Abstract
From the beginning of the year 2020, the world was affected by a coronavirus disease (COVID-19) pandemic, caused by SARS-CoV-2, leading to a shortage of personal protective equipment (PPE) at a global level, and thus generating exposure of health professionals to this extremely contagious virus. Within this context, the present work seeks to present an alternative for the production of face shields “face shields,” in which it recommends its production “in house” through 3D printing, in principle initiated by Prusa Research, where we download your project of support of facial protectors, proceeding with printing through the 3D printer Gtmax3D Core H5. The authors produced a face shield in ABS, in a total time of 3 hours and 44 minutes. Thus, the model presented proved to be feasible, at a low cost, adding to the list of possibilities to produce inputs necessary to maintain the fight against this epidemic.
Keywords: 3D printing, coronavirus, pandemics, personal protective equipment
Coronavirus disease (COVID-19) is caused by SARS-CoV-2, which represents the causative agent of a potentially fatal disease that is a major global public health problem.1 Transmission occurs mainly by close contact to infected individuals’ droplets scattered cough and sneeze. COVID-19 rapidly spreading characteristics led to patients’ isolation, population quarantine and a variety of treatments.2
Disease outbreak represents a higher risk of infection to healthcare professionals,3 mainly due to the increased frequency of exposure.3,4 In order to manage this pandemic challenge, extensive measures to reduce the transmission of COVID-19 from person to person have was implemented, such as the change in individual protection protocols to health professionals, which has generated a massive demand for personal protective equipment (PPE).2,5 In reaction to the acute shortage of PPE in hospitals, due to high demand, a global initiative aroused to manufacture PPE using 3D printers.6,7
In recent years, 3D printing has achieved rapid popularity in the dental field, due to the varied possibilities of using this technology, mainly due to its increased precision and printing speed.8 The most common dental uses for 3D printing are the manufacture of surgical guides, the creation of prostheses, personalized implants, and anatomical prototypes.9,10
In times of the COVID-19 pandemic, one of several challenges is the rapid shortage of personal protective equipment. 3D printing and “maker culture” proved as an alternative in this scenario.6 Thus, this article describes the process of manufacturing face shields using additive 3D printing.
MATERIALS AND METHODS
Description of the 3D Project
Following an international initiative to target 3D prints for PPE production in the face of the COVID-19 pandemic, initiated by Prusa Research (Prague, Czech Republic), the Oral and Maxillofacial team at the General Hospital of Cuiabá reproduced an in-house 3D printing workflow.
In-House Workflow
Prusa designed Faces Shields and the model chosen for the process was RC2, this being adapted to the characteristics of 3D printing, through the Simplify 3D software (Simplify 3D, Cincinnati, OH), for Gtmax3D Core H5 printer (Americana, São Paulo, Brazil). This printer uses fused deposition modeling (FDM) technology and has been used at the department for 3D printing of anatomic models.
The printed filament material was ABS Premium MG94 by Gtmax3D (Americana), with 1.75 mm in diameter (Fig. 1A). The layer thickness was 0.25 mm with 30% internal filling, without the need for support. The temperature of the extruder nozzle and the table of the 3D printer was 230°C and 110°C, respectively. The printing speed was 100%, totaling 3 hours and 44 minutes for each complete pair of face shield structures, which are 2.5 mm thick on the external handle and 2 mm on the internal handle, with 4 reliefs for fitting the transparent acetate visor. A lower stabilizer bar of the visor was also printed, totaling 40 g per face shield (Fig. 1B). The acetate display was dimensioned by laser cutting to ensure a precise fit. An elastic band made the apprehension of the display to the user's head. The approximate cost of the complete face shield, considering the weight of the printed structure, the cost of the acetate display, and the apprehension elastic, was 8 Brazilian Reals (about 2 American dollars) (Fig. 1C).
FIGURE 1.
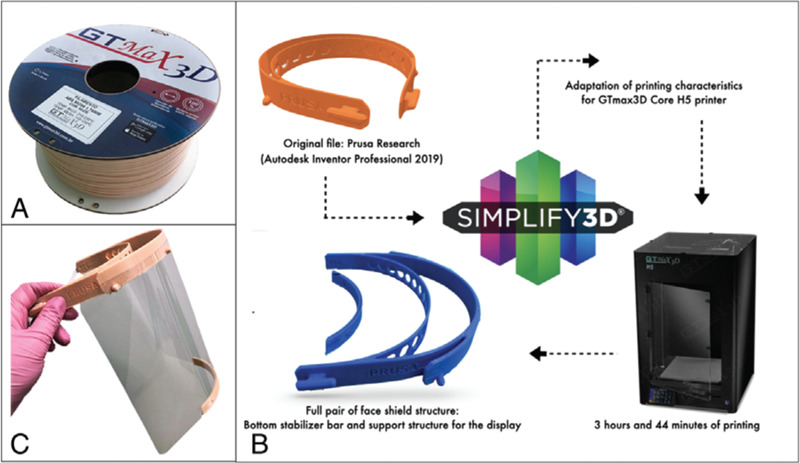
A, ABS Premium MG94 filament by Gtmax3D (Americana, São Paulo, Brazil). B, In-house printing flowchart. C, Full face shield: Printed structure, acetate display and elastic head band.
To disinfect the product, the professional should immerse the face shield in a container of 0.1% sodium hypochlorite or 68% to 71% ethanol for 1 minute. These measures significantly reduce the infectivity of SARS-CoV-2.11
DISCUSSION
COVID-19 is from the SARS-CoV family that affected the world in 2002 to 2003. Like its predecessor, COVID-19 has as its contamination routes the dispersion of aerosol through coughing, sneezing, contact with nasal, oral and ocular mucous membranes,11,12 which makes dental practice and of those who put their professionals and other patients at risk as to the possibility of contamination.12–14
Because of these contamination routes, the World Health Organization (WHO) has developed a guide on which individual protection inputs should be use and what situations. It includes the use of facial protectors in addition to dental use, such as intubations, non-invasive ventilation, tracheostomy, cardiopulmonary resuscitation, manual ventilation before intubation, bronchoscopy.15
The applications of 3D printing in the medical field can vary from simple anatomical pieces to the production of human tissues.16,17 Each different type of 3D printing with its respective materials carries advantages and disadvantages that determine its applicability.7,15,19 However, after the COVID-19 outbreak, there was a rapid shortage of PPE. The use of 3D technologies proved to be a possibility for help.18,19
The company PRUSA made three different models available for printing. The RC1 model allows more pieces printed on a single printing bed. However, if maximum quantity yield is not the priority, the RC2 offers better protection and is more comfortable to use, with a more exceptional wall thickness (slightly more rigid and more durable), with an internal wall of 1.5 mm at 2 mm, and external wall from 2 mm to 2.5 mm. Also, on the RC2 model, the headband is no longer compressed; the visor has been removed from the forehead, allowing a better fit over larger respirators and goggles. In the RC3 model, hexagonal holes were removed for faster print speeds.
The use of facial masks can be beneficial for dentists, doctors, nurses, and technicians. In this world scenario of pandemic and scarcity of protective inputs, the possibility of multicentric manufacturing can mean adjuvant supply, especially in countries less favored, directing the use of printers for this purpose, without this representing exorbitant extra costs.15,19 The main novelty of the proposed 3D printed workflow is directed to the use of the digital dentistry hardware towards a bigger picture, which might be only one of the many possibilities of modern dental practice.
Footnotes
The Foundation for Research Support of the State of Mato Grosso (FAPEMAT) in association with the Brazilian National Council for Scientific and Technological Development (CNPq) partially supported the present research through the grant FAPEMAT-SES/MT-Decit/SCTIE/MS-CNPq- 003/2017.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020; 109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correia IT, Ramos RF, Bathen LC. The surgeons and the COVID-19 pandemic. Rev Col Bras Cir 2020; 47. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood, safety. Transfus Med Rev 2020; 20:30014–30016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003; 348:1967–1976. [DOI] [PubMed] [Google Scholar]
- 5.Sayburn A. Covid-19: PHE upgrades PPE advice for all patient contacts with risk of infection. BMJ 2020; 369:m1391. [DOI] [PubMed] [Google Scholar]
- 6. Prusa Printers. From Design to Mass 3D printing of medical shields in three days. Available at: https://www.prusaprinters.org/prints/25857-prusa-face-shield. Accessed April 12, 2020. [Google Scholar]
- 7.Swenmen GR, Pottel L. Haers PE: Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg 2019; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seol YJ, Kang HW, Lee SJ, et al. Bioprinting technology and its applications. Eur J Cardiothorac Surg 2014; 46:342–348. [DOI] [PubMed] [Google Scholar]
- 9.Ishengoma FR, Mtaho Adam B. 3D printing: developing countries perspectives. Int J Comput Appl 2014; 104:11. [Google Scholar]
- 10.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng 2015; 9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020; 104:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khurshid Z, Asiri FY, al Wadaani H. Human saliva: non-invasive fluid for detecting novel coronavirus (2019-nCoV). Int J Environ Res Public Health 2020; 17:E2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng X, Xu X, Li Y, et al. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020; 12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H, Zhou Y, Liu X, et al. The impact of the COVID-19 epidemic on the utilization of emergency dental services. J Dent Sci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020; 12:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) Available at: https://www.who.int/publications-detail/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages. Accessed April 5, 2020. [Google Scholar]
- 17.Ventola C. Lee. Medical applications for 3D printing: current and projected uses. Pharm Therap 2014; 39:704. [PMC free article] [PubMed] [Google Scholar]
- 18.Schubert C, Van Langeveld MC, Donoso LA. Innovations in 3D printing: a 3D overview from optics to organs. Br J Ophthalmol 2014; 98:159–161. [DOI] [PubMed] [Google Scholar]
- 19.Newman M. Covid-19: doctors’ leaders warn that staff could quit and may die over lack of protective equipment. BMJ (Clinical research ed ) 2020; 368:1257. [DOI] [PubMed] [Google Scholar]


