Abstract
The recent outbreak of a novel coronavirus SARS-CoV-2 has posed a significant global public health threat and caused dramatic social and economic disruptions. A new research direction is attracting a significant amount of attention in the academic community of environmental sciences and engineering, in which rapid community-level monitoring could be achieved by applying the methodology of wastewater based epidemiology (WBE). Given the fact that the development of a mass balance on the total number of viral RNA copies in wastewater samples and the infected stool specimens is the heart of WBE, the result of the quantitative RNA detection in wastewater has to be highly sensitive, accurate, and reliable. Thus, applying effective concentration methods before the subsequent RNA extraction and RT-qPCR detection is a must-have procedure for the WBE. This review provides new insights into the primary concentration methods that have been adopted by the eighteen recently reported COVID-19 wastewater detection studies, along with a brief discussion of the mechanisms of the most commonly used virus concentration methods, including the PEG-based separation, electrostatically charged membrane filtration, and ultrafiltration. In the end, two easy and well-proven concentration strategies are recommended as below, aiming to maximize the practical significance and operational effectiveness of the SARS-CoV-2 virus concentration from wastewater samples.
Strategy1: Prefiltration-Salt addition-Electronegative membrane filtration (for initial volume ≤ 50 mL).
Strategy2: Prefiltration-PEG-based separation-Overnight standing (for initial volume from 50 to 1000 mL).
Keywords: COVID-19 wastewater based epidemiology, Primary concentration, PEG-based separation, Electronegative membrane filtration, Ultrafiltration, Precondition
Graphical abstract
Highlights
-
•
Discuss the potential advantages and disadvantages of commonly used virus concentration methods
-
•
Focus on the recently reported analytical studies of the SARS-CoV-2 RNA in wastewater samples
-
•
Compare the performance of the reported studies based on efficiency, easy to access, and operate
-
•
Recommend two methods of electronegative membrane filtration and PEG-based separation
Nomenclature
- COV1D-19
coronavirus disease 2019
- MgV
mengovirus
- MHV
murine hepatitis virus
- PEDV
porcine epidemic diarrhea virus
- PEG
polyethylene glycol
- RSD
relative standard deviation
- RT-qPCR
real-time quantitative polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- U.S. EPA (or EPA)
U.S. Environmental Protection Agency
- VIRADEL
Viruses ADsorption–ELution
- WBE
wastewater based epidemiology
- WHO
World Health Organization
1. Introduction
The recent outbreak of a novel coronavirus SARS-CoV-2, also known as the COVID-19 disease, has posed a significant global public health threat and causes dramatic social and economic impacts. As of June 19, 2020, the World Health Organization (WHO) has reported more than 14,000,000 infected people globally (WHO, 2020). As a completely novel human coronavirus, the response of the global public health authorities and the academic communities to the COVID-19 demonstrated an inevitable lag due to the lack of understanding of the SARS-CoV-2 virus as well as rapid detection methods. Indeed, the conventional epidemiology highly relies on the systematic diagnosis of excretion samples and clinical symptoms, which is inherently difficult for the detection of a highly infectious disease from a large population in a timely manner.
In 2001, a concept, namely wastewater based epidemiology (WBE), was first proposed to estimate the drug abuse situation in a community by analyzing the pharmaceutical concentration in wastewater (Daughton and Jones-Lepp, 2001). As described by Daughton and Jones-Lepp (2001), any substance excreted by humans in wastewater could be traced back to its initial source concentration, as long as the substance or its metabolites are stable in wastewater to some degree. A similar concept of WBE has been previously adopted to evaluate the prevalence of infectious diseases caused by pathogens. For example, Kazama et al. (2017) successfully conducted a cross-correlation analysis between the concentrations of norovirus GI or GII in weekly collected sewage samples and the reported number of gastroenteritis cases. As a result, applying the methodology of WBE to achieve rapid community-level monitoring of the COVID-19, along with the fast-rising public awareness and emerging scientific studies, has attracted a significant amount of attention. The development of a rapid and economical WBE can facilitate and empower a local public health authority to achieve real-time surveillance and future outbreak prediction of the COVID-19 pandemic (Lesté-Lasserre, 2020; Mallapaty, 2020; Murakami et al., 2020; Sims and Kasprzyk-Hordern, 2020).
There are some critical challenges that we have to overcome before WBE can be applied to accurately reflect the real-time situation of the COVID-19 pandemic within a sewer-delimited community. Among all the challenges, the most immediate one is the relatively low viral particle loadings in large volumes of wastewater (Ikner et al., 2012). Thus, to obtain accurate results with statistical significance, applying effective concentration methods before the subsequent extraction and detection of virus particles is a must-have procedure for WBE. As a result, the primary concentration method is the core process because it has the most significant influence over a complete concentration process. For example, an early method for concentrating poliovirus from various water samples was developed using a soluble material of sodium alginate to build filters to concentrate viral particles (Gaertner, 1967; Nupen, 1970). After filtration, the adsorbed filters will then be dissolved in a small volume of sodium citrate to elute the viral particles and subsequently conducted cell culture infectivity assays for virus detection. It is clear that the development of the soluble sodium alginate filters as the primary concentration method directly determines the next elution step to must adopt sodium citrate as the eluting reagent. Furthermore, due to the nature of soluble alginate filter materials, water samples with high turbidities, such as wastewater, have to conduct pretreatment by passage through a series of filters to reduce the clogging risk during the final virus concentration.
In summary, the characteristics of a primary concentration method can exert significant influence on the overall concentration process by determining if the subsequent extraction and detection method can be successfully applied to fulfill the goals of WBE. According to a recent review, some early developed methods that have been historically used to concentrate enteric viruses from wastewater samples may be subject to some technical issues, due to the SARS-CoV-2 virus is relatively unstable in the environment and is more susceptible to common oxidants (La Rosa et al., 2020a). Therefore, an effective concentration strategy has to be selected or developed in the first place. Given that many relevant techniques have been developed and refined over the past decades, this mini-review discusses the potential advantages and disadvantages of different concentration strategies with an emphasis on the primary concentration methods and the suitability for the COVID-19 WEB. Meanwhile, this mini-review puts a particular focus on several existing analytical studies of the SARS-CoV-2 virus in the wastewater matrix and evaluate their concentration strategies before the subsequent extraction and detection processes. At last, two concentration strategies are recommended to maximize the practical significance and operational effectiveness of the SARS-CoV-2 virus concentration from wastewater samples.
2. Commonly used primary concentration methods
A number of methods have been developed to effectively concentrate viral particles from water samples. Based on various concentrating mechanisms, we can classify all the different primary concentration methods into four broad types, namely (1) two-phase separation/partition precipitation, (2) particle exclusion, (3) VIruses ADsorption–ELution (VIRADEL), and (4) ultrafiltration. It is worth mentioning that the VIRADEL method using electrostatically charged microporous materials as filtration media is the most prevalent technique used today (Ikner et al., 2012), which can be further categorized as electronegative membrane filtration and electropositive membrane filtration based on surface charge differences (see Table 1 ). Along with the continuous evolution of the primary concentration methods, many of early methods have been scarcely used or phased out due to some inherent disadvantages, such as small treatment volumes (hydroextraction), poor recovery performance (cotton gauze pads), and excessive pretreatment processes (soluble membrane filtration). In this section, three of the most commonly used methods (i.e., PEG-based two-phase separation, VIRADEL method, and ultrafiltration) will be briefly discussed and compared on the basis of recovery performance, ease of operation, and consistency of concentrating outcome. Also worth noting is that the PEG-based two-phase separation and the VIRADEL method currently are the only two standard methods adopted by the World Health Organization (WHO/V-B03.03) and the U.S. Environmental Protection Agency (EPA Method 1615) respectively.
Table 1.
Primary concentration method for concentrating viruses from aqueous samples.
| Type | Technique | Advantages | Disadvantages | Representative references |
|---|---|---|---|---|
| Two-phase separation/partition/precipitation | Aqueous polymer separation (PEG) | High recovery rate; useful over a broad spectrum of water quality matrices | Small treatment volumes; inhibition of virus activity | (Adams, 1973; Colombet et al., 2007; Lewis and Metcalf, 1988; WHO, 2003) |
| Multistage ultracentrifugation (1000 to ≥100,000 ×g) | Differentiation of diverse virus types; Non-cytotoxic | Small treatment volumes; large-scale instrument required; excessive processing time | (Fumian et al., 2010; Gias et al., 2008) | |
| Dialysis separation (hydroextraction) | Preferable recovery rate | Small treatment volumes; inhibition of virus activity | (Ramia and Sattar, 1979; Ramia and Sattar, 1980) | |
| Metallic oxides/peroxides/salts precipitation | Large treatment volumes | Pretreatment required for raw aqueous samples; excessive chemical consumptions | (Farrah et al., 1986; Preston and Farrah, 1991) | |
| Particle exclusion | Soluble membrane filtration (alginate) | Non-cytotoxic; preferable recovery rate | Small treatment volumes; pretreatment required for raw aqueous samples | (Gaertner, 1967; Nupen, 1970) |
| Cotton gauze pads filtration | Large treatment volumes; inexpensive | Low recovery rate; poor quantitative performance | (Hill et al., 1971; Liu et al., 1971) | |
| VIRADELa | Electronegative membrane filtration | Large treatment volumes; Preferable recovery rate | Preconditioning required; highly sensitive to organic matters in aqueous samples | (Chalapati Rao et al., 1972; Haramoto et al., 2004; Hata et al., 2015) |
| Electropositive membrane filtration | Large treatment volumes; preferable recovery rate; no need of preconditioning | Highly sensitive to organic matters in aqueous samples | (Hsu et al., 2007; Ikner et al., 2011; Lambertini et al., 2008) | |
| Ultrafiltration | Crossflow/centrifugal | High recovery rate; consistent performance; no need of eluting | Addition of blocking solution required (e.g., glycine or beef extract); backflushing may be needed | (Divizia et al., 1989; Hill et al., 2007; Liu et al., 2012; Rajal et al., 2007; Winona et al., 2001) |
VIRADEL stands for VIruses ADsorption–ELution.
2.1. Polyethylene glycol (PEG) based two-phase separation
Using polyethylene glycol (PEG) based two-phase separation on concentrating viruses between two immiscible aqueous polymer phases was first proposed by Albertsson and Frick (1960). During the 1960s and 1970s, the method of aqueous polymer two-phase separation was continuously refined for the purpose of concentrating enteroviruses over a broad spectrum of water quality matrices, such as river water, tap water, groundwater, and wastewater (Shuval et al., 1969; Wallis et al., 1969). Based on the result reported by Shuval et al. (1969), after mixing with a combination of organic polymers (i.e., dextran and PEG), a sewage sample of several liters can be successfully concentrated for subsequent viruses detection with an average nominal concentration factor of 220-fold and an average recovery efficiency of 72%. The PEG-based separation method was eventually adopted by the WHO as a standard method for conducting environmental surveillance of poliovirus circulation (WHO, 2003). In summary, the PEG-based separation method has been extensively studied to concentrate and purify various viruses (e.g., bacteriophage T2, adenovirus, poliovirus, and ECHO virus) and found to be rapid, inexpensive, consistent, and non-destructive of viruses. In addition, commercial PEG Virus Precipitation Kit (BioVision) has recently become available as a convenient, affordable alternative (~$4–$6 per unit). However, due to the limited size of most separation funnels (maximum for several liters), the PEG-based separation method has an apparent drawback, which is only useful for concentrating samples with a relatively small volume (as shown in Fig. 1a). As a result, a raw water sample must contain a moderate to high viruses loading to ensure enough viruses get concentrated from the limited initial treatment volume.
Fig. 1.

Illustration of (a) polyethylene glycol (PEG) based two-phase separation (image adapted from Shuval et al. (1969)), (b) electropositive membrane filtration system (figure adapted from Fout et al. (2015)), and (c) ultrafiltration system (figure adapted from Shi (2017)) on extracting viruses from water samples.
2.2. Electrostatically charged membranes method
The first attempt of the VIRADEL method to concentrate enterovirus can be traced back to the 1960s when Wallis and Melnick (1967) found the addition of sodium chloride (NaCl) or magnesium chloride (MgCl2) can lead to the adsorption of poliovirus on cellulose nitrate HA membranes (0.45 μm). Superior performances of virus concentration were achieved with much less addition of trivalent aluminum salts (AlCl3 and Al2(SO4)3) when controlling the pH to below 5 (Wallis et al., 1972). This phenomenon is mainly associated with the adsorption of metal ions to the electronegatively charged surface of cellulose nitrate HA membranes, thereby facilitating the attachment of polioviruses via salt-bridging (Ikner et al., 2012). Although the electronegatively charged membranes can effectively extract viruses from water samples, the addition of polyvalent salts makes it difficult for concentrating large volume samples. As a result, a number of early studies tried in situ charge modification of electronegative filters and successfully developed electropositive membrane filtration method, the most common technique (i.e., VIRADEL) currently used for concentrating viruses from water (Farrah and Preston, 1985; Haramoto et al., 2004; Sobsey and Jones, 1979; Zerda et al., 1985). According to the standard method (i.e., EPA Method 1615), electropositive filters (e.g., NanoCeram and 1MDS) are capable of continuously extracting enterovirus and norovirus from groundwater and surface water with the minimum specified volumes of 300 L and 1500 L, respectively (see Fig. 1b) (Fout et al., 2015). However, the presence of organic matter can lead to significantly reduced efficiency of virus concentration due to a preferential attachment of dissolved organic molecules over virus particles. In addition to the presence of organic matter, high water turbidity (≥50 NTU) can directly lead to the failure of sampling when applying the EPA Method 1615 with the recommended NanoCeram virus sampler (Fout et al., 2015). It is, therefore, inappropriate to apply the EPA Method 1615 directly to concentrate virus particles from raw wastewater samples that are usually turbid and contain a substantial amount of dissolved and suspended organic matter.
2.3. Ultrafiltration
Unlike the PEG-based separation and electropositive membrane filtration, using ultrafiltration to extract viruses will not be subject to preconditioning of the water samples, which allows this method to be used over a broad range of water quality (Hill et al., 2007). The earliest study that used ultrafiltration to concentrate poliovirus from a water sample was designed using multiple layers of asymmetric cellulose acetate membranes to separate flow channels for raw water samples and driving solution, and achieved recovery efficiency of 95–100% from a large volume of water of 10 L (Sweet et al., 1971). Later studies using polysulfone hollow fiber ultrafilters demonstrated better resistance to pH and temperature variations, biological degradation, and showed less penetration of target virus particles due to the dense inner surface (Ikner et al., 2012). In terms of the ultrafiltration flow types, tangential crossflow ultrafiltration is a commonly used configuration, which can effectively prevent fouling due to the rapid, tangentially directed crossflow. It is, therefore, particularly suited for extracting viruses from lower quality water samples such as wastewater (Ikner et al., 2012). Although tangential flow crossflow ultrafiltration can provide superior virus recoveries from low-quality water, the equipment required for conducting crossflow ultrafiltration is usually large, immobile, and not field portable (Hill et al., 2007).
3. Enveloped and non-enveloped viruses
It is clear that the primary concentration methods described above are all developed and refined to concentrate the non-enveloped enteric viruses, which are naturally acclimated to the water environment and capable of causing waterborne infectious disease via fecal-oral route (Ikner et al., 2012). Compared with the non-enveloped viruses, the enveloped viruses possess outer surface structures consisting of different functional groups, which may significantly impact their survival and partitioning behavior in an aqueous environment (Ye et al., 2016). An early study focusing on the evaluation of the survivability of an enveloped human coronavirus (HCoV) in the wastewater environment, reported that the time required for the HCoV titer to decrease by 99.9% (T99.9) is substantially shorter than the non-enveloped poliovirus 1 (PV-1) (Gundy et al., 2008). In terms of the primary concentration performance, as shown in Table 2 , under the same conditions, most of the enveloped viruses showed relatively lower recovery rates compared to the non-enveloped viruses. For example, with the optimized ultrafiltration method, Ye et al. (2016) reported mean recoveries of 25.1 ± 3.6% and 18.2 ± 9.5% for the enveloped murine hepatitis virus (MHV) and Pseudomonas phage ϕ6, respectively. Meanwhile, the non-enveloped Enterobacteria phage T3 and MS2 demonstrated significantly higher mean recoveries of 55.6 ± 16.7% and 85.5 ± 24.5%, respectively, when using the same ultrafiltration method. In Randazzo et al.'s (2020) study, the aluminum hydroxide precipitation method showed the closest primary concentration recovery when concentrating wastewater influent samples with spiked porcine epidemic diarrhea virus (PEDV), an enveloped virus member of the Coronaviridae family, and mengovirus (MgV), a non-enveloped member of the Picornaviridae family, of 11 ± 3.5 and 11 ± 2.1%, respectively. However, the overall low recovery rate (~3 to 11%) may hinder this method to be widely adopted for the detection of the SARS-CoV-2 virus.
Table 2.
Recovery performance of concentrating enveloped and non-enveloped viruses.
| Virus (type) | Initial sampling matrix | Concentration method | Recovery rate (%) | Reference |
|---|---|---|---|---|
| SARS-CoVa (enveloped) | Hospital and domestic sewage | Silica gel with Al(OH)3 (Electropositive filtration) | 0–21.4 | (Wang et al., 2005) |
| Bacteriophage f2 (non-enveloped) | 33.6–100 | |||
| MHVb (enveloped) | WWTP influent (prior to primary settling tank) | Centrifugal ultrafiltration | 25.1 ± 3.6 | (Ye et al., 2016) |
| Pseudomonas phage ϕ6 (enveloped) | 18.2 ± 9.5 | |||
| Enterobacteria phage T3 (non-enveloped) | 55.6 ± 16.7 | |||
| Enterobacteria phage MS2 (non-enveloped) | 85.5 ± 24.5 | |||
| PEDVc (enveloped) | WWTP untreated influent | Al(OH)3 precipitation | 11 ± 3.5 | (Randazzo et al., 2020) |
| MgVd (non-enveloped) | 11 ± 2.1 | |||
| PEDV (enveloped) | WWTP secondary & tertiary effluent | 3.3 ± 1.6 | ||
| MgV (non-enveloped) | 6.2 ± 1.0 |
SARS-CoV stands for severe acute respiratory syndrome coronavirus.
MHV stands for murine hepatitis virus.
PEDV stands for porcine epidemic diarrhea virus.
MgV stands for mengovirus.
Due to the characteristics of the enveloped viruses, including the SARS-CoV-2 virus, most of the concentration methods tend to result in a higher decay or inactivation for the enveloped viruses than the non-enveloped viruses. Another risk we face is the reduced viability and infectivity of the virus after a concentrating process. The increased sensitivity of the molecular detection method (i.e., RT-qPCR) has partially alleviated the problem of reducing the viability and infectivity of the viruses because the quantitative determination is no longer based on the traditional cell culture infectivity assays, which heavily relied on the viability, infectivity, and structural integrity of the concentrated viruses (Ikner et al., 2012). However, it is critical to recognize the technical capabilities/limitations of all the processes and techniques used so we can optimize each step involved to help minimize the loss of the original characteristics of the samples.
4. Primary concentration methods used for the COVID-19 WBE
Based on the review of all the currently published COVID-19 studies, there are 18 relevant wastewater SARS-CoV-2 virus detection studies that have been conducted in different epidemic areas over the world, including France, Japan, Israel, Italy, Australia, Netherlands, Spain, India, and Singapore. After carefully identifying the methodologies of these articles, we found the majority of them (17 out of 18) applied primary concentration methods to concentrate the SARS-CoV-2 virus particles or the genomic fragments from the wastewater samples (see Table 3 ).
Table 3.
Primary concentration method for detection of the SARS-CoV-2 virus in wastewater.
| Type | Primary concentration method | Initial volume (L) | Preconditioning method | References |
|---|---|---|---|---|
| PEG-based two-phase separation | PEG-based separation (20 mg/L) followed by ultracentrifugation at 14,000g for 45 min | 0.25–1 | Centrifugation to remove sediment and particles | (Bar Or et al., 2020) |
| PEG-based separation (100 g/L) followed by ultracentrifugation at 14,000g for 30 min | 0.08 | Centrifugation to remove sediment and particles | (Hata et al., 2020) | |
| PEG-based separation (29% (w/w)) followed by stand overnight at 4 °C in a separation funnel | 0.25 | Centrifugation and retaining the resulted pellet for further elution | (La Rosa et al., 2020b) | |
| PEG-based separation (8% (w/v)) followed by ultracentrifugation at 12,000g for 2 h | 0.04 | Prefiltration through 0.2 μm membrane | (Wu et al., 2020) | |
| PEG-based separation (20% (w/v) PEG-6000) | 0.1 | Non-pretreated | (Chavarria-Miró et al., 2020) | |
| (f) PEG-based separation (PEG 10% (w/v)) followed by ultracentrifugation at 10,000g for 30 min | 0.05 | Centrifugation at 10,000g for 20 min, pellet resuspension in beef extract and centrifugation at 10,000g for 10 min | (Ahmed et al., 2020b)a | |
| PEG-based separation (80 g/L of PEG9000) and NaCl (17.5 g/L) followed by ultracentrifugation at 13,000g for 1.5 h | 0.05 | Centrifugation at 4500g followed by filtration of supernatant through 0.22 μm membrane | (Kumar et al., 2020) | |
| Electronegative membrane filtration | Electronegative membrane filtration (HAWP09000 membrane 0.45 μm) | 0.1–0.2 | Adjusting the sample pH to 3.5–4 using 2.0 N HCl | (Ahmed et al., 2020a) |
| Electronegative membrane filtration (cellulose-ester membrane 0.8 μm) | 0.2 | Addition of 2 mL of 2.5 M MgCl2 | (Haramoto et al., 2020) | |
| Electronegative membrane filtration (HAWP04700 membrane 0.45 μm) | 0.05 | (a) Acidification to pH 4.0; (b) non-pretreated; (c) addition of MgCl2 | (Ahmed et al., 2020b)a | |
| Ultrafiltration | Ultrafiltration with 100 kDa molecular weight cut-off | 0.5 | Prefiltration sequentially through mixed membranes (20, 5, 0.45 μm) | (Nemudryi et al., 2020) |
| Centrifugal ultrafiltration with 100 kDa molecular weight cut-off at 1500g for 15 min | 0.1–0.2 | Centrifugation to remove sediment and particles | (Medema et al., 2020) | |
| Centrifugal ultrafiltration with 10 kDa molecular weight cut-off | 0.015 | Prefiltration to remove sediment and particles | (Wu et al., 2020) | |
| (d) Centrifugal ultrafiltration with 30 kDa molecular weight cut-off at 4750g for 10 min; (e) centrifugal ultrafiltration with 10 kDa molecular weight cut-off at 3500g for 30 min | 0.05 | Centrifugation of the sample at 4500g for 10 min at 4 °C to obtain a supernatant | (Ahmed et al., 2020b)a | |
| others | Al(OH)3precipitation (0.009 N) followed by centrifugation at 1700g for 20 min | 0.2 | Adjusting the sample pH to 6.0 | (Randazzo et al., 2020) |
| Ultracentrifugation at 200,000g for 1 h at 4 °C | 0.011 | Homogenization | (Wurtzer et al., 2020) | |
| (g) Ultracentrifugation at 100,000g for 1 h at 4 °C followed by resuspension of pellet in glycine buffer and then ultracentrifugation at 12,000g for 15 min at 4 °C | 0.05 | Non-pretreated | (Ahmed et al., 2020b)a | |
| Not implemented any primary concentration method | 0.5b | Prefiltration sequentially through glass fiber filters of 0.7 and 0.2 μm | (Rimoldi et al., 2020) | |
Underlines are present to emphasize the specific methods that have been adopted.
Murine hepatitis virus (MHV), was used to test the efficiency of seven wastewater virus concentration methods: (a, b, c) electronegative membrane with three different pretreatment options, (d, e) centrifugal ultrafiltration with two molecular weight cut-off, (e) PEG-based two-phase separation, and (g) ultracentrifugation (Ahmed et al., 2020b).
Sampling volume based on the container size mentioned in the reference (Rimoldi et al., 2020).
4.1. The applied PEG-based separation method
The PEG-based separation is the most used technique (7 out of 18) among all concentration methods, and all four studies that adopted this concentration method showed positive results regarding the SARS-CoV-2 detection in wastewater samples (Bar Or et al., 2020; Hata et al., 2020; La Rosa et al., 2020b; Wu et al., 2020). As summarized in Table 3, most of the studies that implemented the PEG-based separation method (6 out of 7) also had additional follow-up processes, such as ultracentrifugation and overnight standing, to further condense the obtained PEG layer. Although ultracentrifugation can significantly reduce the final water content in the obtained PEG layer, the initial cost to obtain an ultracentrifuge may pose a cost-prohibitive obstacle for many analytical laboratories, especially for those in the wastewater treatment plants that typically can only conduct routine wastewater analyses. In comparison, overnight standing at 4 °C in a separation funnel maybe not as effective as the immediate ultracentrifugation. However, this approach is significantly easier to achieve because it only requires a refrigerator, which is readily available in most of the laboratories. In terms of the preconditioning process, most of the studies using the PEG-based separation method (5 out of 7) had applied the centrifugation method to remove sediment and large particles from raw wastewater samples. Similarly, centrifugation is a fast and effective method to precondition a raw wastewater sample, but it requires sizeable centrifuges when handling a relatively large volume of wastewater samples. Thus, we believe the prefiltration method adopted by Wu et al. (2020) using 0.2 μm membranes is more feasible and accessible than centrifugation. It should be noted that the prefiltration method may capture viruses that were previously attached to large particles during the preconditioning process, and therefore, retaining the resulted cake for the subsequent elution together with the separated PEG-layer is highly recommended.
4.2. The applied electronegative membranes filtration method
Among all the SARS-CoV-2 detection studies, the electronegative membranes filtration technique was adopted for primary concentration in 3 of the 18 studies. In terms of the preconditioning method, these three studies are highly representative of the current development of the electronegative membrane filtration technique. Specifically, Ahmed et al. (2020a) used the simplified acidification method to impart a positive electrical double layer around the negatively charged virus particles. Haramoto et al. (2020) added a high concentration of magnesium chloride (MgCl2) to facilitate the attachment of virus particles via salt-bridging. Based on the results reported by Ahmed et al. (2020a), the SARS-COV-2 RNA was occasionally detected in some of the wastewater samples, and none of the wastewater samples showed any RT-qPCR inhibition. In addition, Haramoto et al. (2020) tested the efficiency of the electronegative membranes filtration method by using pepper mild mottle virus (PMMoV) as a detection biomarker and resulted in high RNA results of 2.6 × 106 copies/L. As previously mentioned, using electrostatically charged membranes filtration to concentrate viruses from turbid water, such as raw wastewater, can be subject to a significant reduction of virus recovery efficiency due to the presence of organic matter and high turbidity, which can lead to a preferential attachment to the charged filters and raise the risk of detrimental clogging.
However, in these studies, there is no compelling evidence to suggest that the organic materials from wastewater have a severe impact on the virus adsorption. This phenomenon is probably attributed to the relatively small volume that both studies involved, therefore alleviated the adverse impact from the high organic content in the wastewater samples.
4.3. The applied ultrafiltration method
We found four studies that implemented the ultrafiltration technique as the primary concentration strategy. Among them, two studies chose 100 kDa as the molecular weight cut-off (Medema et al., 2020; Nemudryi et al., 2020), and the other two studied chose to adopt a much smaller molecular weight cut-off, such as 10 kDa and 30 kDa (Ahmed et al., 2020b; Wu et al., 2020). Interestingly, all these studies employed, to some extent, precondition step, which is unnecessary when using the ultrafiltration technique to conduct the primary concentration (Fout et al., 2015; Rajal et al., 2007). For example, Nemudryi et al. (2020) pretreated all wastewater samples by sequentially filtering through 20 μM, 5 μM (Sartorius Biolab Products), and 0.45 μM (Pall Corporation) membrane filters, and then concentrated down the obtained supernatant from 500 mL to 150 μL using ultrafiltration. The extensive amount of preconditioning process not only increased the workload but also increased the chance of experimental batch effect and systematic error. Thus, the necessity of including precondition needs to be carefully identified when choosing ultrafiltration as the primary concentration method. It is worth noting that according to the RT-qPCR result reported by Medema et al. (2020), the detected gene copies in all the wastewater treatment plants showed evident increase along with the increase of the cumulative number of the reported COVID-19 clinical cases. Indeed, the strong correlation between the detected virus concentration in wastewater and the reported clinical cases is determined simultaneously by a combination of various critical factors, such as the viral shedding pattern in stool and viral decay rate in sewage (Hart and Halden, 2020). However, it should not be ignored the ultrafiltration method used by Hart and Halden (2020) is one of the essential requisites which played vital roles in this successful effort.
Collectively, all concentration methods showed positive results regarding the SARS-CoV-2 virus detection in wastewater. In terms of treatment volume, all studies tend to work with a small amount (e.g., less than 1 L), which is likely due to the risk of potential enteric transmission of the COVID-19 in a wastewater sample. Also, handling a large volume of wastewater in an epidemic area may pose a significant infectious risk to the research team members. Another critical factor in the wastewater virus concentration lies in the degree of the primary concentration efficiency (i.e., the recovery rate of the primary concentration step). A superior concentration efficiency indicates a relatively small portion of virus loss during the concentrating procedure, ensuring a higher sensitivity of the overall detection process. Most importantly, the final concentration of the virus in a wastewater sample has to be calculated by dividing the subsequent RT-qPCR result by the primary concentration efficiency. Technically, spiking of a surrogate virus (e.g., F-specific RNA phage) with known concentrations as an internal reference is the most common practice to obtain this critical efficiency information. After screening all the found studies, five different research teams did employ internal reference(s) to facilitate the analysis of the primary concentration efficiency, and the result is presented in Table 4 .
Table 4.
Evaluation of the primary concentration efficiency with different bioindicators.
| Primary concentration type | Selected surrogate virus | Concentration performance (%) | Reference |
|---|---|---|---|
| Al(OH)3 precipitation | PEDV and MgVa | 11 ± 2.1 and 11 ± 3.5b | (Randazzo et al., 2020) |
| Centrifugal ultrafiltration | F-specific RNA phage | 73 ± 50 | (Medema et al., 2020) |
| PEG-based separation | F-specific RNA phage | 57c | (Hata et al., 2020) |
| Electronegative membrane filtration | PMMoVd | 71.6 ± 25.2 | (Haramoto et al., 2020) |
| Electronegative membrane filtration (w/ different pretreatments) | MHVe | 26.7 ± 15.3 (acidification) 60.5 ± 22.2 (non-treatment) 65.7 ± 23.8 (addition of MgCl2) |
(Ahmed et al., 2020b) |
| Centrifugal ultrafiltration (w/ different molecular weight cut-off) | 56.0 ± 32.3 (30 kDa) 28.0 ± 9.10 (10 kDa) |
||
| PEG-based separation | 44.0 ± 27.7 | ||
| Ultracentrifugation | 33.5 ± 12.1 |
PEDV and MgV stand for porcine epidemic diarrhea virus and mengovirus (vMC0).
Recovery data of influent samples.
Relative standard deviation not provided.
PMMoV stands for pepper mild mottle virus.
MHV stands for murine hepatitis virus.
4.4. The efficiency of primary concentration
As of now, we have found only one comprehensive study that systematically compares the efficiency of different primary concentration methods for the COVID-19 wastewater analysis (Ahmed et al., 2020b). As shown in Table 4, four types of primary concentration methods were conducted to concentrate the spiked murine hepatitis virus (MHV) in the wastewater influent. According to the reported concentration efficiencies, the electronegative membrane filtration method with the addition of magnesium chloride resulted in the highest mean recovery rate. It appears that the high recovery rate of the electronegative membrane filtration method was mainly associated with skipping over any prefiltration and pre-centrifugation step, which maximized the internal reference (i.e., the spiked MHV) to be adsorbed from both the liquid and solid fractions of the wastewater sample simultaneously (Ahmed et al., 2020b). Such approach will significantly improve the adaptability of this method when it comes to handling a highly turbid influent sample. Again, the risk of membrane clogging has to be carefully addressed when dealing with a medium-sized turbid influent sample (e.g., 500 mL). This is because the electronegative membrane filtration method requires a sample to pass through a membrane filter, which typically has a small pore size of less than 1.0 μm.
In terms of the PEG-based separation method, both studies showed moderate-to-high recovery efficiencies of 44% and 57%, respectively (Ahmed et al., 2020b; Hata et al., 2020). The relatively higher recovery rate of 57% reported by Hata et al. (2020) was achieved with a single-step pretreatments of centrifugation at 5000g for 5 min to remove large particles from raw influent samples. In comparison, Hata et al. (2020) adopted a more complex pretreatment process of (1) ultracentrifugation at 10,000g for 20 min, (2) resuspension of the obtained pellet in 0.05 M glycine, (3) ultracentrifugation at 10,000g for 10 min, (4) Combination of the supernatants from both ultracentrifugation processes, and (5) pH neutralization by adding 2 M HCl. As the PEG-based separation method have been developed and refined since 1960s, different pretreatments aiming to improve its efficiency and adaptability have also been proposed and applied, which may result in overly complicated procedures. As a result, particular attention should be paid to the additional pretreatment steps used in the overall concentration process because of the possibility of the unforeseeable loss of viruses along with the processing.
As mentioned previously in Section 3, the aluminum hydroxide precipitation method showed low recovery rates (~3 to 11%) in all wastewater samples, and the difference in recovery rate between the spiked enveloped and non-enveloped virus in influent samples is statistically insignificant, indicating the method has a similar efficiency of concentrating enveloped and non-enveloped virus from an influent sample (Randazzo et al., 2020). Comparing to the aluminum hydroxide precipitation method, all other techniques showed better recovery rates. For example, Medema et al. (2020) reported a significantly higher recovery rate of 73 ± 50% by concentrating the F-specific RNA phages with centrifugal ultrafiltration (100 kDa molecular weight cut-off). However, the high recovery rate of 73% was subject to a noticeably large relative standard deviation (RSD) of ±50%, which may reduce the application implication of the centrifugal ultrafiltration method. It should be noted that the issue of a low average recovery rate can be compensated, to some extent, by applying a large initial volume. However, the issue of data scattering (i.e., large RSD) might have a more detrimental effect on the accuracy of the finalized virus concentration estimation because the final virus concentration has to be normalized by a recovery rate, and a large RSD can significantly increase the uncertainty.
By combining the previously published studies of virus detection in wastewater with the most recent reports regarding the COVID-19 WBE, this review will provide some specific suggestions, in the section entitled “5. Recommendations,” for which technique(s) should be chosen and what preconditioning approach(es) should be adopted when conducting the SARS-CoV-2 wastewater detection.
5. Recommendations
In 18 studies that we found relevant to the wastewater SARS-CoV-2 detection, the initial sampling volume was all relatively small. For example, a small volume of 40 mL prefiltered wastewater sample can be successfully concentrated by using the PEG-based separation combined with the ultracentrifugation and subsequently detected via RT-qPCR to result in the SARS-CoV-2 N1 genomic fragments of 50 to 250 copies/mL of raw wastewater (Wu et al., 2020). Furthermore, Ahmed et al. (2020b) reported using 50 mL-aliquot influent samples can result in a superior virus recovery rate up to 65.7 ± 23.8% via the electronegative membrane filtration method. Thus, a large initial concentrating volume seems to have less of an effect on the development of a sufficient concentration method. For the purpose of ease-to-operate, both the PEG-based separation and the electronegative membrane filtration methods have been implemented without large-scale instruments and resulted in desirable recovery efficiencies, such as 57% and 65.7%, respectively (Ahmed et al., 2020b; Hata et al., 2020). Based on what we have summarized above, an ideal method of conducting the primary concentration of the SARS-CoV-2 virus RNA from wastewater samples should have the following performance characteristics, including a small to medium-sized concentrating capacity, an easy to access and operate procedure, a reliable and efficient performance, and, most importantly, strong adaptability to a broad range of water quality.
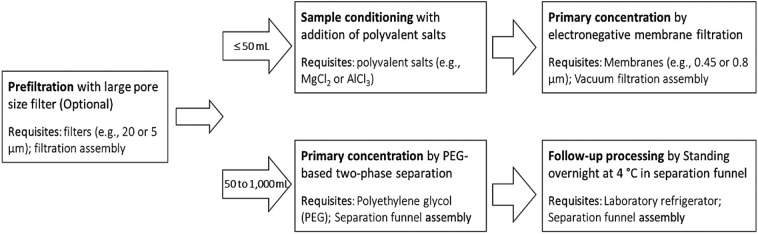
As a result, we highly recommend using the electronegative membrane filtration method with the addition of magnesium chloride as the primary concentration method when dealing with a small initial volume (i.e., less than 50 mL). In case of the potential clogging risk, when handling a medium-sized influent sample (i.e., 50 to 1000 mL), we suggest using the PEG-based separation method followed by overnight standing at 4 °C in a separation funnel as the alternative primary concentration method. Due to the typically high turbidity of an untreated influent sample, the removal of large particles, such as sand, debris, and hair, could be achieved by passing through filters with a large pore size (e.g., 5 or 20 μM), if needed. The recommended two primary concentration processes can be achieved without any large laboratory equipment. At the same time, the main consumables are limited to the filter membranes and chemicals (i.e., magnesium chloride for the electronegative membrane filtration method and polyethylene glycol for the PEG-based separation method, respectively) (see Fig. 2 ). In summary, these two proposed methods are easy to use, free of large laboratory equipment, proven concentration efficiency, and highly accessible over a broad range of research facilities.
Fig. 2.
Recommended two primary concentration methods for simple, effective concentration of the SARS-CoV-2 virus from wastewater samples.
6. Conclusion
Due to the recent outbreak of the COVID-19 pandemic, wastewater based epidemiology starts to attract a significant amount of attention. The fundamental idea is to quantitatively detect the SARS-CoV-2 virus RNA from wastewater samples and then use this information to conduct infection prevalence estimation. To accurately reflect the epidemic situation, the detection technique must be highly sensitive and reliable. Therefore, developing a simple, effective primary concentration method before the SARS-CoV-2 RNA extraction and detection processes is of great significance. This mini-review first discussed several commonly used primary concentration methods, including the PEG-based separation, electrostatically charged membrane filtration, and ultrafiltration. After that, a particular emphasis was put on the eighteen existing SARS-CoV-2 wastewater analytical studies. Based on the primary concentration methods implemented in these studies, we present the following conclusions:
-
1.
This review work highlights the importance of having an easy to access and operate primary concentration method because implementing the real-time wastewater surveillance has to be based on the high availability of wastewater data.
-
2.
Due to the positive results obtained with small initial volumes in all the reported studies, the main drawback (i.e., small concentrating capacity) of the PEG-based separation and the electrostatically charged membrane filtration can, to some extent, be ignored.
-
3.
As of now, the PEG-based separation method is the most prevalent method used for the COVID-19 wastewater based epidemiology.
-
4.
The electronegative membrane filtration method can be carried out without any prefiltration and pre-centrifugation and still produce the most desirable concentration efficiency.
-
5.
Although the electronegative membrane filtration method has been proved experimentally, future studies should be careful about the preferential adsorption of organic matter on the charged membrane surface and the potential risk of clogging when handling turbid samples.
-
6.
Ultrafiltration can provide high recoveries and consistent performance, but an ultrafiltration system is usually large, immobile, and not readily available in most laboratories.
-
7.We recommend two simple and straight-forward primary concentration strategies:
-
(1)Prefiltration – Salt addition – Electronegative membrane filtration (volume ≤ 50 mL).
-
(2)Prefiltration – PEG-based separation – Overnight standing (volume from 50 to 1000 mL).
-
(1)
CRediT authorship contribution statement
Dingnan Lu: Conceptualization, Investigation, Writing - original draft. Zhuangrong Huang: Conceptualization, Investigation, Writing - original draft. Jiayue Luo: Investigation, Writing - original draft. Xiaoqi Zhang: Writing - review & editing. Sha Sha: Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to acknowledge the University of Massachusetts Lowell (UML) and Massachusetts Institute of Technology (MIT) for the acquisition of the latest literature and information regarding the COVID-19 pandemic used in this analysis work.
Editor: Jay Gan
References
- Adams A. Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J. Gen. Virol. 1973;20:391–394. doi: 10.1099/0022-1317-20-3-391. [DOI] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsson P.-å., Frick G. Partition of virus particles in a liquid two-phase system. Biochim. Biophys. Acta. 1960;37:230–237. doi: 10.1016/0006-3002(60)90228-6. [DOI] [PubMed] [Google Scholar]
- Bar Or I., Yaniv K., Shagan M., Ozer E., Erster O., Mendelson E. 2020. Regressing SARS-CoV-2 Sewage Measurements Onto COVID-19 Burden in the Population: A Proof-of-concept for Quantitative Environmental Surveillance.https://www.medrxiv.org/content/10.1101/2020.04.26.20073569v1 reprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalapati Rao V., Chandorkar U., Rao N.U., Kumaran P., Lakhe S.B. A simple method for concentrating and detecting viruses in wastewater. Water Res. 1972;6:1565–1576. doi: 10.1016/0043-1354(72)90080-2. [DOI] [Google Scholar]
- Chavarria-Miró G., Anfruns-Estrada E., Guix S., Paraira M., Galofré B., Sáanchez G. 2020. Sentinel Surveillance of SARS-CoV-2 in Wastewater Anticipates the Occurrence of COVID-19 Cases.https://www.medrxiv.org/content/10.1101/2020.06.13.20129627v1.full.pdf reprint at. [DOI] [Google Scholar]
- Colombet J., Robin A., Lavie L., Bettarel Y., Cauchie H.M., Sime-Ngando T. Virioplankton ‘pegylation’: use of PEG (polyethylene glycol) to concentrate and purify viruses in pelagic ecosystems. J. Microbiol. Methods. 2007;71:212–219. doi: 10.1016/j.mimet.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Daughton C.G., Jones-Lepp T.L. American Chemical Society; Washington, DC: 2001. Pharmaceuticals and Care Products in the Environment Scientific and Regulatory Issues. [Google Scholar]
- Divizia M., Santi A.L., Panà A. Ultrafiltration: an efficient second step for hepatitis A virus and poliovirus concentration. J. Virol. Methods. 1989;23:55–62. doi: 10.1016/0166-0934(89)90089-x. [DOI] [PubMed] [Google Scholar]
- Farrah S.R., Preston D.R. Concentration of viruses from water by using cellulose filters modified by in situ precipitation of ferric and aluminum hydroxides. Appl. Environ. Microbiol. 1985;50:1502–1504. doi: 10.1128/aem.50.6.1502-1504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S.R., Erdos G.W., Toranzos G.A. Virus adsorption to microporous filters modified by in situ precipitation of metallic salts. Water Sci. Technol. 1986;18:141–148. doi: 10.2166/wst.1986.0122. [DOI] [Google Scholar]
- Fout G.S., Cashdollar J.L., Varughese E.A., Parshionikar S.U., Grimm A.C. EPA Method 1615. Measurement of enterovirus and norovirus occurrence in water by culture and RT-qPCR. I. Collection of virus samples. J. Vis. Exp. 2015 doi: 10.3791/52067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumian T.M., Leite J.P., Castello A.A., Gaggero A., Caillou M.S., Miagostovich M.P. Detection of rotavirus A in sewage samples using multiplex qPCR and an evaluation of the ultracentrifugation and adsorption-elution methods for virus concentration. J. Virol. Methods. 2010;170:42–46. doi: 10.1016/j.jviromet.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Gaertner H. Retention and recovery of polioviruses on a soluble ultrafilter. In: Berg G., editor. Transmission of Viruses by the Water Route. Interscience; London: 1967. pp. 121–127. [Google Scholar]
- Gias E., Nielsen S.U., Morgan L.A., Toms G.L. Purification of human respiratory syncytial virus by ultracentrifugation in iodixanol density gradient. J. Virol. Methods. 2008;147:328–332. doi: 10.1016/j.jviromet.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2008;1 doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- Haramoto E., Katayama H., Ohgaki S. Detection of noroviruses in tap water in Japan by means of a new method for concentrating enteric viruses in large volumes of freshwater. Appl. Environ. Microbiol. 2004;70:2154–2160. doi: 10.1128/aem.70.4.2154-2160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Matsumori K., Kitajima M., Katayama H. Concentration of enteric viruses in large volumes of water using a cartridge-type mixed cellulose ester membrane. Food Environ. Virol. 2015;7:7–13. doi: 10.1007/s12560-014-9169-x. [DOI] [PubMed] [Google Scholar]
- Hata A., Honda R., Hara-Yamamura H., Meuchi Y. 2020. Detection of SARS-CoV-2 in Wastewater in Japan by Multiple Molecular Assays-Implication for Wastewater-based Epidemiology (WBE)https://www.medrxiv.org/content/10.1101/2020.06.09.20126417v2 reprint at. [DOI] [Google Scholar]
- Hill W.F., Akin E.W., Benton W.H. Detection of viruses in water: a review of methods and application. Water Res. 1971;5:967–995. doi: 10.1016/0043-1354(71)90033-9. [DOI] [Google Scholar]
- Hill V.R., Kahler A.M., Jothikumar N., Johnson T.B., Hahn D., Cromeans T.L. Multistate evaluation of an ultrafiltration-based procedure for simultaneous recovery of enteric microbes in 100-liter tap water samples. Appl. Environ. Microbiol. 2007;73:4218–4225. doi: 10.1128/AEM.02713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu B.M., Chen C.H., Kung C.M., Wan M.T., Shen S.M. Evaluation of enterovirus recovery in surface water by different adsorption and elution procedures. Chemosphere. 2007;66:964–969. doi: 10.1016/j.chemosphere.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Ikner L.A., Soto-Beltran M., Bright K.R. New method using a positively charged microporous filter and ultrafiltration for concentration of viruses from tap water. Appl. Environ. Microbiol. 2011;77:3500–3506. doi: 10.1128/AEM.02705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikner L.A., Gerba C.P., Bright K.R. Concentration and recovery of viruses from water: a comprehensive review. Food Environ. Virol. 2012;4:41–67. doi: 10.1007/s12560-012-9080-2. [DOI] [PubMed] [Google Scholar]
- Kazama S., Miura T., Masago Y., Konta Y., Tohma K., Manaka T. Environmental surveillance of norovirus genogroups I and II for sensitive detection of epidemic variants. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.03406-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M. 2020. First Proof of the Capability of Wastewater Surveillance for COVID-19 in India Through Detection of Genetic Material of SARS-CoV-2.https://www.medrxiv.org/content/10.1101/2020.06.16.20133215v1.full.pdf reprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini E., Spencer S.K., Bertz P.D., Loge F.J., Kieke B.A., Borchardt M.A. Concentration of enteroviruses, adenoviruses, and noroviruses from drinking water by use of glass wool filters. Appl. Environ. Microbiol. 2008;74:2990–2996. doi: 10.1128/AEM.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesté-Lasserre C. Coronavirus found in Paris sewage points to early warning system. Science. 2020 doi: 10.1126/science.abc3799. [DOI] [Google Scholar]
- Lewis G.D., Metcalf T.G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl. Environ. Microbiol. 1988;54:1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu O., Brashear D., Seraichekas H., Barnick J., Metcalf T. Virus in water: I. A preliminary study on a flow-through gauze sampler for recovering virus from waters. Appl. Environ. Microbiol. 1971;21:405–410. doi: 10.1128/am.21.3.405-410.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Hill V.R., Hahn D., Johnson T.B., Pan Y., Jothikumar N. Hollow-fiber ultrafiltration for simultaneous recovery of viruses, bacteria and parasites from reclaimed water. J. Microbiol. Methods. 2012;88:155–161. doi: 10.1016/j.mimet.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580:176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Murakami M., Hata A., Honda R., Watanabe T. Letter to the editor: wastewater-based epidemiology can overcome representativeness and stigma issues related to COVID-19. Environ. Sci. Technol. 2020;54:5311. doi: 10.1021/acs.est.0c02172. [DOI] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Surya K., Wiegand T., Buyukyoruk M., Wilkinson R. 2020. Temporal Detection and Phylogenetic Assessment of SARS-CoV-2 in Municipal Wastewater.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7276038/ reprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nupen E.M. Virus studies on the Windhoek waste-water reclamation plant (South-West Africa) Water Res. 1970;4:661–672. doi: 10.1016/0043-1354(70)90028-x. [DOI] [Google Scholar]
- Preston D.R., Farrah S.R. Adsorption of viruses by diatomaceous earth coated with metallic oxides and metallic peroxides. Water Sci. Technol. 1991;24:235–240. doi: 10.2166/wst.1991.0065. [DOI] [Google Scholar]
- Rajal V.B., McSwain B.S., Thompson D.E., Leutenegger C.M., Kildare B.J., Wuertz S. Validation of hollow fiber ultrafiltration and real-time PCR using bacteriophage PP7 as surrogate for the quantification of viruses from water samples. Water Res. 2007;41:1411–1422. doi: 10.1016/j.watres.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Ramia S., Sattar S.A. Second-step concentration of viruses in drinking and surface waters using polyethylene glycol hydroextraction. Can. J. Microbiol. 1979;25:587–592. doi: 10.1139/m79-084. [DOI] [PubMed] [Google Scholar]
- Ramia S., Sattar S.A. Concentration of seeded simian rotavirus SA-11 from potable waters by using talc-celite layers and hydroextraction. Appl. Environ. Microbiol. 1980;39:493–499. doi: 10.1128/aem.39.3.493-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D. 2020. Presence and Vitality of SARS-CoV-2 Virus in Wastewaters and Rivers.https://www.medrxiv.org/content/10.1101/2020.05.01.20086009v1 reprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H. Michigan State University; 2017. Membrane-based Concentration and Recovery of Viruses From Complex Water Matrices. [Google Scholar]
- Shuval H.I., Cymbalista B.F.S., Goldblum N. The phase-separation method for the concentration and detection of viruses in water. Water Res. 1969;3:225–240. doi: 10.1016/0043-1354(69)90019-0. [DOI] [Google Scholar]
- Sims N., Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M.D., Jones B.L. Concentration of poliovirus from tap water using positively charged microporous filters. Appl. Environ. Microbiol. 1979;37:588–595. doi: 10.1128/aem.37.3.588-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet B.H., McHale J.S., Hardy K.J., Morton F., Smith J.K., Klein E. Concentration of virus from water by osmotic ultrafiltration — I: biological aspects. Water Res. 1971;5:823–829. doi: 10.1016/0043-1354(71)90018-2. [DOI] [Google Scholar]
- Wallis C., Melnick J.L. Concentration of enteroviruses on membrane filters. J. Virol. 1967;1:472–477. doi: 10.1128/jvi.1.3.472-477.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Grinstein S., Melnick J.L., Fields J.E. Concentration of viruses from sewage and excreta on insoluble polyelectrolytes. Appl. Environ. Microbiol. 1969;18:1007–1014. doi: 10.1128/am.18.6.1007-1014.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Henderson M., Melnick J.L. Enterovirus concentration on cellulose membranes. Appl. Environ. Microbiol. 1972;23:476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. J. Virol. Methods. 2005;128:156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . In: Guidelines for Environmental Surveillance of Poliovirus Circulation. Biologicals DoVa, editor. World Health Organization; Switzerland: 2003. [Google Scholar]
- WHO . World Health Organization; 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 181. [Google Scholar]
- Winona L.J., Ommani A.W., Olszewski J., Nuzzo J.B., Oshima K.H. Efficient and predictable recovery of viruses from water by small scale ultrafiltration systems. Can. J. Microbiol. 2001;47:1033–1041. doi: 10.1139/w01-111. [DOI] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K. 2020. SARS-CoV-2 Titers in Wastewater are Higher Than Expected From Clinically Confirmed Cases.https://www.medrxiv.org/content/10.1101/2020.06.15.20117747v2 reprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.-M., Maday Y., Teyssou R., Richard E. 2020. Evaluation of Lockdown Impact on SARS-CoV-2 Dynamics Through Viral Genome Quantification in Paris Wastewaters.https://www.medrxiv.org/content/10.1101/2020.04.12.20062679v2 reprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Zerda K.S., Gerba C.P., Hou K., Goyal S. Adsorption of viruses to charge-modified silica. Appl. Environ. Microbiol. 1985;49:91–95. doi: 10.1128/aem.49.1.91-95.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]