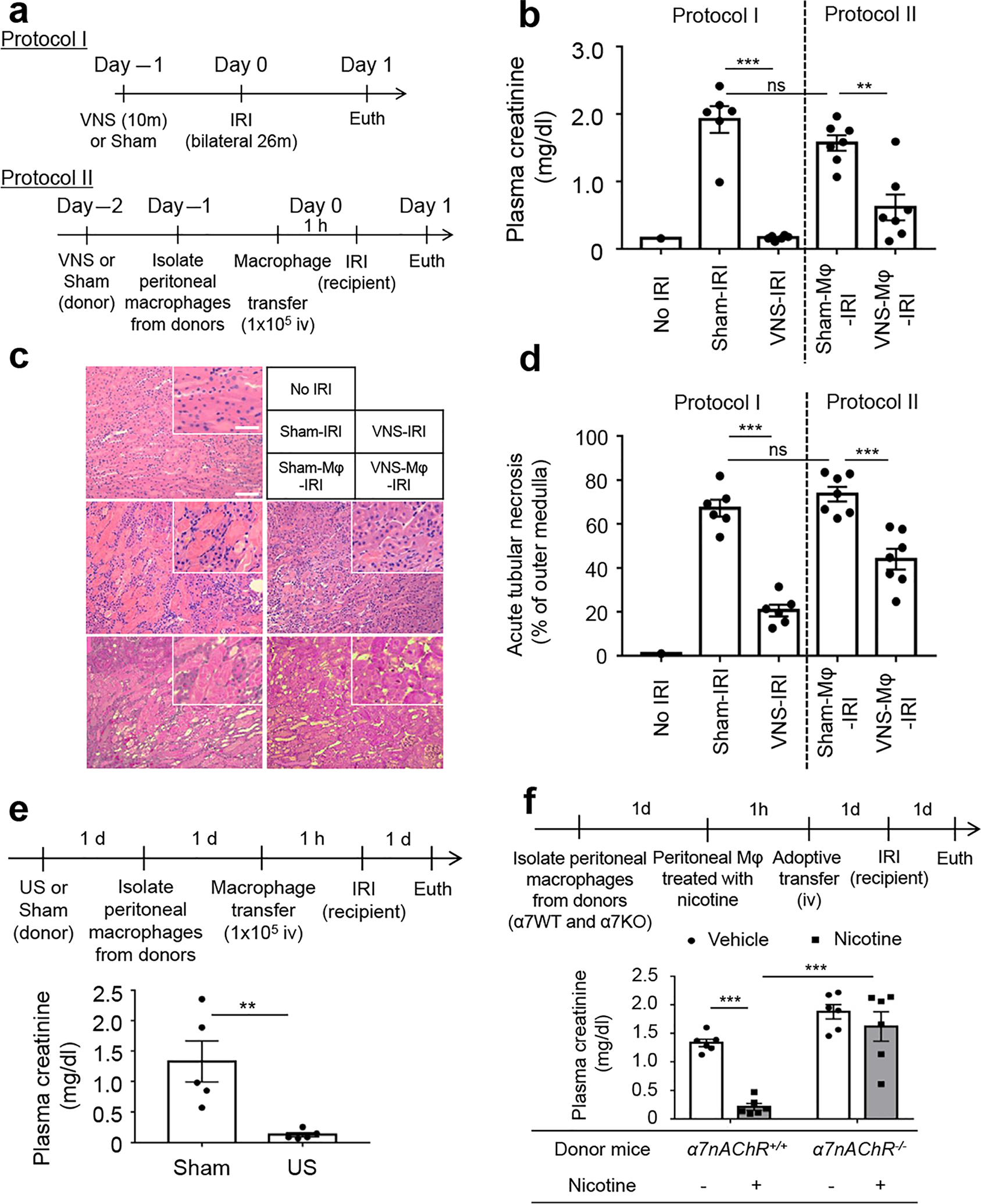
(a) Experimental design for b–d. Adoptive transfer of 1 × 105 peritoneal macrophages from VNS-treated mice to recipient mice, or prior VNS alone, protected kidneys from ischemia-reperfusion injury (IRI) as shown by (b) plasma creatinine, (c) tissue morphology (representative hematoxylin and eosin staining of kidney outer medulla), and (d) acute tubular necrosis (ATN; scored from hematoxylin and eosin–stained samples). (e) Adoptive transfer of peritoneal macrophages from US-treated mice protected recipient mice from IRI. (f) Adoptive transfer of 1 × 105 nicotine-treated peritoneal macrophages (MΦ) from α7nAChR+/+ (α7WT; progeny control) but not from α7nAChR−/− (α7KO) mice protected kidneys of recipient mice from IRI. n = 6–7 each in b–d; n = 5 in e; n = 6 in f. Data in b and d were analyzed using 1-way analysis of variance (ANOVA), and data in f were analyzed with 2-way ANOVA. Means were compared by post hoc multiple comparison test (Tukey’s). Data in e were analyzed with Student’s t-test (2-tailed). **P < 0.01 and ***P < 0.001. Bar = 100 μm in main panel and 50 μm in inset. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Hes1 is a new mediator in the CAP
Macrophages can mediate anti-inflammatory effects, such as suppression of tumor necrosis factor (TNF), when the CAP is activated. We observed previously that VNS treatment before IRI required α7nAChR to maintain expression of arginase1 (Arg1), an anti-inflammatory marker of M2 macrophages that is suppressed by AKI, in the infiltrated macrophages in the kidney.11 Although some mechanisms related to the anti-inflammatory effects of α7nAChR stimulation in the CAP, such as inhibition of the nuclear translocation of NF-κB,19 Jak2-Stat3,20 and microRNA-124,21 are known, further investigations of molecular mechanisms in the CAP are needed. Here we focused on the downstream signaling of α7nAChR in macrophages. Peritoneal macrophages isolated from α7nAChR+/+ and α7nAChR−/− mice were treated with nicotine (10 μM) and/or lipopolysaccharide (LPS; 100 ng/ml), as shown in Figure 2a, then RNA sequencing (RNA-seq) was performed. Figure 2b is a heat map created based on the top 30 genes highly induced by LPS treatment in α7nAChR+/+-derived peritoneal macrophages, and this includes inflammatory cytokines such as interleukin-1β, interleukin-6, and TNF-α (details are shown in Supplementary Table S1). We then focused on nicotine-induced genes in α7nAChR+/+-derived cells that might modulate inflammation (modeled by LPS treatment). Genes were selected that had more than 4-fold relative gene expression in nicotine-treated cells compared with the control group (vehicle-treated). The same calculation was applied to nicotine + LPS–treated cells and LPS-treated cells, and 264 genes were identified as genes commonly induced by nicotine based on these 2 comparisons (Figure 2c, left). Eighteen α7nAChR-dependent nicotine-induced genes were then selected whose expressions were lower (<1/2) in α7nAChR−/−-than in α7nAChR+/+-derived peritoneal macrophages (Figure 2c, right; Supplementary Table S2). Among these, we focused on the role of Hes1 in the CAP, given its reported anti-inflammatory role in macrophages.22,23 Gene expression analysis by RNA-seq around the Hes1 gene (Figure 2d) was confirmed by real-time polymerase chain reaction (Figure 2e). Hes1 expression was induced by nicotine treatment in both α7nAChR+/+- and α7nAChR−/−-derived peritoneal macrophages, but its induction was significantly lower in α7nAChR−/−-derived cells (Figure 2d–e), indicating that Hes1 expression is regulated through α7nAChR. Nicotine also induced Hes1 in RAW 264.7 cells, a macrophage cell line (Supplementary Figure S2).
Figure 2 |. Genome-wide analysis identified that Hes1 is downstream of α7 nicotinic receptors.
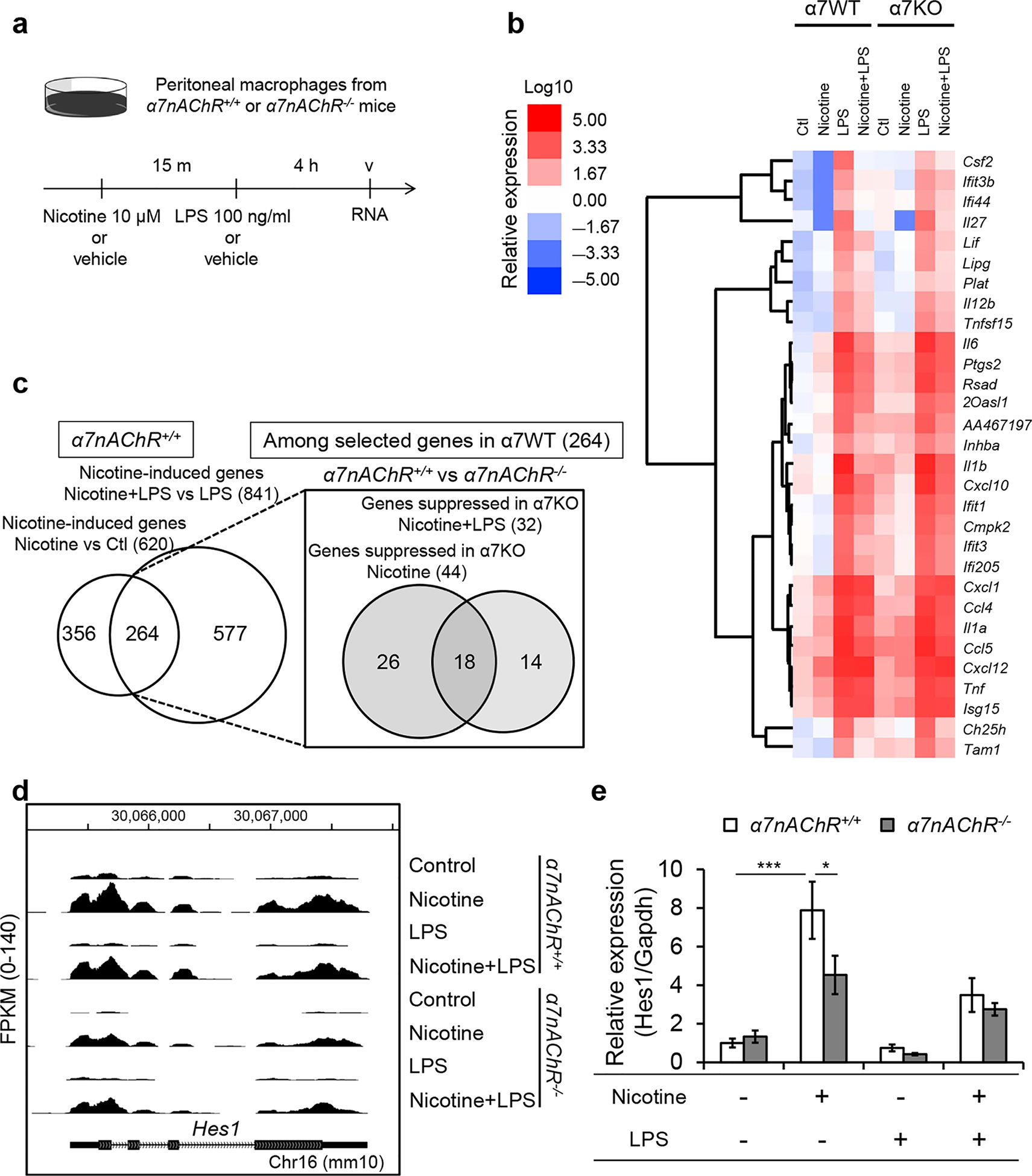
(a) Experimental design for b–d. RNA was isolated from α7nAChR+/+ or α7nAChR−/− peritoneal macrophages treated with vehicle (saline) or nicotine (10 μM; 15 minutes) then with vehicle (saline) or lipopolysaccharide (LPS) (100 ng/ml; 4 hours). (b) A heat map of the genes highly induced by LPS in peritoneal macrophages. RNA sequencing was performed and the relative expression was calculated based on FPKM (fragments per kilobase of exon per million mapped fragments). Relative gene expressions compared with control (Ctl) group were calculated, and the top 30 genes induced by LPS in α7nAChR+/+-derived cells were selected (raw data in Supplementary Table S1). Clustering was performed to generate a heat map based on FPKM. (c) RNA sequencing revealed nicotine-induced genes selected based on the comparison of nicotine versus control and nicotine + LPS versus LPS in cells from α7nAChR+/+ mice (left). Among the selected genes, nicotine-induced genes whose expressions were lower (<1/2 compared with α7nAChR+/+-derived cells) in α7nAChR−/−-derived peritoneal macrophages were extracted (right). (d) Gene expression from analysis of RNA sequencing data around Hes1 gene. (e) Quantitative real-time polymerase chain reaction confirmed nicotine-induced Hes1 expression in both α7nAChR+/+- and α7nAChR−/−-derived peritoneal macrophages, but its induction was significantly lower in α7nAChR−/−-derived cells. n = 12 in e. Data in e were analyzed with 2-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). *P< 0.05 and ***P < 0.001.
We then focused on the function of Hes1 in the context of the CAP. TNF induction by LPS and its suppression by nicotine treatment, as observed previously in peritoneal macrophages,3 were confirmed by enzyme-linked immunosorbent assay in peritoneal macrophages (Supplementary Figure S3) and RAW 264.7 cells (Figure 3a). Nicotine-induced TNF reduction was weakened when Hes1 expression was suppressed by small, interfering RNA (Figure 3b; Supplementary Figure S4 shows small, interfering RNA efficiency). In addition, the induction of TNF expression by LPS was reduced by overexpression of Hes1 (Figure 3c). Hes1 overexpression induced mostly macrophage M2 markers (anti-inflammatory markers), as expected, although Nos2 (iNos, an M1 marker) expression was also upregulated by Hes1 overexpression (Figure 3d). Furthermore, Hes1 expression in peritoneal macrophages was induced by VNS or US in vivo (Figure 3e), but the induction was not observed in F4/80+ splenocytes or F4/80+ cells in the kidney (Supplementary Figure S5). As to M1 and M2 markers, US suppressed TNF expression and VNS induced Chil3 (Ym1) expression (an M2 marker) in peritoneal macrophages (Supplementary Figure S6), which supports an anti-inflammatory phenotype of peritoneal macrophages. Lastly, when Hes1-overexpressing RAW 264.7 cells were adoptively transferred into naïve mice prior to renal IRI, the kidneys were protected (Figure 3f). These results reveal a new and important anti-inflammatory role of Hes1 in the CAP.
Figure 3 |. Hes1 is a new mediator in the cholinergic anti-inflammatory pathway (CAP).
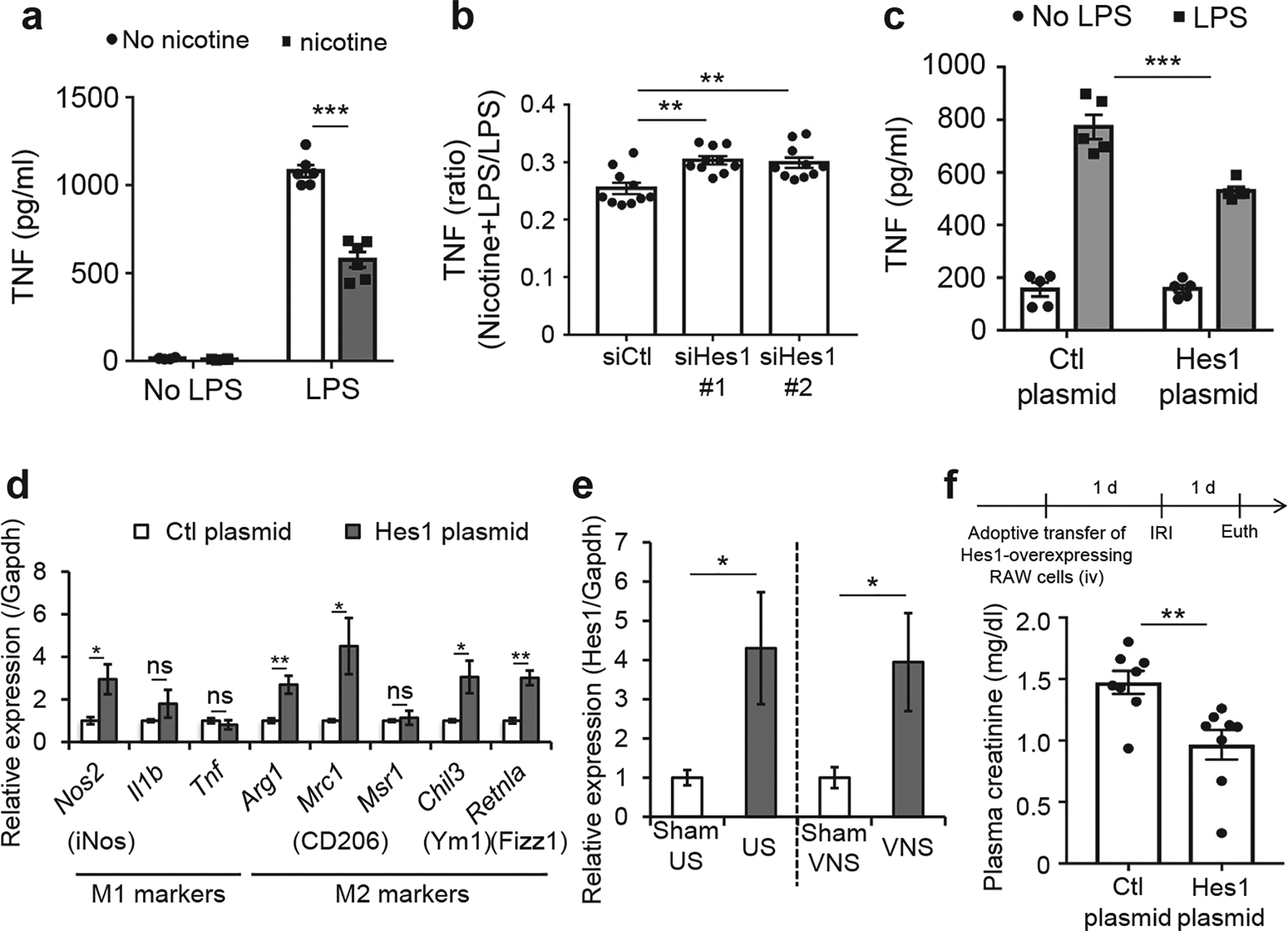
(a) Tumor necrosis factor (TNF) induction by lipopolysaccharide (LPS) is suppressed by nicotine treatment in RAW 264.7 cells. RAW 264.7 cells were treated with LPS (100 ng/ml) for 4 hours after nicotine (10 μM) treatment for 15 minutes. Then TNF was measured in the medium by enzyme-linked immunosorbent assay. (b) Small, interfering RNA (siRNA) against Hes1 inhibits nicotine-induced TNF suppression. (c) Hes1 overexpression suppresses LPS-stimulated TNF induction in RAW 264.7 cells. Ctl, control. (d) Hes1 overexpression in RAW 264.7 cells mainly induces M2 markers (anti-inflammatory). (e) Ultrasound (US) or vagus nerve stimulation (VNS) increased Hes1 expression in peritoneal macrophages isolated 24 hours after US or VNS and incubated overnight. (f) Adoptive transfer of Hes1-overexpressing RAW 264.7 cells protected the kidney from IRI. Arg1, arginase 1; Chil3, chitinase-like 3; Il1b, interleukin 1-β; Mrc1 (CD206), mannose receptor, C type1; Msr1, macrophage scavenger receptor 1; Nos2 (iNos), nitric oxide synthase 2; Retnla (Ym1 or Fizz1), resistin-like alpha; Tnf, tumor necrosis factor. n = 6 in a; n = 10 in b; n = 5 in c and d; n = 10 in e; and n = 8 in f. Data in a and c were analyzed using 2-way analysis of variance. Data in b were analyzed with 1-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). Data in d–f were analyzed with Student’s t-test (2-tailed). *P < 0.05, **P < 0.01, and ***P < 0.001.
β2-Adrenergic receptors on CD4 + splenocytes are important for the renal protection induced by VNS or US
We demonstrated that prior activation of the CAP using 3 different experimental procedures (electrical VNS,11 US,9,10 or C1 neuron photostimulation12) protects the kidney from IRI (26 minutes of bilateral ischemia, 24 hours of reperfusion), one of the most common models of AKI.24 However, the pathway and mechanisms by which these 3 procedures protect the kidney seem to differ slightly (Supplementary Figure S1). C1 neurons located in the medulla oblongata of brainstem regulate autonomic function by responding to various stresses and stimuli.25 Selective C1 neuron stimulation activates CAP via a sympathetic rather than vagal route (parasympathetic) and through adrenergic receptor-positive CD4+ T cells.12 US also requires CD4+ T cells for their protective effect,9 but the importance of β2-adrenergic receptors expressed by CD4+ T cells in CAP-mediated protection from IRI has not yet been established in vivo. To evaluate the role of β2-adrenergic receptor–positive CD4+ T cells in the CAP activated by US or VNS, a β2-adrenergic receptor antagonist (butoxamine, 15 mg/kg, i.p.) was administered prior to US or VNS treatment in IRI. Butoxamine abolished the renal protective effect of US (Figure 4a–c) and VNS (Figure 4d). Conversely, salbutamol alone (15 mg/kg, i.p.), a β2-adrenergic receptor agonist, protected the kidneys from IRI, thereby reproducing the effect of US or VNS (Figure 4e).
Figure 4 |. β2-adrenergic receptors are important for the renal protection induced by vagus nerve stimulation (VNS) or ultrasound (US).
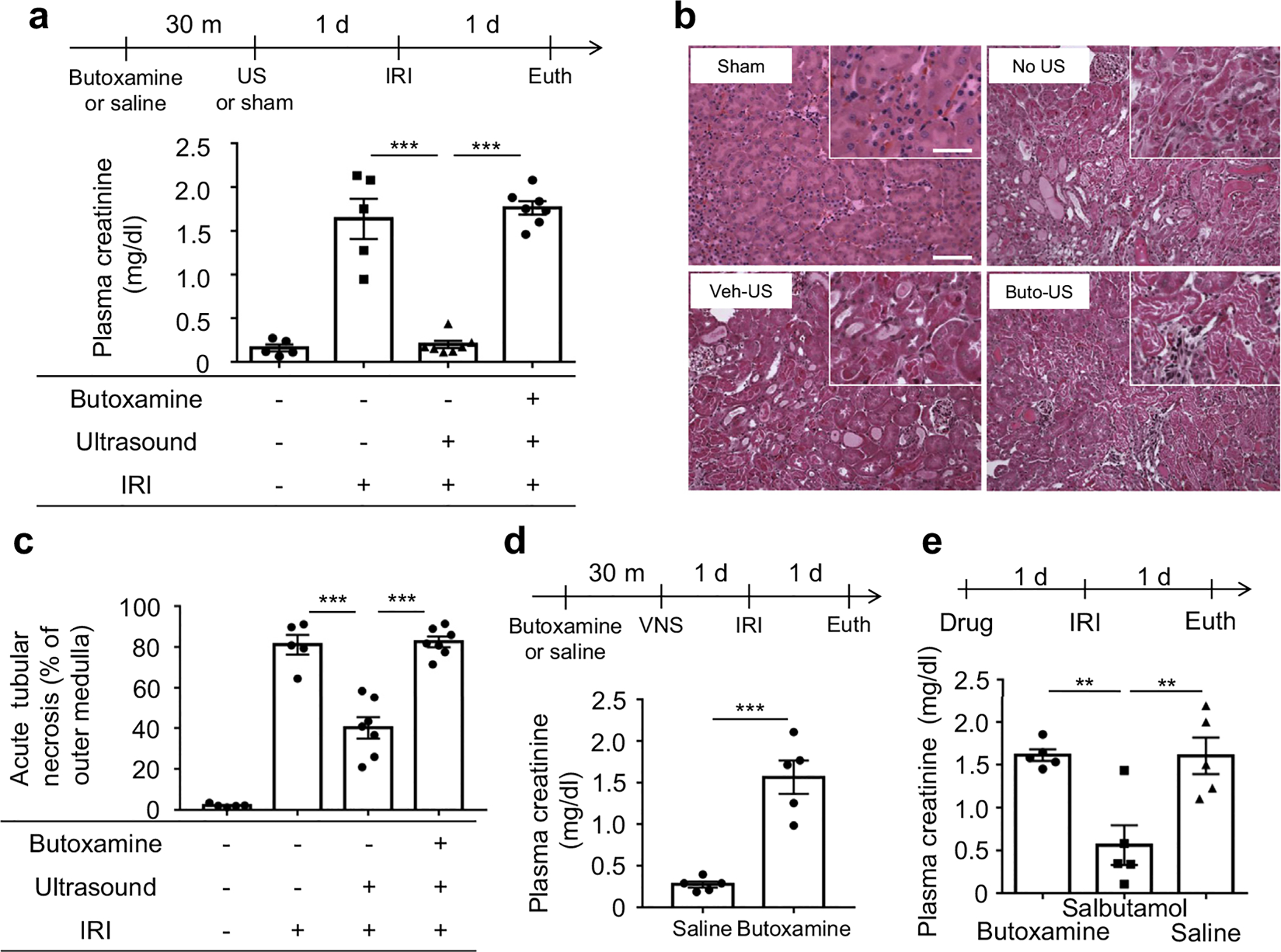
(a–c) Ischemia-reperfusion injury (IRI; 26 minutes ischemia and 24 hours reperfusion) was performed 24 hours after US (bilateral for 2 minutes) treatment. Prior administration of β2-adrenergic receptor antagonist (butoxamine, Buto, 15 mg/kg, i.p.) abolished the renal protective effect of US as shown by (a) plasma creatinine, (b) tissue morphology (representative hematoxylin and eosin staining of kidney sections, outer medulla), and (c) acute tubular necrosis (ATN; scored from hematoxylin and eosin–stained samples). (d) Butoxamine (15 mg/kg) abolished the renal protective effect of VNS (5 Hz, 1 ms, 50 μA for 10 minutes). (e) Systemic β2-adrenergic receptor agonist (salbutamol, 15 mg/kg, i.p.) protects the kidney from IRI. n = 5–7 each in a–c; n = 5 each in d and e. Data were analyzed using 1-way analysis of variance in a, c, and e. Means were compared by post hoc multiple comparison test (Tukey’s). Data in d were analyzed with Student’s t-test (2-tailed). **P < 0.01 and ***P < 0.001. Bar = 100 μm in main panel and 50 μm in inset. Euth, euthanize; Veh, vehicle (saline). To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
When CD4+ splenocytes from US-(Figure 5a) or VNS-treated donor mice (Figure 5b) were adoptively transferred into recipient mice, the kidneys of recipient mice were protected from IRI. Furthermore, kidneys were protected when salbutamol-treated CD4+ splenocytes were transferred to naïve recipients 24 hour before IRI (Figure 5c). In summary, activation of β2-adrenergic receptors expressed by CD4+ splenocytes is an essential step underlying the CAP-mediated protective effect of US and VNS in IRI.
Figure 5 |. β2-adrenergic receptors on CD4-positive splenocytes are critical in the cholinergic anti-inflammatory pathway (CAP).
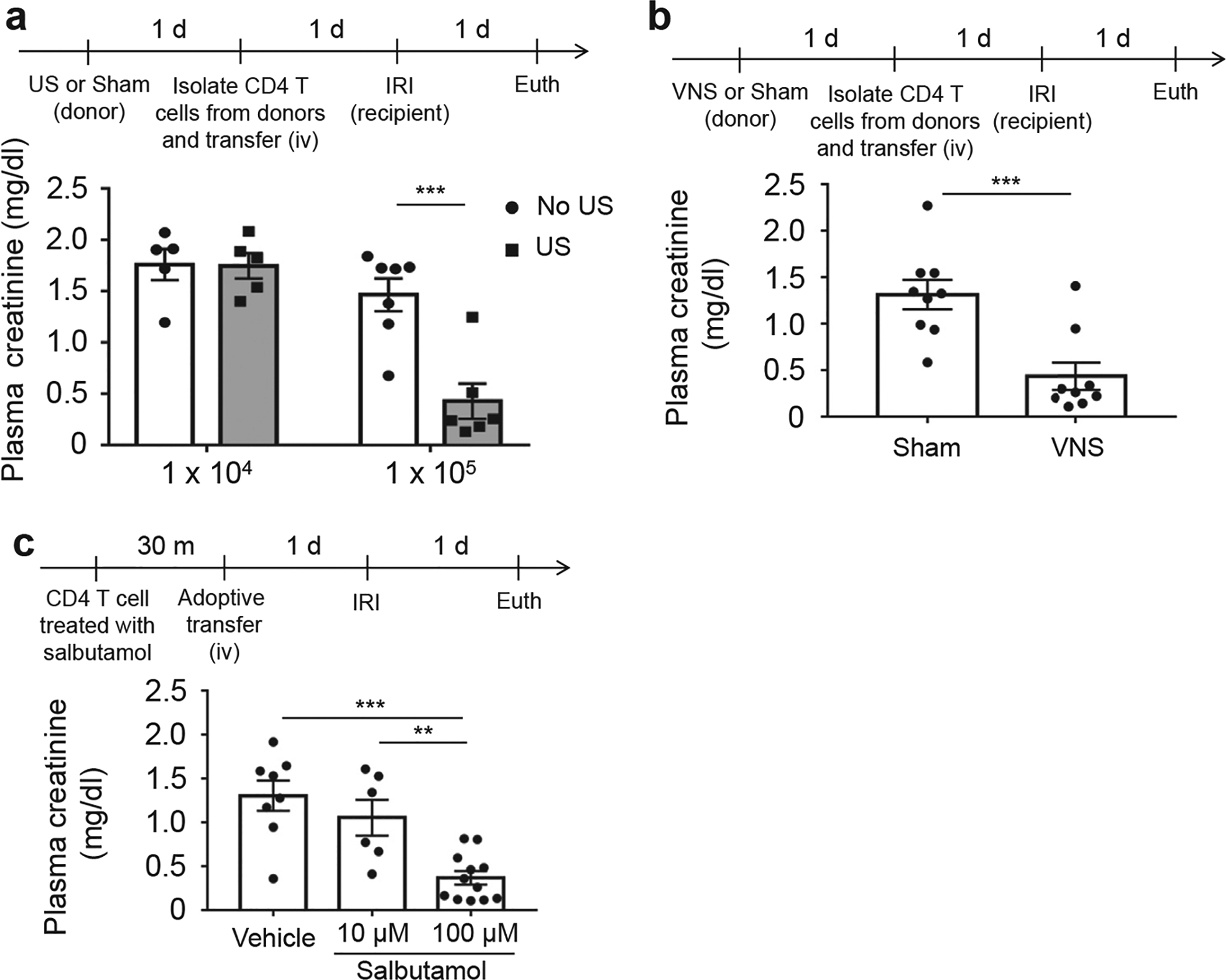
Adoptive transfer of 1 × 105 CD4-positive splenocytes from (a) ultrasound (US)-treated or (b) vagus nerve stimulation (VNS)-treated donor mice protects the kidneys in recipient mice from ischemia-reperfusion injury (IRI). (c) Kidneys were protected when β2-adrenergic receptor agonist–treated CD4-positive splenocytes were transferred 24 hours before IRI. n = 5–7 each in a; n = 9 each in b; and n = 6–12 in c. Data were analyzed using 2-way analysis of variance in a. Data in c were analyzed with 1-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). Data in b were analyzed with Student’s t-test (2-tailed). **P < 0.01 and ***P < 0.001.
Activated peritoneal macrophages need the spleen or other immune cells such as T cells and/or B cells to provide organ protection
The importance of spleen as an integral component of the CAP was initially shown more than 10 years ago.26 Although ACh levels in the spleen are increased by VNS,27 little or no direct cholinergic innervation from the vagus has been found in the spleen.28,29 The splenic sympathetic (noradrenergic) nerve innervates the spleen, and CD4+ ACh-producing T cells that respond to norepinephrine released by splenic sympathetic nerve terminals appear to be the source of ACh that links VNS and α7nAChR-expressing macrophages.27 Although the idea has been postulated that VNS activates macrophages through CD4+ T cells, little is known about the interaction between these 2 cell types in the CAP. Thus, we developed 2 experimental plans to explore this interaction (Figure 6a). To focus on the interaction between peritoneal macrophages and splenocytes, including CD4+ T cells, we removed the spleen of recipient mice before adoptive transfer of peritoneal macrophages (Figure 6b and c). The protective effect of VNS-treated (Figure 6b) or nicotine-treated peritoneal macrophage transfer (Figure 6c) was eliminated or reduced by prior splenectomy. In addition, the protective effect of nicotine-treated peritoneal macrophage transfer was lost in Rag1-deficient mice that do not have B cells and T cells (Figure 6d). Somewhat surprisingly, the protection was not rescued by reconstitution of Rag1-deficient mice with CD4+ T cells (Figure 6e), and norepinephrine-treated CD4+ T cells were not protective in Rag1-deficient mice (Figure 6f). Taken together, the organ protective effect of peritoneal macrophages activated in the CAP requires the spleen or splenocytes including T cells and other cells. These results indicate that CD4+ T cells and macrophages, and other immune cells, might interact in more complex ways than originally thought to produce a tissue-protective anti-inflammatory effect via the CAP (Figure 7).
Figure 6 |. Activated peritoneal macrophages need the spleen or other immune cells such as T cells and/or B cells to protect the kidney from injury.
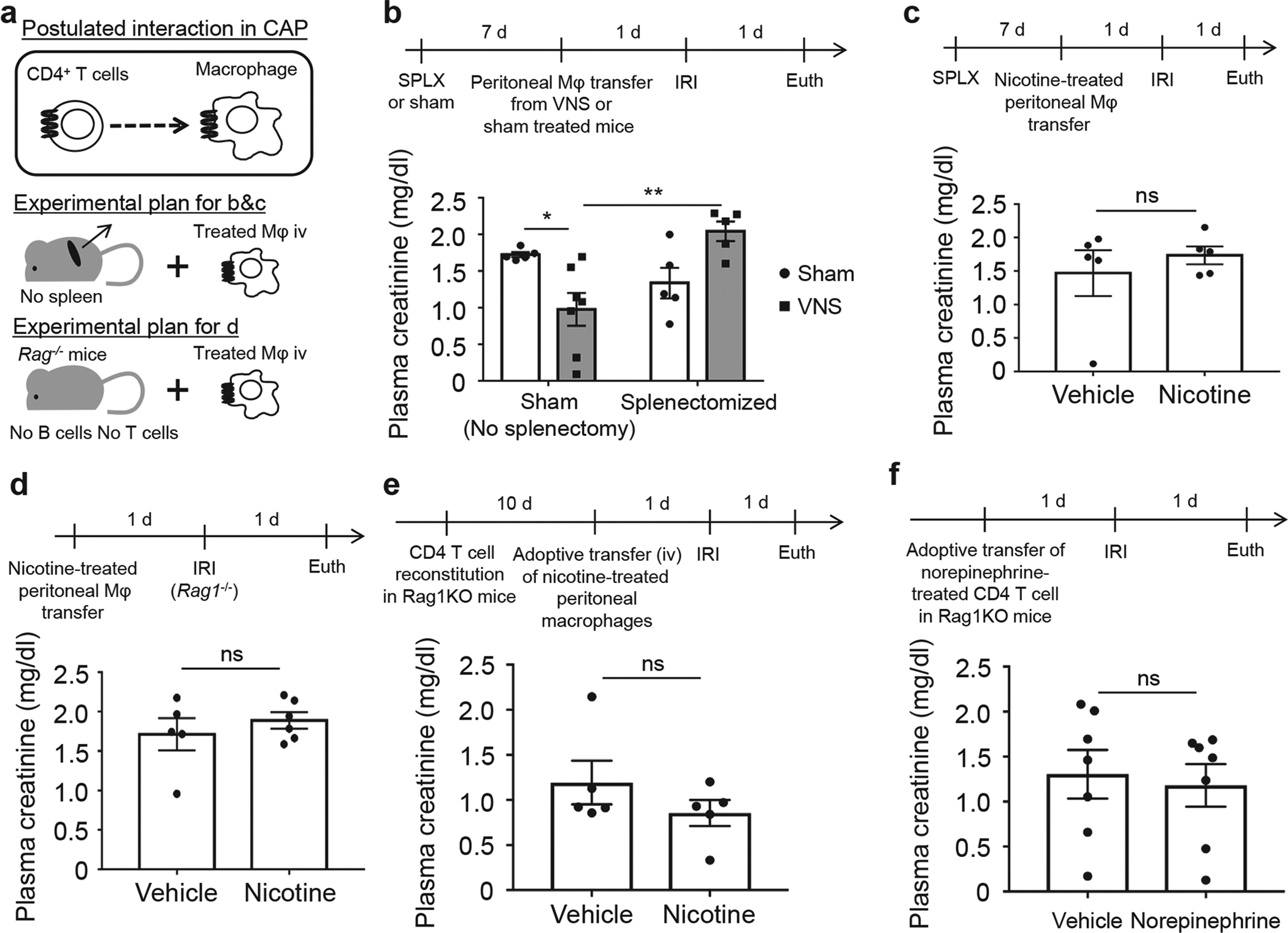
(a) Hypothesis and experimental plan. The protective effect by (b) vagus nerve stimulation (VNS)–treated peritoneal macrophage transfer or (c) nicotine-treated peritoneal macrophage transfer was eliminated or reduced by prior splenectomy (SPLX) as demonstrated by plasma creatinine. (d) The protective effect by nicotine-treated peritoneal macrophage transfer was lost in Rag1-deficient mice. (e) Reconstitution of Rag1-deficient mice with 1 × 106 wild-type CD4-positive T cells (i.v.) 10 days before adoptive transfer did not restore the protective effect of nicotine-treated peritoneal macrophages in the mice challenged with ischemia-reperfusion injury (IRI). (f) The protective effect by norepinephrine-treated CD4-positive T cell transfer was lost in Rag1-deficient mice. n = 5–7 each in b; n = 5 in c and e, n = 5–6 in d, and n = 7 in f. Data in b were analyzed with 2-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). Data in c–f were analyzed with Student’s t-test (2-tailed). *P < 0.05 and **P <0.01.
Figure 7 |. Summary of our new findings on the cholinergic anti-inflammatory pathway.
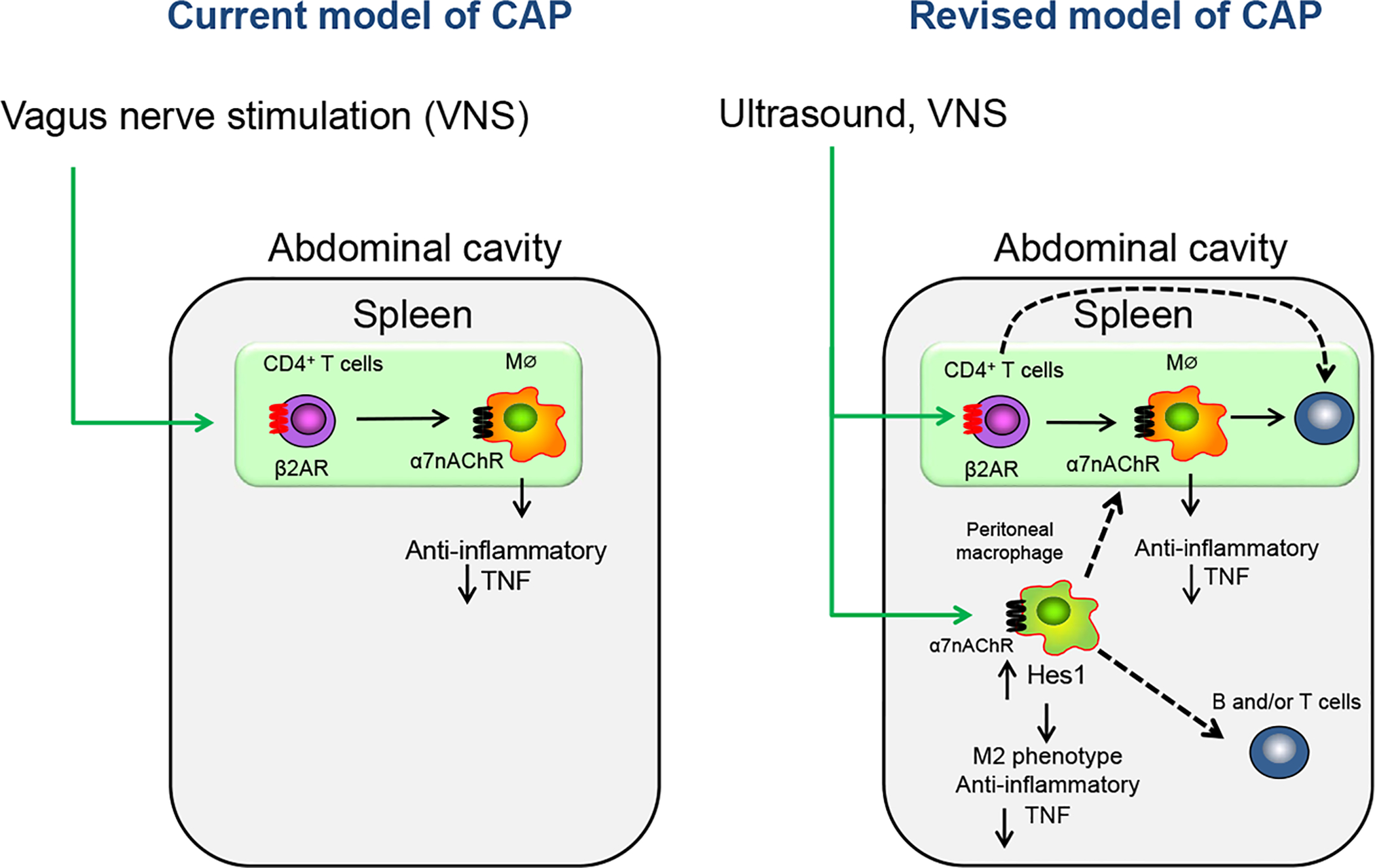
The left panel shows the conventional concepts of the efferent arm of the cholinergic anti-inflammatory pathway (CAP). The efferent vagus nerve stimulates CD4-positive (CD4+) T cells in spleen via the splenic sympathetic nerve. Norepinephrine, released from the sympathetic nerve, binds to β2-adrenergic receptors (β2ARs) on CD4+ T cells, which then elicits release of ACh from these choline acetyltransferase-expressing T cells. Acetylcholine (Ach) binding to alpha 7 nicotinic acetylcholine receptors (α7nAChRs) on macrophages (MΦ) produces an anti-inflammatory response, such as tumor necrosis factor (TNF)–α suppression. The right panel summarizes our new findings on the CAP and suggests that the CAP-stimulated anti-inflammatory response may be mediated by a more complex interaction of immune cells than previously appreciated. Ultrasound- or VNS-induced protection of kidneys from ischemia-reperfusion injury (IRI) mediated by CD4+ T cells is β2AR-dependent. Ultrasound or VNS activates peritoneal macrophages that protect the kidney from IRI. Kidney protection by activated α7nAChR-expressing peritoneal macrophages is dependent on α7nAChRs. Hes1 expression in macrophages and the induction of Hes1 further elicits an M2 phenotype and produces an anti-inflammatory response. These studies identify Hes1 as a new anti-inflammatory mediator in this pathway and signaling molecule downstream of α7nAChRs, but other genes were also identified by RNA sequencing that will require further investigation. The finding that spleen or lymphocytes (B and/or T cells) are required for the anti-inflammatory effect of activated peritoneal macrophages in vivo and other results using Rag1-deficient mice introduce an expanded view of the mechanisms involved in CAP-induced protection from acute kidney injury and perhaps from injury of other organs, as well. Dashed lines, pathways not yet defined.
DISCUSSION
The new findings from our studies are as follows and are summarized in Figure 7. The protective effect of α7nAChR-expressing macrophages in the CAP was demonstrated in vivo for the first time using peritoneal macrophages. We demonstrated that activation of β2-adrenergic receptors expressed by CD4+ splenocytes is necessary for the protection from kidney injury provided by ultrasound treatment or VNS. A new directional interaction between macrophages and other immune cells that might include CD4+ T cells in the CAP was identified. While activation of β2AR-positive CD4+ splenocytes is necessary for tissue protection, it may not solely precede macrophage activation as previously thought. US- or VNS-stimulated peritoneal macrophages may also produce a protective effect that is independent of acetylcholine release from CD4+ splenocytes but requires a population of T or B cells that have not yet been characterized. Hes1, which is induced by VNS or US, was newly identified as an important molecule downstream of α7nAChR on macrophages in the CAP.
Similarities between US- and VNS-induced kidney protection mechanisms through the CAP
Based on our previous findings,9–11 we hypothesized that the CAP is activated by both US and VNS through a common pathway. In parallel experiments applied to US-related and VNS-related studies (Figure 1, 3–5), we found comparable mechanisms of protection for both US and VNS. Administration of β2-adrenergic receptor antagonist (butoxamine) prior to US treatment or VNS and IRI abolished kidney protection induced by US or VNS, indicating a common requirement for β2-adrenergic receptors (Figure 4a–d). Adoptive transfer of either peritoneal macrophages or CD4 T cells from US- or VNS-treated donors to naïve recipients protected kidneys of recipient mice from IRI (Figure 1a–e and Figure 5a–c). These experiments revealed that US and VNS activate peritoneal macrophages and CD4 T cells to protect kidneys from IRI. Although the mechanism underlying US-induced activation of the CAP and potential similarities to VNS-induced CAP activation are unknown, there are clearly common mechanisms that involve immune cell-mediated protection.
Peritoneal macrophages are new key contributors in vivo in the CAP
Considering the role of α7nAChR-positive peritoneal macrophages in vitro,3 the discovery of the importance of the spleen,26 and the recognition of the essential role of ACh-synthesizing T cells in the spleen,27 it was suggested that macrophages in the spleen have an important role in the CAP. This concept is supported by our findings that α7nAChR-positive splenocytes are necessary for VNS-related kidney protection11; however, the importance of α7nAChR-positive splenic macrophages in vivo has never been proved. Nonsplenic, peritoneal macrophages have been employed extensively for unveiling the molecular mechanisms of the CAP in vitro.3,20 In adoptive transfer studies we demonstrated for the first time in vivo that peritoneal macrophages stimulated by US or VSN can relay an anti-inflammatory signal to naïve recipient mice to protect their kidneys from IRI and that α7nAChRs on peritoneal macrophages are necessary for a tissue protective effect in the CAP (Figure 1). Thus, US or VNS can activate peritoneal macrophages in the CAP in vivo (Figure 7), although the underlying mechanisms remain unknown. Anatomical proximity between the vagus nerve and peritoneal cavity might indicate that US or VNS can activate the peritoneal macrophages directly via acetylcholine and its receptors.17,18 Further experiments will be needed to explore direct cholinergic effects on macrophages as well as the possibility that peritoneal macrophages were activated through second-order mechanisms, given that they were isolated 24 hours after treatment of mice with US or VNS. These experiments do not exclude the possibility that US or VNS also activates α7nAChR-positive macrophages in the spleen, and the relative contributions or interactions of these populations in CAP-mediated tissue protection are as yet unknown.
Peritoneal macrophages are one of the most studied macrophage populations, and recently 2 physically, functionally, and developmentally different peritoneal macrophage subsets have been described.30 The small peritoneal macrophages (SPMs) (F4/80lowCD11blowMHCIIhigh) are bone marrow-derived and can be recruited from circulating monocytes into the peritoneum under inflammatory conditions. By contrast, the large peritoneal macrophages (LPMs) (F4/80highCD11bhighMHCIIlow) are yolk sac–derived and the major peritoneal macrophage population under steady-state conditions. LPMs are responsible for phagocytosis of apoptotic cells and tissue repair.30,31 Further characterization of the peritoneal macrophages revealed that LPMs, but not SPMs, selectively express the zinc finger transcription factor GATA-binding protein 6 (GATA6).32,33 Very recently a reservoir of mature F4/80highGATA6+ peritoneal macrophages was reported to infiltrate rapidly into the afflicted tissue directly across the mesothelium following a sterile injury in liver, and the phenotypic change toward an alternative (M2), anti-inflammatory phenotype of the invaded macrophages was observed.34 Thus, understanding of peritoneal macrophage subset characterization, origin, and functions has been enhanced. Considering the recent advances and our findings, there is a possibility that US or VNS might enhance the anti-inflammatory function of LPMs through α7nAChR. The phenotypic change of macrophages and the recruitment of macrophages into the kidney that we previously observed11 might also be related with GATA6 expression. Further detailed analysis might accelerate our understanding of the role of peritoneal macrophages in the CAP.
Hes1 as a new downstream mediator of α7nAChR on macrophages in the CAP
We identified a new signaling molecule downstream of α7nAChR on macrophages in the CAP using genome-wide analysis (Figures 2 and 3). Hes1 belongs to a family of basic helix-loop-helix DNA-binding proteins and plays a critical role in the development of multiple organs in processes such as neurogenesis, cell differentiation, and development of immunity.35–37 Hes1 is a transcription factor that acts as a transcriptional repressor, and it is activated by both canonical and noncanonical pathways; Notch represents one of the prominent canonical pathways.37 Our analysis of peritoneal macrophages using RNA-seq revealed Hes1 as a new downstream mediator of α7nAChR in macrophages, and we connected the anti-inflammatory role of Hes1 in macrophages and the CAP for the first time by showing nicotine-mediated α7nAChR-dependent induction of Hes1 in vitro and US- or VNS-induced increases of Hes1 expression in vivo (Figures 2 and 3). Although Hes1 knockdown only partially weakened nicotine-induced TNF reduction (Figure 3b), this can be explained by the existence of known or unknown other factors regulated through α7nAChR. Hes1-overexpression nicely suppressed TNF induction by LPS (Figure 3c) and shifted macrophage polarization toward an anti-inflammatory (M2) phenotype (Figure 3d). Similarly, US and/or VNS shifted polarization to an anti-inflammatory M2 phenotype, although quantitatively these results (Supplementary Figure S6) were different from those depicted in Figure 3d. There are several possibilities that might cause the differences seen in Figure 3d and Supplementary Figure S6. First, in Figure 3d, the isolated effect of Hes1 overexpression in macrophages (RAW 264.7) was observed independent of any systemic or local microenvironmental effects that are likely to occur with peritoneal macrophages isolated following VNS, as was done in Supplementary Figure S6. Second, RAW 264.7 cells were studied in Figure 3d as opposed to peritoneal macrophages used in Supplementary Figure S6. Third, the in vivo activation of Hes1 with VNS/US may lead to quantitative differences in Hes1 expression.
Taken together, we discovered a key molecule in the CAP and a cellular biomarker of US or VNS treatment. Hes1-deficient mice exhibited severe neurulation defects and died during gestation or just after birth,38 and tissue-specific deletion or transgenic mice will be needed for further study, especially for exploring the more detailed function of Hes1 in the CAP in vivo.
In addition to Hes1, there are other candidate genes that might be downstream of α7nAChR on macrophages based on our RNA-seq data (Figure 2c and Supplementary Table S2). Although heme oxygenase 1 (Hmox1) would be a very logical candidate gene to study, because of its anti-inflammatory function39 and its protective role in acute kidney injury,40 the link between Hmox1 and the CAP has already been reported.41 Other candidate genes might also have important roles in the CAP, and potential complex interactions among the known mechanisms, including Jak2-Stat320 and micro-RNA-124,21 and the candidate genes might be critical in the CAP. Comprehensive analysis will be needed to reveal the complicated downstream pathways through α7nAChR in macrophages in the CAP.
The role of β2-adrenergic receptors on CD4+ splenocytes in mediating an anti-inflammatory effect in vivo
We previously showed that optogenetic photostimulation of C1 neurons (located in the medulla oblongata) exerts a kidney protective effect mainly through sympathetic nerve activation12. This was further supported by results showing that butoxamine (β2-selective antagonist) and hexamethonium (ganglionic blocker that blocks both sympathetic and para-sympathetic nerve activity), but not subdiaphragmatic vagotomy, attenuate C1 stimulation-induced kidney protection.12 Supporting the idea of sympathetic activation driving the CAP, Vida et al. showed that β2-adrenergic receptors on CD4+CD25- T cells are essential for the anti-inflammatory effect of the CAP in the LPS model,42 and we found that US-induced kidney protection requires CD4+ splenocytes.9 In the current study we took this one step further by showing that β2-adrenergic receptors on CD4+ splenocytes are needed for the kidney protective effect of US and VNS in acute kidney injury (Figures 4 and 5). Rosas-Ballina et al. showed that ACh-synthesizing CD4+CD44highCD62Llow splenocytes relay neural signals to macrophages in a vagus nerve circuit.27 Our previous data indicate that afferent VNS causes kidney protection through an identified pathway in the brain11 (Supplementary Figure S1). Combined with our findings on C1 neuron-stimulated kidney protection through sympathetic nerve, there is a possibility that vagus afferent stimulation might activate splenic sympathetic nerve through C1 neurons. Furthermore this implies that US might possibly stimulate vagus afferent nerve in the abdomen, leading to the activation of CD4+ splenocytes via β2-adrenergic receptors.
A new directional interaction between CD4+ T cells and macrophages and other interactions among immune cells in the cholinergic anti-inflammatory pathway are suggested
As shown in Supplementary Figure S1, the current consensus on the CAP posits that ACh-synthesizing T lymphocytes activate α7nAChR-positive macrophages in the spleen. Rosas-Ballina et al. elegantly showed that adoptive transfer of ACh-synthesizing T lymphocytes into nude mice, which do not have functional T cells, partially restores the VNS-induced anti-inflammatory effect.27 Based on their findings, 1-directional activation (CD4 T cells → macrophages) in the CAP was postulated. This implies that macrophages are the final key cellular components in the CAP. However, we showed that adoptive transfer of activated peritoneal macrophages into splenectomized mice or Rag1-deficient mice, which do not have B and T cells, did not protect the kidneys from IRI (Figure 6a–d). This indicates that macrophages are not the terminal cellular mediator that is activated in the CAP or that peritoneal macrophages behave differently from splenic macrophages in the CAP. In addition, adoptive transfer of nicotine-treated peritoneal macrophages into Rag1-deficient mice with CD4+ T cells reconstituted did not protect the kidney (Figure 6e). When Rag1-deficient mice received norepinephrine-treated CD4+ T cells, the protection was not observed (Figure 6f). These data imply that other immune cells such as B cells and CD8 T cells might also be involved in the CAP in mediating renal protection. Thus, our studies support modification of the current cholinergic anti-inflammatory paradigm and suggest that US- or VNS-induced macrophage activation requires subsequent lymphocyte participation (and perhaps the spleen as a specialized niche) in mediating the protective effect of the CAP (Figure 7).
In summary, we believe that our findings further elucidate our understanding of the CAP and suggest additional mechanisms that may be important in neural-immune regulation of disease. Based on our study, Hes1 activation could be a new pharmacological strategy to prevent acute kidney injury. Targeting Hes1 might block inflammation and preserve organ function by activating the CAP and might be also applied to various inflammation-related disorders.
METHODS
Mice and reagents
Male mice (8–12 weeks of age, 20–25 g) were used for all experiments. Wild-type C57Bl/6 mice were purchased from the National Cancer Institute (Frederick, MD), α7nAChR−/− mice (B6.129S7-Chrna7tm1Bay/J) were obtained from Jackson Laboratories (Bar Harbor, ME), and WT (α7nAChR+/+) progeny were used as controls in Figures 1 and 2. Rag1−/− mice (B6.129S7-Rag1tm1Mom/J) were obtained from Jackson Laboratories. Butoxamine (β2-adrenergic receptor antagonist), salbutamol (β2-adrenergic receptor agonist), nicotine (nicotinic acetylcholine receptor agonist), and LPS (from Escherichia coli O111:B4) were purchased from Sigma-Aldrich (St. Louis, MO).
Ultrasound treatment
Mice were anesthetized with an i.p. injection of a mixture of ketamine (120 mg/kg) and xylazine (12 mg/kg) and received ultrasound treatment, as described previously.9 A clinical Sequoia 512 ultrasound machine with a 15L8w transducer (Acuson, Malvern, PA) was used for ultrasound application. Fur on the back was shaved and removed using a depilatory. Mice were then placed on a modified microscope stage in a face-down posture. Prewarmed ultrasound gel was then placed on the depilated skin for ultrasound application. Mouse body temperature was monitored via rectal probe (Fine Science Tools, Foster City, CA) and maintained at 35.5°C with a heating pad and heat lamp. Once the animal’s body temperature was stabilized, the left kidney was localized in real time using conventional B-mode imaging. Then the ultrasound treatment consisted of ultrasound pulses administered with a bursting mechanical index of 1.2. Ultrasound pulses were 1 second in duration and were applied once every 6 seconds for 2 minutes (20 seconds of exposure in total per kidney). After 2 minutes, the same procedure was applied to the right kidney. Control animals underwent the same preparation procedures but were not exposed to ultrasound. Total ultrasound exposure was approximately 5 minutes, with small variations in the time required to stabilize body temperature and localize the kidneys. After ultrasound treatment, animals were allowed to recover from the anesthetic in a temperature-controlled incubator.
Vagus nerve stimulation
Mice were anesthetized with an i.p. injection of ketamine (120 mg/kg) and xylazine (12 mg/kg) and underwent VNS, as described previously.11 The left cervical vagus nerve was isolated via a midline cervical incision and placed on a bipolar silver wire electrode for stimulation (AS633; Cooner Wire, Chatsworth, CA). Electrical stimulation (square wave; intensity: 50 μA; frequency: 5 Hz; duration: 1 ms) was applied for 10 minutes using a Grass model S88 stimulator and stimulus isolation unit (AstroNova, Inc., West Warwick, RI) or Isostim A320 (WPI, Sarasota, FL). In sham-operated animals, the vagus nerve was exposed but not stimulated. Mice received buprenorphine (0.15 mg/kg) as a postoperative analgesic.
Peritoneal macrophage preparation and adoptive transfer studies
After the mouse was killed, it was sprayed with 70% ethanol and was mounted on its back. The skin on the ventral surface was cut with a scissors and forceps, and the parietal peritoneum lining the peritoneal cavity was exposed with the skin being gently pulled. Then 8 ml of ice-cold sterile phosphate-buffered saline (PBS) was injected into the peritoneal cavity using a 19-gauge needle with a 10-ml syringe. After injection, the peritoneum was gently massaged and as much of the injected fluid was collected as possible. The collected cell suspension was centrifuged at 500 g for 5 minutes, and the supernatant was discarded. The cells were resuspended in culture medium (RPMI, 10% FBS, 1% glutamine, 1% penicillin-streptomycin) at a concentration of 1 × 106 cells/ml and were plated in 24-well cell culture plates. The cells were washed with PBS 1 hour after being plated, and PBS was replaced with culture medium. The next day the cells were washed with PBS, counted, and used for further experiments.
For adoptive transfer studies using peritoneal macrophages or RAW 264.7 cells, IRI was performed 1 hour after the injection of peritoneal macrophages. To enhance the purity of peritoneal macrophages after isolating peritoneal cells requires at least overnight incubation and washing as described above, so we chose the time course to minimize the time between VNS (or US) treatment and IRI. It takes 48 hours between VNS (or US) and IRI with this time course, and we confirmed that VNS (or US) 48 hours prior to IRI protects the kidney.
We tested dose dependency for the number of cells using CD11b+−enriched cells from the spleen and for nicotine concentration using peritoneal macrophages. Based on the preliminary experiments, we used 1 × 105 peritoneal macrophages treated with 10 μM nicotine for our study (Supplementary Figures S7 and S8). In additional preliminary experiments nicotine-treated RAW 264.7 cells protected kidneys from IRI (Supplementary Figure S9), therefore providing evidence for the feasibility of using Hes1-overexpressing RAW 264.7 cells for adoptive transfer studies.
Macrophage enrichment from the spleen and kidney
Spleens were harvested, and single cell suspensions were generated by passing whole spleen through 40 μm filters into PBS. Kidneys were weighed, minced, and incubated with collagenase type IA (10 μg/ml; Sigma-Aldrich) and DNAseI in cold Roswell Park Memorial Institute buffer with penicillin and streptomycin, L-glutamine, 10% fetal calf serum and N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (pH 7.4, 25 mM) for 40 minutes at 37°C. The digested kidney tissue suspension was teased sequentially through a 53-μm and 35-μm cell strainer (Endecotts Ltd.) by using the rubber end of a 5-ml syringe plunger. The cell pellet either from the spleen or kidney was collected by centrifugation (500 × g for 5 minutes) and then treated with red blood cell lysis buffer (Biolegend, San Diego, CA) for 3 minutes according to the manufacturer’s protocol. The samples were centrifuged after cell lysis, and the resulting cell pellet was diluted with buffer (PBS, 0.5% bovine serum albumin and 2 mM ethylenediamine tetraacetic acid). Then F4/80-positive cells were isolated by MACS separation using Anti-F4/80 MicroBeads UltraPure for mouse (Miltenyi Biotec, Bergisch Gladbach, Germany).
CD4+ splenocyte enrichment and adoptive transfer studies
Spleens were harvested, and single cell suspensions were generated by passing whole spleen through 40-μm filters into PBS. The cell pellet was collected by centrifugation (500 × g for 5 minutes) and then treated with red blood cell lysis buffer (Biolegend) for 3 minutes according to the manufacturer’s protocol. The samples were centrifuged after cell lysis, and the resulting cell pellet was diluted with buffer (PBS, 0.5% bovine serum albumin and 2 mM ethylenediamine tetraacetic acid). Then CD4+ T cells were isolated using the Dynabeads Untouched Mouse CD4 Cells Kit (Thermo Fisher Scientific, Asheville, NC).
CD4+ cells were isolated from donor mice 24 hours after US (Figure 5a) or VNS (Figure 5b), and 1 × 104 or 1 × 105 cells (in 200 μl PBS) were injected into recipient mice (naive WT C57BL/6 mice) via tail vein 24 hours before IRI. CD4+ T cells isolated from naive WT (C57BL/6) mice were treated with salbutamol (Sigma-Aldrich; 10 or 100 μM; Figure 5c) or norepinephrine (Sigma-Aldrich; 10 μM, as used in our previous study12; Figure 6f) for 30 minutes at 37° C. After washing the cells with PBS, CD4+ T cells were resuspended in PBS, and 1 × 105 cells (200 μl) were injected into recipient mice (naive WT C57BL/6 mice) via tail vein 24 hours before IRI.
Ischemia-reperfusion injury and splenectomy
Mice were anesthetized with an i.p. injection of ketamine (120 mg/kg) and xylazine (12 mg/kg) and underwent renal ischemia-reperfusion injury (IRI), as described previously.9 Bilateral renal IRI was performed through flank incisions by clamping the renal blood vessels for 26 minutes. The clamps were then removed and the wound sutured after restoration of blood flow was visually observed. Sham-operated mice underwent the same procedure except that the renal vessels were not clamped. Mice were killed for collection of tissue samples after 24 hours of reperfusion. For splenectomy, mice were anesthetized with an i.p. injection of ketamine (120 mg/kg) and xylazine (12 mg/kg). The splenic vasculature was then ligated and the spleen removed through a small flank incision. Sham-treated mice underwent the same procedure with the exception of splenic artery ligation and removal of the spleen. Sham-treated and splenectomized mice were allowed to recover for 7 days prior to IRI studies. Mice received buprenorphine (0.15 mg/kg) as a postoperative analgesic for both IRI and splenectomy.
Plasma creatinine and stereological analysis of tissue morphology
Plasma was prepared by centrifuging heparinized blood at 7000 g for 5 minutes. Plasma creatinine (mg/dl) was determined by using an enzymatic method with minor modifications from the manufacturer’s protocol (using double the sample volume; Diazyme Laboratories, Poway, CA) that has been verified by LC-MS.11
Kidneys were dissected and the capsule was removed. A center transverse section was cut and placed in 4% periodate-lysineparaformaldehyde (4% paraformaldehyde/1.4% DL-lysine/0.2% sodium periodate in 0.1 M sodium phosphate buffer, pH 7.4) for 24 hours and then stored in 70% EtOH until paraffin embedding (UVA Research Histology Core). Paraffin sections (5 μm) were cut and stained with hematoxylin and eosin. The sections were viewed by light microscopy (Zeiss AxioImager Z1/Apotome microscope, Carl Zeiss Microscopy, Thornwood, NY). Photographs were taken with an AxioCam MRc camera (Zeiss) and brightness/contrast and white balance adjustments were made using StereoInvestigator software (MBF Bioscience, Williston, VT).
The extent of acute tubular necrosis was assessed in an unbiased, systematic manner using design-based stereology to achieve statistically accurate random sampling of kidney sections and yielding the percentage of total area of the section occupied by injured tubules, as described previously.11 The investigator was blinded to the experimental identity of the sections. Sections were imaged by using a Zeiss Axio Imager Z1/Apotome Microscope fitted with motorized focus drives and motorized XYZ microscope stage and integrated to a workstation running Stereo Investigator software. The area fraction fractionator probe was used for stereological analysis of the fractional area of the section occupied by tubular necrosis. The following parameters were defined: counting frame, 400 × 400 μm; sample grid, 800 × 800 μm; grid spacing, 85 μm. These values were determined empirically such that adequate numbers of sample sites were visited and adequate numbers of markers (indicating injured tubules) were acquired, in keeping with accepted counting rules for stereology. Injured tubules were identified based on the presence of cast formation, tubule dilation, and/or tubular epithelial denucleation. A total of 271 ± 5.7 (mean ± SEM) grid sites was evaluated per section.
RNA-seq
Peritoneal macrophages, isolated from α7nAChR−/− and α7nAChR+/+ mice as described in the peritoneal macrophage preparation section, were treated with nicotine (10 μM) or vehicle for 15 minutes and then stimulated with LPS (100 ng/ml) or vehicle for 4 hours at 37°C. RNA was isolated from the peritoneal macrophages using the RNeasy Micro Kit (Qiagen, Valencia, CA). Poly(A)-containing mRNA molecules were isolated from total RNA, then converted to cDNA with poly A primers using a TruSeq RNA Sample Preparation kit v2 (Illumina). Sequenced paired-end reads were mapped onto the mouse genome build mm10 using TopHat version 2.0.13,43 and the FPKM (fragments per kilobase of transcript per million mapped fragments) was calculated as an indicator of gene expression level using Cufflinks version 2.2.144 along with the default parameter settings.45 High-throughput sequencing of mRNA was performed using a Hiseq2500 (Illumina) system. For analysis and visualization of the data generated by Cufflinks, we used the R package cummeRbund (http://compbio.mit.edu/cummeRbund/). Based on FPKM data, clustering was performed using Cluster 3.0,46 and a heat map was created with Java TreeView 1.1.47
Availability of data
Data are available from the Gene Expression Omnibus. The RNA-seq data sets are available at accession number GSE103047.
Real-time polymerase chain reaction
RNeasy Mini plus kit (Qiagen) was used for RNA isolation, and RNA concentration was determined based on spectrophometric determination of 260:280 ratio. cDNA was generated from the resultant RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) as described by the manufacturer. Resultant cDNA was then used to determine relative mRNA expression of various genes by real-time polymerase chain reaction using the iTAC Universal SYBR Green Supermix (Bio-Rad). Primer sequences are shown in Supplementary Table S3.
Gene knockdown by small, interfering RNA
RAW 264.7 cells were plated in 24-well cell culture plates with 500 μl medium (DMEM, 10% fetal bovine serum, 1% penicillin-streptomycin) at a density of 3.0 × 105 cells/ml and cultured overnight. The cells were transfected with Silencer Select for Hes1 (Thermo Fisher Scientific, s67463 and s201454) or Silencer Select Negative Control No. 1 siRNA (Thermo Fisher Scientific, 4390843) at a concentration of 5 nM using TransIT-X2 reagent according to the manufacturer’s protocol. At 24 hours after transfection, the cells were used for further analysis. The knockdown efficiencies of Hes1 were validated by quantitative real-time polymerase chain reaction (CFX96, Bio-Rad) using the same primers described in Table S3 (Supplementary Figure S4).
Gene overexpression
RAW 264.7 cells were plated in 24-well cell culture plates with 500 μl medium (DMEM, 10% FBS, 1% penicillin-streptomycin) at a density of 3.0 × 105 cells/ml and cultured overnight. The cells were transfected with Hes1 plasmid (GFP-tagged) (Origene Technologies, Inc., Rockville, MD, MG203818) or empty plasmid (Thermo Fisher Scientific, pcDNA3.1) at a concentration of 1 μg/ml using TransIT-2020 reagent according to the manufacturer’s protocol. At 48 hours after transfection, the cells were used for further analysis. The overexpression of Hes1 was validated by immunocytochemistry.
TNF measurement Plasma
TNF concentration was measured using the mouse TNF alpha ELISA Ready-SET-Go kit (Affymetrix, Santa Clara, CA) as described by the manufacturer. EL× 405 (BioTek Instruments, Winooski, VT) was used as an enzyme-linked immunosorbent assay plate washer. Synergy HTX (BioTek Instruments) was used as an enzyme-linked immunosorbent assay plate reader.
Statistical analysis
Data were analyzed using 1-way or 2-way analysis of variance and Student’s t-test (2-tailed) for experiments with only 2 subgroups and with a significant difference defined as P < 0.05. Repeated experiments were analyzed as a randomized complete block design. Means were compared by post hoc multiple-comparison test (Tukey’s or Sidak’s), and all values are presented as mean ± SEM and as individual values in dot plots. Sham is included only for reference in Figure 1b. All the analyses were performed with GraphPad Prism version 7 (GraphPad Software Inc., La Jolla, CA).
Study approval
All animals were handled and procedures were performed in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the University of Virginia Institutional Animal Care and Use Committee.
Supplementary Material
Figure S1. Summary of our previous findings on protection from acute kidney injury by stimulation of the cholinergic anti-inflammatory pathway. Efferent vagus nerve stimulation (VNS), ultrasound (US), and stimulation of brainstem C1 neurons (either through direct electrical or optogenetic photostimulation or indirectly following acute stress-induced stimulation) applied 24 hours before an ischemic episode protect the kidney from ischemia-reperfusion injury by activating the cholinergic anti-inflammatory pathway (CAP). Vagal afferent stimulation also protects the kidney but through a different and unidentified pathway. C1 stimulation activates the CAP both by activation of the vagus nerve and via a different as yet unidentified pathway. The protective effects by all 3 modalities are lost in splenectomized mice, demonstrating the requirement for the spleen, or in mice deficient in alpha7-nicotinic acetylcholine receptors (α7nAChR–/– mice); protection by US requires CD4+ T cells. Despite similarities in the requirements for the protective effect of the three treatments, the precise target of US is not known, nor is the mechanism by which it activates the CAP. Considerable data from studies of the cholinergic anti-inflammatory pathway support the concept that norepinephrine released from the splenic nerve activates β2-adrenergic receptors (β2ARs) on CD4+ T cells that synthesize the neurotransmitter acetylcholine and that stimulation of these cells results in release of acetylcholine in the spleen. Activation of α7nAChR on macrophages reduces tumor necrosis factor release, hence providing a possible anti-inflammatory mechanism. However, the precise downstream mechanisms by which activation of the cholinergic anti-inflammatory pathway protects kidneys from ischemia-reperfusion injury, including detailed understanding of downstream signaling of α7nAChR in macrophages, are not known. In addition, the requirement for β2ARs in this pathway in kidney protection has not been demonstrated, and possible bidirectional interactions between immune cells have not been investigated. These open questions form the basis for some of the experiments in the current study.
Figure S2. Hes1 expression is induced by nicotine in RAW 264.7 cells. RAW 264.7 cells were treated with lipopolysaccharide (LPS; 100 ng/ml) for 4 hours after nicotine (10 μM) treatment for 15 minutes, and Hes1 expression was evaluated using real-time polymerase chain reaction. n = 5. Data were analyzed using 2-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). *P < 0.05.
Figure S3. Tumor necrosis factor (TNF) induction by lipopolysaccharide (LPS) is suppressed by nicotine treatment in peritoneal macrophages. Peritoneal macrophages from wild-type mice were treated with LPS (100 ng/ml) for 4 hours after nicotine (10 μM) treatment for 15 minutes. Then TNF was measured in the medium by enzyme-linked immunosorbent assay. n = 9. Data were analyzed using 2-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). ***P < 0.001.
Figure S4. The efficiency of small, interfering RNA (siRNA) against Hes1. RAW 264.7 cells were transfected with siRNA against Hes1 for 24 hours, and the efficiency of siRNA was evaluated with real-time polymerase chain reaction.
Figure S5. Hes1 expression is not induced in macrophages from the spleen and kidney after vagus nerve stimulation (VNS). Macrophages were isolated from the spleen and kidney 24 hours after VNS, then Hes1, Arg1, and Chil3 expression were evaluated with real-time polymerase chain reaction. n = 5. Data were analyzed with Student’s t-test (2-tailed). **P < 0.01.
Figure S6. Ultrasound (US) suppresses tumor necrosis factor (TNF) expression and vagus nerve stimulation (VNS) induces expression of an M2 marker in peritoneal macrophages. Peritoneal cells were isolated 24 hours after US or VNS treatment. After peritoneal macrophages were enriched, real-time polymerase chain reaction was performed. US suppressed TNF expression and VNS induced Chil3 (Ym1) expression (an M2 marker) in peritoneal macrophages. n = 10. Data were analyzed with Student’s t-test (2-tailed). *P < 0.05.
Figure S7. Adoptive transfer of 1 × 105 CD11b+ splenocytes from vagus nerve stimulation (VNS)-treated mice protected the kidney from IRI. CD11b+ splenocytes (MACS-enriched) were isolated 24 hours after VNS treatment and 1–10 × 105 cells were adoptively transferred. One day later bilateral ischemia-reperfusion injury (IRI) was performed in the recipient mice. n = 3–6. Data were analyzed using 2-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). **P < 0.01.
Figure S8. Adoptive transfer of nicotine (100 μM)-treated peritoneal macrophages protected the kidney from ischemia-reperfusion injury (IRI). After peritoneal macrophages were treated with different concentrations of nicotine, the cells were adoptively transferred. One day later bilateral IRI was performed in the recipient mice. n = 3. To determine if adoptively transferred cells are subsequently found in spleen or kidney, we injected (i.v.) 1 × 105 peritoneal macrophages labeled with CFSE, performed bilateral IRI 1 day later, and then 24 hours later flow cytometry was performed to determine the number of CFSE-positive cells in the spleen and injured kidney. Unfortunately, CFSE-positive cells were not observed in either organ. In addition, we also did similar experiments using i.p. injection of 1 × 106 peritoneal cells and then assessed CFSE-positive cells over time (4, 12, and 24 hours after IRI). In the latter experiments we also did not observe CFSE-positive cells in the spleen or kidney in any of the experimental conditions. This issue is often raised when considering the marked protective effect of adoptively transferred immune cells; however, our group has found in numerous circumstances that the transferred cells are not detected in the kidney. In similar prior experiments from our laboratory injection of 5 × 106-labeled dendritic cells resulted in localization primarily to the spleen with little to no detectable signal in kidney (Bajwa A, Huang L, Kurmaeva E, et al. Sphingosine 1-phosphate receptor 3-deficient dendritic cells modulate splenic responses to ischemia-reperfusion injury. J Am Soc Nephrol. 2016;27:1076–1090).
Figure S9. Adoptive transfer of nicotine-treated RAW cells protected the kidney from ischemia-reperfusion injury (IRI). RAW 264.7 cells were treated with nicotine (100 μM) and the cells were adoptively transferred. One day later bilateral IRI was performed in the recipient mice. n = 5. Data were analyzed with Student’s t-test (2-tailed). *P < 0.05.
Table S2. 18 genes induced by nicotine in α7WT and suppressed in α7KO mice.
Table S3. Primer sequences for real-time quantitative polymerase chain reaction.
Table S1. Genes highly induced by lipopolysaccharide (top 30).
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) under award nos. R01DK085259, U18EB02178, and R01DK062324 (to MDO), by 2 Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowships for Research Abroad (awarded separately to TI and CA), JSPS KAKENHI 15K21745 (to CA), and the Takeda Science Foundation (to CA). The stereology data described here was gathered on an “MBF Bioscience and Zeiss microscope system for stereology and tissue morphology” funded by NIH grant no. 1S10RR026799-01 (to MDO). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank the University of Virginia Research Histology Core for their assistance in preparation of histology slides, and Akashi Taguchi and Mika Kobayashi at the University of Tokyo for their assistance in preparation of RNA-seq. We also thank Patrice G. Guyenet, Department of Pharmacology, University of Virginia, for very useful discussion about the project.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
Supplementary material is linked to the online version of the paper at www.kidney-international.org.
REFERENCES
- 1.Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. 2017;20:156–166. [DOI] [PubMed] [Google Scholar]
- 2.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421: 384–388. [DOI] [PubMed] [Google Scholar]
- 4.Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113:8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonaz B, Sinniger V, Hoffmann D, et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28:948–953. [DOI] [PubMed] [Google Scholar]
- 6.Goverse G, Stakenborg M, Matteoli G. The intestinal cholinergic anti-inflammatory pathway. J Physiol. 2016;594:5771–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Opland D, Tsai S, et al. Pten deletion in RIP-Cre neurons protects against type 2 diabetes by activating the anti-inflammatory reflex. Nat Med. 2014;20:484–492. [DOI] [PubMed] [Google Scholar]
- 8.Carnevale D, Perrotta M, Pallante F, et al. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat Commun. 2016;7:13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gigliotti JC, Huang L, Ye H, et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol. 2013;24:1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gigliotti JC, Huang L, Bajwa A, et al. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI. J Am Soc Nephrol. 2015;26:2470–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue T, Abe C, Sung SS, et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alpha7nAChR+ splenocytes. J Clin Invest. 2016;126:1939–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe C, Inoue T, Inglis MA, et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nature Neurosci. 2017;20:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. [DOI] [PubMed] [Google Scholar]
- 14.Mehta RL, Burdmann EA, Cerda J, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387:2017–2025. [DOI] [PubMed] [Google Scholar]
- 15.Vida G, Pena G, Deitch EA, et al. alpha7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186:4340–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matteoli G, Gomez-Pinilla PJ, Nemethova A, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63:938–948. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Matsugami T, Chiba T. The origin of sensory innervation of the peritoneum in the rat. Anat Embryol (Berl) 2002;205:307–313. [DOI] [PubMed] [Google Scholar]
- 18.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10: 1216–1221. [DOI] [PubMed] [Google Scholar]
- 20.de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Li Q, Gui H, et al. MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of proinflammatory cytokines. Cell Res. 2013;23:1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, Chung AY, Wu I, et al. Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity. 2008;29:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shang Y, Coppo M, He T, et al. The transcriptional repressor Hes1 attenuates inflammation by regulating transcription elongation. Nat Immunol. 2016;17:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramesh G, Ranganathan P. Mouse models and methods for studying human disease, acute kidney injury (AKI). Methods Mol Biol. 2014;1194: 421–436. [DOI] [PubMed] [Google Scholar]
- 25.Guyenet PG, Stornetta RL, Bochorishvili G, et al. C1 neurons: the body’s EMTs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R187–R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huston JM, Ochani M, Rosas-Ballina M, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosas-Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellinger DL, Lorton D, Hamill RW, et al. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204. [DOI] [PubMed] [Google Scholar]
- 29.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun. 2007;21:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosn EE, Cassado AA, Govoni GR, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107:2568–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassado Ados A, D’Imperio Lima MR, Bortoluci KR. Revisiting mouse peritoneal macrophages: heterogeneity, development, and function. Front Immunol. 2015;6:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosas M, Davies LC, Giles PJ, et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Kubes P. A Reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell. 2016;165: 668–678. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T, Kageyama R. Expression dynamics and functions of Hes factors in development and diseases. Curr Top Dev Biol. 2014;110:263–283. [DOI] [PubMed] [Google Scholar]
- 36.Dhanesh SB, Subashini C, James J. Hes1: the maestro in neurogenesis. Cell Mol Life Sci. 2016;73:4019–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rani A, Greenlaw R, Smith RA, et al. HES1 in immunity and cancer. Cytokine Growth Factor Rev. 2016;30:113–117. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi M, Ang SL, Shiota K, et al. Targeted disruption of mammalian hairy and enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. [DOI] [PubMed] [Google Scholar]
- 39.Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys. 2014;564:83–88. [DOI] [PubMed] [Google Scholar]
- 40.Bolisetty S, Zarjou A, Agarwal A. Heme oxygenase 1 as a therapeutic target in acute kidney injury. Am J Kidney Dis. 2017;69: 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsoyi K, Jang HJ, Kim JW, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 2011;14: 2057–2070. [DOI] [PubMed] [Google Scholar]
- 42.Vida G, Pena G, Kanashiro A, et al. beta2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 2011;25:4476–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trapnell C, Williams BA, Pertea G, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Hoon MJ, Imoto S, Nolan J, et al. Open source clustering software. Bioinformatics. 2004;20:1453–1454. [DOI] [PubMed] [Google Scholar]
- 47.Saldanha AJ. Java Treeview–extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Summary of our previous findings on protection from acute kidney injury by stimulation of the cholinergic anti-inflammatory pathway. Efferent vagus nerve stimulation (VNS), ultrasound (US), and stimulation of brainstem C1 neurons (either through direct electrical or optogenetic photostimulation or indirectly following acute stress-induced stimulation) applied 24 hours before an ischemic episode protect the kidney from ischemia-reperfusion injury by activating the cholinergic anti-inflammatory pathway (CAP). Vagal afferent stimulation also protects the kidney but through a different and unidentified pathway. C1 stimulation activates the CAP both by activation of the vagus nerve and via a different as yet unidentified pathway. The protective effects by all 3 modalities are lost in splenectomized mice, demonstrating the requirement for the spleen, or in mice deficient in alpha7-nicotinic acetylcholine receptors (α7nAChR–/– mice); protection by US requires CD4+ T cells. Despite similarities in the requirements for the protective effect of the three treatments, the precise target of US is not known, nor is the mechanism by which it activates the CAP. Considerable data from studies of the cholinergic anti-inflammatory pathway support the concept that norepinephrine released from the splenic nerve activates β2-adrenergic receptors (β2ARs) on CD4+ T cells that synthesize the neurotransmitter acetylcholine and that stimulation of these cells results in release of acetylcholine in the spleen. Activation of α7nAChR on macrophages reduces tumor necrosis factor release, hence providing a possible anti-inflammatory mechanism. However, the precise downstream mechanisms by which activation of the cholinergic anti-inflammatory pathway protects kidneys from ischemia-reperfusion injury, including detailed understanding of downstream signaling of α7nAChR in macrophages, are not known. In addition, the requirement for β2ARs in this pathway in kidney protection has not been demonstrated, and possible bidirectional interactions between immune cells have not been investigated. These open questions form the basis for some of the experiments in the current study.
Figure S2. Hes1 expression is induced by nicotine in RAW 264.7 cells. RAW 264.7 cells were treated with lipopolysaccharide (LPS; 100 ng/ml) for 4 hours after nicotine (10 μM) treatment for 15 minutes, and Hes1 expression was evaluated using real-time polymerase chain reaction. n = 5. Data were analyzed using 2-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). *P < 0.05.
Figure S3. Tumor necrosis factor (TNF) induction by lipopolysaccharide (LPS) is suppressed by nicotine treatment in peritoneal macrophages. Peritoneal macrophages from wild-type mice were treated with LPS (100 ng/ml) for 4 hours after nicotine (10 μM) treatment for 15 minutes. Then TNF was measured in the medium by enzyme-linked immunosorbent assay. n = 9. Data were analyzed using 2-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). ***P < 0.001.
Figure S4. The efficiency of small, interfering RNA (siRNA) against Hes1. RAW 264.7 cells were transfected with siRNA against Hes1 for 24 hours, and the efficiency of siRNA was evaluated with real-time polymerase chain reaction.
Figure S5. Hes1 expression is not induced in macrophages from the spleen and kidney after vagus nerve stimulation (VNS). Macrophages were isolated from the spleen and kidney 24 hours after VNS, then Hes1, Arg1, and Chil3 expression were evaluated with real-time polymerase chain reaction. n = 5. Data were analyzed with Student’s t-test (2-tailed). **P < 0.01.
Figure S6. Ultrasound (US) suppresses tumor necrosis factor (TNF) expression and vagus nerve stimulation (VNS) induces expression of an M2 marker in peritoneal macrophages. Peritoneal cells were isolated 24 hours after US or VNS treatment. After peritoneal macrophages were enriched, real-time polymerase chain reaction was performed. US suppressed TNF expression and VNS induced Chil3 (Ym1) expression (an M2 marker) in peritoneal macrophages. n = 10. Data were analyzed with Student’s t-test (2-tailed). *P < 0.05.
Figure S7. Adoptive transfer of 1 × 105 CD11b+ splenocytes from vagus nerve stimulation (VNS)-treated mice protected the kidney from IRI. CD11b+ splenocytes (MACS-enriched) were isolated 24 hours after VNS treatment and 1–10 × 105 cells were adoptively transferred. One day later bilateral ischemia-reperfusion injury (IRI) was performed in the recipient mice. n = 3–6. Data were analyzed using 2-way analysis of variance. Means were compared by post hoc multiple comparison test (Tukey’s). **P < 0.01.
Figure S8. Adoptive transfer of nicotine (100 μM)-treated peritoneal macrophages protected the kidney from ischemia-reperfusion injury (IRI). After peritoneal macrophages were treated with different concentrations of nicotine, the cells were adoptively transferred. One day later bilateral IRI was performed in the recipient mice. n = 3. To determine if adoptively transferred cells are subsequently found in spleen or kidney, we injected (i.v.) 1 × 105 peritoneal macrophages labeled with CFSE, performed bilateral IRI 1 day later, and then 24 hours later flow cytometry was performed to determine the number of CFSE-positive cells in the spleen and injured kidney. Unfortunately, CFSE-positive cells were not observed in either organ. In addition, we also did similar experiments using i.p. injection of 1 × 106 peritoneal cells and then assessed CFSE-positive cells over time (4, 12, and 24 hours after IRI). In the latter experiments we also did not observe CFSE-positive cells in the spleen or kidney in any of the experimental conditions. This issue is often raised when considering the marked protective effect of adoptively transferred immune cells; however, our group has found in numerous circumstances that the transferred cells are not detected in the kidney. In similar prior experiments from our laboratory injection of 5 × 106-labeled dendritic cells resulted in localization primarily to the spleen with little to no detectable signal in kidney (Bajwa A, Huang L, Kurmaeva E, et al. Sphingosine 1-phosphate receptor 3-deficient dendritic cells modulate splenic responses to ischemia-reperfusion injury. J Am Soc Nephrol. 2016;27:1076–1090).
Figure S9. Adoptive transfer of nicotine-treated RAW cells protected the kidney from ischemia-reperfusion injury (IRI). RAW 264.7 cells were treated with nicotine (100 μM) and the cells were adoptively transferred. One day later bilateral IRI was performed in the recipient mice. n = 5. Data were analyzed with Student’s t-test (2-tailed). *P < 0.05.
Table S2. 18 genes induced by nicotine in α7WT and suppressed in α7KO mice.
Table S3. Primer sequences for real-time quantitative polymerase chain reaction.
Table S1. Genes highly induced by lipopolysaccharide (top 30).
Data Availability Statement
Data are available from the Gene Expression Omnibus. The RNA-seq data sets are available at accession number GSE103047.


