Abstract
Early-life exposures to environmental insults can misprogram development and increase metabolic disease risk in a sex-dependent manner by mechanisms that remain poorly characterized. Modifiable factors of increasing public health relevance, such as diet, psychological stress, and endocrine-disrupting chemicals, can affect glucocorticoid receptor signaling during gestation and lead to sex-specific postnatal metabolic derangements. Evidence from humans and animal studies indicate that glucocorticoids crosstalk with sex steroids by several mechanisms in multiple tissues and can affect sex-steroid–dependent developmental processes. Nonetheless, glucocorticoid sex-steroid crosstalk has not been considered in the glucocorticoid-induced misprogramming of metabolism. Herein we review what is known about the mechanisms by which glucocorticoids crosstalk with estrogen, androgen, and progestogen action. We propose that glucocorticoid sex-steroid crosstalk is an understudied mechanism of action that requires consideration when examining the developmental misprogramming of metabolism, especially when assessing sex-specific outcomes.
Keywords: glucocorticoid, estrogen, androgen, progestogen, crosstalk, sex-specific, metabolism, diabetes, developmental programming, endocrine-disrupting chemicals
As proposed by the Developmental Origins of Health and Disease (DOHaD) hypothesis, the environment is known to affect fetal development and induce physiological changes that can increase disease risk later in life. Central to organismal survival, normal physiology is dependent on glucose homeostasis, yet glucose regulation is susceptible to developmental misprogramming through disruption of multiple tissues and hormonal axes [1, 2]. Fetal programming of glucose intolerance was initially documented as a result of famine in the early studies that supported the DOHaD hypothesis [3]. Subsequent research has shown that fetal exposures to less-severe environmental insults increase metabolic disease risk later in life and can do so in a sex-dependent manner by mechanisms that remain insufficiently characterized [1, 4]. Prenatal insults may alter the development and postnatal function of key metabolic tissues, or can alter the development of tissues that secondarily affect tissues that regulate glucose homeostasis. Work detailing the epigenetic mechanisms by which environmental insults disrupt cellular development is ongoing [5]. Understanding the mechanistic origins of this developmental misprogramming is essential because the long-term metabolic health of those who are developmentally compromised is likely more susceptible to the panoply of modern metabolic disease risk factors, including unhealthy diets, sedentary lifestyles, circadian disruptions, and environmental pollution. Currently, nearly 10% of the US population has diabetes [6], and approximately 463 million people suffer from the disease globally [7]. Additionally, the contribution of insulin resistance and diabetes to the pathogenesis of other devastating diseases with growing incidence and societal burden is becoming increasingly apparent, including cancer [8] and Alzheimer disease [9]. Thus, understanding the mechanistic bases of metabolic misprogramming holds immense potential for early risk assessment, mitigation, disease treatment, and even prevention of multigenerational disease inheritance.
1. Misprogramming of Metabolism by Glucocorticoids
Regulated in a circadian, ultradian, and stress-related manner, glucocorticoids (GCs) play an important role in maintaining various metabolic and homeostatic functions essential for life. GCs elicit their actions in large measure by binding to the glucocorticoid receptor (GR), a member of the nuclear receptor superfamily that is encoded by the NR3C1 gene, which through alternative splicing and translation gives rise to several GR isoforms [10]. Whereas less is known about many of the translation isoforms, GRα is the most studied splicing isoform and will be the focus of this review. GR signaling is an important target for the developmental misprogramming of metabolism because GCs adjust fetal development in response to adverse environmental conditions to maximize survival [11]. Late in gestation, GCs promote the maturation of fetal tissues, including those that control glucose and lipid homeostasis postnatally, such as the liver, adipose tissue, pancreatic β cells, and skeletal muscle [12, 13]. Animal models of developmental overexposure to GCs demonstrate numerous metabolic derangements, including glucose intolerance, decreased insulin sensitivity, reduced β-cell mass, alterations in circulating lipids and adipokines, increased hepatic lipid accumulation, and exaggerated hepatic glucose production (reviewed in [11, 14]). Furthermore, many traditional intrauterine growth restriction animal models have been used to study the developmental origins of metabolic disease result in fetal overexposure to GCs; these include models employing calorie restriction, protein restriction, and uterine artery ligation (reviewed in [15]). Collectively, these data indicate that multiple perturbations can disrupt regulated endogenous GC action, potentially resulting in alterations in metabolic homeostasis that promote derangements in glucose and lipid homeostasis later in life.
A. Public Health Relevance of Developmental Glucocorticoid Receptor Disruption
Aberrant overactivation of GR signaling during fetal development by factors of public health relevance are increasingly associated with the developmental misprogramming of metabolism. For example, human studies and animal models both reveal that chronic psychological stress during pregnancy leads to fetal GC excess and increases the later-life risk of diabetes and obesity in offspring (reviewed in [1]). Antenatal exposure to pharmacological GCs administered to accelerate lung development and augment the survival of preterm infants has been suggested to lower HOMA-β (homeostatic model assessment-β) during early adulthood [16] and reduce insulin sensitivity [17], and thus may increase the offspring’s long-term metabolic disease risk as has been documented in animal models [11]. More studies are needed to comprehensively assess the extent to which antenatal GC treatment affects the metabolic health of aged adults, especially because pharmacological GC treatment during pregnancy is also used in some elective cesarean deliveries and as prophylaxis for women with certain previous pregnancy complications [18, 19].
The potential influence of environmental anthropogenic GR modulators is also being increasingly recognized. The relevance of environmental GR modulators is supported by studies that have found widespread GR-modulating activity from household dust samples [20] as well as water samples from various countries [21-25]. This GR-modulating activity has been attributed both to the presence of widely prescribed pharmacological GR agonists that migrate into the environment as well as to environmental toxicants with GR-modulating activity. Endocrine-disrupting chemicals (EDCs) are defined as exogenous chemicals, or mixtures of chemicals, that interfere with any aspect of hormone action [26]. Developmental exposures to a variety of EDCs have been shown to promote later-life metabolic derangements in human and animal studies, including glucose intolerance, insulin resistance, altered β-cell function, obesity, and hepatic lipid accumulation [27]. Whereas the capacity of EDCs to modulate sex-steroid and thyroid hormone action has been recognized for decades [26], the ability of several of these toxicants to modulate GR signaling is only now becoming clear [28]. A recent study found that a mouse model of perinatal exposure to the GR-activating fungicide tolylfluanid increased hepatic phosphoenolpyruvate carboxykinase expression and hepatic glucose production selectively in male offspring [29]. An extensive assessment of EDCs that disrupt GR activity and GC homeostasis has recently been published [30]. Collectively, these data indicate that multiple factors of public health relevance may induce long-term adverse effects on metabolic health by aberrantly overactivating GR signaling during fetal development.
B. Developmental Glucocorticoid Exposure Promotes Sex-Specific Metabolic Misprogramming
Whereas most studies assessing metabolic outcomes following prenatal GC overexposure in animal models have examined outcomes in male offspring only, the few studies that have interrogated offspring in males and females have found evidence for sex-specific outcomes. Observed male-specific outcomes following prenatal treatment with the synthetic glucocorticoid dexamethasone (DEX) include increased expression of phosphoenolpyruvate carboxykinase [31] and higher circulating insulin levels [31, 32]. One study found that prenatal DEX exposure potentiated diet-induced hepatic steatosis mediated in part by an underactive growth-hormone axis, an effect observed in female offspring only [33]. Although more studies are needed to assess the extent of sex-specificity in outcomes related to glucose and lipid homeostasis following prenatal GC overexposure, these examples of sex-specific metabolic derangements coupled with numerous reports of sex differences in other end points following prenatal GC overexposure (eg, hypothalamic-pituitary-adrenal responsivity and cardiovascular function; reviewed in [34]) suggest there is sex-specificity in the misprogramming of development by GCs that merits further investigation.
C. Missing Links: Glucocorticoid Sex-Steroid Crosstalk in Metabolic Programming
Critically, GC-induced misprogramming of metabolism has predominantly been studied in the context of GR activation, with minimal consideration given to crosstalk with sex-steroid hormones. This neglect belies clear evidence of interactions between these endocrine axes. First, GCs modify circulating sex-steroid levels during fetal development and adulthood, which in turn modulates sex-steroid effects by altering activation of their own receptors. Second, GCs can directly alter androgen, estrogen, and progestogen action by modulating cellular sex-steroid receptor signaling and gene transcription, as shown in adult-derived human and murine tissues in vitro and ex vivo (Figs. 1-3). Importantly, a substantial number of studies have shown that disrupting androgen or estrogen action during fetal development by treatment with native sex steroids, exposure to EDCs, or via other stressors that modulate endogenous sex-steroid levels all can result in later-life metabolic derangements, which often show sex specificity [35-38]. Thus, GC sex-steroid crosstalk has important implications relevant to DOHaD and the in utero programming of metabolic disease risk.
Figure 1.
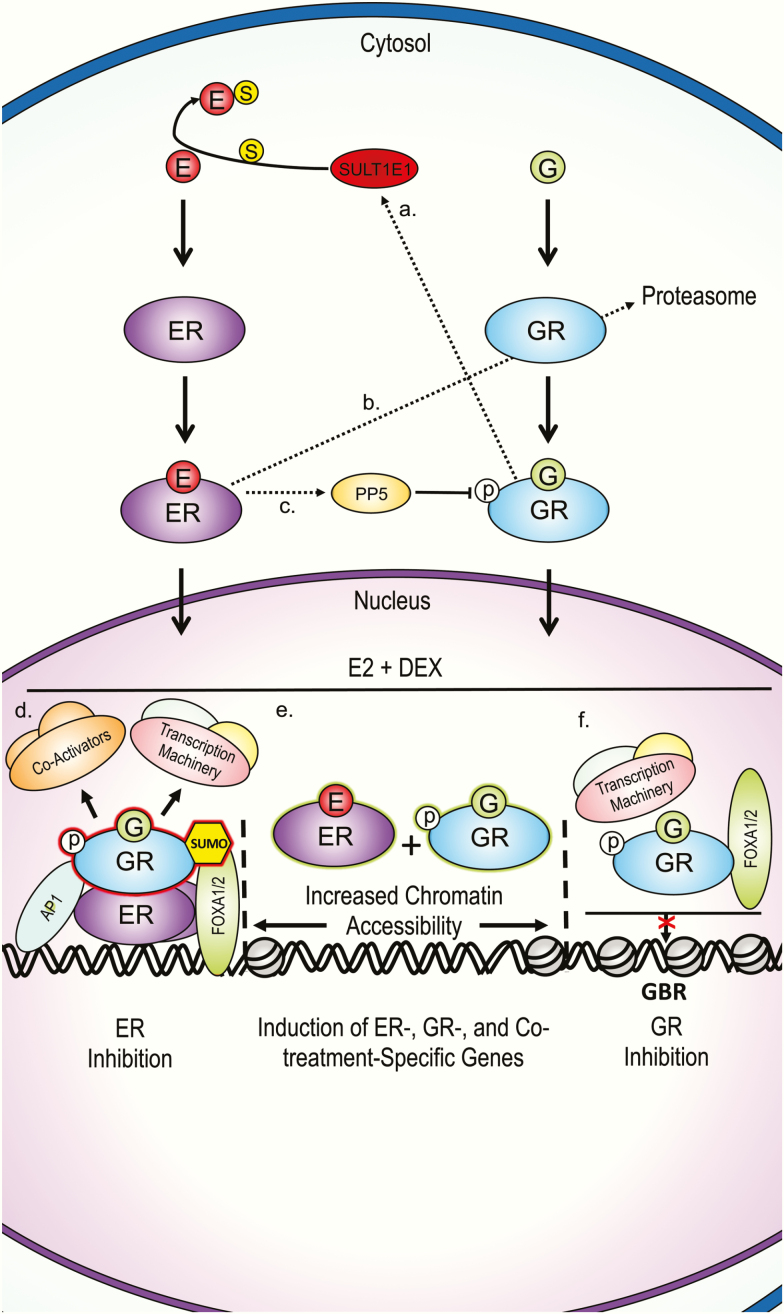
Summary of proposed G and estrogen cellular crosstalk mechanisms from multiple tissues. A, Gs upregulate SULTE1, which reduces estrogen bioavailability by sulfation in human and mouse hepatocytes, and human breast cancer MCF-7 cells [43]. B, Estrogen promotes GR proteasomal degradation by upregulating p53 and the E3 ubiquitin ligase Mdm2 in MCF-7 cells [44]. C, Estrogen upregulates PP5, which reduces G action by dephosphorylating GR Ser-211 in breast cancer cell lines [45]. D, GR inhibits ER transcription assembly for some genes. DNA-bound ER is bound by GRs DBD. AP-1 and FOXA1 assist in GR-ER association in breast cancer cell lines [47, 49]. GR SUMOylation is needed for destabilization of transcription complexes in certain enhancers [49]. E, Co-treatment results in increased chromatin accessibility and unique ER and GR genome binding and consequent gene expression not observed during either single hormone treatment in breast and uterine cancer cell lines [50, 51, 62]. F, Estrogen inhibits GR target gene expression for some genes, characterized by lower chromatin accessibility and consequent lower GR, FOXA1/2, and transcription machinery binding to GBRs in human uterine cells [59, 60]. DBD, DNA-binding domain; DEX, dexamethasone; E, estrogen; ER, estrogen receptor; G, glucocorticoid; GBR, glucocorticoid-binding region, GR, glucocorticoid receptor; p, phosphate; PP5, protein phosphatase 5; SULT1E1, estrogen sulfotransferase; S, sulfate.
Figure 3.
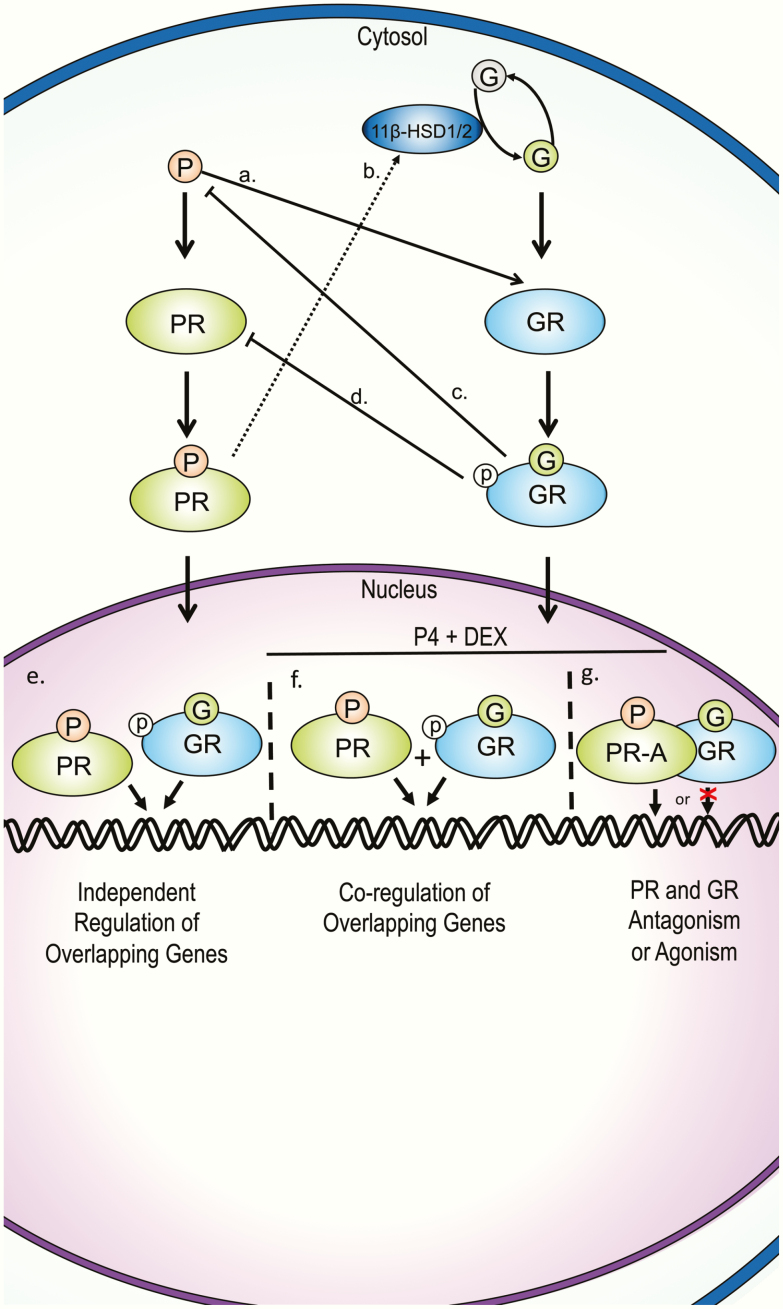
Summary of proposed glucocorticoid and progesterone cellular crosstalk mechanisms from multiple tissues. A, P can bind to GR and act as an agonist or antagonist, depending on G bioavailability [104-133]. B, PR can modulate G bioavailability by regulating the expression of 11β-HSD1 and 11β-HSD2 [137-139]. C, GR action can suppress progesterone production by upregulating placental CRH [140, 141]. D, GR can downregulate PR-A expression [136]. E, GR and PR can bind and regulate common genes separately [102, 135]. F, GR and PR can co-regulate common genes when both reports are activated [102]. G, GR and PR-A may physically interact as a form of antagonism or agonism [102, 125, 136]. CRH, corticotropin-releasing hormone; DEX, dexamethasone; G, glucocorticoid; GR, glucocorticoid receptor; p, phosphate; P, progesterone; PR, progesterone receptor.
The mechanisms by which crosstalk between GCs and sex steroids during fetal development contribute to the misprogramming of metabolic physiology have not been directly explored, but these interactions are likely significant because fetal GC overexposure disrupts sex-steroid action in the developing fetus and has lasting effects on reproductive parameters in animal models [39] (Fig. 4). Importantly, how GC-induced misprogramming of the hypothalamic-pituitary-gonadal (HPG) axis contributes to metabolic misprogramming has not been directly tested. The reported sex-specific metabolic outcomes following developmental GC overexposures suggests that disruption of sex hormone action is one potential mediator of GC programming of sex-specific outcomes. Herein we review what is known about the mechanisms by which GCs modulate estrogen, androgen, and progestogen signaling. We propose that GC sex-steroid crosstalk is an understudied endocrine mechanism of toxicity that needs to be considered in studies assessing developmental misprogramming, especially when assessing sex-specific metabolic phenotypes.
Figure 4.
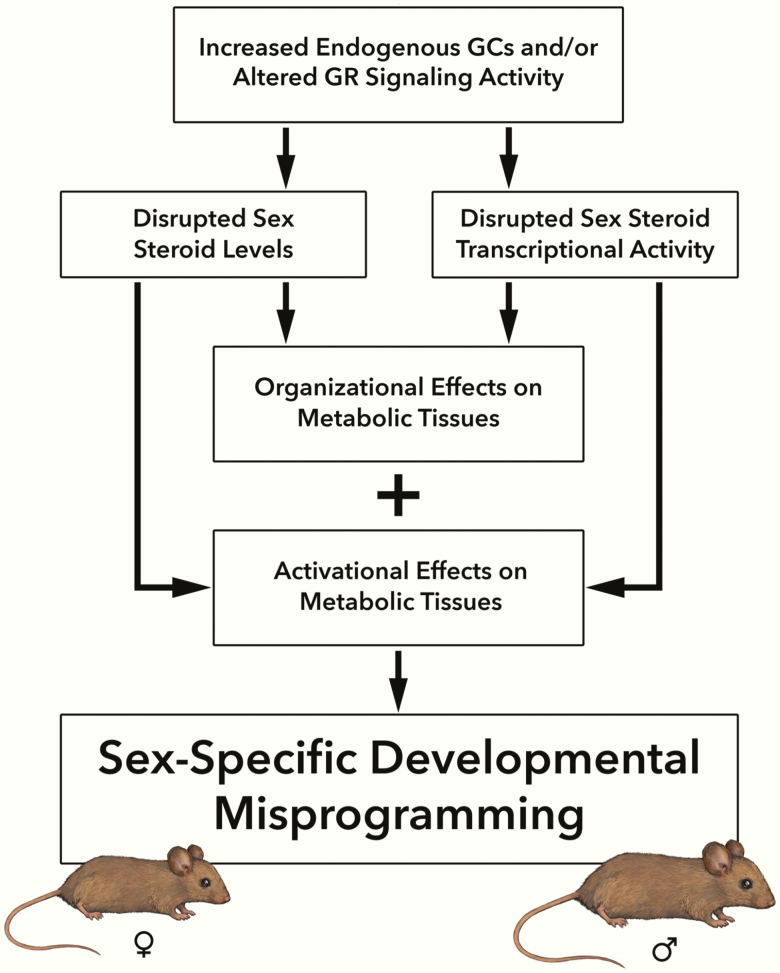
Schema of proposed sex-specific developmental misprogramming following developmental GR disruption. Exposure to psychological stress, pharmacological GCs, or GR-disrupting endocrine-disrupting chemicals during fetal development lead to increased endogenous GCs and/or alter GR signaling activity in the developing fetus. This leads to disrupted fetal sex-steroid levels and/or disrupted sex-steroid transcriptional activity, which can have organizational effects on metabolic tissues during development and activational effects on metabolic tissues later in life. The end results of these effects are sex-specific developmental outcomes in the offspring. GC, glucocorticoid; GR, glucocorticoid receptor.
2. Glucocorticoid and Estrogen Receptor Crosstalk
Crosstalk between GR and estrogen receptor (ER) signaling has been established in numerous cell types from different species, including humans, rats, and mice. Although 3 estrogen receptors have been described, including ERα, ERβ, and the G protein-coupled ER, this section will focus on ERα because most crosstalk studies to date have focused on ERα. ER and GR have been shown to affect each other’s action both by altering receptor and ligand availability as well as by modulating each other’s genomic binding and transcriptional end points. This section outlines the current state-of-knowledge regarding GR and ERα crosstalk to contextualize how a common transcriptional mechanism of hormonal communication that is currently understudied during fetal development can lead to a better understanding of developmental misprogramming by aberrant GR signaling.
Work describing the nature of GR-ER crosstalk reviewed herein is based on breast cancer cell models as well as uterine and hepatic tissue, although GR/ERα crosstalk in different brain regions has also been reported [40-42]. Despite clear differences in function and developmental origin among these tissues, evidence of crosstalk between GR and ER has consistently been evident. In the ERα-positive breast cancer cell line MCF-7, along with mouse livers and human hepatocytes, GCs inhibit estradiol (E2) from binding to ERs by upregulating estrogen sulfotransferase (SULT1E1) and inducing estrogen inactivation via sulfation [43]. Likewise, E2 promotes the proteasomal degradation of GR in MCF-7 cells by upregulating p53 and the E3 ubiquitin ligase Mdm2 [44]. E2 can further reduce GR activity by decreasing the activating phosphorylation of GR at Ser-211 via upregulation of protein phosphatase 5 in an ERα-dependent manner [45].
Apart from crosstalk at the ligand and receptor level of action, GR and ERα also influence each other’s binding to chromatin and consequential control of gene expression. Indeed, a large overlap of DNA-binding sites for ERα, androgen receptor (AR), and GR have been identified in male breast tumors [46]. The potent pharmacological GR agonist DEX inhibits E2-mediated MCF-7 proliferation and downregulates ERα target gene expression by promoting GR recruitment to ERα-binding regions, causing the destabilization of the ERα transcriptional complex [47]. The observed direct interaction between GR and ERα is mediated through the GR DNA-binding domain (DBD), and the binding of GR to ER binding sites was shown to be mediated by activator protein 1 (AP-1) and the pioneer factor Forkhead Box A1 (FoxA1) [47]. The widespread expression of FoxA1 and AP-1 during development suggests that GR binding to ER binding sites may occur during development as well. Another study found that the co-regulator interaction domain of the ERα ligand-binding domain (LBD) was necessary for the co-recruitment of GR to the estrogen response element (ERE)-rich array in an in vitro model, suggesting that co-regulator proteins also contribute to GR-ERα crosstalk [48]. Furthermore, ligand-bound SUMOylated GR can repress ER-activated genes by inhibiting the recruitment of the mega transcription factor complex to ERα-bound enhancers [49].
DEX has the ability both to inhibit and potentiate ERα target gene expression, indicating that the transcriptional outcome of GR-ERα crosstalk is gene specific [48]. Co-treatment with E2 and DEX resulted in ERα-assisted loading of GR that was dependent on AP-1 [50]. Another study found similar results in MCF-7 cells, in which ERα and GR co-activation promoted GR chromatin association with ER and AP-1 response elements as well as with FoxO response elements [51]. This study provided important evidence of DEX and E2 co-treatment enhancing ERα target gene expression [51]. Interestingly, the extent of ERα and GR crosstalk may go beyond altering known genes regulated by each hormone receptor alone. For example, in addition to reducing ER chromatin binding at some sites, DEX and E2 co-treatment also gave rise to GR and ERα binding at sites previously not identified in mouse mammary epithelial cell lines in the absence of co-treatment [50]. Although this study did not assess gene expression, another study in human uterine endometrial cancer cells showed that simultaneous activation of GR and ERα gave rise to differentially expressed genes that were unique to the DEX and E2 co-exposure condition [52].
In addition to crosstalk in liver tissue and breast cancer cells, GCs are known to antagonize uterotrophic estrogen action [53-55]. DEX decreases estrogen-stimulated insulin-like growth factor 1 (IGF-1) gene expression [56] and inhibits the proinflammatory and bactericidal activity of E2 in the rat uterus [57, 58]. Reciprocally, E2 can prevent GR from binding to gene promoters and consequently inhibit gene expression by promoting ERα binding to GREs and decreasing polymerase 2 occupancy [59] as well as by reducing recruitment of pioneer factors FoxA1/2 to GREs in human endometrial cancer cell lines [60]. In human uterine leiomyoma and myometrium cell types, approximately 97% of the examined genes that were simultaneously regulated by DEX and E2 had similar expression patterns, while a few genes were identified as antagonistically regulated by DEX and E2 [61]. Likewise, co-treatment of DEX and E2 in the human uterine endometrial cancer cell line ECC1 resulted in only 5.2% of the co-regulated genes antagonistically regulated [52]. In the human endometrial adenocarcinoma Ishikawa cell line, DEX and E2 co-treatment resulted in a transcriptional profile that was most similar to that of E2, in part because GR adopted a chromatin-binding profile more similar to that of ERα [62]. Thus, GCs and estrogens crosstalk in numerous ways to antagonize each other’s actions or to cooperate and drive transcription.
Emerging evidence suggests there is crosstalk between GR and other ERs besides ERα. In the ERα-negative A549 lung epithelial cell line, bisphenol A–induced suppression of the GR target gene ENaCγ was attenuated with the ER antagonist ICI 182780, suggesting that ERβ mediated this inhibitory effect, although more definitive studies are needed to validate this conclusion [63]. Furthermore, DEX has been shown to downregulate ERα and upregulate ERβ in cultured human adipose tissue [64].
The mechanisms explaining these different gene- and tissue-specific end points remain to be characterized, and the findings described in cancer cell lines need to be validated in metabolic tissues. However, the diverse evidence of GR-ERα crosstalk in multiple tissues suggests that GR-ERα crosstalk may be a common method of controlling the function of tissues sensitive both to GCs and estrogens. Whereas the individual developmental impact of GCs and estrogens have been studied extensively in the context of DOHaD, the implications of GR-ERα crosstalk for DOHaD is a fertile area for exploration given that ERs and GR are expressed in fetal tissues critical for metabolic function, including skeletal muscle, liver, and adipose tissue [65-68].
3. Glucocorticoid and Androgen Receptor Crosstalk
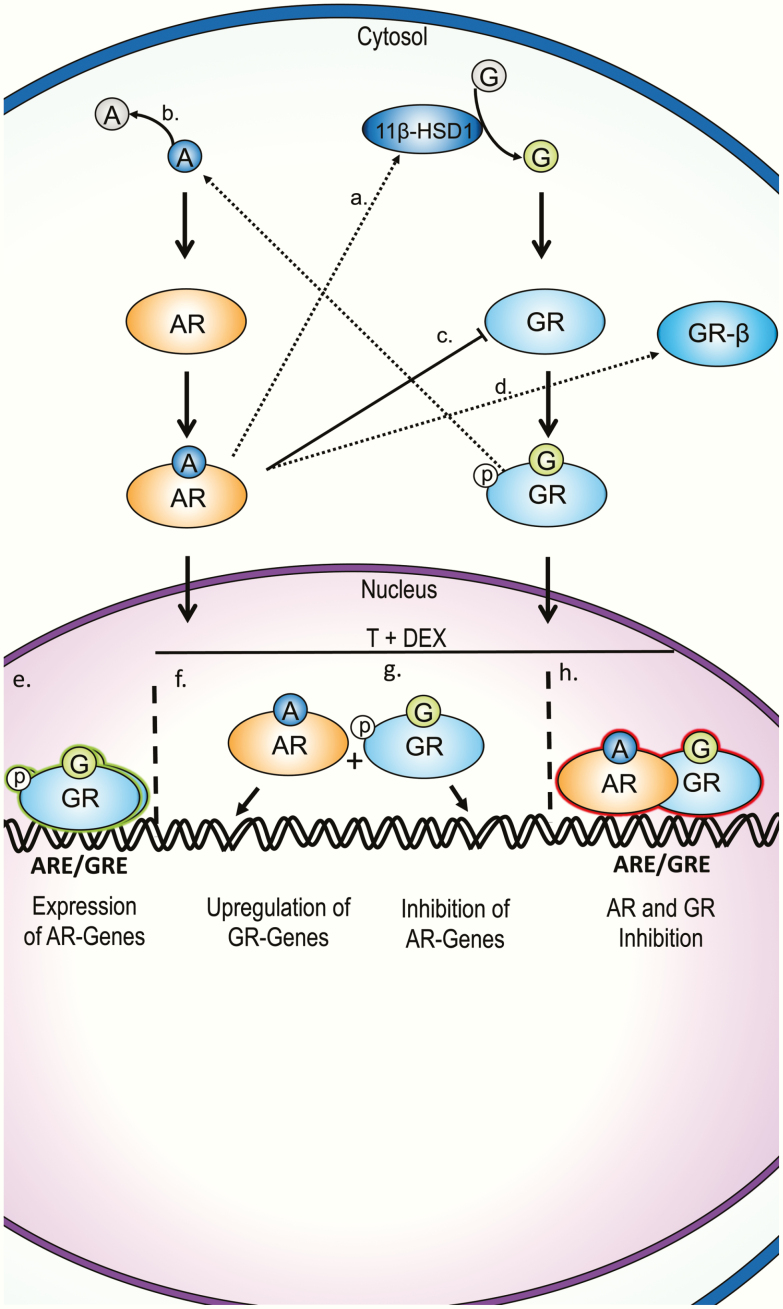
GCs crosstalk with androgen signaling by lowering circulating androgen levels as well as by directly modulating cellular AR transcriptional end points. GCs suppress circulating testosterone (T) levels in men when administered exogenously [69-72] as well as in men with Cushing syndrome [73, 74]. In addition to suppressing the HPG axis, there are numerous cellular mechanisms by which GCs modulate androgen action (Fig. 2). The DBDs of the AR and GR have a high degree of amino acid sequence similarity, including a conserved P-Box, which allows them to bind similar, sometimes even identical, hormone response elements [75-77]; however, GR is unable to bind a subset of androgen-response elements [76, 78]. The functional overlap between AR and GR is evident in castration-resistant prostate cancer, in which GR activity regulates a different yet considerably overlapping transcriptome that renders androgen-deprivation therapy ineffective [79]. About one-third to one-half of the AR-binding regions overlap with GR-binding regions in antiandrogen-resistant xenograft tumors and GR-expressing LNCaP-1F5 cells [79, 80]. The presence of ligand-bound AR also influences genomic GR binding activity; liganded GR can antagonize AR transcription in the presence of androgens, but GR can promote AR transcriptional end points in the absence of androgens [77, 80]. Although the extent of overlap in chromatin binding and transcription between GR and AR in non–prostate cancer tissues needs to be assessed, these results coupled with the structural similarities between the receptors suggest that GR has the ability to crosstalk with AR at the genomic level.
Figure 2.
Summary of proposed G and androgen cellular crosstalk mechanisms from multiple tissues. A, T can increase G bioavailability by upregulating 11β-HSD1 gene expression in omental adipose tissue from children [94]. B, DEX promotes the inactivation of DHT into 3α/β-diol in human preadipocytes [92, 93]. C, AR signaling suppresses GR gene expression in human prostate cancer and suppresses GR transactivation in CV-1 monkey kidney fibroblasts [86, 87]. D, DHEA upregulates and preferentially directs splicing of GR messenger RNA toward the β isoform in human promyelocytic THP-1 cells [85]. E, GR upregulates AR-target genes in absence of T in prostate cancer cells [79, 80]. F, AR promotes GR-dependent gene expression in 3T3-L1s, brown adipose, and prostate cancer cells [80, 82, 95]. G, GR interferes with some AR-transcriptional end points in co-treatment in prostate cancer cells [80]. H, AR and GR may inhibit each other by forming heterodimers [83]. A, androgen; AR, androgen receptor; ARE, androgen response element; DEX, dexamethasone; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; G, glucocorticoid; GR, glucocorticoid receptor; GRE, glucocorticoid response element; p, phosphate; T, testosterone.
Depending on the DNA binding sequence, both AR and GR can either promote transcription or interfere with transcriptional activity [81, 82]. GR’s inhibitory effects on AR transcription is probably not due to competition for DNA binding, because DEX and dihydrotestosterone (DHT) co-treatment actually results in increased AR chromatin binding [80]. One possible way by which AR and GR inhibit each other’s transcriptional activity at specific genes may be by forming heterodimers at GREs [83]. Coactivators for each hormone receptor likely also play a role in crosstalk between GR and AR because SRC-1, the coactivator for several steroid hormone receptors including GR, can inhibit AR transactivation [84]. Another possible mechanism of AR-mediated GR antagonism is through alternative splicing, as has been shown in monkey kidney fibroblasts in which the weak androgen dehydroepiandrosterone was shown to upregulate and preferentially direct splicing of GR messenger RNA into the β isoform, which is known to inhibit the expression of some GRα-regulated genes [85]. Apart from genomic crosstalk, AR signaling suppresses GR gene expression in prostate cancer [86]; however, whether AR suppresses GR expression in other tissues requires testing. The anabolic steroid oxandrolone antagonized GR transactivation in an in vitro monkey kidney CV-1 cell luciferase model without affecting cortisol binding to GR [87]. This effect was AR dependent, and interestingly, the dichlorodiphenyltrichloroethane metabolite dichlorodiphenyldichloroethylene, a known antiandrogenic EDC, also suppressed GR transactivation. This suggests that AR-modulating EDCs may affect GR end points as well.
Crosstalk between AR and GR has also been reported in metabolic tissues such as pancreatic β cells, adipose tissue, and the liver. One study suggested that AR decreases DEX-induced β-cell apoptosis in the INS-1 model [88]. GR was shown to upregulate AR expression and promote nuclear AR translocation during adipogenesis in human preadipocytes while concurrently decreasing AR transcriptional activity [89]. It is possible that the DEX-mediated upregulation of AR during adipogenesis results from the prodifferentiation effects of GR, and that DEX-mediated repression of AR transcriptional activity is needed to promote fat cell development because androgens inhibit adipocyte differentiation [90, 91]. In support of this, DEX promotes the inactivation of DHT into 3α/3β-androstanediol during human preadipocyte differentiation [92, 93]. This suggests that some crosstalk mechanisms may be specific to certain developmental windows. T can upregulate 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) in omental adipose tissue from children [94], suggesting that AR crosstalk with GR can also be mediated by altering tissue GC availability because 11β-HSD1 catalyzes the activation of GCs from inactive precursors. DHT and corticosterone co-treatment in white and brown adipose tissues resulted in amplified upregulation of GR-dependent genes that were not upregulated with DHT alone, whereas AR antagonism decreased GR transcriptional activity in adipose and the liver [95]. Finally, androgens were shown to sensitize mice to GC-mediated lipid accumulation and insulin resistance, suggesting that androgen action cross-talks with GCs in metabolically important tissues [96].
Overall, multiple mechanisms of crosstalk between GCs and androgens have been reported in several tissues in mouse models and humans (Fig. 2). Although GR-AR crosstalk is a current clinical focus in prostate cancer research, not much is known about the physiological relevance of GR-AR crosstalk during fetal development or its relevance to DOHaD, even though GR and AR are both co-expressed in multiple fetal tissues, including the fetal liver and muscle among others [97, 98]. It is possible that GC-mediated disruption of normal androgen-dependent end points during the male fetal T surge may contribute to sex differences in the development of multiple tissues, including those that regulate glucose homeostasis postnatally.
4. Glucocorticoid and Progesterone Receptor Crosstalk
Progestogens are known to oppose GC action in different physiological settings, including in bone formation and lactation; however, they can also induce GC-like effects, such as immunosuppression during pregnancy [99]. These cell-type specific effects can be attributed to several crosstalk mechanisms that depend on the similarity of GR and the progesterone receptor (PR) and are likely influenced by cellular bioavailability of GCs. PR has 2 known nuclear isoforms, PR-A and PR-B, in addition to several alternatively spliced truncated isoforms whose function is not well understood [100]. PR-B possesses 164 additional amino acids at the amino terminus compared to PR-A, and is known to act as an activator of transcription, whereas PR-A has been shown to function as a dominant negative transrepressor of PR-B in a cell- and gene-specific context; however, it may also induce transcription in some contexts [100, 101]. Progesterone (P4) can also act through membrane progestogen receptors and PR membrane components [100].
PR is the sex-steroid receptor with the highest similarity to GR. The sequence homology between the DBD of PR and GR is 90%, which allows both receptors to share chromatin-binding sites [102, 103]. The LBDs are 55% homologous, allowing P4 to bind GR with significantly higher affinity when compared to T or E2, but with relatively weak affinity in comparison to GCs [104]. As such, P4 and synthetic PR modulators can elicit cellular effects by binding to GR [100, 105-107]. Synthetic GCs (including DEX) and endogenous GCs (eg, corticosterone) can also bind to PR [105, 108, 109]. Although the binding affinity of cortisol appears to be weaker compared to these GCs, it may still induce biological effects through PR [110].
Evidence from in vitro experiments employing doses of P4 in the micromolar range in the absence of GCs suggest that P4 is able to act as a GR agonist in physiological settings when endogenous P4 levels are the highest, such as pregnancy. Evidence for P4 acting as a GR agonist in female reproductive tissues has been shown in human primary myometrial cells in which P4 inhibits interleukin-1β–driven cyclooxygenase 2 (COX-2) expression [111, 112], and in rat luteal cells in which it inhibits 20α-hydroxysteroid dehydrogenase [113]. GR agonism by P4 is thought to contribute to immune suppression during pregnancy [114]. This concept is supported by studies showing GR-mediated suppression of several immune processes by P4, such as nitric oxide production in murine peripheral mononuclear cells and macrophages [115, 116], interferon-γ expression in mouse natural killer cells and human uterine natural killer cells [117], and IL-6 levels in bone marrow–derived dendritic cells [118] as well as by inducting murine T-cell apoptosis [119]. One study found that P4 upregulated the proinflammatory cytokine IL-12p40 in the human ectocervical epithelial cell line Ect1/E6E7 by activating GR, suggesting that the direction of P4 immunomodulatory effects are cell specific [120]. While the results of these studies are likely relevant to tissues with high 11β-HSD2 expression and consequently low GC bioavailability, studies showing that P4 can antagonize GR action when coadministered with GCs also emphasize the capacity of P4 to modulate GR signaling in the presence of endogenous GCs.
As a partial agonist for GR, P4 can antagonize GC action by preventing full GR induction by GCs [104, 121, 122]. Evidence that sufficiently high concentrations of P4 outcompete GCs for GR binding has been suggested as one potential mechanism by which P4 prevents GC-mediated bone loss [123]. In human chorion, amnion, and placental tissue, competition for GR by P4 has been suggested to antagonize GC-mediated prostaglandin synthesis by maintaining prostaglandin dehydrogenase activity and inhibiting COX-2 expression [42, 124, 125]. P4 competition for GR has also been suggested to antagonize GC action in rat fetal and human lung epithelial cells [126, 127] and in adipose tissue [128-133]. More studies are needed to test the extent to which high endogenous P4 levels during gestation or during the luteal phase of the menstrual cycle interfere with GC by preventing GC-GR binding, rather than by other mechanisms.
Studies using the human breast cancer cell line T47D/A1-2, which has been transfected with GR to induce similar expression levels as PR, have shown that PR and GR can regulate common genes by distinct mechanisms [102, 134, 135]. Independent treatments of DEX or the synthetic PR ligand R-5020 both upregulated and downregulated common genes in addition to regulating distinct genes [135]. These results suggest that PR and GR regulate distinct sets of genes that overlap; however, the extent that GR induction by R-5020 contributed to the overlapping gene set was not assessed. ChIP-sequencing after treatment with equal concentrations of either hormone showed that, in addition to having unique chromatin binding sites, PR and GR share common binding sites [102]. Co-treatment experiments in this study revealed various examples of gene-specific regulation, including additive chromatin binding of each receptor and consequent gene upregulation, GR-mediated inhibition of PR genomic binding and gene expression, and reciprocal decreases in chromatin binding of both receptors. More studies are needed to assess the extent to which PR and GR crosstalk by these mechanisms in non-cancer tissues that express normal levels of both receptors.
Additional mechanisms of crosstalk have been proposed in a variety of human and murine tissues. In CV-1 fibroblasts, overexpression of PR-A inhibited GR activity by mechanisms that did not require P4 binding to PR-A or PR-A binding to DNA [101]. Limited evidence suggests that PR-A and GR can physically interact as a form of antagonism or agonism [102, 125, 136]. GR-mediated downregulation of PR-A expression may be one common mechanism of crosstalk, as shown in mouse lung [136]. Modulation of circulating P4 and GC levels may be another mechanism of crosstalk because P4 can regulate GC metabolizing enzymes such as 11β-HSD1 [137, 138] and 11β-HSD2 [139], whereas GC-induced placental corticotropin-releasing hormone production can reduce human trophoblast P4 production [140, 141]. Like endogenous GCs, P4 can also bind to corticosteroid-binding globulin [142], potentially altering GC levels and bioavailability.
The overall evidence of GCs and P4 crosstalk in several tissues by numerous mechanisms suggests that aberrant GC action during fetal development has the potential to disrupt developmental PR action. More studies are needed to understand the role that PR plays in the development of key metabolic tissues and how developmental disruption of PR by aberrant GC exposure alters PR-mediated developmental processes. Because PR is also known to crosstalk with AR and ER [99], studies are needed to test how GC-mediated disruption of fetal T levels or ER activity can disrupt PR action during fetal development.
5. Sex-Steroid and Glucocorticoid Action During Fetal Development
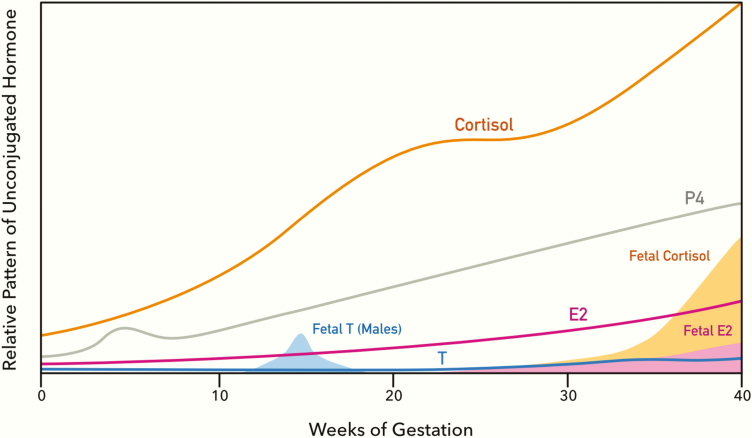
Levels of maternal estrogens, P4, and cortisol increase during gestation and peak near term to maintain a successful pregnancy (Fig. 5) [143, 144]. Fetal levels of sex steroids and cortisol peak at different gestational periods and induce drastic organizational changes in the developing fetus (Fig. 5) [11, 145-147]. Fetal GCs are known to peak toward the end of gestation in a variety of mammalian species, including humans, when they stimulate the maturation of tissues in preparation for postnatal life [11, 147]. Aberrant GC activation during development consistently leads to later life adverse health outcomes, including metabolic defects in numerous species (reviewed in [11, 148, 149]).
Figure 5.
Maternal and fetal patterns of cortisol, P4, E2, and T during human pregnancy. Levels of each hormone are shown relative to its basal gestation levels. Maternal patterns are shown in solid lines; fetal patterns are shown as shaded areas. This figure was modified [11, 143-147] to capture normal patterns of maternal and fetal hormone changes during gestation, disruptions that may alter endocrine crosstalk that results in sex-specific misprogramming of development and potential long-term health consequences. E2, estradiol; P4, progesterone; T, testosterone.
The action of sex steroids on the developing fetus is known to be instrumental in the establishment of physiological sex differences. With the exception of fetuses affected by gonadal dysgenesis, fetal T in males peaks during midgestation in humans and toward the end of gestation in rats [150]. Increased T levels in male fetuses during the masculinization window and during neonatal development are known to establish sexually dimorphic traits in reproductive organs and the brain (reviewed in [151, 152]). Although T levels are markedly higher in developing males, tissue-specific aromatization of androgens to estrogens is common during development and mediates sex-specific differences in developmental outcomes [153]. Thus, androgen and estrogen action are both important for sex-specific development. Although male and female fetuses are exposed to the same amount of P4, sex differences in fetal P4 action may contribute to sex differences in brain development because PR expression has been reported to be higher in the male hypothalamus, including the medial preoptic area (MPOA) in neonatal mice and rats, and the ventromedial nucleus (VMN) in neonatal mice [154]. The higher PR expression in the neonatal male hypothalamus has been attributed to upregulation by ERα action induced by aromatized T [155]. Thus, disruption of either sex steroid class during fetal development may alter sex-specific end points. Given the extensive evidence of GC crosstalk with AR, ERα, and PR, aberrant GR activation during these early developmental windows may be an underappreciated disruptor of sex-steroid–dependent developmental outcomes, which may lead to sex-specific consequences.
6. The Role of Sex Steroids and Glucocorticoids in Sex-Specific Fetal Programming of Metabolism
GR-driven developmental misprogramming of the HPG axis may alter how sex steroids regulate metabolism in a sex-specific manner. Sex differences in fat distribution, lipid metabolism, insulin sensitivity, and glucose metabolism are well known and have been reviewed extensively [152, 156]. Although numerous factors contribute to sex differences in metabolism, including societal pressures and sex chromosome complement [157, 158], this section will focus on differences in circulating sex steroids [151]. Sex steroids regulate numerous aspects of glucose and lipid homeostasis (reviewed in [159, 160]). E2 is suggested to protect against insulin resistance in women by increasing insulin sensitivity. Conversely, lower T levels in men are associated with increased visceral adiposity and decreased insulin sensitivity. Although its effects on metabolism are less clear, P4 has been shown to modulate β-cell insulin secretion alone or by modulating E2 action, and P4 has also been shown to decrease insulin sensitivity [161]. Thus, developmental insults that disrupt the function of the HPG axis later in life, such as elevated fetal GC levels or inappropriate GR signaling activation, have the potential to disrupt glucose homeostasis by altering the metabolism-regulating activational effects of sex steroids. Additionally, GC-mediated disruption of sex-steroid action on peripheral tissues during development may lead to sex-dependent disruption in the organization of metabolic tissues.
Sex steroids are known to regulate the sexual differentiation of reproductive organs and the brain, yet little is known about how sex steroids influence the development of peripheral metabolic tissues. However, recent work suggests that androgens and estrogens contribute to the development of key metabolic tissues [162-164]. One recent study showed that sex differences in hepatic metabolism in mice can be programmed by neonatal estrogen action [162]. Furthermore, girls on average have a lower body weight, more adiposity, and increased circulating insulin compared to newborn boys [165-168], suggesting that peripheral tissues undergo sex-specific organizational changes in utero and are thus sensitive to hormonal disruptions during development. As such, the molecular machinery for sex-steroid action is present during fetal and neonatal development. ARs are expressed in the human fetal liver [169], and ER expression displays sex-specific differences in the mouse liver late in gestation and during neonatal life [162, 163]. Additionally, estrogen signaling in white adipose progenitors inhibit differentiation into brown adipose [164], further suggesting that estrogen action during early development can induce organizational changes in adipose tissue in a sex-dependent manner. Whether estrogen action is involved in the early development of other tissues that control glucose homeostasis needs further study, but work in zebrafish demonstrates ER activation in the developing pancreas [65]. Additionally, sex-steroid–metabolizing enzymes such as aromatase [169-171], SULT1E1 [172, 173], and enzymes responsible for synthesizing the more potent androgen DHT [169] are expressed in the fetal liver. Whether tissue-specific disruption of sex-steroid metabolism contributes to the differential programming of peripheral metabolic tissues requires further study. Importantly, GCs upregulate aromatase in fetal hepatocytes [174, 175], whereas DEX upregulates SULT1E1 expression in adult mouse and human hepatocytes, and consequently decreases circulating E2 in mice [43]. Collectively this suggests that fetal GC overexposure may affect fetal circulating estrogen levels and impact estrogen-dependent fetal development.
Interestingly, disruption of estrogen, androgen, or GC signaling results in adverse metabolic outcomes later in life (reviewed in [35]), further emphasizing the importance of these hormone classes in the normal development of metabolic physiology. Inhibition of ERα activity during fetal development by inhibiting aromatase in baboons results in later life insulin resistance [176, 177], whereas prenatal exposure to ERα- and AR-disrupting EDCs is associated with adverse metabolic outcomes in population-based and animal studies [27]. Prenatal overexposure to androgens leads to metabolic perturbations in female offspring characterized by increased adiposity, impairments in insulin secretion, and/or insulin resistance in mice, rats, sheep, and monkeys [178-182]. Further work is needed to determine how aberrant sex-steroid action leads to these metabolic perturbations. Direct AR, ERα, or PR modulation in key metabolic tissues could lead to organizational misprogramming that results in disease. Alternatively, aberrant sex-steroid action could alter the development of reproductive or neuronal end points that increase susceptibility to metabolic disease by changing eating behavior or sex hormone levels during adulthood. Nonetheless, there is robust evidence showing that aberrant GR activation disrupts sex-steroid action during development. Whether GR disruption results in sex-specific adverse outcomes by altering sex-steroid action during the establishment of sex differences of key metabolic tissues remains to be studied.
The potential effects of developmental disruption of PR action on metabolic misprogramming is largely speculative because of limited understanding of the role that PR plays in the development of key metabolic tissues. Higher neonatal expression of PR in male rodent MPOA and VMN, hypothalamic areas involved in maintaining lipid and glucose homeostasis in addition to controlling sexual behavior and other functions [1, 183], suggests that developmental PR disruption may alter the development of these regions and promote sex-specific alterations in postnatal metabolism. Because higher PR expression in the MPOA and VMN of male fetuses has been attributed to increased ERα action by aromatized fetal T, disrupting fetal T levels or ERα expression by aberrant GC action may alter the male development of these hypothalamic areas [155]. Whether there are sex-specific differences in PR expression in other developing tissues critical for controlling postnatal metabolism requires further study. Additionally, the effects that gestational GC overexposure have on adult P4 levels needs further investigation because gestational GC overexposures have been associated with increased apoptosis in fetal ovaries and lasting alterations in the HPG axis as discussed later. Thus, abnormal programming of P4 secretion in women may alter glucose homeostasis during adulthood given its influence on insulin secretion and insulin sensitivity [161]. Studies are needed to verify these hypotheses and to assess the extent to which developmental PR disruption can contribute to sex-specific misprogramming of metabolism.
7. Evidence for Developmental Sex-Steroid Disruption by Aberrant Glucocorticoid Receptor Activation
Early-life GC overexposure from psychological stress or pharmacological GC treatments in animal models disrupts the normal T surge during early development and alters androgen-dependent developmental outcomes in a sex-dependent manner [39]. Early studies showed that maternal exposure to psychological stress or exogenous GCs during the last trimester decreased fetal androgen action, as evidence by decreased anogenital distance and lowered testes weight at birth as well as “feminized” sexual behavior during adulthood in male offspring [184-186]. Subsequent studies suggested that these phenotypes were established during fetal development since prenatal treatments with pharmacological GCs (DEX, betamethasone, or prednisone) as well as chronic stress during late gestation blunted gestational T in mice and rats [187-190]. Critically, the 2 studies that longitudinally measured fetal T during pregnancy showed that stress increased fetal T at gestational day 17 and decreased T during gestational days 18 to 19, when fetal T typically peaks [188, 189]. The impact of these time-dependent alterations in fetal T on development need more detailed assessments. Subsequent studies showed that gestational GC overexposure inhibited male genital development in fetal sheep and mice [191-193], suggesting that gestational GC overexposure can interfere with the development of reproductive organs by altering fetal T and/or by inappropriately activating GR signaling in these tissues directly. Interestingly, as opposed to males, gestational GC overexposure appears to have androgenic effects on female fetuses. Stressful life events have been associated with increased anogenital distance at birth in female infants [194] and “masculinized” play behavior in young girls [195]. In animal studies, prenatal stress has been shown to increase fetal T in female mice [196]. The nature behind sexually dimorphic effects of gestational GC overexposure on fetal androgenic action requires further study.
Critically, the GC-induced fetal misprogramming of reproductive organs appears to have effects on the HPG axis that persist into adulthood. Developmental overexposure to GCs altered anogenital distance [197-201], delayed puberty [202, 203], and affected reproductive organ weights, function, and sperm parameters [204-206] in rats. Importantly, the most consistent finding from all these studies was that the different GC overexposure paradigms resulted in approximately 40% to 50% reductions in circulating T in male offspring during adulthood. Notably, prenatal DEX exposure during late gestation in rats amplified the developmental alterations in male reproductive organs relative to antiandrogenic phthalate exposure alone, including the severity of hypospadias, incidence of cryptorchidism, reductions in anogenital distance, and lower plasma T concentrations [207]. Although none of these studies assessed metabolic parameters in the affected offspring, low T in males is known to impair glucose homeostasis based on the various roles that androgens play in glucose and lipid control [160]. Similar GC overexposure paradigms in studies that tested for metabolic end points have demonstrated reduced insulin sensitivity and impaired glucose tolerance [31, 208-211]. Thus, reductions in later-life circulating T in males may be one mechanism by which developmental GC overexposures can derange metabolism in a sex-specific manner.
Unfortunately, few studies have assessed circulating sex steroids in female offspring following developmental overexposure to GCs. One study showed that maternal stress in guinea pigs increased T levels in female offspring during adulthood [212], whereas prenatal DEX led to reduced circulating follicle-stimulating hormone and luteinizing hormone in peripubertal rats [213] and lower serum E2 in adult female offspring [214]. The most consistent results reported in female offspring were alterations in the onset of puberty and variations in the length of estrus stages [215-220] as well as decreased numbers of healthy primordial follicles [221, 222], likely due to the proapoptotic action of DEX reported on human and rat fetal ovaries [223, 224]. Thus, developmental GC overexposure has the ability to disrupt later-life processes in female offspring that depend on sex-steroid action. Critically, decreased circulating T in men, in contrast with increased circulating T in women, has been associated with type 2 diabetes risk [225, 226]. Given that prenatal GC overexposure in animal models has been shown to decrease T in adult males and increase T in adult females, thorough assessments should test whether these outcomes are consistent in humans. Acute stressful events during gestation in humans and rodent models decrease circulating P4 levels, and can induce premature birth and or miscarriage [227-229]. Interestingly, male offspring have a higher rate of premature birth [230]. The links between GR/PR crosstalk and prematurity should be explored, especially, because premature birth is associated with a higher risk of metabolic disease later in life [231, 232].
Work from other animal models that resulted in fetal GC overexposure further supports the idea that abnormal crosstalk between GCs and sex steroids during development leads to the misprogramming of metabolism. For example, gestational caloric and protein restriction increase fetal GC exposure while also leading to derangements in metabolic and reproductive health (reviewed in [1, 15, 233]). These gestational exposures likely misprogram development differently from psychological stress or pharmacological GC treatments because they increase gestational T levels [234-236]. More work is needed to fully understand how these dietary models misprogram metabolism in the offspring, but given that reproductive perturbations are observed throughout the lifespan of the offspring, they likely involve alterations of the HPG axis (reviewed in [233]). Thus, several animal models that increase fetal GC exposure lead to similar metabolic and reproductive phenotypes in the offspring, some of which exhibit sex specificity. Further work is needed to understand the mechanisms by which GCs and sex steroids crosstalk during development and how these interactions contribute to adverse metabolic phenotypes.
8. Misprogramming of Neuroendocrine Pathways Controlling Sex-Specific Metabolic Outcomes
GC-mediated disruption of sex-steroid action in the hypothalamus and/or anterior pituitary may lead to postnatal sex-specific derangements in metabolism. The neuroendocrine system undergoes substantial sex-specific organizational changes induced by sex steroids during early development, including in regions that control glucose and lipid homeostasis such as the hypothalamus and anterior pituitary [4, 237]. For example, differences in growth hormone (GH) secretion by sex are established by organizational effects of neonatal sex-steroid exposure and are then activated by sex hormones after the onset of puberty (reviewed in [238, 239]). After puberty, females continuously secrete GH, which leads to persistent activation of hepatic signal transducer and activator protein 5b (STAT5b), whereas males display pulsatile GH secretion leading to differences in downstream GH signaling and consequential sex differences in hepatic gene expression and metabolic function (reviewed in [240]). Partial masculinization of the GH-IGF-1 axis and consequential liver function have been reported in female mice developmentally overexposed to T [241, 242]. In contrast, estrogen is known to masculinize neural pathways [243]. Intriguingly, exposure to the estrogenic EDC bisphenol A also results in the “partial masculinization” of the GH/IGF-1 axis [244], underscoring how disruption of sex-steroid action can disrupt a developmental pathway that regulates sex-specific metabolic function postnatally. It is possible that overactivation of GR signaling during early development can alter the sex-specific development of neuroendocrine pathways that are mediated by sex steroids and lead to sex-specific outcomes in glucose and lipid homeostasis, although this hypothesis needs further testing. Interestingly, one study showed that prenatal DEX exposure increased hepatic steatosis in female rat offspring only, at least in part via a reduction in hypothalamic GH-releasing hormone and consequential GH action [33]. More work is needed to assess how GR signaling overactivation affects sex-steroid action in neuroendocrine regions that control postnatal metabolism and how these alterations can lead to defects in glucose and lipid homeostasis.
9. Future Directions
In line with previous analyses [245], the evidence outlined herein presents the current understanding of the multiple mechanisms by which GCs crosstalk with sex steroids in various tissues. In developed tissues and adult animals, GC sex-steroid crosstalk leads to physiologically important effects on reproductive capacity, cancer risk, and metabolic function. Our understanding of how GR sex-steroid crosstalk operates during fetal development is mainly limited to GC overexposure studies that have shown reductions in fetal T levels, alterations in reproductive development, and persistent effects on the HPG axis. We suggest that abnormal GR signaling activation can disrupt sex-steroid action in key metabolic tissues during development as well, either by altering sex-steroid levels or by directly interfering with AR, ER, or PR transcriptional end points, ultimately resulting in organizational changes that affect metabolic health later in life. Given that sex-specificity in metabolic outcomes following developmental GC overexposure is a commonly observed phenotype that remains incompletely understood, further studies examining GC sex-steroid crosstalk are essential for enhancing our understanding of sex-specific metabolic programming. The exciting findings that tissue co-stimulation with GCs and either androgens or estrogens alters the regulation of genes that are not regulated by either hormone alone warrants further study to clarify the molecular mechanisms by which co-exposures modulate gene expression. Furthermore, the ability of GCs and sex steroids to induce unique gene signatures only in the presence of each other warrants investigating how sex steroids and GCs synergize in fetal and neonatal tissues to regulate development. This is especially important since our basic understanding of how sex steroids regulate the development of peripheral metabolic tissues remains limited [89, 162-164, 246]. Thus, studying GC sex-steroid crosstalk is imperative to advancing our understanding of basic mammalian development, but it is also important to better appreciate how disruptions in each of these hormone classes affects development. Further work is needed to understand the outcomes of prenatal GC overexposures in fetuses with atypical sex-steroid profiles, as seen with children born with differences in sexual development. While this review focuses on metabolic misprogramming, interrogating how environmental and pharmacological GCs as well as prenatal stress crosstalk with sex steroids is essential for understanding the development of outcomes in other areas of health that have been shown to exhibit sex-specific differences resulting from perinatal insults.
From the perspective of environmental health, consideration of GC sex-steroid crosstalk is especially salient for EDC screens and regulatory assessments of chemical safety. In animal models, careful and uniform management of animal stress, a trigger of endogenous GC release, is essential for studying EDCs affecting estrogenic and androgenic end points given the antagonistic effects of GCs that have been reported. Further, in vitro screens for sex-steroid– and GC-disrupting activity should account for transcriptional crosstalk. The genomic binding and transcriptional overlap that GR exhibits with AR, ER, and PR as well as the ability of AR and ER to promote assisted loading with GR during co-exposures could result in significantly higher activity than when studying one chemical or pathway in isolation. Critically, the dose-response of co-treatments should be evaluated to ascertain how GCs and sex steroids fundamentally alter transcriptional end points and physiological outcomes across exposure ranges. This is relevant to EDC screening efforts because the activity of one type of EDC (eg, estrogenic) in the environmental context of a high- or low-interacting hormone (eg, GC) may elicit very different outcomes. For example, higher EDC exposures in animal models can possibly raise endogenous GC levels and obscure sex-steroid–dependent phenotypes if these endogenous GCs antagonize sex-steroid action. Thus, measurements of endogenous GCs and GC-related outcomes in animal models examining sex hormone–disrupting EDCs should be a standardized practice to better understand nonlinear responses relevant to assessing safe and acceptable levels of EDC exposures [247, 248].
Furthermore, crosstalk of EDCs that exhibit affinity both to GR and a sex-steroid receptor with an opposing function should be considered because it is possible that one chemical can have strong endocrine-disrupting actions at lower doses mediated by one receptor that may be blunted by antagonism through a separate nuclear receptor at higher concentrations. The similarities between the PR and GR LBDs and the resulting potential for ligands to bind both receptors with different affinities should be accounted for when testing GR- or PR-active EDCs in tissues expressing both receptors. This same logic applies to the mineralocorticoid receptor, which has even greater similarity to GR than PR but is beyond the scope of the present review [103]. In all of these instances, crosstalk between these hormone classes or differential activation of different receptors may be possible mechanisms for the nonmonotonic dose-response relationships for some EDCs. In addition, the issue of GC sex-steroid crosstalk is fundamentally important for understanding the biological impact of exposure to chemical mixtures in which various components may modulate signaling through these intersecting signaling cascades. Furthermore, given the increasing recognition that allostatic load plays an important role in the pathogenesis of metabolic diseases [249], models of concordant exposure to EDCs modulating estrogenic or androgenic signaling with excess endogenous, environmental, or pharmacological GCs may illuminate metabolic misprogramming events that promote the development of human diseases and their sex-specific manifestations.
10. Conclusions
Evidence from animal studies strongly support the capacity of GCs to disrupt sex-steroid action during fetal development. Interestingly, the same exposure doses or stress paradigms that have resulted in altered sexual development have been shown to induce metabolic dysfunction during adulthood in animals, and these metabolic outcomes often show differences by sex. How the sex-steroid–disrupting actions of prenatal GC overexposure lead to sex differences in metabolic outcomes has not been explored. Studies focused on metabolic end points generally do not measure sex hormone end points in their studies, making it difficult to unravel how disruptions of sexual reproduction and metabolism are connected, despite the definite influence of sex steroids on insulin sensitivity, insulin secretion, and adiposity [159, 160]. The role that sex steroids play in the development of organs that are essential in regulating glucose and lipid homeostasis remains incompletely understood, yet estrogen- and androgen-overexposure studies consistently show adverse metabolic outcomes in animal studies. Furthermore, EDCs that disrupt estrogen and/or androgen action have been shown to misprogram metabolism in animal studies and some epidemiological studies. Considering hormone crosstalk during development is necessary to mechanistically illuminate how metabolism is misprogrammed by EDC exposures and how sex-specific outcomes arise.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (R21 ES021354, R01 ES028879, T32 HD007009, and P30 ES027792). We would like to thank Amy Koehler for contributing her artwork to this manuscript.
Glossary
Abbreviations
- AP-1
activator protein 1
- AR
androgen receptor
- CBD
corticosteroid-binding globulin
- COX
cyclooxygenase
- DBD
DNA-binding domain
- DEX
dexamethasone
- DHT
dihydrotestosterone
- DOHaD
Developmental Origins of Health and Disease
- E2
estradiol
- EDCs
endocrine-disrupting chemicals
- ER
estrogen receptor
- ERE
estrogen response element
- FoxA1
Forkhead Box A1
- GC
glucocorticoid
- GH
growth hormone
- GR
glucocorticoid receptor
- HPG
hypothalamic-pituitary-gonadal
- 11β-HSD
11β-hydroxysteroid dehydrogenase
- IGF-1
insulin-like growth factor-1
- IL
interleukin
- LBD
ligand-binding domain
- MPOA
medial preoptic area
- NK
natural killer
- P4
progesterone
- PCBs
polychlorinated biphenyls
- PR
progesterone receptor
- SULT1E1
estrogen sulfotransferase
- T
testosterone
- VMN
ventromedial nucleus
Additional Information
Disclosure Summary: R.M.S. declares honoraria from the American Medical Forum and CVS/Health. The other authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References and Notes
- 1. Bouret S, Levin BE, Ozanne SE. Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol Rev. 2015;95(1):47-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21(4):199-205. [DOI] [PubMed] [Google Scholar]
- 3. Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351(9097):173-177. [DOI] [PubMed] [Google Scholar]
- 4. Dearden L, Bouret SG, Ozanne SE. Sex and gender differences in developmental programming of metabolism. Mol Metab. 2018;15:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavalli G, Heard E. Advances in epigenetics link genetics to the environment and disease. Nature. 2019;571(7766):489-499. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 7. International Diabetes Federation. IDF Diabetes Atlas. 9th ed 2019. http://www.diabetesatlas.org. Accessed December 14, 2019. [Google Scholar]
- 8. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60(4):207-221. [DOI] [PubMed] [Google Scholar]
- 9. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64-74. [DOI] [PubMed] [Google Scholar]
- 10. Cain DW, Cidlowski JA. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29(4):545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fowden AL, Valenzuela OA, Vaughan OR, Jellyman JK, Forhead AJ. Glucocorticoid programming of intrauterine development. Domest Anim Endocrinol. 2016;56(Suppl):S121-S132. [DOI] [PubMed] [Google Scholar]
- 12. Fowden AL, Forhead AJ. Glucocorticoids as regulatory signals during intrauterine development. Exp Physiol. 2015;100(12):1477-1487. [DOI] [PubMed] [Google Scholar]
- 13. Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57(1):113-122. [DOI] [PubMed] [Google Scholar]
- 14. Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(Suppl 3):U49-U62. [DOI] [PubMed] [Google Scholar]
- 15. Fowden AL, Forhead AJ. Hormones as epigenetic signals in developmental programming. Exp Physiol. 2009;94(6):607-625. [DOI] [PubMed] [Google Scholar]
- 16. Kelly BA, Lewandowski AJ, Worton SA, et al. Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics. 2012;129(5):e1282-e1290. [DOI] [PubMed] [Google Scholar]
- 17. Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856-1862. [DOI] [PubMed] [Google Scholar]
- 18. Jobe AH, Goldenberg RL. Antenatal corticosteroids: an assessment of anticipated benefits and potential risks. Am J Obstet Gynecol. 2018;219(1):62-74. [DOI] [PubMed] [Google Scholar]
- 19. Jobe AH. Antenatal corticosteroids—a concern for lifelong outcomes. J Pediatr. 2020;217:184-188. [DOI] [PubMed] [Google Scholar]
- 20. Suzuki G, Tue NM, Malarvannan G, et al. Similarities in the endocrine-disrupting potencies of indoor dust and flame retardants by using human osteosarcoma (U2OS) cell-based reporter gene assays. Environ Sci Technol. 2013;47(6):2898-2908. [DOI] [PubMed] [Google Scholar]
- 21. Stavreva DA, George AA, Klausmeyer P, et al. Prevalent glucocorticoid and androgen activity in US water sources. Sci Rep. 2012;2:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conley JM, Evans N, Cardon MC, et al. Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environ Sci Technol. 2017;51(9):4781-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van der Linden SC, Heringa MB, Man HY, et al. Detection of multiple hormonal activities in wastewater effluents and surface water, using a panel of steroid receptor CALUX bioassays. Environ Sci Technol. 2008;42(15):5814-5820. [DOI] [PubMed] [Google Scholar]
- 24. Macikova P, Groh KJ, Ammann AA, Schirmer K, Suter MJ. Endocrine disrupting compounds affecting corticosteroid signaling pathways in Czech and Swiss waters: potential impact on fish. Environ Sci Technol. 2014;48(21):12902-12911. [DOI] [PubMed] [Google Scholar]
- 25. Jia A, Wu S, Daniels KD, Snyder SA. Balancing the budget: accounting for glucocorticoid bioactivity and fate during water treatment. Environ Sci Technol. 2016;50(6):2870-2880. [DOI] [PubMed] [Google Scholar]
- 26. Zoeller RT, Brown TR, Doan LL, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from the Endocrine Society. Endocrinology. 2012;153(9):4097-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heindel JJ, Blumberg B, Cave M, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mimoto MS, Nadal A, Sargis RM. Polluted pathways: mechanisms of metabolic disruption by endocrine disrupting chemicals. Curr Environ Health Rep. 2017;4(2):208-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruiz D, Regnier SM, Kirkley AG, et al. Developmental exposure to the endocrine disruptor tolylfluanid induces sex-specific later-life metabolic dysfunction. Reprod Toxicol. 2019;89:74-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J, Yang Y, Liu W, Schlenk D, Liu J. Glucocorticoid and mineralocorticoid receptors and corticosteroid homeostasis are potential targets for endocrine-disrupting chemicals. Environ Int. 2019;133(Pt A):105133. [DOI] [PubMed] [Google Scholar]
- 31. O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287(5):E863-E870. [DOI] [PubMed] [Google Scholar]
- 32. Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. Eur J Endocrinol. 2001;145(4):529-539. [DOI] [PubMed] [Google Scholar]
- 33. Carbone DL, Zuloaga DG, Hiroi R, Foradori CD, Legare ME, Handa RJ. Prenatal dexamethasone exposure potentiates diet-induced hepatosteatosis and decreases plasma IGF-I in a sex-specific fashion. Endocrinology. 2012;153(1):295-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldstein JM, Hale T, Foster SL, Tobet SA, Handa RJ. Sex differences in major depression and comorbidity of cardiometabolic disorders: impact of prenatal stress and immune exposures. Neuropsychopharmacology. 2019;44(1):59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cardoso RC, Padmanabhan V. Prenatal steroids and metabolic dysfunction: lessons from sheep. Annu Rev Anim Biosci. 2019;7:337-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang G, Cherkerzian S, Loucks EB, et al. Sex differences in the prenatal programming of adult metabolic syndrome by maternal androgens. J Clin Endocrinol Metab. 2018;103(11):3945-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mauvais-Jarvis F. Developmental androgenization programs metabolic dysfunction in adult mice: clinical implications. Adipocyte. 2014;3(2):151-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xita N, Tsatsoulis A. Fetal origins of the metabolic syndrome. Ann N Y Acad Sci. 2010;1205:148-155. [DOI] [PubMed] [Google Scholar]
- 39. Barrett ES, Swan SH. Stress and androgen activity during fetal development. Endocrinology. 2015;156(10):3435-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haynes LE, Lendon CL, Barber DJ, Mitchell IJ. 17 Beta-oestradiol attenuates dexamethasone-induced lethal and sublethal neuronal damage in the striatum and hippocampus. Neuroscience. 2003;120(3):799-806. [DOI] [PubMed] [Google Scholar]
- 41. Ooishi Y, Mukai H, Hojo Y, et al. Estradiol rapidly rescues synaptic transmission from corticosterone-induced suppression via synaptic/extranuclear steroid receptors in the hippocampus. Cereb Cortex. 2012;22(4):926-936. [DOI] [PubMed] [Google Scholar]
- 42. Patel FA, Funder JW, Challis JR. Mechanism of cortisol/progesterone antagonism in the regulation of 15-hydroxyprostaglandin dehydrogenase activity and messenger ribonucleic acid levels in human chorion and placental trophoblast cells at term. J Clin Endocrinol Metab. 2003;88(6):2922-2933. [DOI] [PubMed] [Google Scholar]
- 43. Gong H, Jarzynka MJ, Cole TJ, et al. Glucocorticoids antagonize estrogens by glucocorticoid receptor-mediated activation of estrogen sulfotransferase. Cancer Res. 2008;68(18):7386-7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kinyamu HK, Archer TK. Estrogen receptor-dependent proteasomal degradation of the glucocorticoid receptor is coupled to an increase in Mdm2 protein expression. Mol Cell Biol. 2003;23(16):5867-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Y, Leung DY, Nordeen SK, Goleva E. Estrogen inhibits glucocorticoid action via protein phosphatase 5 (PP5)-mediated glucocorticoid receptor dephosphorylation. J Biol Chem. 2009;284(36):24542-24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Severson TM, Kim Y, Joosten SEP, et al. Characterizing steroid hormone receptor chromatin binding landscapes in male and female breast cancer. Nat Commun. 2018;9(1):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karmakar S, Jin Y, Nagaich AK. Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) α and activator protein 1 (AP1) in dexamethasone-mediated interference of ERα activity. J Biol Chem. 2013;288(33):24020-24034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bolt MJ, Stossi F, Newberg JY, Orjalo A, Johansson HE, Mancini MA. Coactivators enable glucocorticoid receptor recruitment to fine-tune estrogen receptor transcriptional responses. Nucleic Acids Res. 2013;41(7):4036-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang F, Ma Q, Liu Z, et al. Glucocorticoid receptor:MegaTrans switching mediates the repression of an ERα-regulated transcriptional program. Mol Cell. 2017;66(3):321-331.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miranda TB, Voss TC, Sung MH, et al. Reprogramming the chromatin landscape: interplay of the estrogen and glucocorticoid receptors at the genomic level. Cancer Res. 2013;73(16):5130-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. West DC, Pan D, Tonsing-Carter EY, et al. GR and ER coactivation alters the expression of differentiation genes and associates with improved ER+ breast cancer outcome. Mol Cancer Res. 2016;14(8):707-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Whirledge S, Xu X, Cidlowski JA. Global gene expression analysis in human uterine epithelial cells defines new targets of glucocorticoid and estradiol antagonism. Biol Reprod. 2013;89(3):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bever AT, Hisaw FL, Velardo JT. Inhibitory action of desoxycorticosterone acetate, cortisone acetate, and testosterone on uterine growth induced by estradiol-17β. Endocrinology. 1956;59(2):165-169. [DOI] [PubMed] [Google Scholar]
- 54. Bitman J, Cecil HC. Differential inhibition by cortisol of estrogen-stimulated uterine responses. Endocrinology. 1967;80(3):423-429. [DOI] [PubMed] [Google Scholar]
- 55. Campbell PS. The mechanism of the inhibition of uterotrophic responses by acute dexamethasone pretreatment. Endocrinology. 1978;103(3):716-723. [DOI] [PubMed] [Google Scholar]
- 56. Sahlin L. Dexamethasone attenuates the estradiol-induced increase of IGF-I mRNA in the rat uterus. J Steroid Biochem Mol Biol. 1995;55(1):9-15. [DOI] [PubMed] [Google Scholar]
- 57. Rhen T, Grissom S, Afshari C, Cidlowski JA. Dexamethasone blocks the rapid biological effects of 17beta-estradiol in the rat uterus without antagonizing its global genomic actions. FASEB J. 2003;17(13):1849-1870. [DOI] [PubMed] [Google Scholar]
- 58. Rhen T, Cidlowski JA. Estrogens and glucocorticoids have opposing effects on the amount and latent activity of complement proteins in the rat uterus. Biol Reprod. 2006;74(2):265-274. [DOI] [PubMed] [Google Scholar]
- 59. Whirledge S, Cidlowski JA. Estradiol antagonism of glucocorticoid-induced GILZ expression in human uterine epithelial cells and murine uterus. Endocrinology. 2013;154(1):499-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Whirledge S, Kisanga EP, Taylor RN, Cidlowski JA. Pioneer factors FOXA1 and FOXA2 assist selective glucocorticoid receptor signaling in human endometrial cells. Endocrinology. 2017;158(11):4076-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Whirledge S, Dixon D, Cidlowski JA. Glucocorticoids regulate gene expression and repress cellular proliferation in human uterine leiomyoma cells. Horm Cancer. 2012;3(3):79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vahrenkamp JM, Yang CH, Rodriguez AC, et al. Clinical and genomic crosstalk between glucocorticoid receptor and estrogen receptor α in endometrial cancer. Cell Rep. 2018;22(11):2995-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hijazi A, Guan H, Yang K. Bisphenol a suppresses glucocorticoid target gene (ENaCγ) expression via a novel ERβ/NF-κB/GR signalling pathway in lung epithelial cells. Arch Toxicol. 2017;91(4):1727-1737. [DOI] [PubMed] [Google Scholar]
- 64. Kamble PG, Pereira MJ, Almby K, Eriksson JW. Estrogen interacts with glucocorticoids in the regulation of lipocalin 2 expression in human adipose tissue. Reciprocal roles of estrogen receptor α and β in insulin resistance? Mol Cell Endocrinol. 2019;490:28-36. [DOI] [PubMed] [Google Scholar]
- 65. Bondesson M, Hao R, Lin CY, Williams C, Gustafsson JÅ. Estrogen receptor signaling during vertebrate development. Biochim Biophys Acta. 2015;1849(2):142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rando G, Tan CK, Khaled N, et al. Glucocorticoid receptor-PPARα axis in fetal mouse liver prepares neonates for milk lipid catabolism. Elife. 2016;5:e11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ballard PL, Baxter JD, Higgins SJ, Rousseau GG, Tomkins GM. General presence of glucocorticoid receptors in mammalian tissues. Endocrinology. 1974;94(4):998-1002. [DOI] [PubMed] [Google Scholar]
- 68. Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11beta-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin Ii receptor in neonatal sheep. Endocrinology. 2001;142(7):2854-2864. [DOI] [PubMed] [Google Scholar]
- 69. Schaison G, Durand F, Mowszowicz I. Effect of glucocorticoids on plasma testosterone in men. Acta Endocrinol (Copenh). 1978;89(1):126-131. [DOI] [PubMed] [Google Scholar]
- 70. MacAdams MR, White RH, Chipps BE. Reduction of serum testosterone levels during chronic glucocorticoid therapy. Ann Intern Med. 1986;104(5):648-651. [DOI] [PubMed] [Google Scholar]
- 71. Cumming DC, Quigley ME, Yen SS. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab. 1983;57(3):671-673. [DOI] [PubMed] [Google Scholar]
- 72. Doerr P, Pirke KM. Cortisol-induced suppression of plasma testosterone in normal adult males. J Clin Endocrinol Metab. 1976;43(3):622-629. [DOI] [PubMed] [Google Scholar]
- 73. Smals AG, Kloppenborg PW, Benraad TJ. Plasma testosterone profiles in Cushing’s syndrome. J Clin Endocrinol Metab. 1977;45(2):240-245. [DOI] [PubMed] [Google Scholar]
- 74. Vierhapper H, Nowotny P, Waldhäusl W. Production rates of testosterone in patients with Cushing’s syndrome. Metabolism. 2000;49(2):229-231. [DOI] [PubMed] [Google Scholar]
- 75. Chang CS, Kokontis J, Liao ST. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci U S A. 1988;85(19):7211-7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Claessens F, Alen P, Devos A, Peeters B, Verhoeven G, Rombauts W. The androgen-specific probasin response element 2 interacts differentially with androgen and glucocorticoid receptors. J Biol Chem. 1996;271(32):19013-19016. [DOI] [PubMed] [Google Scholar]
- 77. Pihlajamaa P, Sahu B, Jänne OA. Determinants of receptor- and tissue-specific actions in androgen signaling. Endocr Rev. 2015;36(4):357-384. [DOI] [PubMed] [Google Scholar]
- 78. Denayer S, Helsen C, Thorrez L, Haelens A, Claessens F. The rules of DNA recognition by the androgen receptor. Mol Endocrinol. 2010;24(5):898-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155(6):1309-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sahu B, Laakso M, Pihlajamaa P, et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013;73(5):1570-1580. [DOI] [PubMed] [Google Scholar]
- 81. Yen PM, Liu Y, Palvimo JJ, et al. Mutant and wild-type androgen receptors exhibit cross-talk on androgen-, glucocorticoid-, and progesterone-mediated transcription. Mol Endocrinol. 1997;11(2):162-171. [DOI] [PubMed] [Google Scholar]
- 82. List HJ, Lozano C, Lu J, Danielsen M, Wellstein A, Riegel AT. Comparison of chromatin remodeling and transcriptional activation of the mouse mammary tumor virus promoter by the androgen and glucocorticoid receptor. Exp Cell Res. 1999;250(2):414-422. [DOI] [PubMed] [Google Scholar]
- 83. Chen SY, Wang J, Yu GQ, Liu W, Pearce D. Androgen and glucocorticoid receptor heterodimer formation a possible mechanism for mutual inhibition of transcriptional activity. J Biol Chem. 1997;272(22):14087-14092. [DOI] [PubMed] [Google Scholar]
- 84. Ikonen T, Palvimo JJ, Jänne OA. Interaction between the amino-and carboxyl-terminal regions of the rat androgen receptor modulates transcriptional activity and is influenced by nuclear receptor coactivators. J Biol Chem. 1997;272(47):29821-29828. [DOI] [PubMed] [Google Scholar]
- 85. Buoso E, Galasso M, Ronfani M, et al. Role of spliceosome proteins in the regulation of glucocorticoid receptor isoforms by cortisol and dehydroepiandrosterone. Pharmacol Res. 2017;120:180-187. [DOI] [PubMed] [Google Scholar]
- 86. Xie N, Cheng H, Lin D, et al. The expression of glucocorticoid receptor is negatively regulated by active androgen receptor signaling in prostate tumors. Int J Cancer. 2015;136(4):E27-E38. [DOI] [PubMed] [Google Scholar]
- 87. Zhao J, Bauman WA, Huang R, Caplan AJ, Cardozo C. Oxandrolone blocks glucocorticoid signaling in an androgen receptor-dependent manner. Steroids. 2004;69(5):357-366. [DOI] [PubMed] [Google Scholar]
- 88. Harada N, Katsuki T, Takahashi Y, et al. Androgen receptor silences thioredoxin-interacting protein and competitively inhibits glucocorticoid receptor-mediated apoptosis in pancreatic β-cells. J Cell Biochem. 2015;116(6):998-1006. [DOI] [PubMed] [Google Scholar]
- 89. Hartig SM, He B, Newberg JY, et al. Feed-forward inhibition of androgen receptor activity by glucocorticoid action in human adipocytes. Chem Biol. 2012;19(9):1126-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Singh R, Artaza JN, Taylor WE, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147(1):141-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144(11):5081-5088. [DOI] [PubMed] [Google Scholar]
- 92. Blouin K, Nadeau M, Mailloux J, et al. Pathways of adipose tissue androgen metabolism in women: depot differences and modulation by adipogenesis. Am J Physiol Endocrinol Metab. 2009;296(2):E244-E255. [DOI] [PubMed] [Google Scholar]
- 93. Veilleux A, Côté JA, Blouin K, et al. Glucocorticoid-induced androgen inactivation by aldo-keto reductase 1C2 promotes adipogenesis in human preadipocytes. Am J Physiol Endocrinol Metab. 2012;302(8):E941-E949. [DOI] [PubMed] [Google Scholar]
- 94. Zhu L, Hou M, Sun B, et al. Testosterone stimulates adipose tissue 11β-hydroxysteroid dehydrogenase type 1 expression in a depot-specific manner in children. J Clin Endocrinol Metab. 2010;95(7):3300-3308. [DOI] [PubMed] [Google Scholar]
- 95. Spaanderman DC, Nixon M, Buurstede JC, et al. Androgens modulate glucocorticoid receptor activity in adipose tissue and liver. J Endocrinol. 2018;JOE-18-0503.R1. Doi:10.1530/JOE-18-0503 [DOI] [PubMed] [Google Scholar]
- 96. Gasparini SJ, Swarbrick MM, Kim S, et al. Androgens sensitise mice to glucocorticoid-induced insulin resistance and fat accumulation. Diabetologia. 2019;62(8):1463-1477. [DOI] [PubMed] [Google Scholar]
- 97. Stubbs AP, Engelman JL, Walker JI, Faik P, Murphy GM, Wilkinson ML. Measurement of androgen receptor expression in adult liver, fetal liver, and Hep-G2 cells by the polymerase chain reaction. Gut. 1994;35(5):683-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120(1):51-57. [DOI] [PubMed] [Google Scholar]
- 99. Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18(4):502-519. [DOI] [PubMed] [Google Scholar]
- 100. Islam S, Afrin S, Jones SI, Segars J.. Selective progesterone receptor modulators-mechanisms and therapeutic utility. Endoc Rev. 2020;41(5):bnaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell-and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7(10):1244-1255. [DOI] [PubMed] [Google Scholar]
- 102. Ogara MF, Rodríguez-Seguí SA, Marini M, et al. The glucocorticoid receptor interferes with progesterone receptor-dependent genomic regulation in breast cancer cells. Nucleic Acids Res. 2019;47(20):10645-10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240(4854):889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. von Langen J, Fritzemeier KH, Diekmann S, Hillisch A. Molecular basis of the interaction specificity between the human glucocorticoid receptor and its endogenous steroid ligand cortisol. Chembiochem. 2005;6(6):1110-1118. [DOI] [PubMed] [Google Scholar]
- 105. Ojasoo T, Doré JC, Gilbert J, Raynaud JP. Binding of steroids to the progestin and glucocorticoid receptors analyzed by correspondence analysis. J Med Chem. 1988;31(6):1160-1169. [DOI] [PubMed] [Google Scholar]
- 106. Allen TK, Nazzal MN, Feng L, Buhimschi IA, Murtha AP. Progestins inhibit tumor necrosis factor α–induced matrix metalloproteinase 9 activity via the glucocorticoid receptor in primary amnion epithelial cells. Reprod Sci. 2019;26(9):1193-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kontula K, Paavonen T, Luukkainen T, Andersson LC. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem Pharmacol. 1983;32(9):1511-1518. [DOI] [PubMed] [Google Scholar]
- 108. Issar M, Sahasranaman S, Buchwald P, Hochhaus G. Differences in the glucocorticoid to progesterone receptor selectivity of inhaled glucocorticoids. Eur Respir J. 2006;27(3):511-516. [DOI] [PubMed] [Google Scholar]
- 109. Al-Khouri H, Greenstein BD. Role of corticosteroid-binding globulin in interaction of corticosterone with uterine and brain progesterone receptors. Nature. 1980;287(5777):58-60. [DOI] [PubMed] [Google Scholar]
- 110. Leo JC, Guo C, Woon CT, Aw SE, Lin VC. Glucocorticoid and mineralocorticoid cross-talk with progesterone receptor to induce focal adhesion and growth inhibition in breast cancer cells. Endocrinology. 2004;145(3):1314-1321. [DOI] [PubMed] [Google Scholar]
- 111. Lei K, Chen L, Georgiou EX, et al. Progesterone acts via the nuclear glucocorticoid receptor to suppress IL-1β-induced COX-2 expression in human term myometrial cells. PloS One. 2012;7(11):e50167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lei K, Georgiou EX, Chen L, et al. Progesterone and the repression of myometrial inflammation: the roles of MKP-1 and the AP-1 system. Mol Endocrinol. 2015;29(10):1454-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sugino N, Telleria CM, Gibori G. Progesterone inhibits 20alpha-hydroxysteroid dehydrogenase expression in the rat corpus luteum through the glucocorticoid receptor. Endocrinology. 1997;138(10):4497-4500. [DOI] [PubMed] [Google Scholar]
- 114. Shah NM, Lai PF, Imami N, Johnson MR. Progesterone-related immune modulation of pregnancy and labor. Front Endocrinol (Lausanne). 2019;10:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jones LA, Anthony JP, Henriquez FL, et al. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology. 2008;125(1):59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wolfson ML, Schander JA, Bariani MV, Correa F, Franchi AM. Progesterone modulates the LPS-induced nitric oxide production by a progesterone-receptor independent mechanism. Eur J Pharmacol. 2015;769:110-116. [DOI] [PubMed] [Google Scholar]
- 117. Guo W, Li P, Zhao G, Fan H, Hu Y, Hou Y. Glucocorticoid receptor mediates the effect of progesterone on uterine natural killer cells. Am J Reprod Immunol. 2012;67(6):463-473. [DOI] [PubMed] [Google Scholar]
- 118. Jones LA, Kreem S, Shweash M, Paul A, Alexander J, Roberts CW. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. J Immunol. 2010;185(8):4525-4534. [DOI] [PubMed] [Google Scholar]
- 119. Hierweger AM, Engler JB, Friese MA, et al. Progesterone modulates the T-cell response via glucocorticoid receptor-dependent pathways. Am J Reprod Immunol. 2019;81(2):e13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Louw-du Toit R, Hapgood JP, Africander D.. Medroxyprogesterone acetate differentially regulates interleukin (IL)-12 and IL-10 in a human ectocervical epithelial cell line in a glucocorticoid receptor (GR)-dependent manner. J Biol Chem. 2014;289(45):31136-31149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang S, Jonklaas J, Danielsen M. The glucocorticoid agonist activities of mifepristone (RU486) and progesterone are dependent on glucocorticoid receptor levels but not on EC50 values. Steroids. 2007;72(6-7):600-608. [DOI] [PubMed] [Google Scholar]
- 122. Hackney JF, Holbrook NJ, Grasso RJ. Progesterone as a partial glucocorticoid agonist in L929 mouse fibroblasts: effects on cell growth, glutamine synthetase induction and glucocorticoid receptors. J Steroid Biochem. 1981;14(10):971-977. [DOI] [PubMed] [Google Scholar]
- 123. Prior JC. Progesterone as a bone-trophic hormone. Endocr Rev. 1990;11(2):386-398. [DOI] [PubMed] [Google Scholar]
- 124. Patel FA, Clifton VL, Chwalisz K, Challis JR. Steroid regulation of prostaglandin dehydrogenase activity and expression in human term placenta and chorio-decidua in relation to labor. J Clin Endocrinol Metab. 1999;84(1):291-299. [DOI] [PubMed] [Google Scholar]
- 125. Guo CM, Zhu XO, Ni XT, Yang Z, Myatt L, Sun K. Expression of progesterone receptor A form and its role in the interaction of progesterone with cortisol on cyclooxygenase-2 expression in amnionic fibroblasts. J Clin Endocrinol Metab. 2009;94(12):5085-5092. [DOI] [PubMed] [Google Scholar]
- 126. Schmidt C, Klammt J, Thome UH, Laube M. The interaction of glucocorticoids and progesterone distinctively affects epithelial sodium transport. Lung. 2014;192(6):935-946. [DOI] [PubMed] [Google Scholar]
- 127. Kunzmann S, Ottensmeier B, Speer CP, Fehrholz M. Progesterone antagonizes dexamethasone-regulated surfactant proteins in vitro. Reprod Sci. 2019;26(8):1062-1070. [DOI] [PubMed] [Google Scholar]
- 128. Schmidt M, Renner C, Löffler G. Progesterone inhibits glucocorticoid-dependent aromatase induction in human adipose fibroblasts. J Endocrinol. 1998;158(3):401-407. [DOI] [PubMed] [Google Scholar]
- 129. Pedersen SB, Børglum JD, Møller-Pedersen T, Richelsen B. Characterization of nuclear corticosteroid receptors in rat adipocytes. Regional variations and modulatory effects of hormones. Biochim Biophys Acta. 1992;1134(3):303-308. [DOI] [PubMed] [Google Scholar]
- 130. Pedersen SB, Jønler M, Richelsen B. Characterization of regional and gender differences in glucocorticoid receptors and lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab. 1994;78(6):1354-1359. [DOI] [PubMed] [Google Scholar]
- 131. Olefsky JM. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975;56(6):1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xu XF, Hoebeke J, Björntorp P. Progestin binds to the glucocorticoid receptor and mediates antiglucocorticoid effect in rat adipose precursor cells. J Steroid Biochem. 1990;36(5): 465-471. [DOI] [PubMed] [Google Scholar]
- 133. Pedersen SB, Kristensen K, Richelsen B. Anti-glucocorticoid effects of progesterone in vivo on rat adipose tissue metabolism. Steroids. 2003;68(6):543-550. [DOI] [PubMed] [Google Scholar]
- 134. Nordeen SK, Kühnel B, Lawler-Heavner J, Barber DA, Edwards DP. A quantitative comparison of dual control of a hormone response element by progestins and glucocorticoids in the same cell line. Mol Endocrinol. 1989;3(8):1270-1278. [DOI] [PubMed] [Google Scholar]
- 135. Wan Y, Nordeen SK. Overlapping but distinct gene regulation profiles by glucocorticoids and progestins in human breast cancer cells. Mol Endocrinol. 2002;16(6):1204-1214. [DOI] [PubMed] [Google Scholar]
- 136. Shao R, Egecioglu E, Weijdegård B, et al. Developmental and hormonal regulation of progesterone receptor A-form expression in female mouse lung in vivo: interaction with glucocorticoid receptors. J Endocrinol. 2006;190(3):857-870. [DOI] [PubMed] [Google Scholar]
- 137. Mark PJ, Augustus S, Lewis JL, Hewitt DP, Waddell BJ. Changes in the placental glucocorticoid barrier during rat pregnancy: impact on placental corticosterone levels and regulation by progesterone. Biol Reprod. 2009;80(6):1209-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lei K, Chen L, Cryar BJ, et al. Uterine stretch and progesterone action. J Clin Endocrinol Metab. 2011;96(6):E1013-E1024. [DOI] [PubMed] [Google Scholar]
- 139. Darnel AD, Archer TK, Yang K. Regulation of 11beta-hydroxysteroid dehydrogenase type 2 by steroid hormones and epidermal growth factor in the Ishikawa human endometrial cell line. J Steroid Biochem Mol Biol. 1999;70(4-6):203-210. [DOI] [PubMed] [Google Scholar]
- 140. Jeschke U, Mylonas I, Richter DU, et al. Regulation of progesterone production in human term trophoblasts in vitro by CRH, ACTH and cortisol (prednisolone). Arch Gynecol Obstet. 2005;272(1):7-12. [DOI] [PubMed] [Google Scholar]
- 141. Yang R, You X, Tang X, Gao L, Ni X. Corticotropin-releasing hormone inhibits progesterone production in cultured human placental trophoblasts. J Mol Endocrinol. 2006;37(3):533-540. [DOI] [PubMed] [Google Scholar]
- 142. Mickelson KE, Forsthoefel J, Westphal U. Steroid-protein interactions. Human corticosteroid binding globulin: some physicochemical properties and binding specificity. Biochemistry. 1981;20(21):6211-6218. [DOI] [PubMed] [Google Scholar]
- 143. Albrecht ED, Pepe GJ. Placental endocrinology. In: Skinner, MK. Encyclopedia of Reproduction. 2nd ed. Cambridge, MA:Academic Press;2018:491-501. [Google Scholar]
- 144. Kerlan V, Nahoul K, Le Martelot MT, Bercovici JP. Longitudinal study of maternal plasma bioavailable testosterone and androstanediol glucuronide levels during pregnancy. Clin Endocrinol (Oxf). 1994;40(2):263-267. [DOI] [PubMed] [Google Scholar]
- 145. Reyes FI, Boroditsky RS, Winter JS, Faiman C. Studies on human sexual development. II. Fetal and maternal serum gonadotropin and sex steroid concentrations. J Clin Endocrinol Metab. 1974;38(4):612-617. [DOI] [PubMed] [Google Scholar]
- 146. Hill M, Pařízek A, Cibula D, et al. Steroid metabolome in fetal and maternal body fluids in human late pregnancy. J Steroid Biochem Mol Biol. 2010;122(4):114-132. [DOI] [PubMed] [Google Scholar]
- 147. Donaldson A, Nicolini U, Symes EK, Rodeck CH, Tannirandorn Y. Changes in concentrations of cortisol, dehydroepiandrosterone sulphate and progesterone in fetal and maternal serum during pregnancy. Clin Endocrinol (Oxf). 1991;35(5):447-451. [DOI] [PubMed] [Google Scholar]
- 148. Jellyman JK, Valenzuela OA, Fowden AL. Horse species symposium: glucocorticoid programming of hypothalamic-pituitary-adrenal axis and metabolic function: animal studies from mouse to horse. J Anim Sci. 2015;93(7):3245-3260. [DOI] [PubMed] [Google Scholar]
- 149. Fowden AL, Giussani DA, Forhead AJ. Endocrine and metabolic programming during intrauterine development. Early Hum Dev. 2005;81(9):723-734. [DOI] [PubMed] [Google Scholar]
- 150. Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30(7):883-925. [DOI] [PubMed] [Google Scholar]
- 151. Mauvais-Jarvis F, Arnold AP, Reue K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab. 2017;25(6):1216-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol. 2010;31(3):341-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wagner CK. The many faces of progesterone: a role in adult and developing male brain. Front Neuroendocrinol. 2006;27(3):340-359. [DOI] [PubMed] [Google Scholar]
- 156. Varlamov O, Bethea CL, Roberts CT Jr. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne). 2014;5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zore T, Palafox M, Reue K. Sex differences in obesity, lipid metabolism, and inflammation—a role for the sex chromosomes? Mol Metab. 2018;15:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chen X, McClusky R, Chen J, et al. The number of X chromosomes causes sex differences in adiposity in mice. PloS Genet. 2012;8(5):e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring). 2015;23(4):713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Handgraaf S, Philippe J. The role of sexual hormones on the enteroinsular axis. Endocr Rev. 2019;40(4):1152-1162. [DOI] [PubMed] [Google Scholar]
- 162. Della Torre S, Mitro N, Meda C, et al. Short-term fasting reveals amino acid metabolism as a major sex-discriminating factor in the liver. Cell Metab. 2018;28(2):256-267.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Della Torre S, Rando G, Meda C, Ciana P, Ottobrini L, Maggi A. Transcriptional activity of oestrogen receptors in the course of embryo development. J Endocrinol. 2018;238(3):165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lapid K, Lim A, Clegg DJ, Zeve D, Graff JM. Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nat Commun. 2014;5:5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ibáñez L, Sebastiani G, Lopez-Bermejo A, Díaz M, Gómez-Roig MD, de Zegher F. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: relation to prenatal growth. J Clin Endocrinol Metab. 2008;93(7):2774-2778. [DOI] [PubMed] [Google Scholar]
- 166. Hammami M, Koo W, Hockman EM. Body composition of neonates from fan beam dual energy x-ray absorptiometry measurement. J Parent Enter Nutr. 2003;27(6):423-426. [DOI] [PubMed] [Google Scholar]
- 167. Shields BM, Knight B, Hopper H, et al. Measurement of cord insulin and insulin-related peptides suggests that girls are more insulin resistant than boys at birth. Diabetes Care. 2007;30(10): 2661-2666. [DOI] [PubMed] [Google Scholar]
- 168. Verkauskiene R, Beltrand J, Claris O, et al. Impact of fetal growth restriction on body composition and hormonal status at birth in infants of small and appropriate weight for gestational age. Eur J Endocrinol. 2007;157(5):605-612. [DOI] [PubMed] [Google Scholar]
- 169. O’Shaughnessy PJ, Monteiro A, Bhattacharya S, Fraser MJ, Fowler PA. Steroidogenic enzyme expression in the human fetal liver and potential role in the endocrinology of pregnancy. Mol Hum Reprod. 2013;19(3):177-187. [DOI] [PubMed] [Google Scholar]
- 170. Toda K, Simpson ER, Mendelson CR, Shizuta Y, Kilgore MW. Expression of the gene encoding aromatase cytochrome P450 (CYP19) in fetal tissues. Mol Endocrinol. 1994;8(2):210-217. [DOI] [PubMed] [Google Scholar]
- 171. Price T, Aitken J, Simpson ER. Relative expression of aromatase cytochrome P450 in human fetal tissues as determined by competitive polymerase chain reaction amplification. J Clin Endocrinol Metab. 1992;74(4):879-883. [DOI] [PubMed] [Google Scholar]
- 172. Duanmu Z, Weckle A, Koukouritaki SB, et al. Developmental expression of aryl, estrogen, and hydroxysteroid sulfotransferases in pre- and postnatal human liver. J Pharmacol Exp Ther. 2006;316(3):1310-1317. [DOI] [PubMed] [Google Scholar]
- 173. Dubaisi S, Caruso JA, Gaedigk R, et al. Developmental expression of the cytosolic sulfotransferases in human liver. Drug Metab Dispos. 2019;47(6):592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lanoux MJ, Cleland WH, Mendelson CR, Carr BR, Simpson ER. Factors affecting the conversion of androstenedione to estrogens by human fetal hepatocytes in monolayer culture. Endocrinology 1985;117(1):361-368. [DOI] [PubMed] [Google Scholar]
- 175. Zhao Y, Mendelson CR, Simpson ER. Characterization of the sequences of the human CYP19 (aromatase) gene that mediate regulation by glucocorticoids in adipose stromal cells and fetal hepatocytes. Mol Endocrinol. 1995;9(3):340-349. [DOI] [PubMed] [Google Scholar]
- 176. Maniu A, Aberdeen GW, Lynch TJ, et al. Estrogen deprivation in primate pregnancy leads to insulin resistance in offspring. J Endocrinol. 2016;230(2):171-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Pepe GJ, Maniu A, Aberdeen G, et al. Insulin resistance elicited in postpubertal primate offspring deprived of estrogen in utero. Endocrine. 2016;54(3):788-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85(3):1206-1210. [DOI] [PubMed] [Google Scholar]
- 179. Hakim C, Padmanabhan V, Vyas AK. Gestational hyperandrogenism in developmental programming. Endocrinology. 2017;158(2):199-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207(2):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab. 2008;295(2):E262-E268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Recabarren SE, Padmanabhan V, Codner E, et al. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab. 2005;289(5):E801-E806. [DOI] [PubMed] [Google Scholar]
- 183. Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276(6):R1569-R1578. [DOI] [PubMed] [Google Scholar]
- 184. Dahlöf LG, Hård E, Larsson K. Sexual differentiation of offspring of mothers treated with cortisone during pregnancy. Physiol Behav. 1978;21(4):673-674. [DOI] [PubMed] [Google Scholar]
- 185. Dahlöf LG, Hård E, Larsson K. Influence of maternal stress on offspring sexual behaviour. Anim Behav. 1977;25(4):958-968. [DOI] [PubMed] [Google Scholar]
- 186. Ward IL. Prenatal stress feminizes and demasculinizes the behavior of males. Science. 1972;175(4017):82-84. [DOI] [PubMed] [Google Scholar]
- 187. Lalau JD, Aubert ML, Carmignac DF, Grégoire I, Dupouy JP. Reduction in testicular function in rats. Neuroendocrinology. 1990;51(3):284-288. [DOI] [PubMed] [Google Scholar]
- 188. Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114(5):1635-1644. [DOI] [PubMed] [Google Scholar]
- 189. Ward IL, Weisz J. Maternal stress alters plasma testosterone in fetal males. Science. 1980;207(4428):328-329. [DOI] [PubMed] [Google Scholar]
- 190. Yun HJ, Lee JY, Kim MH. Prenatal exposure to dexamethasone disturbs sex-determining gene expression and fetal testosterone production in male embryos. Biochem Biophys Res Commun. 2016;471(1):149-155. [DOI] [PubMed] [Google Scholar]
- 191. Pedrana G, Sloboda DM, Pérez W, Newnham JP, Bielli A, Martin GB. Effects of pre-natal glucocorticoids on testicular development in sheep. Anat Histol Embryol. 2008;37(5):352-358. [DOI] [PubMed] [Google Scholar]
- 192. Pedrana G, Viotti M, Souza E, et al. Apoptosis-related protein expression during pre- and post-natal testicular development after administration of glucocorticoid in utero in the sheep. Reprod Domest Anim. 2013;48(5):795-802. [DOI] [PubMed] [Google Scholar]
- 193. Yucel S, Desouza A, Baskin LS. In utero prednisone exposure affects genital development. J Urol. 2004;172(4 Pt 2):1725-1730; discussion 1730. [DOI] [PubMed] [Google Scholar]
- 194. Barrett ES, Parlett LE, Sathyanarayana S, et al. Prenatal exposure to stressful life events is associated with masculinized anogenital distance (AGD) in female infants. Physiol Behav. 2013;114-115:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Barrett ES, Redmon JB, Wang C, Sparks A, Swan SH. Exposure to prenatal life events stress is associated with masculinized play behavior in girls. Neurotoxicology. 2014;41:20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. vom Saal FS, Quadagno DM, Even MD, Keisler LW, Keisler DH, Khan S. Paradoxical effects of maternal stress on fetal steroids and postnatal reproductive traits in female mice from different intrauterine positions. Biol Reprod. 1990;43(5):751-761. [DOI] [PubMed] [Google Scholar]
- 197. Holson RR, Gough B, Sullivan P, Badger T, Sheehan DM. Prenatal dexamethasone or stress but not ACTH or corticosterone alter sexual behavior in male rats. Neurotoxicol Teratol. 1995;17(4):393-401. [DOI] [PubMed] [Google Scholar]
- 198. Pereira OC, Arena AC, Yasuhara F, Kempinas WG. Effects of prenatal hydrocortisone acetate exposure on fertility and sexual behavior in male rats. Regul Toxicol Pharmacol. 2003;38(1):36-42. [DOI] [PubMed] [Google Scholar]
- 199. Gerardin DC, Pereira OC, Kempinas WG, Florio JC, Moreira EG, Bernardi MM. Sexual behavior, neuroendocrine, and neurochemical aspects in male rats exposed prenatally to stress. Physiol Behav. 2005;84(1):97-104. [DOI] [PubMed] [Google Scholar]
- 200. Piffer RC, Garcia PC, Pereira OCM. Adult partner preference and sexual behavior of male rats exposed prenatally to betamethasone. Physiol Behav. 2009;98(1-2):163-167. [DOI] [PubMed] [Google Scholar]
- 201. Pallarés ME, Adrover E, Baier CJ, et al. Prenatal maternal restraint stress exposure alters the reproductive hormone profile and testis development of the rat male offspring. Stress. 2013;16(4):429-440. [DOI] [PubMed] [Google Scholar]
- 202. Pereira OC, Piffer RC. Puberty installation and adrenergic response of seminal vesicle from rats exposed prenatally to hydrocortisone. Life Sci. 2005;77(12):1381-1390. [DOI] [PubMed] [Google Scholar]
- 203. Borges CS, Dias AF, Rosa JL, et al. Alterations in male rats following in utero exposure to betamethasone suggests changes in reproductive programming. Reprod Toxicol. 2016;63:125-134. [DOI] [PubMed] [Google Scholar]
- 204. Piffer RC, Garcia PC, Gerardin DC, Kempinas WG, Pereira OC. Semen parameters, fertility and testosterone levels in male rats exposed prenatally to betamethasone. Reprod Fertil Dev. 2009;21(5):634-639. [DOI] [PubMed] [Google Scholar]
- 205. Borges CdS, Dias AFMG, Silva PV, et al. Long-term adverse effects on reproductive function in male rats exposed prenatally to the glucocorticoid betamethasone. Toxicology. 2017;376:15-22. [DOI] [PubMed] [Google Scholar]
- 206. Pereira OC, Yasuhara F, Arena AC. Cholinergic responses of seminal vesicles isolated from rats exposed perinatally to hydrocortisone. Pharmacol Res. 2003;48(1):91-95. [PubMed] [Google Scholar]
- 207. Drake AJ, van den Driesche S, Scott HM, Hutchison GR, Seckl JR, Sharpe RM. Glucocorticoids amplify dibutyl phthalate-induced disruption of testosterone production and male reproductive development. Endocrinology. 2009;150(11):5055-5064. [DOI] [PubMed] [Google Scholar]
- 208. Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101(10):2174-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Somm E, Vauthay DM, Guérardel A, et al. Early metabolic defects in dexamethasone-exposed and undernourished intrauterine growth restricted rats. PloS One. 2012;7(11):e50131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Buhl ES, Neschen S, Yonemitsu S, et al. Increased hypothalamic-pituitary-adrenal axis activity and hepatic insulin resistance in low-birth-weight rats. Am J Physiol Endocrinol Metab. 2007;293(5):E1451-E1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Pantaleão LC, Murata G, Teixeira CJ, et al. Prolonged fasting elicits increased hepatic triglyceride accumulation in rats born to dexamethasone-treated mothers. Sci Rep. 2017;7(1):10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Kaiser S, Sachser N. The social environment during pregnancy and lactation affects the female offsprings’ endocrine status and behaviour in guinea pigs. Physiol Behav. 1998;63(3):361-366. [DOI] [PubMed] [Google Scholar]
- 213. Ristić N, Severs W, Nestorović N, et al. Effects of prenatal dexamethasone on the rat pituitary gland and gonadotropic cells in female offspring. Cells Tissues Organs. 2016;201(2):148-158. [DOI] [PubMed] [Google Scholar]
- 214. Lv F, Wan Y, Chen Y, et al. Prenatal dexamethasone exposure induced ovarian developmental toxicity and transgenerational effect in rat offspring. Endocrinology. 2018;159(3):1401-1415. [DOI] [PubMed] [Google Scholar]
- 215. McCoy SJ, Shirley BA. Effects of prenatal administration of testosterone and cortisone on the reproductive system of the female rat. Life Sci. 1992;50(9):621-628. [DOI] [PubMed] [Google Scholar]
- 216. Borges CS, Pacheco TL, Guerra MT, et al. Reproductive disorders in female rats after prenatal exposure to betamethasone. J Appl Toxicol. 2017;37(9):1065-1072. [DOI] [PubMed] [Google Scholar]
- 217. Smith JT, Waddell BJ. Increased fetal glucocorticoid exposure delays puberty onset in postnatal life. Endocrinology. 2000;141(7):2422-2428. [DOI] [PubMed] [Google Scholar]
- 218. Gandelman R, Rosenthal C. Deleterious effects of prenatal prednisolone exposure upon morphological and behavioral development of mice. Teratology. 1981;24(3):293-301. [DOI] [PubMed] [Google Scholar]
- 219. Harvey PW, Chevins PF. Androstenedione or corticosterone treatment during pregnancy alters estrous cycle of adult female offspring in mice. Experientia. 1985;41(4):492-494. [DOI] [PubMed] [Google Scholar]
- 220. Iwasa T, Matsuzaki T, Murakami M, et al. Delayed puberty in prenatally glucocorticoid administered female rats occurs independently of the hypothalamic Kiss1-Kiss1r-GnRH system. Int J De Neurosci. 2011;29(2):183-188. [DOI] [PubMed] [Google Scholar]
- 221. Ristić N, Nestorović N, Manojlović-Stojanoski M, et al. Maternal dexamethasone treatment reduces ovarian follicle number in neonatal rat offspring. J Microsc. 2008;232(3):549-557. [DOI] [PubMed] [Google Scholar]
- 222. Hułas-Stasiak M, Dobrowolski P, Tomaszewska E. Prenatally administered dexamethasone impairs folliculogenesis in spiny mouse offspring. Reprod Fertil Dev. 2016;28(7):1038-1048. [DOI] [PubMed] [Google Scholar]
- 223. Ristić N, Nestorović N, Manojlović-Stojanoski M, et al. Adverse effect of dexamethasone on development of the fetal rat ovary. Fund Clin Pharmacol. 2019;33(2):199-207. [DOI] [PubMed] [Google Scholar]
- 224. Poulain M, Frydman N, Duquenne C, et al. Dexamethasone induces germ cell apoptosis in the human fetal ovary. J Clin Endocrinol Metab. 2012;97(10):E1890-E1897. [DOI] [PubMed] [Google Scholar]
- 225. Ruth KS, Day FR, Tyrrell J, et al. ; Endometrial Cancer Association Consortium Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Rasmussen JJ, Selmer C, Frøssing S, et al. Endogenous testosterone levels are associated with risk of type 2 diabetes in women without established comorbidity. J Endocr Soc. 2020;4(6):bvaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Solano ME, Kowal MK, O’Rourke GE, et al. Progesterone and HMOX-1 promote fetal growth by CD8+ T cell modulation. J Clin Invest. 2015;125(4):1726-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Arck PC, Rücke M, Rose M, et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod Biomed Online. 2008;17(1):101-113. [DOI] [PubMed] [Google Scholar]
- 229. Joachim R, Zenclussen AC, Polgar B, et al. The progesterone derivative dydrogesterone abrogates murine stress-triggered abortion by inducing a Th2 biased local immune response. Steroids. 2003;68(10-13):931-940. [DOI] [PubMed] [Google Scholar]
- 230. Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4(1):19-30. [DOI] [PubMed] [Google Scholar]
- 231. Hofman PL, Regan F, Jackson WE, et al. Premature birth and later insulin resistance. N Engl J Med. 2004;351(21):2179-2186. [DOI] [PubMed] [Google Scholar]
- 232. Kajantie E, Osmond C, Barker DJ, Eriksson JG. Preterm birth—a risk factor for type 2 diabetes? The Helsinki Birth Cohort Study. Diabetes Care. 2010;33(12):2623-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Zambrano E, Guzmán C, Rodríguez-González GL, Durand-Carbajal M, Nathanielsz PW. Fetal programming of sexual development and reproductive function. Mol Cell Endocrinol. 2014;382(1):538-549. [DOI] [PubMed] [Google Scholar]
- 234. Zambrano E, Rodríguez-González GL, Guzmán C, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563(Pt 1):275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Mossa F, Carter F, Walsh SW, et al. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol Reprod. 2013;88(4):92. [DOI] [PubMed] [Google Scholar]
- 236. Rae MT, Rhind SM, Fowler PA, Miller DW, Kyle CE, Brooks AN. Effect of maternal undernutrition on fetal testicular steroidogenesis during the CNS androgen-responsive period in male sheep fetuses. Reproduction. 2002;124(1):33-39. [PubMed] [Google Scholar]
- 237. Chowen JA, Freire-Regatillo A, Argente J. Neurobiological characteristics underlying metabolic differences between males and females. Prog Neurobiol. 2019;176:18-32. [DOI] [PubMed] [Google Scholar]
- 238. Chowen JA, Frago LM, Argente J. The regulation of GH secretion by sex steroids. Eur J Endocrinol. 2004;151(Suppl 3):U95-U100. [DOI] [PubMed] [Google Scholar]
- 239. Brie B, Ramirez MC, De Winne C, et al. Brain control of sexually dimorphic liver function and disease: the endocrine connection. Cell Mol Neurobiol. 2019;39(2):169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20(11):2613-2629. [DOI] [PubMed] [Google Scholar]
- 241. Ramirez MC, Luque GM, Ornstein AM, Becu-Villalobos D. Differential neonatal testosterone imprinting of GH-dependent liver proteins and genes in female mice. J Endocrinol. 2010;207(3):301-308. [DOI] [PubMed] [Google Scholar]
- 242. Ramirez MC, Zubeldía-Brenner L, Wargon V, Ornstein AM, Becu-Villalobos D. Expression and methylation status of female-predominant GH-dependent liver genes are modified by neonatal androgenization in female mice. Mol Cell Endocrinol. 2014;382(2):825-834. [DOI] [PubMed] [Google Scholar]
- 243. Wu MV, Manoli DS, Fraser EJ, et al. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139(1):61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Ramirez MC, Bourguignon NS, Bonaventura MM, Lux-Lantos V, Libertun C, Becu-Villalobos D. Neonatal xenoestrogen exposure alters growth hormone-dependent liver proteins and genes in adult female rats. Toxicol Lett. 2012;213(3):325-331. [DOI] [PubMed] [Google Scholar]
- 245. Kroon J, Pereira AM, Meijer OC. Glucocorticoid sexual dimorphism in metabolism: dissecting the role of sex hormones. Trends Endocrinol Metab. 2020;31(5):357-367. [DOI] [PubMed] [Google Scholar]
- 246. Zheng D, Wang X, Antonson P, Gustafsson JÅ, Li Z. Genomics of sex hormone receptor signaling in hepatic sexual dimorphism. Mol Cell Endocrinol. 2018;471:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Vandenberg LN. Low dose effects challenge the evaluation of endocrine disrupting chemicals. Trends Food Sci Technol. 2019;84:58-61. [Google Scholar]
- 249. Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat Rev Endocrinol. 2014;10(5):303-310. [DOI] [PubMed] [Google Scholar]