Abstract
Significance: Optimal skin wound healing is crucial for maintaining tissue homeostasis, particularly in response to an injury. The skin immune system is under regulation of mediators such as bioactive lipids and cytokines that can initiate an immune response with controlled inflammation, followed by efficient resolution. However, nutritional deficiency impacts wound healing by hindering fibroblast proliferation, collagen synthesis, and epithelialization, among other crucial functions. In this way, the correct nutritional support of bioactive lipids and of other essential nutrients plays an important role in the outcome of the wound healing process.
Recent Advances and Critical Issues: Several studies have revealed the potential role of lipids as a treatment for the healing of skin wounds. Unsaturated fatty acids such as linoleic acid, α-linolenic acid, oleic acid, and most of their bioactive products have shown an effective role as a topical treatment of chronic skin wounds. Their effect, when the treatment starts at day 0, has been observed mainly in the inflammatory phase of the wound healing process. Moreover, some of them were associated with different dressings and were tested for clinical purposes, including pluronic gel, nanocapsules, collagen films and matrices, and polymeric bandages. Therefore, future research is still needed to evaluate these dressing technologies in association with different bioactive fatty acids in a wound healing context.
Future Directions: This review summarizes the main results of the available clinical trials and basic research studies and provides evidence-based conclusions. Together, current data encourage the use of bioactive fatty acids for an optimal wound healing resolution.
Keywords: fatty acids, skin, wound healing, inflammation, bioactive compounds

Eliana Pereira de Araújo, PhD
Scope and Significance
Lipids are involved in several aspects of the inflammatory process such as vascular contraction, chemotaxis, adhesion, diapedesis, cell death, and tissue restoration. Many studies have analyzed the effects of several lipids, particularly fatty acids, and their bioproducts, in skin inflammation. In this study, the inflammatory response and the involvement of fatty acids in skin wound healing were assessed. In addition, we discussed the main effects of fatty acid treatment, including various cellular features for healing, the activation of specific fatty acid receptors, their inflammatory and immune functions in the skin, as well as their possible applications in clinical practice.
Translational Relevance
In the past decade, many experimental studies investigated the role of fatty acids in skin regeneration. Basic research and clinical trials have shown the effectiveness of fatty acids as therapeutic or preventive approaches. However, these findings have not been fully incorporated in clinical practice. The translational relevance of this review is that topical treatment with bioactive fatty acids may provide novel therapeutic strategies for the treatment of acute or chronic wounds, as well as to prevent pressure ulcers.
Clinical Relevance
Chronic wounds are defined as having full-thickness and a slow healing propensity. They represent a silent epidemic affecting about 1–2% of the world's population, impacting negatively on the quality of life and resulting in a high cost for public and private health systems. Chronic inflammation, decreased angiogenesis, an imbalance between mature and immature collagen synthesis, extracellular matrix (ECM) deposition and degradation, and dysfunction in cellular migration and proliferation are some of the mechanisms responsible for abnormal healing. Understanding the effects of low-cost bioactive fatty acids on healing may provide insights into therapeutic approaches and improve their applicability to accelerate wound closure.
An Overview of Wound Healing Process
The skin is the largest organ of humans and its main function is to serve as a protective barrier against the environment. As a barrier, the skin is highly exposed to distinct external stressors (physical, chemical, or biological), which results in frequent damage.1 To withstand these injuries, evolution has provided the skin several complex mechanisms aimed at restoring tissue integrity. The wound healing process involves the activation of platelets, neutrophils, macrophages, endothelial cells, keratinocytes, and fibroblasts, as well as the secretion of growth factors, cytokines, chemokines, and other substances needed to coordinate tissue repair.2 The healing process is divided into three overlapping phases: inflammation, proliferation, and remodeling.3
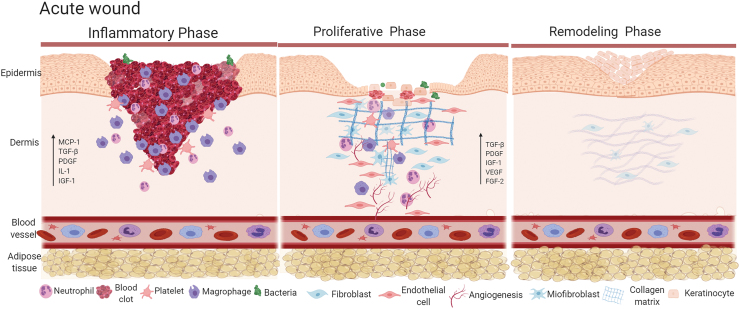
The inflammatory phase (Fig. 1) is characterized by vasodilation and subsequent increase in vessel permeability nearby the wound. This facilitates the migration of monocytes and neutrophils attracted by chemokines, growth factors, and cytokines primarily secreted by platelets aggregated at the lesion's site during the development of the hemostatic clot.2–4 An important aspect of this phase is monocyte infiltration that occurs in response to specific chemoattractants such as protein fragments of the ECM and monocyte chemoattractant protein 1 (MCP-1). Thereafter, monocytes differentiate into active macrophages that release growth factors involved in tissue granulation development.5,6 Transforming growth factor β (TGF-β) and platelet-derived growth factor (PDGF) are some of the most remarkable substances secreted during this phase6,7 as they are responsible for the formation of new ECM, blood vessels, and activate fibroblasts, which develop at the edges of the lesion. In addition, activated fibroblasts secrete interleukin-1 (IL-1) and insulin-like growth factor (IGF-I), which play important roles in the initiation of the proliferative phase.6–8
Figure 1.
Acute wound healing. Left-hand panel, in acute wound healing, inflammatory phase is characterized by clot formation, chemotaxis of neutrophils and monocytes followed by the production of chemokines, cytokines and growth factors. Middle panel, as wound healing progresses, proliferative phase is characterized by intense angiogenesis, proliferation of fibroblasts, differentiation of myofibroblasts and collagen deposition; during this phase there is production of growth factors. Right-hand panel, during remodeling phase there is intense deposition of, initially type III collagen and then, type I collagen; this is accompanied by reepithelization. Color images are available online.
During proliferative phase (Fig. 1), the continuous macrophage secretion of TGF-β, MCP-1, PDGF, IGF-1, and fibroblast growth factor-2 results in ECM replacement by a stronger and elastic connective tissue.6–8 The main events taking place during this phase are the establishment of a permeability barrier in the new tissue, the formation of adequate blood supply and the strengthening of the wounded tissue.6–8 At the end of the healing process ECM becomes mature and remodeled. At the remodeling phase (Fig. 1), the scar develops its highest tensile force as strong collagen deposition begins. Initially there is type III collagen that forms disorganized fibers, which is followed by type I collagen deposition in an organized manner providing the final aspect of the scar.6–9
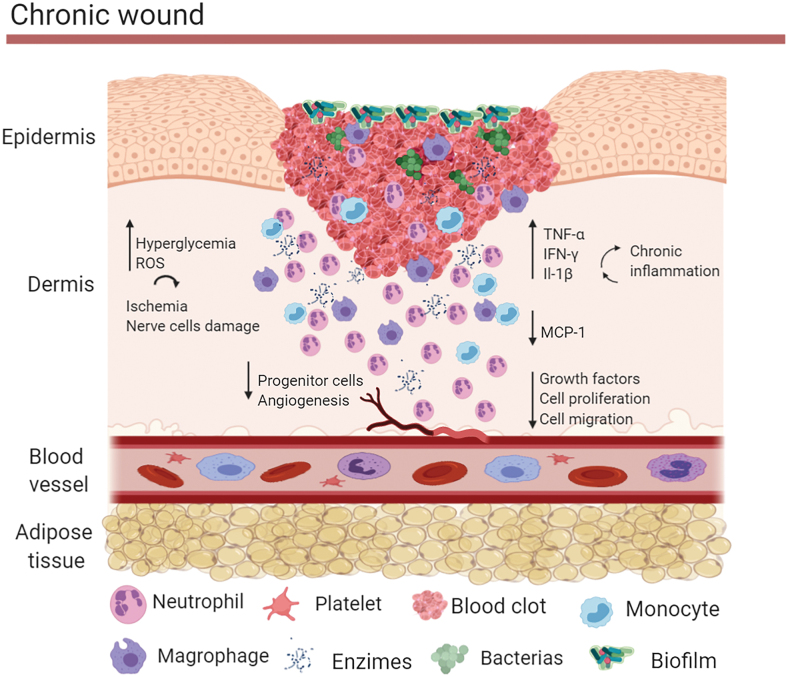
However, pathological factors can disturb healing leading to the development of chronic wounds (Fig. 2). These factors vary widely and include abnormalities in blood supply, metabolic disorders, immune function, and others.10–14 Diabetes and vascular disease are the main medical conditions leading to development of chronic wounds.15–17 The main causes of diabetic foot ulcers are peripheral neuropathy and peripheral vascular disease. These abnormalities are caused by chronic hyperglycemia, which promotes formation of advanced glycation end-products and activation of the polyol and hexosamine pathways reducing the normal conduction of neurons.18,19 In addition, hyperglycemia leads to an increase of reactive oxygen species and interruption of nitric oxide synthesis. Thus, there is an increase in oxidative stress in the nerve cells and an increase in vasoconstriction leading to ischemia, which will promote further nerve cells damage and eventually cell death.20 Diabetes also impairs progenitor cells influx, proliferation, and release of growth factors at the wound site. In addition, it leads to a chronic inflammatory state and impaired new vessel formation.10 There is a delay in the production of chemokines and cytokines such as MCP-1 in the wound bed, which delays the chemotaxis of monocytes and the activation of macrophages,10,21 resulting in persistent accumulation of debris, apoptotic cells, and neutrophils. In addition, chronic wounds often suffer the damaging effects of microbes, such as Streptococcus pyogenes, Enterococcus spp., and Pseudomonas aeruginosa, which colonize and reproduce in the wound bed.22 These microbes can form thick biofilms, which increase wound adhesion, immune deficiency, and local resistance to antibiotics.22,23 All these features contribute to chronicity and refractoriness of wounds.23
Figure 2.
Chronic wound healing. In chronic wounds there is increased reactive oxygen species production and interruption of nitric oxide synthase, leading to ischemia and nerve cell damage. There is also reduction of progenitor cells influx, proliferation, and release of growth factors, accompanied by a chronic inflammatory state and impaired new vessel formation. There is also a delay in the production of chemokines, increased of cytokines and abnormal activation of macrophages. In addition, chronic wounds often suffer the damaging effects of microbes that can form thick biofilms, which increase wound adhesion, immune deficiency, and local resistance to antibiotics. Color images are available online.
Taken together, these data suggest that the reversion of the abnormal wound microenvironment could mitigate cell damage and restore optimal healing. Thus, due to their bioactive properties, unsaturated fatty acids (monounsaturated fatty acids, MUFAs and polyunsaturated fatty acids, PUFAs), can play an important role in the chronic wound healing process.
Bioactive lipids as inflammatory mediators in skin
Nutritional deficiency is involved with impairments in wound repair and other inflammatory skin conditions.24–26 Thus, malnourished patients can develop pressure ulcers, infections, and delayed wound healing that result in chronic nonhealing wounds. In this context, attempts to recover the nutritional status are commonly used in clinical practice as an important therapeutic approach in wound healing; this includes the prescription of dietary supplements. Accordingly, the nutritional support should include bioactive compounds together with all the other essential nutrients.27–29
Eicosanoids, endocannabinoids, and sphingolipids are examples of bioactive lipids involved in skin biology as well as in the regulation of skin inflammation and immunity.30 The structural and protective skin lipids are diverse and contribute to whole epidermal barrier integrity; nevertheless, if some insult results on its disruption, another source of lipids may be helpful for its complete recovery and remodeling. To explore why some lipid-derived inflammatory mediators have been studied for accelerating wound healing repair, it is important to briefly review their definition, classification, and main functions.
Lipids: definition, classification, and functions
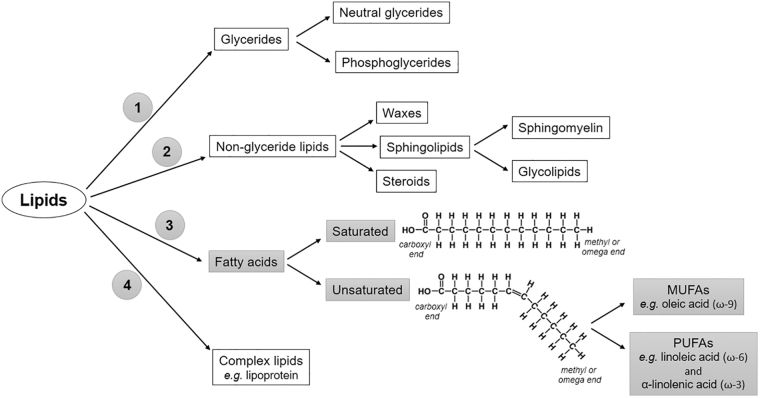
Lipids correspond to a large heterogeneous group of hydrophobic organic molecules composed of hydrogen, carbon, and oxygen. In addition to supplying energy, they have other vital and structural functions. They are widely distributed in nature, being found as constituents of cell membranes (glycerophospholipids, sphingolipids, and cholesterol), steroids hormones (estradiol, testosterone, and aldosterone), and cortisol, fat-soluble vitamins (A, D, E, and K), among others. Due to their wide heterogeneity, lipids can be classified according to distinct features. The most common classification relies on the separation into four main subgroups: (1) glycerides (glycerol-containing lipids), (2) nonglycerides (sphingolipids, waxes, and steroids), (3) fatty acids (saturated and unsaturated), and (4) complex lipids (lipoproteins).31 A schematic representation of the lipid classification is shown in Fig. 3.
Figure 3.
Schematic overview of the most common lipids classification. Highlighted in gray, fatty acids can be classified according to the presence of double covalent bonds in the chain. Besides, unsaturated fatty acids are classified in MUFAs, for example, oleic-acid from omega-9 family, or PUFAs, for example, linoleic acid and α-linolenic acid, from omega-6 and -3 families, respectively. MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
The most abundant lipid found in the human diet is triglyceride, which consists of a glycerol molecule linked to fatty acids. The number of fatty acids bonded to one molecule of glycerol can vary between one and three, as well as its binding position (sn-1, sn-2, or sn-3). Fatty acids, in turn, are carboxylic acids with long aliphatic chains, which may be straight or branched. The generic structure of all fatty acids corresponds to a hydrocarbon chain with a reactive carboxyl group at one end and a methyl group at the other end. According to the length of the chain, they can be classified into short-chain (SCFA) (2–6 carbon atoms), medium-chain (MCFA) (6–12 carbon atoms), long-chain (LCFA) (13–21 carbon atoms), and very-long-chain fatty acids (VLCFA) (more than 22 carbon atoms).31,32
Fatty acid chains can also be classified into saturated or unsaturated depending on the presence of double covalent bonds (-C = C-) between the carbon atoms. If saturated, there is only a simple bond between the carbon atoms (-C-C-). A fatty acid chain is monounsaturated if it contains one double bond and polyunsaturated if it contains more than one double bond.31 The chain structure of the fatty acids, as well as their classification, is depicted in Fig. 3.
In addition to the number of carbons and double bonds, the location of the double-bond position and its geometric configuration (cis or trans) are determinants for the fatty acid metabolic rate. The double-bond position in the chain, defined by the number of carbon atoms from the terminal methyl group (omega or ω) (H3C), determines the “metabolic family” to which the fatty acid belongs. This occurs because the human body has enzymes capable of inserting carbon atoms into the fatty acid chain, in a position next to the carboxyl group, changing the chain length and generating other fatty acids according to the demand. Because of the large variety in nature, some unsaturated fatty acids have been thoroughly studied due to their potential benefits for health.
Monounsaturated fatty acids
Oleic acid (OA) (18:1) and erucic acid (22:1) are the main MUFAs. Both belong to the omega-9 (ω-9 or n-9) family and have only one double bond in their carbon chain, making them more resistant to oxidation. Because of this, oils which are rich in MUFAs, such as extra-virgin olive oil (EVOO), present antioxidant properties which promote higher stability and longer shelf life compared to PUFA-enriched oils.33 Although erucic acid is largely found in rapeseed, an important constituent of canola oil, OA is the main MUFA in the human diet with highly bioactive properties.
As the main component of EVOO, OA represents up to 80% of its total lipid composition.34 Due to its antioxidant role, during the past decade, the effects of OA have been widely studied in the Mediterranean style diet, which is rich in EVOO, showing a potential reduction in cholesterol levels and on cardiovascular risk factors.35,36 However, more recently, a new paradigm has emerged with the demonstration that EVOO consumption has effects on many other conditions, such as inflammation, oxidative stress, coagulation, platelet aggregation, fibrinolysis, endothelial function, and lipid metabolism.37 Since the wound healing process depends on the orchestrated functioning of these mechanisms, it is important to briefly cite here some of the main benefits attributed to EVOO.
In the literature, most of the studies about the functions of OA, specifically, have been conducted from a classic study in the field known as PREDIMED (Prevención con Dieta Mediterránea): a large, parallel-group, multicenter, controlled, randomized 5-year clinical trial that aimed to characterize the effects of the Mediterranean diet on the primary prevention of cardiovascular disease.38 Considering that EVOO shows high levels of OA beyond other phenolic compounds, which are also strong antioxidants and radical scavengers, it is hard to isolate their effects. In this way, we have described in the following paragraphs some of the OA-related anti-inflammatory roles, since most of the EVOO properties already mentioned can be related both to OA and phenolic compounds.
In the literature, the anti-inflammatory effects attributed to OA have been observed after the chronic consumption of EVOO, which reduces the serum levels of C-reactive protein, IL-6, IL-7, and IL-18; in addition to raising adiponectin levels in inflamed human adipocytes through the attenuation of JNK-mediated peroxisome proliferator-activated receptor (PPAR) suppression.39–41
Although some groups have already studied the benefits of EVOO on wound healing acceleration, the positive effects observed can be due to the phenolic compounds and not to OA. In this context, to provide a deeper understanding of the main effects of OA treatment in skin wounds, studies should be conducted isolating it from EVOO, thus, eliminating any bias from other antioxidant molecules also found in this oil.
Polyunsaturated fatty acids
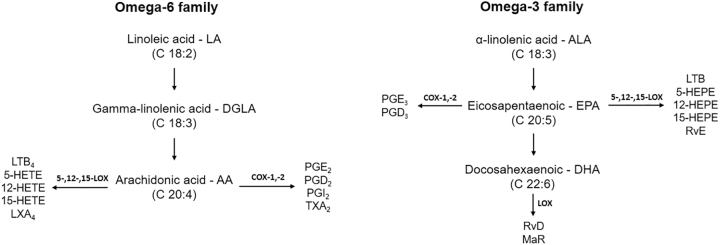
From a nutritional point of view, the most important PUFAs are those from the omega-3 (ω-3 or n-3) and omega-6 (ω-6 or n-6) families, which have the first double bond in the third and the sixth carbon, respectively, counting from the terminal methyl group. α-linolenic acid (ALA) belongs to the ω-3 family and is an important precursor of other fatty acids with bioactive properties, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). On the contrary, linoleic acid (LA) belongs to the ω-6 family and is a precursor of arachidonic acid (AA), an important fatty acid with several metabolic effects, since it originates other eicosanoids from the ω-6 family, such as leukotriene, prostaglandins, and thromboxane. The detailed production of fatty acids derived from LAs and ALAs are depicted in Fig. 4.
Figure 4.
Schematic overview of the production of eicosanoids and other inflammatory mediators from PUFAs omega-3 and -6. LA is metabolized to DGLA, which is also converted to AA. Depending on which pathway is activated: COX or LOX, several eicosanoids are originated. Oxygenated eicosanoids derived from AA are predominantly pro-inflammatory, while those from EPA and DHA are predominantly anti-inflammatory. LA, linoleic acid; DGLA, gamma-linolenic acid; AA, arachidonic acid; COX, cyclooxygenase; LOX, lipoxygenase; LT, leukotriene; HETE, hydroxy-eicosatetraenoic acid; LX, lipoxin; PG, prostaglandin; TX, thromboxane; HEPE, hydroxy-eicosapentaenoic acid; Rv, resolving; MaR, maresin.
Even though the human body can produce saturated and unsaturated fatty acids from carbohydrates and proteins, it is not able to synthesize ALA and LA. In addition, some specific cells or tissues, like epidermal keratinocytes, do not express Δ5 and Δ6 desaturase42; therefore, they rely on the systemic provision of essential PUFAs originated from ALA and LA oxidation. Plants, on the contrary, synthesize both these PUFAs, and because of this, they are found in many seeds, nuts, seed oils, and seed oil-derived products. EPA and DHA, from the omega-3 family, are largely found in seafood, for example, tuna, salmon, and sardines, among others. AA, from the omega-6 family, is found in red meat, eggs, and visceral organs, such as the liver.
The information about which are the sources of fatty acids is important not only because many bioactive compounds are generated from these essential lipids, as already mentioned, but also because they are precursors of several structural constituents and eicosanoids with hormone-like functions, which will be discussed in the following topics.
Role of omega-3 and omega-6 polyunsaturated fatty acids in the inflammatory process
The interest in studying the health benefits from PUFAs emerged in the early 1970s when researchers observed the association between high levels of cold-water fish consumption and a low incidence of heart-related diseases in Eskimos from Greenlandic West-Coast.43 The authors have also noted a lower incidence of autoimmune and inflammatory-related diseases in this population. These data have triggered a series of follow-up studies over the next few decades about the lipid content of these fish and how it could prevent the development of several diseases. Currently, it is known that the protective effect provided by the Eskimo diet is largely attributed to the high level of omega-3 and -6 PUFAs.
Generally speaking, these PUFAs are capable of modulating the systemic inflammatory response regulating several events in the inflammation and immune response, which includes leukocyte chemotaxis, adhesion molecule expression, leukocyte–endothelial adhesion interactions, platelet aggregation, and vasodilatation, among others.44 Therefore, the main role of PUFAs in the inflammatory process is largely due to eicosanoid production.
Eicosanoids are 20-carbon bioactive products that are originated from omega-6 and -3 PUFA metabolism, such as prostaglandins, leukotrienes, thromboxanes, and lipoxins, which modulate the inflammatory response unevenly.45 Eicosanoids from omega-6 PUFA metabolism, produced by AA oxidation, are potent inflammatory mediators, involved in infection, inflammation, tissue damage, immune system modulation, and platelet aggregation, and are directly linked to tumor development, growth, and metastases. On the contrary, ALA (omega-3 family) can be converted into EPA and DHA, which compete with AA for the enzymatic pathways of cyclooxygenase (COX) and lipoxygenase (LOX), also giving rise to eicosanoids, with a predominant anti-inflammatory role. DHA can also originate specialized proresolving mediators known as resolvins, protectins, and maresins.32,46 A schematic overview of the production of eicosanoids and inflammatory mediators from PUFAs omega-3 and -6 is also shown in Fig. 4.
More specifically, mechanisms underlying the anti-inflammatory actions of EPA and DHA include changes in the phospholipid composition of the cell membrane, resulting in the synthesis of lipid mediators with a lower inflammatory potential than omega-6 fatty acid-derived mediators. Moreover, both EPA and DHA can act as agonists of PPAR-γ, whose activation exerts anti-inflammatory effects, and also can stabilize the NF-kB/IkB complex, suppressing the activation of the genes involved in the inflammatory process.44
The expression of eicosanoid receptors is cell and tissue-specific. Thus, eicosanoids have different physiological roles depending on the cell or tissue they bind to and according to the intracellular signaling pathway activated in each situation. Because of this, they can switch between proinflammatory and anti-inflammatory molecules, which makes the knowledge about their function a little confusing. For example, in neurons, when prostaglandin E2 (PGE2) binds to its receptor (EP), it can lead to pain associated with inflammation. Conversely, autocrine EP signaling by PGE2 in macrophages and leukocytes decreases tumor necrosis factor-alpha (TNF-α) synthesis and increases the expression of anti-inflammatory IL-10, thus attenuating the inflammatory response.46 Hence, the inflammatory profile of eicosanoids depends on the prevalence of many terminal prostanoid synthases, which can generate series-2 and series-3 eicosanoids from the oxidation of AA and EPA, respectively. The role of the main eicosanoids specific in the inflammatory processes of the skin is depicted below.
Effects of main PUFAs-derived eicosanoids in skin inflammation
Independently of the uneven role of eicosanoids from different classes, they modulate many cellular functions related to improvements in tissue repair. Eicosanoids originated from omega-3 and -6 oxidation, such as PGE2, PGE1, PGE3, PGD2, PGF2α, PGI2, and TXB2, are produced by several cells in the skin.30,47,48
Among these, PGE2 is considered the most important COX-2 product during skin wound healing. Also known as dinoprostone, PGE2 is a product of AA (omega-6 family) oxidation through the COX pathway. All PGE2 effects are mediated through four integral membrane G protein-coupled prostanoid receptors: EP1, EP2, EP3, and EP4.46,49–51
In the skin, PGE2 is produced by epidermal keratinocytes and dermal fibroblasts and has proinflammatory actions, resulting in vasodilation, cell proliferation, and modulating the immune response.52–54 As a potential inflammatory mediator, PGE2 elicits vascular permeability and edema formation via EP3 on mast cells (MCs) and facilitates Th1 differentiation and Th17 expansion via EP4 on T cells and dendritic cells (DCs).55 Beyond vasodilation and vascular leakage, PGE2 also regulates fibroblast migration and collagen contraction, two crucial components of the fibrotic phase of dermal repair.56 Furthermore, PGE2 has an antifibrotic effect by increasing the expression of matrix metalloproteinase (MMP) 2 and 9, which are a type IV collagenase found at elevated levels in chronic wounds and inhibiting TGF-β1-induced collagen synthesis of dermal fibroblasts. The antifibrotic mechanism of PGE2 is partly due to its termination of the TGF-β1/Smad pathway by the activation of EP2 receptors.57 PGE2 is also involved in keratinocyte proliferation and differentiation, in the chemotaxis of keratinocytes, and in the modulation of dermal fibroblasts.49,58–60
In addition to PGE2, other eicosanoids involved in the inflammatory response of the skin are generated through the action of COX enzymes, such as other prostaglandins (PGD2 and PGI2), prostacyclin (PGI2), and thromboxane A2 (TXA2).61,62 For example, PGD2, for example, is mainly produced by Langerhans cells and dermal MCs. Since PGD2 is a potent antiproliferative and anti-inflammatory mediator, its biological effects are related to immune and allergic responses.52,63 Its effects are mediated through CRTH2 and DPs receptors expressed in several skin cells, including keratinocytes.64,65 Still, from the same family, prostacyclin (PGI2) is produced by dermal fibroblasts. Its biological effects are the inhibition of platelet activation and vasodilatation. TXA2 is produced by epidermal keratinocytes and has prothrombotic properties. Both PGI2 and TXA2 exert their biological effects through prostacyclin receptors (IP) and thromboxane receptors (TP), respectively, that are expressed by keratinocytes and other skin cells.66,67
Other inflammatory mediators generated from AA are the leukotrienes. Different from the previous ones, leukotrienes are synthesized through the 5-LOX pathway. The skin expresses not only 5- but also 12- and 15-LOX.54 From leukotriene A4 (LTA4), other inflammatory mediators can be generated, such as LTB4, LTC4, LTD4, and LTE4. As prostaglandins and thromboxanes, leukotrienes also exert their effects through G protein-coupled transmembrane receptors, including LTB4 receptor type-1 (BLT1) and type 2 (BLT2).68 There is not enough evidence about the cellular source of LTB4 in the skin; however, recently, Zhu et al. showed that epidermal keratinocytes can produce low levels of LTB4.69 Despite this, the major source of LTB4 in the skin is infiltrating neutrophils.70
Another product of the 5-LOX pathway is peroxide 5-hydroperoxy-eicosatetraenoic acid (HPETE). Since it is unstable, it is quickly reduced to 5-eicosatetraenoic (HETE) acid and 5-oxo-eicosatetraenoic (ETE) acid.71 Both leukotrienes and 5-oxo-ETE are potent chemoattractants that can contribute to cutaneous inflammation and allergy.54
12-HETE, a product of the 12-LOX pathway, is a potent proinflammatory chemotactic mediator that acts through the 12-HETE receptor (12-HETER).72,73 It is synthesized by epidermal keratinocytes and dermal fibroblasts, and it is involved in cutaneous wound healing and inflammatory disease.74 An in vitro study on keratinocytes indicated that 12-LOX pathway activity can be inhibited by 15-HETE; a product of the 15-LOX pathway.75 15-HETE, in turn, has an important anti-inflammatory role, reducing polymorphonuclear leukocytes infiltration in addition to acting as a biochemical precursor of lipoxin A4 (LXA4).76 Its effects rely on the activation of PPAR-γ. A summary of the biological effects of the main eicosanoids involved in the inflammatory process in the skin, which can be applied to wounds, is shown in Table 1.
Table 1.
Summary of the metabolites from essential PUFAs involved in the inflammatory processes in the skin
| Metabolite | Pathway | Receptors | Main Biological Effects | Cellular Origin in the Skin |
|---|---|---|---|---|
| PGE2 | COX | EP1, EP2, EP3, and EP4 | Vasodilatation, chemotaxis, cell proliferation, and modulation of immune response | Epidermal keratinocytes and dermal fibroblasts |
| PGD2 | COX | CRTH2, DP2 or GPR44 | Immunomodulation (platelet aggregation) | Epidermal keratinocytes, mast cells, and Langerhans cells |
| PGI2 | COX | IP | Vasodilatation and inhibition of platelet aggregation | Dermal fibroblasts |
| TXA2 | COX | TP | Vasoconstriction and platelet aggregation | Epidermal keratinocytes |
| LTB4 | 5-LOX | BLT1 and BLT2 | Chemotaxis | Infiltrating neutrophils and epidermal keratinocytes (lower levels) |
| 12-HETE | 12-LOX | 12-HETER | Chemotaxis, leucocyte migration, cell proliferation | Epidermal keratinocytes and dermal fibroblasts |
| 15-HETE | 15-LOX | PPAR-γ | Counteract 12-HETE and LTB4 effects, reduces polymorphonuclear leukocytes infiltration, and act as biochemical precursor of LXA4 | Epidermal keratinocytes, and dermal fibroblasts |
In brief, the majority of oxygenated products obtained from AA (omega-6 family) through COX and LOX pathways are predominantly proinflammatory, while those from ALA and LA (omega-3 family) are mainly anti-inflammatory. Hence, they can improve or impair the mechanisms of wound healing depending on its phase: inflammatory, proliferative, or remodeling. Based on this, for the development of new topical treatments, additional work is still needed to identify the physiological roles of all eicosanoids and other bioactive lipids in each phase of wound healing. Moreover, it is important to provide advance to elucidate which intracellular mechanisms are involved in each signaling pathway activated by those lipids in the skin.
Discussion of Findings and Relevant Literature
Receptor-mediated fatty acids intracellular signaling in the skin
Several studies have explored cellular mechanisms involving intracellular signaling activated by fatty acid receptors during skin wound healing. However, most of these experimental studies have not provided a precise description of how the activation of classical signaling pathways involving lipids and their bioactive products can modulate intracellular signaling. To clarify this subject, we searched for some intracellular signaling pathways involving fatty acid receptors in the skin, already experimentally described in the wound healing process (Table 2).
Table 2.
Summary of the cellular mechanisms involving the intracellular signaling activated by fatty acid receptors during skin wound healing
| Title | Author | Year | Agonist | Pathway | Resollution |
|---|---|---|---|---|---|
| New Peroxisome Proliferator-Activated Receptor Agonist (GQ-11) Improves Wound Healing in Diabetic Mice | Silva | 2019 | GQ-11 | PPARγ | Accelerates wound closure in diabetic mice by upregulated anti-inflammatory/pro-healing factors Il-10, Arg-1 and Tgf-b Il-10, Vegf, downregulated Il-1b, IL-6 and Tnf-a. |
| Macrophage PPARγ and impaired wound healing in type 2 diabetes. | Mirza | 2015 | Prostaglandin J2 | PPARγ | Improved healing in diabetic mice, decreased expression of IL-1β, TNF-α, IL-6 and increased VEGF, IGF and TGF-β via NF-kβ signaling in wound diabetic mice |
| Topical Docosahexaenoic Acid (DHA) Accelerates Skin Wound Healing in Rats and Activates GPR120. | Arantes | 2016 | Docosahezaenoic (DHA) | FFAR4/GPR120 | Wound healing was accelerated, reduction in the expression of interleukin (IL) 1β and an increase in the expression of IL-6, increase in expression of transforming growth factor β (TGF-β) and the keratinocyte marker involucrum |
| 12-Hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. | Liu | 2014 | Arachidonic acid | BLT2 | 12-HHT/BLT2 axis promotes wound healing by accelerating keratinocyte migration through NF-kB–Dependent upregulation of TNF and MMP9 |
| Prostaglandin E2 differentially modulates human fetal and adult dermal fibroblast migration and contraction: implication for wound healing. | Sandulache | 2006 | Prostaglandin E2 | EP2/EP4 | Inhibits fetal and adult fibroblast migration |
| Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. | Nelson | 2013 | Prostaglandin E2 | gpr44 | Decreased WIHN |
| Time heals all wounds—but 12-HHT is faster. | Gus-Brautbar | 2014 | 12-hydroxyheptadecatrienoic acid 12-HHT | BLT2 | Synthetic BLT2 agonist accelerated wound closure in a mouse diabetes model |
| Upregulation of prostaglandin EP4 receptor messenger RNA in fetal rabbit skin wound. | Li | 2000 | Prostaglandin EP4 | EP4 | In normal skin, the EP4 receptor messenger RNA levels were higher in adults than in fetuses. Twelve hours after wounding, the EP4 receptor transcript was remarkably induced in fetal skin wounds but repressed in adult skin wounds |
| Effects of TS-022, a newly developed prostanoid DP1 receptor agonist, on experimental pruritus, cutaneous barrier disruptions and atopic dermatitis in mice. | Arai | 2007 | Prostaglandin D2 | Prostanoid DP(1) receptor | Suppressive effect on scratching and its effect of accelerating repair of the disrupted cutaneous barrier |
The data previously published have shown the participation of several G protein-coupled receptors (GPCRs) in this process.77 GPCRs have a structure consisting of seven alpha helices capable of recognizing different ligands and thus altering their structural compliance of the receptors. G protein activation stimulates α-subunit and βγ-dimer dissociation by initiating intracellular signaling cascades involved in regulating the expression of genes related to survival, proliferation, differentiation, and other cellular processes. G proteins are classified according to its α subunit structure and sequence. The three main isoforms are Gs, Gq, and Gi; however, other isoforms, such as Gt (transducin protein), Go, and GK, also play important roles in calcium and potassium channel regulation.78
Generally speaking, when activated, Gq subtype receptors increase intracellular Ca2+ mobilization, Gs subtype receptors stimulate adenylyl cyclase and elicit the activation of phosphoinositide 3-kinase (PI3K) via the β-arrestin pathway and Gi subtype receptors; on the contrary, they inhibit adenylyl cyclase, decreasing intracellular cAMP levels.
Just after being synthesized, each prostanoid is released from the cells and acts on specific receptors on neighboring cells, which are coupled to specific G proteins. PGE2, for example, acts on four receptor subtypes (EP1–EP4), each of which has distinct signal transduction properties, and exert diverse physiological functions. PGD2 also acts on two different receptors, DP1 and DP2.77 Based on this, we have listed below the main fatty acid receptors in the skin, including those ones from the GPCR family, and a brief description of their intracellular mechanisms during the healing process.
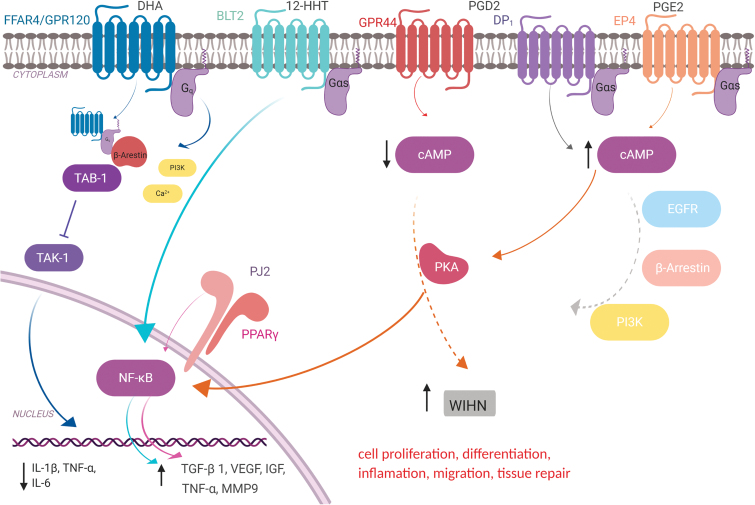
GPR120 or FFAR4
Many families of GPCRs are characterized in depth. GPR120, also known as free fatty acid receptor 4 (FFAR4), is a member of the rhodopsin family of GPCRs with seven alpha-helices that cross the cell membrane. They are regulated by accessory proteins that influence guanine nucleotide-binding, guanosine triphosphate hydrolysis, or subunit interactions.79
GPR120 ligands perform their action through a different mechanism from the GPCRs' classic mechanism. GPR120 signaling is dependent on β-arrestin-2 protein. Thus, the binding of an agonist activates GPR120, promoting the association of β-arrestin-2 to the receptor. Subsequently, there is an internalization of the GPR120/β-arrestin-2 complex. In the cytoplasm, β-arrestin-2 can bind to TAB1 protein, blocking the association of TAB1 with TAK1, and consequently activates TAK1. The lack of activation of this receptor prevents the signal transduction of the inflammatory pathway IκB/NFκB, thus blocking the inflammatory response. Because of this, GPR120 has recently emerged as a potential therapeutic target for several inflammatory disorders.80–83
There is still a lack of knowledge in the field of wound care about the role of the lipid activation of GPR120 and the reduction of the inflammatory phase. However, a study has shown that DHA is able to speed up wound healing in mice, reducing the expression of IL-1β and increasing the expression of IL-6, TGF-β, and the keratinocyte marker involucrum, probably by GPR120 activation.84
DP1 and DP2
D prostanoid receptor 1 (DP1) is a member of the prostanoid family and was the first receptor identified for PGD2. It belongs to a Gs subtype; thus, its activation leads to a rise in the intracellular levels of cAMP. DP1 is expressed by vascular smooth muscle and platelets causing vasodilatation.85,86 In the skin, PGD2-DP1 signaling plays an important role in MC maturation and MC-mediated inflammation.77 DP1 is also expressed by DCs and invariant NKT (iNKT) cells and can reduce the production of IFN-γ through protein kinase A (PKA) activation.87
Prostaglandin D2 receptor 2 (DP2) or the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2), is also known as GPR44 and was originally identified as an orphan receptor. DP2 is a Gi receptor with seven transmembrane alpha-helices that are preferentially expressed in CD4+ effector T helper 2 (Th2) cells,88 which act by reducing intracellular cAMP production,89 mediating the chemotaxis of eosinophils, basophils, and Th2 lymphocytes generated during an inflammatory response. In the skin, PGD2 can inhibit hair follicle regeneration through the GPR44 receptor, indicating that the inhibition of PGD2 production or GPR44 signaling could participate in skin regeneration.90
EP1, EP2, EP3, and EP4
EP4 is a PGE2 receptor that also belongs to the family of GPCRs. This receptor plays a variety of roles through cAMP effectors such as PKA, increasing cAMP levels. Recent emerging evidence has revealed that, in addition to cAMP and its downstream signaling, EP4 also modulates a variety of signaling pathways, such as PI3K, β-arrestin, and the transactivation of EGFR.91 Similar to EP4, the EP2 receptor is also a Gs receptor, which increases cAMP levels after PGE2 binding. On the contrary, EP3 is a G receptor, thus acts decreasing cAMP levels after its activation by PGE2. EP1, from a different phylogenetic origin, acts through increasing intracellular Ca2+ levels.
PGE2 is the major inflammatory mediator associated with dermal wound healing.56,91 Recent studies have revealed that PGE2 may facilitate Th1 differentiation and Th17 expansion via the EP4 receptor on T cells and DCs during chronic inflammation.90,92,93 Moreover, PGE2 increases vascular permeability and edema formation via EP3 on MCs and may raise the blood flow by eliciting vasodilatation via EP2/EP4 receptors on smooth muscle cells.77
BLT1 and BLT2
Leukotriene B4 (LTB4) is a potent inflammatory mediator derived from AA. Two GPCRs for LTB4 have been identified: BLT1 and BLT2, both Gi-like G proteins, which can induce cell migration.94 They are also receptors for 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid (12-HHT). BLT1 is found in several helper T cells, DCs, osteoclasts, granulocytes, eosinophils, and macrophages, while BLT2 is mainly expressed in keratinocytes.94,95
12-HHT is found in the wound fluid of mice, and BLT2-deficient mice exhibited impaired reepithelialization and delayed wound closure after skin punching.95 The activation of BLT2 receptors facilitates wound healing by accelerating keratinocyte migration through NF-kB-dependent upregulation of TNF-α and MMP9 during the inflammatory phase of healing.95,96
Peroxisome proliferator-activated receptors γ
Different from the other receptors described in this section, PPARs are nuclear receptors that regulate a large number of genes involved in differentiation and lipid metabolism. They are localized in macrophages and are essential for switching these cells into an anti-inflammatory state. In the skin, PPARs control the expression of genes involved in cell proliferation, differentiation, and also the inflammatory response. Also, PPARs appear to be essential for maintaining skin barrier permeability, inhibiting keratinocyte cell growth, promoting keratinocyte terminal differentiation, and regulating the skin inflammatory response.97
PUFAs such as 15-hydroxy-eicosatetraenoic acids, 13-hydroxy-octadecadienoic acid, 15-Deoxy-Δ-12, and 14-Prostaglandin J2 (15d-PGJ2) can activate PPAR-γ receptors. The mechanisms of activation negatively regulate the gene expression of proinflammatory genes in a ligand-dependent manner by antagonizing the activities of transcription factors such as members of the nuclear factor κB (NF-κB) and activator protein-1 (AP-1) families.98 Activation of these pathways by 15d-PGJ2 reduces the levels of proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 in the wound, and slightly increases the levels of prohealing growth factors, such as VEGF, IGF, and TGF-β1. Besides, PPAR-γ signaling activation downregulates the proinflammatory phenotype exhibited by macrophages isolated from wounds.99,100
A schematic overview of the major receptors for fatty acids and lipid-derived substances presents in the skin and downstream proteins implicated in their intracellular signaling is shown in Fig. 5.
Figure 5.
Schematic overview of the main receptors for fatty acids and lipid-derived substances presents in the skin and the downstream proteins involved in their intracellular signaling. FFAR4/GPR120, Free fatty acid receptor 4/G protein-coupled receptor 120 ; DHA, Docosahexaenoic acid; BLT2, leukotriene B4 receptor 2; 12-HHT, 12-hydroxyheptadecatrenoic acid; GPR44, G protein-coupled receptor 44; PGD2, prostaglandin D2; DP1, prostaglandin D2 receptor 1 ; EP4, prostaglandin E2 receptor 4; PGE2, prostaglandin E2; Gq and Gas, subtypes of the Ga protein; β-Arrestin, beta-arrestin; TAB-1, TGF-beta activated kinase 1 (MAP3K7) binding protein 1; TAK-1, mitogen-activated protein kinase 7; NF-kB, factor nuclear kappa B; Pj2, PPAR γ ligand PJ (2); PPAR-γ, peroxisome proliferator-activated receptor gamma; c-AMP, cyclic-AMP ; EGFR, epidermal growth factor receptor; PI3K, phosphatidylinositol-3-kinase; WIHN, wound-induced hair neogenesis. Color images are available online.
Bioactive Fatty Acids used for Wound Healing: A Systematic Review
To identify the main fatty acids that have been tested for skin repair, in August 2019, we performed a systematic review with “Fatty Acids”, “Skin”, and “Wound Healing” as the keywords on PubMed database using: “Fatty Acids”[Mesh] AND “Skin”[Mesh] AND “Wound Healing”[Mesh] strategy. A second search was performed with the keywords “Bandages” and “Fatty Acids” also on the PubMed database using: “Bandages”[Mesh] AND “Fatty Acids”[Mesh] strategy. The inclusion criteria were original articles, full text available, English language, published at any time, any animal model, intervention using topical fatty acids applied on wound bed, comparison based in the control group, and articles describing outcomes in “wound contraction,” “wound healing,” or “wound area”. The inclusion criteria were searched in every abstract, methodology, and results of each article.
Results
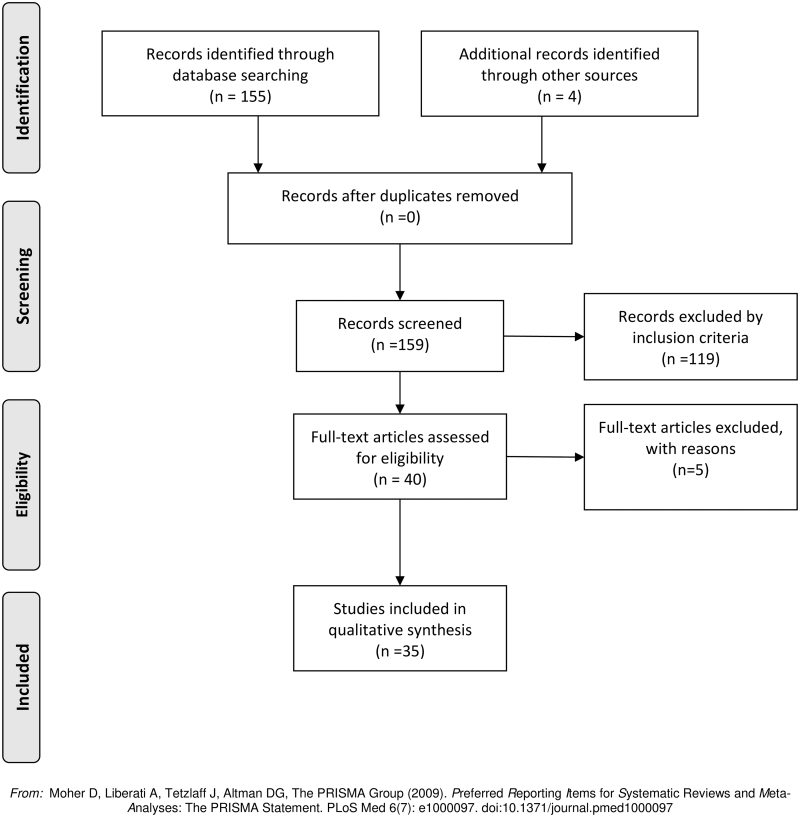
We analyzed the abstracts, methodologies, and results of 159 original articles. One hundred twenty eight articles were excluded for not meeting the inclusion criteria. In the end, 35 original articles were selected for the review (Fig. 6). The main result of the review was that topical treatment using fatty acids or their bioproducts accelerate the wound healing process, independently of the omega family origin (Table 3). Their effect, when the treatment starts on day 0, was observed mainly in the inflammatory phase of the wound healing process.
Figure 6.
Systematic Review workflow. One hundred fifty-five articles were identified through database searching. Four additional articles were added from other sources. One hundred nineteen articles were excluded for not meeting the inclusion criteria. Finally, 35 articles were included for the review.
Table 3.
Summary of systematic review
| Year | Fatty acids | Start | Outcome | Treatment | Model | Author |
|---|---|---|---|---|---|---|
| 2019 | 16% Linseed oil, 13% evening primrose oil, 16% olive oil | D0 | Speed up WH | Topical | Healthy rat | Ishak103 |
| 2018 | 100 ng of RvD1, RvD2, or RvD4 | D0 | Speed up WH | Topical | Healthy mice | Hellmann120 |
| 2018 | 100 μM palmitoleic acid | D0 | Speed up WH | Topical | Healthy rat | Weimann121 |
| 2017 | 50 μM 16,16-dimethyl PG E2 | D0 | Restored WIHN | injected | Healthy mice | Zhu69 |
| 2017 | 2% jasmonic acid derivative | D0 | Speed up WH | Topical | Human suction blister | Henriet130 |
| 2017 | 60–70% linoleic acid from hyperoxygenated fatty acids | D0 | Prevented facial PU | Topical | RCT high risk of PU | Otero123 |
| 2016 | 30 μM of Docosahexaenoic Acid | D0 | Speed up WH | Topical | Healthy rat | Arantes84 |
| 2016 | 100 μM alprostadil | D0 | Speed up WH | Topical | Healthy mice | Naska110 |
| 2015 | 10 μM Arachidonic acid | D0 | Speed up WH | Topical | Healthy mice | Oh113 |
| 2015 | 2 mM T26A PG transporter inhibitor | D0 | Speed up WH | Topical | non- and Diabetic Rat | Liu117 |
| 2015 | 50 μM PG J2 in F-127 pluronic gel | D0 | Speed up WH | Topical | Diabetic mice | Mirza99 |
| 2015 | Olive oil vs. hyperoxygenated fatty acid | ND | ND preventing PU | Topical | RCT, high risk of PU | Lupianez-Perez126 |
| 2014 | 10 μM Leukotriene B4 | ND | Speed up WH | Topical | non- and Diabetic Rat | Liu95 |
| 2014 | Extra-virgin olive oil vs. hyperoxygenated fatty acids | ND | ND preventing PU | Topical | RCT, risk of PU | Díaz-Valenzuela125 |
| 2013 | 2.5 mg/mL Lipoic Acid–loaded nanocapsules or LA | D0 | Speed up WH | Topical | Healthy rat | Külkamp-Guerreiro127 |
| 2013 | 10% oleic-linoleic mixture in collagen-based films | ND | Speed up WH | Topical | Healthy rat | Santos102 |
| 2013 | 10 μg PG D2 | D7 | Decreased WIHN | Topical | Healthy mice | Nelson90 |
| 2013 | hyperoxygenated fatty acids vs. triisostearin | ND | Prevented PU | Topical | RCT, high risk of PU | Torra i Bou124 |
| 2012 | 30 mg/g α-lipoic acid | D0 | Speed up WH | Topical | Healthy mice | Leu128 |
| 2012 | 0.003% PGE1 | D0 | Speed up WH | Topical | Rabbit ear | Arai118 |
| 2012 | 100 μg PGE1 | D0 | Speed up WH | injected | Healthy rat | Takikawa109 |
| 2012 | 2 mM PG transporter inhibitor | D0 | Speed up WH | Topical | Diabetic mice | Syeda118 |
| 2012 | oleic acid based polymeric bandage | D0 | Speed up WH | Topical | Healthy rat | Mohanty106 |
| 2011 | 50 ng/wound 14S,21R-dihydroxy-DHA | D2 | Speed up WH | Topical | LOX-deficient Mice | Tian115 |
| 2011 | 50 ng/wound 14S,21R-dihydroxy-DHA | ND | Speed up WH | Topical | Diabetic mice | Tian116 |
| 2011 | 30 μM linolenic or 30 μM oleic acids | D0 | OA Speed up WH | Topical | Healthy mice | Cardoso104 |
| 2010 | 50 ng 14S,21-diHDHA or 14R,21-diHDHA | D0 | Speed up WH | Topical | Healthy mice | Lu114 |
| 2010 | 0.1 g fatty acid extracts of dried Lucilia sericata larvae | D0 | Speed up WH | Topical | Healthy rat | Zhang129 |
| 2008 | 99% 300 μL linoleic acid or 99% 300 μL oleic acid | D0 | Increased WBC | Topical | Healthy rat | Pereira101 |
| 2008 | 99 mg/g oleic acid or 48 mg/g icosapentaenoic acid | D0 | EPA Speed up OA | Topical | Healthy Pig | Shingel107 |
| 2008 | 23% PA and 21% EPA or 19% AA and 16% PA | ND | Speed up WH | Topical | Burned Rat | Badiu122 |
| 2004 | 30 μM linolenic, 30 μM linoleic, or 30 μM oleic acids | D0 | OA Speed up LA Speed down WH | Topical | Healthy mice | Cardoso105 |
| 2003 | 8 μg PGE2 | D0 | Restored WIHN | Topical | COX inhibited Mice | Kämpfer119 |
| 2001 | 10 μg PGE1 in collagen matrix | D0 | Speed up WH | Topical | Healthy Rabbits | Ono111 |
| 1999 | 100 μg/g prostacyclin analogue SM-10902 | D0 | Speed up WH | Topical | Diabetic mice | Yamamoto112 |
Fatty acids and by-products used in wound healing.
All outcomes p < 0.05.
WH, wound healing; PU, pressure ulcers; LOX, lipoxygenase; WBC, white blood cell; AA, arachidonic acid; PA, palmitic acid; OA, oleic acid; LA, linoleic acid; EPA, eicosapentaenoic acid; WIHN, wound-induced hair neogenesis, start ND, not described; outcome ND, no difference; RCT, randomized controlled trial.
Data from included studies
Together, the data already published have shown that the topical use of fatty acids modulates inflammatory and immune responses during wound healing. We found that topical OA (omega-9 family) treatment significantly increases the number of neutrophils present in the wound bed.101 A similar effect has been shown in the commercial formulation of the OA-LA mixture with acceleration of the wound healing rate and augmentation of neutrophils infiltrate.102
We also identified that topical treatment with olive oil (up to 80% of OA) and primrose oil (77% of LA) showed a higher wound closure rate than the control group.103 In the same way, topical treatment with OA (omega-9) accelerated the wound closure when compared to the α-linolenic (omega 3) treated group, and modulated the inflammatory phase of wound healing, beyond enhancing the reparative response.104 When studied separately, the topical application of OA induced faster wound closure than LA (omega-6).104 However, treatment with ALA (omega-3) showed slower wound healing.105 OA-based polymeric bandages speed up the wound healing time.106 On the contrary, solid emulsion gel, as a vehicle for the delivery of the EPA-DHA mixture, achieved better speed closure than OA.107 These data indicate that linoleic and ALA, per se, may not be effective to accelerate the wound closure, but their bioactive products, generated by COX and LOX actions, such as prostaglandins, thromboxanes, and leukotrienes, could be more effective.
In fact, some prostaglandins have also been shown to enhance wound closure. PGE1 by itself,108–110 or in a collagen matrix,111 speeds up wound healing time. Moreover, accelerated wound closure and epithelization were increased using an agonist of PPAR-γ, which is activated by 15-deoxy-Δ-12,14-prostaglandin J2 (15d-PGJ2), in diabetic animals.99 This article observed that this agonist raised the chemotaxis of CD31+ cells in the wound, and raised VEGF concentration, which probably acted together to facilitate the acceleration of wound closure in these diabetic animals. Another study with prostanoids showed that topical treatment with an analog (SM-10902) of prostacyclin, also known as PGI2, increased skin blood flow, the formation of new vessels, and decreased the wound lesion area in diabetic mice.112 Even treatment with AA has been shown to be effective for accelerating wound closure.113 In addition, Leukotriene B4, produced by the oxidation of AA via the LOX pathway in neutrophils and macrophages, also accelerated wound closure in diabetic animals.95 Treatment with 14,21-dihydroxy-DHA, a novel bioproduct from the omega-3 family, has been shown to accelerate wound epithelialization, increase the number of new vessels, and increase chemotaxis of CD31+ cells both in healthy and diabetic rodents.114–116
The data published also indicate that not only treatment with fatty acids is effective, but inhibition of prostaglandin transporter (PGT), for example, also improves wound healing in diabetes and healthy animals, decreasing the wound area during the proliferative phase.117,118 This occurs because PGT inhibition modulates arterial blood flow, mobilizes endothelial progenitor cells and human epidermal keratinocytes, and leads to vascularization and epithelialization in wound healing by regulating vasodilatory and proangiogenic prostaglandins.
Wound-induced hair neogenesis (WIHN), that is, the de novo generation of hair follicles, is a rare phenomenon in adult animals. Interestingly, we found that topical or wound-bed injected PGE2 increased new hair follicles in the scar tissue.69,119 Also, several histological differences were described in wounds treated with DHA. The topical application of DHA in wounded skin facilitates the formation of a new hair follicles-like structure in the scar tissue.84 On the contrary, the topical use of PGD2 decreased wound-induced hair follicle neogenesis.90 WIHN using PGE2 and DHA started when the treatment was performed during the inflammatory phase of wound healing, unlike PGD2, where the treatment started 7 days after the wound creation. Altogether, this evidence suggests that prostaglandins could modulate WIHN and wound closure in a time-dependent manner.
Resolvins also could modulate the wound healing velocity. Topical application of resolvin D1, D2, or D4 has improved epithelialization during skin wound healing in healthy animals.120 It is important for clinical practice since resolvin receptors are highly expressed in epidermal keratinocytes.
From another omega family, topical treatment using palmitoleic acid, an omega-7 MUFA, also decreased the wound area in healthy and burned rats.121,122 These authors have attributed the anti-inflammatory activity of palmitoleic acid for healing, especially in the stages of granulation tissue formation and remodeling, although this fatty acid has inhibited LPS-stimulated neutrophil migration, accelerating wound healing via an anti-inflammatory effect.
It is well known that fatty acid treatment is used to prevent the development of pressure ulcers in bed-bound patients. Consistently with the prohealing benefits shown in basic research, one study has described that olive oil is more efficient than hyperoxygenated fatty acids (HOFA) for preventing pressure ulcers in bedridden patients.123,124 On the contrary, another study performed a comparison of topical treatment using olive oil versus HOFA, it showed that there is no difference between olive oil and HOFA for preventing pressure ulcers.125,126
Other lipids, not fatty acids, have also demonstrated some benefits in the wound healing process of healthy animals. Alpha-lipoic acid, a natural compound with antioxidant properties and other potential health benefits, when topically applied, accelerated wound healing in healthy animals.127,128 Also, unsaturated fatty acid extracts from dried Lucilia sericata larvae increased wound contraction and VEGF quantification in healthy animals.129 More recently, the topical application of jasmonic acid, which has a similar chemical structure to prostaglandins, has improved skin healing by accelerating epithelial repair in healthy volunteers who received a suction blister induction.130
Summary
Concluding remarks and future directions
Many efforts have been made in the field of wound care to attenuate the suffering of patients. Although wound healing is a physiological process, various disorders can delay it, which makes the injury chronic and more susceptible to infections and other related complications, especially in the skin. Different treatments have been tested in experimental and clinical studies, as well as new products have been designed, but the problem remains unsolved.
In the past few decades, lipids have emerged as a potential therapeutic target for the treatment of wounds. Due to their bioactive properties, unsaturated fatty acids (MUFAs and PUFAs) are the most studied and have been showing great results. However, most of the studies are still from basic research and, although they have shown effective results, the molecular mechanisms involved are not completely clear. It happens, in part, because fatty acids can bind to different receptors, and each receptor can activate different intracellular pathways, resulting in several possible physiologic and metabolic changes.
In this way, many experimental attempts have been performed using analog substances of lipids, or even testing receptor agonists, which can show different results from the ones observed when fatty acid is used for the treatment of wounds directly. In these cases, despite the fact that the chemical structure of the synthetic molecule is similar to the fatty acid chain, the bioactive properties cannot be mimicked completely. Besides, when fatty acids bind to their receptor, they can meddle other intracellular pathways.
Furthermore, the clinical use of these lipids depends on their stability, since they can quickly oxidize, and of their metabolization, since many enzymatic pathways such as LOX and COX can be activated, changing the course of the fatty acid. This is an explanation for the increased use of eicosanoids and other metabolized products instead of AA or EPA/DHA in experimental studies; this should be considered especially in studies with oil blend or olive oil. These oils present different percentages of fatty acids, but their action also depends on other bioactive compounds. Olive oil, particularly, has shown many benefits in several metabolic processes, but its effect is due not only to OA but also to phenolic compounds.
Finally, the use of bioactive lipids in clinical practice can improve wound care, but there is still a lack of knowledge of the action of each fatty acid in wound repair. In this way, future studies must be conducted to clarify this subject and thus, enable the development of new effective therapeutic approaches.
Take-Home Messages
Skin wound healing is an orchestrated physiological process, which occurs after the onset of a cutaneous lesion to restore the damaged tissue.
Wound closure involves a series of events that can be divided into three phases: inflammatory, proliferative, and remodeling.
Lipids are involved in the whole process involving wound resolution such as vascular contraction, chemotaxis, cell adhesion, and tissue restoration.
Due to their bioactive properties, unsaturated fatty acids have emerged as a potential therapeutic target in the field of wound care.
Oleic (ω-9), linoleic (ω-6), and α-linolenic (ω-3) acids, as well as their bioactive products, are the most common fatty acids tested in skin wounds, showing an effective role in accelerating the wound closure.
Topical treatment with fatty acids' analogs as well as their receptors' agonist has also been demonstrating similar positive effects.
Bioactive lipids' effects, when the treatment starts on day 0, occur mainly in the inflammatory phase of the wound healing process.
Different bandages associated with lipid formulations have been developed and tested in clinical practice, but the incidence of complications associated with late wound closure persists.
Future studies should be conducted for elucidating the main intracellular mechanisms of each bioactive lipid in each wound healing phase.
Other alternatives and more efficient treatments will be better designed from this novel knowledge.
Abbreviations and Acronyms
- 12-HETE
12-Hydroxyeicosatetraenoic acid
- 12-HETER
12-HETE receptor
- 12-HHT
12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid
- 15d-PGJ2
15-Deoxy-Δ-12,14-Prostaglandin J2
- 15-HETE
15-Hydroxyeicosatetraenoic acid
- AA
arachidonic acid
- ALA
α-linolenic acid
- AP-1
activator protein-1
- BLT
leukotriene receptor
- cAMP
cyclic adenosine monophosphate
- COX
cyclooxygenase
- CRTH2
chemoattractant receptor-homologous molecule expressed on Th2 cells
- DC
dendritic cell
- DHA
docosahexaenoic acid
- DP
D prostanoid receptor
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EP
prostaglandin receptor
- EP
prostanoid receptor
- EPA
eicosapentaenoic acid
- ETE
5-oxo-eicosatetraenoic
- EVOO
extra-virgin olive oil
- FFAR4
free fatty acid receptor 4
- FGF-2
fibroblast growth factor 2
- GPCR
G protein-coupled receptor
- GPCR
G protein-coupled receptor
- HETE
5-eicosatetraenoic
- HIF-1
hypoxia-inducible factor-1
- HOFA
hyperoxygenated fatty acids
- HPETE
5-hydroperoxy-eicosatetraenoic acid
- IFN-γ
interferon-gamma
- IGF-1
insulin-like growth factor 1
- IkB
inhibitor of kB
- IL
interleukin
- JNK
c-Jun N-terminal kinase
- LA
linoleic acid
- LOX
lipoxygenase
- LT
leukotriene
- LXA4
lipoxin A4
- MC
mast cell
- MCP-1
monocyte chemoattractant protein 1
- MMP
metalloproteinases
- MUFA
monounsaturated fatty acid
- NFkB
factor nuclear kappa B
- OA
oleic acid
- PDGF
platelet-derived growth factor
- PG
prostaglandin
- PGT
prostaglandin transporter
- PI3K
phosphoinositide 3-kinase
- PKA
protein kinase A
- PPAR
peroxisome proliferator-activated receptor
- PUFA
polyunsaturated fatty acids
- Rv
resolvin
- TGF-β1
transforming growth factor β1
- TIMPs
tissue inhibitors of metalloproteinases
- TNF-α
tumor necrosis factor-alpha
- TP
thromboxane receptor
- TX
tromboxane
- VEGF
vascular endothelial growth factor
- WIHN
wound-induced hair neogenesis
Acknowledgments and Funding Sources
The review and wound healing research in the authors' laboratory were supported by Coordination for the Improvement of Higher Education Personnel (1744875 and 88882.434715/2019–01) and The São Paulo Research Foundation (FAPESP: 2016/17810–3) funding.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Carlos P. Jara is a clinical and research nurse, Master in Health Institution Management, Master in Health Science, and PhD Student in Health Sciences at Faculty of Nursing in State University of Campinas. His expertise are skin repair and healing process, both in vitro and in animal models.
Natalia F Mendes is a Nutritionist, Master in Nutrition, Sports, and Metabolism, and PhD Student in Health Sciences at Faculty of Nursing, University of Campinas. Her research interests are the molecular mechanisms involved in the central and peripheral inflammation mediated by fatty acids, particularly neuroinflammation and gliosis in the hypothalamus.
Thais P. Prado has graduation and degree in nurse, Master in Health Science, and PhD Student in Health Sciences at Faculty of Nursing in State University of Campinas. Her research interests are wound healing, in vitro and in vivo animal models and humans.
Eliana P. de Araújo, MSC, PhD, is a Professor of the Nursing School, and research of the Obesity and Comorbidities Research Center, University of Campinas, Brazil. Her research focuses on molecular and cellular mechanisms of tissue repair and regeneration and mechanisms involved in the central and peripheral inflammation mediated by fatty acids.
References
- 1. Martin P. Wound healing—aiming for perfect skin regeneration. Science 1997;276:75–81 [DOI] [PubMed] [Google Scholar]
- 2. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007;117:1219–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark RA. Biology of dermal wound repair. Dermatol Clin 1993;11:647–666 [PubMed] [Google Scholar]
- 4. Bennett JS, Berger BW, Billings PC. The structure and function of platelet integrins. J Thromb Haemost 2009;7 Suppl 1:200–205 [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Sakuma M, Chen Z, et al. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation 2005;112:2993–3000 [DOI] [PubMed] [Google Scholar]
- 6. Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 2011;13:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pakyari M, Farrokhi A, Maharlooei MK, et al. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle) 2013;2:215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canedo-Dorantes L, Canedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflam 2019;2019:3706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tracy LE, Minasian RA, Caterson EJ. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv Wound Care (New Rochelle) 2016;5:119–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moura J, Madureira P, Leal EC, et al. Immune aging in diabetes and its implications in wound healing. Clin Immunol 2019;200:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu SC, Lan CE. High-glucose environment disturbs the physiologic functions of keratinocytes: Focusing on diabetic wound healing. J Dermatol Sci 2016;84:121–127 [DOI] [PubMed] [Google Scholar]
- 12. Singh K, Agrawal NK, Gupta SK, et al. Increased expression of TLR9 associated with pro-inflammatory S100A8 and IL-8 in diabetic wounds could lead to unresolved inflammation in type 2 diabetes mellitus (T2DM) cases with impaired wound healing. J Diabetes Complications 2016;30:99–108 [DOI] [PubMed] [Google Scholar]
- 13. Tang Y, Zhang MJ, Hellmann J, et al. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 2013;62:618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacLeod AS, Mansbridge JN. The innate immune system in acute and chronic wounds. Adv Wound Care (New Rochelle) 2016;5:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deiters U, Barsig J, Tawil B, et al. The macrophage-activating lipopeptide-2 accelerates wound healing in diabetic mice. Exp Dermatol 2004;13:731–739 [DOI] [PubMed] [Google Scholar]
- 16. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol 2015;173:370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loots MA, Lamme EN, Zeegelaar J, et al. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998;111:850–857 [DOI] [PubMed] [Google Scholar]
- 18. Piaggesi A. Research development in the pathogenesis of neuropathic diabetic foot ulceration. Curr Diab Rep 2004;4:419–423 [DOI] [PubMed] [Google Scholar]
- 19. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei W, Liu Q, Tan Y, et al. Oxidative stress, diabetes, and diabetic complications. Hemoglobin 2009;33:370–377 [DOI] [PubMed] [Google Scholar]
- 21. Jhamb S, Vangaveti VN, Malabu UH. Genetic and molecular basis of diabetic foot ulcers: Clinical review. J Tissue Viability 2016;25:229–236 [DOI] [PubMed] [Google Scholar]
- 22. Kalan LR, Brennan MB. The role of the microbiome in nonhealing diabetic wounds. Ann N Y Acad Sci 2019;1435:79–92 [DOI] [PubMed] [Google Scholar]
- 23. Zhao G, Usui ML, Lippman SI, et al. Biofilms and inflammation in chronic wounds. Adv Wound Care (New Rochelle) 2013;2:389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract 2010;25:61–68 [DOI] [PubMed] [Google Scholar]
- 25. Kavalukas SL, Barbul A. Nutrition and wound healing: an update. Plast Reconstr Surg 2011;127 Suppl 1:38S–43S [DOI] [PubMed] [Google Scholar]
- 26. Molnar JA, Underdown MJ, Clark WA. Nutrition and chronic wounds. Adv Wound Care (New Rochelle) 2014;3:663–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collins CE, Kershaw J, Brockington S. Effect of nutritional supplements on wound healing in home-nursed elderly: a randomized trial. Nutrition 2005;21:147–155 [DOI] [PubMed] [Google Scholar]
- 28. Thompson C, Fuhrman MP. Nutrients and wound healing: still searching for the magic bullet. Nutr Clin Pract 2005;20:331–347 [DOI] [PubMed] [Google Scholar]
- 29. van Anholt RD, Sobotka L, Meijer EP, et al. Specific nutritional support accelerates pressure ulcer healing and reduces wound care intensity in non-malnourished patients. Nutrition 2010;26:867–872 [DOI] [PubMed] [Google Scholar]
- 30. Kendall AC, Pilkington SM, Massey KA, et al. Distribution of bioactive lipid mediators in human skin. J Invest Dermatol 2015;135:1510–1520 [DOI] [PubMed] [Google Scholar]
- 31. Nelson DL, Cox MM. Lehninger Principles of Biochemistry. New York: Worth Publishers, 2000. [Google Scholar]
- 32. Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol 2011;668 Suppl 1:S50–S58 [DOI] [PubMed] [Google Scholar]
- 33. Owen RW, Mier W, Giacosa A, et al. Phenolic compounds and squalene in olive oils: the concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignansand squalene. Food Chem Toxicol 2000;38:647–659 [DOI] [PubMed] [Google Scholar]
- 34. Tripoli E, Giammanco M, Tabacchi G, et al. The phenolic compounds of olive oil: structure, biological activity and beneficial effects on human health. Nutr Res Rev 2005;18:98–112 [DOI] [PubMed] [Google Scholar]
- 35. Covas MI, Konstantinidou V, Fito M. Olive oil and cardiovascular health. J Cardiovasc Pharmacol 2009;54:477–482 [DOI] [PubMed] [Google Scholar]
- 36. Martinez-Gonzalez MA, Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol 2014;25:20–26 [DOI] [PubMed] [Google Scholar]
- 37. Yubero-Serrano EM, Lopez-Moreno J, Gomez-Delgado F, et al. Extra virgin olive oil: More than a healthy fat. Eur J Clin Nutr 2019;72:8–17 [DOI] [PubMed] [Google Scholar]
- 38. Estruch R, Martinez-Gonzalez MA, Corella D, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 2006;145:1–11 [DOI] [PubMed] [Google Scholar]
- 39. Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004;292:1440–1446 [DOI] [PubMed] [Google Scholar]
- 40. Estruch R. Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc 2010;69:333–340 [DOI] [PubMed] [Google Scholar]
- 41. Scoditti E, Massaro M, Carluccio MA, et al. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic Acid and hydroxytyrosol in human adipocytes. PLoS One 2015;10:e0128218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chapkin RS, Ziboh VA. Inability of skin enzyme preparations to biosynthesize arachidonic acid from linoleic acid. Biochem Biophys Res Commun 1984;124:784–792 [DOI] [PubMed] [Google Scholar]
- 43. Bang HO, Dyerberg J, Nielsen AB. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet 1971;1:1143–1145 [DOI] [PubMed] [Google Scholar]
- 44. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 2017;45:1105–1115 [DOI] [PubMed] [Google Scholar]
- 45. Kendall AC, Nicolaou A. Bioactive lipid mediators in skin inflammation and immunity. Prog Lipid Res 2013;52:141–164 [DOI] [PubMed] [Google Scholar]
- 46. Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol 2015;15:511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ziboh VA. Prostaglandins, leukotrienes, and hydroxy fatty acids in epidermis. Semin Dermatol 1992;11:114–120 [PubMed] [Google Scholar]
- 48. Sugimoto M, Arai I, Futaki N, et al. Role of COX-1 and COX-2 on skin PGs biosynthesis by mechanical scratching in mice. Prostaglandins Leukot Essent Fatty Acids 2006;75:1–8 [DOI] [PubMed] [Google Scholar]
- 49. Konger RL, Malaviya R, Pentland AP. Growth regulation of primary human keratinocytes by prostaglandin E receptor EP2 and EP3 subtypes. Biochim Biophys Acta 1998;1401:221–234 [DOI] [PubMed] [Google Scholar]
- 50. Honda T, Matsuoka T, Ueta M, et al. Prostaglandin E(2)-EP(3) signaling suppresses skin inflammation in murine contact hypersensitivity. J Allergy Clin Immunol 2009;124:809–818 e2. [DOI] [PubMed] [Google Scholar]
- 51. Scott G, Leopardi S, Printup S, et al. Proteinase-activated receptor-2 stimulates prostaglandin production in keratinocytes: analysis of prostaglandin receptors on human melanocytes and effects of PGE2 and PGF2alpha on melanocyte dendricity. J Invest Dermatol 2004;122:1214–1224 [DOI] [PubMed] [Google Scholar]
- 52. Harris SG, Padilla J, Koumas L, et al. Prostaglandins as modulators of immunity. Trends Immunol 2002;23:144–150 [DOI] [PubMed] [Google Scholar]
- 53. Rhodes LE, Gledhill K, Masoodi M, et al. The sunburn response in human skin is characterized by sequential eicosanoid profiles that may mediate its early and late phases. FASEB J 2009;23:3947–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nicolaou A. Eicosanoids in skin inflammation. Prostaglandins Leukot Essent Fatty Acids 2013;88:131–138 [DOI] [PubMed] [Google Scholar]
- 55. Kawahara K, Hohjoh H, Inazumi T, et al. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim Biophys Acta 2015;1851:414–421 [DOI] [PubMed] [Google Scholar]
- 56. Sandulache VC, Parekh A, Li-Korotky HS, et al. Prostaglandin E2 differentially modulates human fetal and adult dermal fibroblast migration and contraction: implication for wound healing. Wound Repair Regen 2006;14:633–643 [DOI] [PubMed] [Google Scholar]
- 57. Zhao J, Shu B, Chen L, et al. Prostaglandin E2 inhibits collagen synthesis in dermal fibroblasts and prevents hypertrophic scar formation in vivo. Exp Dermatol 2016;25:604–610 [DOI] [PubMed] [Google Scholar]
- 58. Sato T, Kirimura Y, Mori Y. The co-culture of dermal fibroblasts with human epidermal keratinocytes induces increased prostaglandin E2 production and cyclooxygenase 2 activity in fibroblasts. J Invest Dermatol 1997;109:334–339 [DOI] [PubMed] [Google Scholar]
- 59. Honma Y, Arai I, Hashimoto Y, et al. Prostaglandin D2 and prostaglandin E2 accelerate the recovery of cutaneous barrier disruption induced by mechanical scratching in mice. Eur J Pharmacol 2005;518:56–62 [DOI] [PubMed] [Google Scholar]
- 60. Parekh A, Sandulache VC, Singh T, et al. Prostaglandin E2 differentially regulates contraction and structural reorganization of anchored collagen gels by human adult and fetal dermal fibroblasts. Wound Repair Regen 2009;17:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hirata T, Narumiya S. Prostanoids as regulators of innate and adaptive immunity. Adv Immunol 2012;116:143–174 [DOI] [PubMed] [Google Scholar]
- 62. Sivamani RK. Eicosanoids and Keratinocytes in Wound Healing. Adv Wound Care (New Rochelle) 2014;3:476–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ikai K, Imamura S. Prostaglandin D2 in the skin. Int J Dermatol 1988;27:141–149 [DOI] [PubMed] [Google Scholar]
- 64. Satoh T, Moroi R, Aritake K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol 2006;177:2621–2629 [DOI] [PubMed] [Google Scholar]
- 65. Kanda N, Ishikawa T, Watanabe S. Prostaglandin D2 induces the production of human beta-defensin-3 in human keratinocytes. Biochem Pharmacol 2010;79:982–989 [DOI] [PubMed] [Google Scholar]
- 66. Nakajima S, Honda T, Sakata D, et al. Prostaglandin I2-IP signaling promotes Th1 differentiation in a mouse model of contact hypersensitivity. J Immunol 2010;184:5595–5603 [DOI] [PubMed] [Google Scholar]
- 67. Andoh T, Nishikawa Y, Yamaguchi-Miyamoto T, et al. Thromboxane A2 induces itch-associated responses through TP receptors in the skin in mice. J Invest Dermatol 2007;127:2042–2047 [DOI] [PubMed] [Google Scholar]
- 68. Toda A, Yokomizo T, Shimizu T. Leukotriene B4 receptors. Prostaglandins Other Lipid Mediat 2002;68–69:575–585 [DOI] [PubMed] [Google Scholar]
- 69. Zhu AS, Li A, Ratliff TS, et al. After skin wounding, noncoding dsRNA coordinates prostaglandins and Wnts to promote regeneration. J Invest Dermatol 2017;137:1562–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oyoshi MK, He R, Li Y, et al. Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity 2012;37:747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grant GE, Rokach J, Powell WS. 5-Oxo-ETE and the OXE receptor. Prostaglandins Other Lipid Mediat 2009;89:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nguyen CH, Stadler S, Brenner S, et al. Cancer cell-derived 12(S)-HETE signals via 12-HETE receptor, RHO, ROCK and MLC2 to induce lymph endothelial barrier breaching. Br J Cancer 2016;115:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Napolitano M. The role of the 12(S)-HETE/GPR31/12-HETER axis in cancer and ischemia-reperfusion injury. Biochem Soc Trans 2019;47:743–754 [DOI] [PubMed] [Google Scholar]
- 74. Ruzicka T. The role of the epidermal 12-hydroxyeicosatetraenoic acid receptor in the skin. Eicosanoids 1992;5 Suppl:S63–S65 [PubMed] [Google Scholar]
- 75. Kragballe K, Pinnamaneni G, Desjarlais L, et al. Dermis-derived 15-hydroxy-eicosatetraenoic acid inhibits epidermal 12-lipoxygenase activity. J Invest Dermatol 1986;87:494–498 [DOI] [PubMed] [Google Scholar]
- 76. Serhan CN, Jain A, Marleau S, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol 2003;171:6856–6865 [DOI] [PubMed] [Google Scholar]
- 77. Hohjoh H, Inazumi T, Tsuchiya S, et al. Prostanoid receptors and acute inflammation in skin. Biochimie 2014;107 Pt A:78–81 [DOI] [PubMed] [Google Scholar]
- 78. Ferre S. The GPCR heterotetramer: challenging classical pharmacology. Trends Pharmacol Sci 2015;36:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cismowski MJ, Lanier SM. Activation of heterotrimeric G-proteins independent of a G-protein coupled receptor and the implications for signal processing. Rev Physiol Biochem Pharmacol 2005;155:57–80 [DOI] [PubMed] [Google Scholar]
- 80. Milligan G, Alvarez-Curto E, Hudson BD, et al. FFA4/GPR120: Pharmacology and Therapeutic Opportunities. Trends Pharmacol Sci 2017;38:809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oh DY, Olefsky JM. Omega 3 fatty acids and GPR120. Cell Metab 2012;15:564–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cintra DE, Ropelle ER, Moraes JC, et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One 2012;7:e30571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dragano NRV, Solon C, Ramalho AF, et al. Polyunsaturated fatty acid receptors, GPR40 and GPR120, are expressed in the hypothalamus and control energy homeostasis and inflammation. J Neuroinflammation 2017;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Arantes EL, Dragano N, Ramalho A, et al. Topical Docosahexaenoic Acid (DHA) Accelerates Skin Wound Healing in Rats and Activates GPR120. Biol Res Nurs 2016;18:411–419 [DOI] [PubMed] [Google Scholar]
- 85. Arai I, Takaoka A, Hashimoto Y, et al. Effects of TS-022, a newly developed prostanoid DP1 receptor agonist, on experimental pruritus, cutaneous barrier disruptions and atopic dermatitis in mice. Eur J Pharmacol 2007;556:207–214 [DOI] [PubMed] [Google Scholar]
- 86. Cheng K, Wu TJ, Wu KK, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A 2006;103:6682–6687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Torres D, Paget C, Fontaine J, et al. Prostaglandin D2 inhibits the production of IFN-gamma by invariant NK T cells: consequences in the control of B16 melanoma. J Immunol 2008;180:783–792 [DOI] [PubMed] [Google Scholar]
- 88. Nagata N, Iwanari H, Kumagai H, et al. Generation and characterization of an antagonistic monoclonal antibody against an extracellular domain of mouse DP2 (CRTH2/GPR44) receptors for prostaglandin D2. PLoS One 2017;12:e0175452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hata AN, Zent R, Breyer MD, et al. Expression and molecular pharmacology of the mouse CRTH2 receptor. J Pharmacol Exp Ther 2003;306:463–470 [DOI] [PubMed] [Google Scholar]
- 90. Nelson AM, Loy DE, Lawson JA, et al. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. J Invest Dermatol 2013;133:881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yokoyama U, Iwatsubo K, Umemura M, et al. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Rev 2013;65:1010–1052 [DOI] [PubMed] [Google Scholar]
- 92. Marchese A, Sawzdargo M, Nguyen T, et al. Discovery of three novel orphan G-protein-coupled receptors. Genomics 1999;56:12–21 [DOI] [PubMed] [Google Scholar]
- 93. Yao C, Sakata D, Esaki Y, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med 2009;15:633–640 [DOI] [PubMed] [Google Scholar]
- 94. Yokomizo T. Two distinct leukotriene B4 receptors, BLT1 and BLT2. J Biochem 2015;157:65–71 [DOI] [PubMed] [Google Scholar]
- 95. Liu M, Saeki K, Matsunobu T, et al. 12-Hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J Exp Med 2014;211:1063–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gus-Brautbar Y, Panigrahy D. Time heals all wounds—but 12-HHT is faster. J Exp Med 2014;211:1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mirza RE, Fang MM, Ennis WJ, et al. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013;62:2579–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bertin B, Dubuquoy L, Colombel JF, et al. PPAR-gamma in ulcerative colitis: a novel target for intervention. Curr Drug Targets 2013;14:1501–1507 [DOI] [PubMed] [Google Scholar]
- 99. Mirza RE, Fang MM, Novak ML, et al. Macrophage PPARgamma and impaired wound healing in type 2 diabetes. J Pathol 2015;236:433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Silva JC, Pitta MGR, Pitta IR, et al. New peroxisome proliferator-activated receptor agonist (GQ-11) improves wound healing in diabetic mice. Adv Wound Care (New Rochelle) 2019;8:417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pereira LM, Hatanaka E, Martins EF, et al. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem Funct 2008;26:197–204 [DOI] [PubMed] [Google Scholar]
- 102. Santos CG, Nascimento MF, Oliveira CR, et al. Bioassay-guided evaluation of wound healing effect of fatty acids-incorporated collagen-based films. Acta Cir Bras 2013;28:346–352 [DOI] [PubMed] [Google Scholar]
- 103. Ishak WMW, Katas H, Yuen NP, et al. Topical application of omega-3-, omega-6-, and omega-9-rich oil emulsions for cutaneous wound healing in rats. Drug Deliv Transl Res 2019;9:418–433 [DOI] [PubMed] [Google Scholar]
- 104. Cardoso CR, Favoreto S Jr., Oliveira LL, et al. Oleic acid modulation of the immune response in wound healing: a new approach for skin repair. Immunobiology 2011;216:409–415 [DOI] [PubMed] [Google Scholar]
- 105. Cardoso CR, Souza MA, Ferro EA, et al. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen 2004;12:235–243 [DOI] [PubMed] [Google Scholar]
- 106. Mohanty C, Das M, Sahoo SK. Sustained wound healing activity of curcumin loaded oleic acid based polymeric bandage in a rat model. Mol Pharm 2012;9:2801–2811 [DOI] [PubMed] [Google Scholar]
- 107. Shingel KI, Faure MP, Azoulay L, et al. Solid emulsion gel as a vehicle for delivery of polyunsaturated fatty acids: implications for tissue repair, dermal angiogenesis and wound healing. J Tissue Eng Regen Med 2008;2:383–393 [DOI] [PubMed] [Google Scholar]
- 108. Arai K, Yamazaki M, Maeda T, et al. Influence of various treatments including povidone-iodine and healing stimulatory reagents in a rabbit ear wound model. Int Wound J 2013;10:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Takikawa M, Sumi Y, Tanaka Y, et al. Protective effect of prostaglandin E(1) on radiation-induced proliferative inhibition and apoptosis in keratinocytes and healing of radiation-induced skin injury in rats. J Radiat Res 2012;53:385–394 [DOI] [PubMed] [Google Scholar]
- 110. Naska S, Yuzwa SA, Johnston AP, et al. Identification of drugs that regulate dermal stem cells and enhance skin repair. Stem Cell Rep 2016;6:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ono I, Zhou LJ, Tateshita T. Effects of a collagen matrix containing prostaglandin E(1) on wound contraction. J Dermatol Sci 2001;25:106–115 [DOI] [PubMed] [Google Scholar]
- 112. Yamamoto T, Horikawa N, Komuro Y, et al. Effect of topical application of a stable prostacyclin analogue, SM-10902 on wound healing in diabetic mice. Eur J Pharmacol 1996;302:53–60 [DOI] [PubMed] [Google Scholar]
- 113. Oh SY, Lee SJ, Jung YH, et al. Arachidonic acid promotes skin wound healing through induction of human MSC migration by MT3-MMP-mediated fibronectin degradation. Cell Death Dis 2015;6:e1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lu Y, Tian H, Hong S. Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing. J Lipid Res 2010;51:923–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tian H, Lu Y, Shah SP, et al. 14S,21R-dihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds. J Biol Chem 2011;286:4443–4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tian H, Lu Y, Shah SP, et al. Autacoid 14S,21R-dihydroxy-docosahexaenoic acid counteracts diabetic impairment of macrophage prohealing functions. Am J Pathol 2011;179:1780–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu Z, Benard O, Syeda MM, et al. Inhibition of prostaglandin transporter (PGT) promotes perfusion and vascularization and accelerates wound healing in non-diabetic and diabetic rats. PLoS One 2015;10:e0133615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Syeda MM, Jing X, Mirza RH, et al. Prostaglandin transporter modulates wound healing in diabetes by regulating prostaglandin-induced angiogenesis. Am J Pathol 2012;181:334–346 [DOI] [PubMed] [Google Scholar]
- 119. Kämpfer H, Brautigam L, Geisslinger G, et al. Cyclooxygenase-1-coupled prostaglandin biosynthesis constitutes an essential prerequisite for skin repair. J Invest Dermatol 2003;120:880–890 [DOI] [PubMed] [Google Scholar]
- 120. Hellmann J, Sansbury BE, Wong B, et al. Biosynthesis of D-series resolvins in skin provides insights into their role in tissue repair. J Invest Dermatol 2018;138:2051–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Weimann E, Silva MBB, Murata GM, et al. Topical anti-inflammatory activity of palmitoleic acid improves wound healing. PLoS One 2018;13:e0205338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Badiu DL, Balu AM, Barbes L, et al. Physico-chemical characterisation of lipids from Mytilus galloprovincialis (L.) and Rapana venosa and their healing properties on skin burns. Lipids 2008;43:829–841 [DOI] [PubMed] [Google Scholar]
- 123. Otero DP, Dominguez DV, Fernandez LH, et al. Preventing facial pressure ulcers in patients under non-invasive mechanical ventilation: a randomised control trial. J Wound Care 2017;26:128–136 [DOI] [PubMed] [Google Scholar]
- 124. Torra i Bou J-E, Segovia Gomez T, Verdu Soriano J, et al. The effectiveness of a hyperoxygenated fatty acid compound in preventing pressure ulcers. J Wound Care 2005;14:117–121 [DOI] [PubMed] [Google Scholar]
- 125. Diaz-Valenzuela A, Garcia-Fernandez FP, Carmona Fernandez P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: Multicentre, controlled, randomised, and double-blinded clinical trial. Int Wound J 2019;16:1314–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lupianez-Perez I, Uttumchandani SK, Morilla-Herrera JC, et al. Topical olive oil is not inferior to hyperoxygenated fatty aids to prevent pressure ulcers in high-risk immobilised patients in home care. Results of a multicentre randomised triple-blind controlled non-inferiority trial. PLoS One 2015;10:e0122238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Külkamp-Guerreiro IC, Souza MN, Bianchin MD, et al. Evaluation of lipoic acid topical application on rats skin wound healing. Acta Cir Bras 2013;28:708–715 [DOI] [PubMed] [Google Scholar]
- 128. Leu JG, Chen SA, Chen HM, et al. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and alpha-lipoic acid. Nanomedicine 2012;8:767–775 [DOI] [PubMed] [Google Scholar]
- 129. Zhang Z, Wang S, Diao Y, et al. Fatty acid extracts from Lucilia sericata larvae promote murine cutaneous wound healing by angiogenic activity. Lipids Health Dis 2010;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Henriet E, Jager S, Tran C, et al. A jasmonic acid derivative improves skin healing and induces changes in proteoglycan expression and glycosaminoglycan structure. Biochim Biophys Acta Gen Subj 2017;1861:2250–2260 [DOI] [PubMed] [Google Scholar]