Abstract
Ticks and tick-borne diseases are on the rise world-wide and vaccines to prevent transmission of tick-borne diseases is an urgent public health need. Tick transmission of pathogens to the mammalian host occurs during tick feeding. Therefore, it is reasoned that vaccine targeting of tick proteins essential for feeding would thwart tick feeding and consequently prevent pathogen transmission. The phenomenon of acquired tick-immunity, wherein, repeated tick infestations of non-natural hosts results in the development of host immune responses detrimental to the tick feeding has served as a robust paradigm in the pursuit of tick salivary antigens that may be vaccine targeted. While several salivary antigens have been identified, immunity elicited against these antigens have only provided modest tick rejection. This has raised the possibility that acquired tick-immunity is directed against tick components other than tick salivary antigens. Using Ixodes scapularis, the blacklegged tick, that vectors several human pathogens, we demonstrate that immunity directed against tick salivary glycoproteins is indeed sufficient to recapitulate the phenomenon of tick-resistance. These observations emphasize the utility of tick salivary glycoproteins as viable vaccine targets to thwart tick feeding and direct our search for anti-tick vaccine candidates.
Keywords: Ixodes scapularis, acquired tick-immunity, salivary proteins, salivary glycoproteins
Introduction
Ixodes scapularis, the blacklegged tick, vectors many human pathogens in North America including Borrelia burgdorferi sensu stricto, the agent of Lyme disease (Barbour and Fish, 1993). Incidences of diseases transmitted by Ixodes ticks are on the rise and vaccines to control the vast majority of the tick-borne human diseases are currently not available (Paules et al., 2018). While vaccines targeting specific pathogens is the traditional approach (Plotkin and Plotkin, 2011), it also requires the development of multiple tick-borne pathogen-specific vaccines. The growing molecular understanding of the critical role of tick saliva in facilitating tick feeding (Hovius et al., 2008; Kotal et al., 2015; Ribeiro and Francischetti, 2003) and in assisting pathogen transmission (Nuttall and Labuda, 2004; Simo et al., 2017) has directed a paradigm shift in our approach to vaccines against tick-transmitted diseases (Embers and Narasimhan, 2013). Hard ticks such as I. scapularis, remain attached to the host for several days in order to feed to repletion, a process essential for the tick to complete its life cycle (Anderson and Magnarelli, 2008). The pharmacologically active salivary components introduced into the feeding lesion modulate diverse host responses and are central for the tick to engorge successfully (Kazimirova and Stibraniova, 2013; Ribeiro et al., 2006; Wikel, 2013). Microbes transmitted by an infected tick are deposited into the bite-site along with tick saliva (Wikel, 2013). These microorganisms sometimes exploit components of tick saliva to enhance entry and survival in the host (Ramamoorthi et al., 2005; Schuijt et al., 2011a). Therefore, salivary proteins that facilitate tick feeding and pathogen transmission have emerged as attractive vaccine targets (Murfin and Fikrig, 2017) and offer a parsimonious approach to control multiple tick-transmitted diseases.
Over the last two decades, mining of the tick sialome by biochemical and by insilico methods has revealed that the tick salivary proteome is complex (Francischetti et al., 2009; Kim et al., 2016; Narasimhan et al., 2007; Perner et al., 2018) and is composed of a dynamic array of proteins whose expressions are modulated by physiological changes that occur in the tick during feeding, and by concomitant host immune responses. Hence, identifying critical tick salivary proteins that can serve as vaccine targets has posed a challenge (Mulenga et al., 2000b). Seminal observations made by William Trager 80 years ago showed that guinea pigs or rabbits repeatedly infested with Dermacentor variabilis, the dog tick, develop robust acquired immunity to D. variabilis resulting in rapid tick rejection (Trager, 1939). Interestingly, repeated infestations of mice, which are natural hosts of I. scapularis, does not provoke tick-resistance by mechanisms that remain to be deciphered (Anderson et al., 2017; Narasimhan et al., 2019). The phenomenon of acquired tick resistance has since been observed on other tick-non-natural host models (Brown et al., 1984; Wada et al., 2010) including in the I. scapularis-guinea pig model (Nazario et al., 1998). More importantly, acquired resistance to nymphal I. scapularis also resulted in decreased transmission of B. burgdorferi to the host (Narasimhan et al., 2007; Nazario et al., 1998). Acquired tick-resistance is characterized by recruitment of inflammatory cells, predominantly basophils, to the tick bite-site followed by degranulation of basophils that is suggested to be detrimental to tick feeding (Askenase, 1977; Brown and Askenase, 1981; Tabakawa et al., 2018). While a molecular and mechanistic understanding of the phenomenon of acquired tick-resistance is not available, initial studies invoke both cellular and humoral immune responses directed, presumably, at salivary antigens critical for tick feeding (Brossard and Wikel, 2004; Brown and Askenase, 1983). These insights have bolstered the assumption that critical salivary antigens may be targeted to block tick feeding and pathogen transmission, and has facilitated the identification of immuno-reactive salivary antigens (Das et al., 2001; Lewis et al., 2015; Radulovic et al., 2014; Schuijt et al., 2011b). However, immunity to these antigens or antigen subsets has only modestly recapitulated the tick-resistance phenotype. This raises the possibility that the robust tick-resistance phenotype observed upon repeated tick infestations is likely driven by multiple factors other than, or in addition to, tick salivary components. In this study we provide experimental proof that tick saliva is indeed sufficient to elicit a robust tick-resistance phenotype. We also assess the relative contributions of protein and non-protein components of tick saliva in eliciting the tick-resistance phenotype and provide a new thrust to tick salivary antigen-based tick vaccine efforts.
Materials and Methods
Ethics statement
Animal care and housing followed the rules described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA. The protocols described below for the use of mice and guinea pigs were reviewed and approved by the Yale University Institutional Animal Care and Use Committee (YUIACUC) and the approved Protocol number is 2018–07941. All animal experiments were conducted in a Biosafety Level 2 animal facility according to YUIACUC rules. All data generated in this work will be readily shared and available upon request.
Ticks and animals
I. scapularis adults, nymphs and larvae were obtained from a tick colony at the Oklahoma State University, Stillwater, OK, and maintained in an incubator at 23°C and 85% relative humidity under a 14-hour light, 10-hour dark photoperiod. 4–5-weeks old female Hartley guinea pigs (Charles River, MA) were used to feed nymphal ticks. Female New Zealand white rabbits (Charles River, MA) were used to feed female adult ticks essentially as described earlier (Schuijt et al., 2011b). Adult tick saliva was collected from engorged adult female ticks using Pilocarpine as described by Patton et al (2012). Approximately 10 μl saliva/adult tick was obtained and saliva from 40–50 fed adults was pooled, aliquoted and stored at −80°C prior to use.
Immunization of guinea pigs against tick saliva
4–5-weeks old female Hartley guinea pigs (Charles River, MA) were immunized subcutaneously with 20 μl ( ~ 4 μg of protein) of tick saliva in the absence of added adjuvant. The animals were boosted twice at 3-week intervals with 20 μl of tick saliva in the absence of adjuvant. Control animals were immunized with 4μg of Ovalbumin (Ova) and boosted twice at 3-week intervals in the absence of adjuvant. The animals were bled retro-orbitally 2 weeks after the last boost to obtain 500 μl of blood and the serum separated for use in ELISA experiments to assess saliva-specific antibody titers. At least 2 animals were used in each group and experiments repeated three times. Immunizations with saliva or Ova was also performed in incomplete Freund’s adjuvant following the same immunization regimen as described above to determine if the oil-water emulsion-based delivery of saliva would enhance immunity elicited by saliva.
To assess the dose-dependent impact of saliva immunization on tick-resistance, guinea pigs were immunized subcutaneously with 1 μl(~200 ng), or 0.1μl (~ 20 ng) 0r 0.01μl (~ 2 ng) of tick saliva without added adjuvant following the same regimen as described above using at least two animals /group.
Glycosidase, protease, phosphorylase or lipase treatment of tick saliva
To remove glycosylations, 20 μl of tick saliva was incubated with a cocktail of glycosidases that removes both N and O-glycosylations and provided in the EDGLY deglycosidase kit (Sigma, MO). Deglycosylation reaction was conducted under denaturing conditions as recommended by the manufacturer. For protease treatment, saliva was digested with 2.5 Units of proteinase K (Sigma-Alrich) in CutSmart buffer (NEB) and 1% SDS for 1 hour at 37°C. For dephosphorylation saliva was incubated in 1× CutSmart buffer, 5 μl calf intestine alkaline phosphatase and PBS at 37°C for one hour. For lipase treatment saliva was mixed with 5 μl porcine lipase A(1mg/ml)and 265 μl of PBS and incubated at 37°C for one hour. Enzymatically-treated saliva was frozen at −80 °C overnight, and thawed prior to immunization of guinea pigs using immunization regimens described above in the absence of adjuvant. Control animals were similarly immunized with untreated saliva in respective buffers.
Generation of recombinant salivary proteins (Salps)
RNA was isolated from salivary glands dissected I. scapularis from ticks fed to repletion and cDNA synthesized according to the manufacture’s protocol (iScript cDNA synthesis kit, Bio-RAD). Gene specific primers was used to amplify the mRNA region encoding the mature protein of each Salp listed in Suppl Tables 1 and 2. Purified amplicons were then cloned into pMT-Bip-V5-HisA vector and recombinant DNA sequenced at the Keck sequencing facility, Yale University, to validate the clones. Recombinant proteins of each Salp was generated using the Drosophila expression system as described earlier (Anguita et al., 2002) and according to the manufacturer’s protocol (Invitrogen, CA). To generate recombinant Salp14 and TSLPI in the mammalian expression system (henceforth referred to as Salp14-m and TSLPI-m), the respective amplicons encoding the mature proteins were subcloned into the pEZT-DLUX vector (Addgene,MA) and recombinant DNA sequenced at the Keck sequencing facility, Yale University, to validate the clones Expression. and protein purification of Salp14-m and TSLPI-m were performed using the Expi293 expression system (Thermo Scientific, MA). Protein purity was assessed by SDS-PAGE using 4–20% gradient precast gels (Biorad, CA) and quantified using the BCA protein estimation kit (Thermo Scientific, MA).
Immunization of guinea pigs against recombinant Salivary proteins (Salps)
4–5-week-old female Hartley guinea pigs (Charles River, MA) were immunized subcutaneously with two individual cocktails of recombinant Salp proteins (listed in Suppl Table 1, Cocktail 1 and Cocktail 2) in IFA. The animals were boosted twice at 3-week intervals. Control animals were immunized with Ovalbumin (Ova) and boosted twice at 3-week intervals in the absence of adjuvant. The animals were bled retro-orbitally 2 weeks after the last boost to obtain 500μl of blood and serum separated for use in ELISA experiments to assess rSalp-specific antibody titers.
ELISA assessment of saliva-specific or recombinant Salp-specific lpG levels
To assess saliva-specific humoral response 96-well ELISA plates were coated overnight with 500 ng of saliva prepared as described above and incubated with guinea pig anti-saliva sera collected 2-weeks post last immunization and prior to tick challenge at1:500 or 1:5000 dilution. Bound antibody was detected with HRP-conjugated goat anti-guinea pig IgG and TMB substrate solution (Thermo Scientific, IL). Guinea pig anti-Ova sera collected 2-weeks post last immunization and prior to tick challenge served as control sera. Salp-specific humoral response was similarly assessed using 500 ng of each of the recombinant Salps (Supplemental Table 1) to coat the 96-well ELISA plates and seroreactivity to guinea pig anti-saliva sera or guinea pig anti recombinant Salp cocktail sera.
Uninfected tick challenge of guinea pigs
Immunized or naïve guinea pigs were anesthetized by intramuscular injection of ketamine and xylazine mixture and then challenged with 30 nymphal I. scapularis ticks by placing ticks on their shaved backs. Ticks were allowed to attach prior to housing guinea pigs individually in wire-bottom cages with 3 layers of tick containment involving a pan of water below the wire-bottom, a hopper-inclusive lid, and Vaseline grease around the outer edges of the cage. Guinea pigs were monitored daily to monitor the numbers of tick feeding, erythema in skin and to collect any fallen ticks from the water pan and the numbers of ticks obtained was used to calculate percent recovery. Erythema at the tick bite-sites were assessed by two researchers blinded to the experimental groups and scored based on percentage of erythematous tick bite-sites as follows: redness at < 10 % of tick bite sites: 0.5; redness at 20–50 % of tick bite-sites: 1; redness at 50–80 % of tick bite sites: 2; redness at =/>80% of tick bite-sites: 3. Repleted ticks were individually weighed using a Sartorius balance to measure engorgement weights as a measure of feeding success.
Statistical analysis
In scoring for seroreactivity to saliva or specific salivary antigens, erythema, and rate of tick detachment, the significance of the difference between the mean values of control and experimental groups was analysed by 2-way ANOVA and Tukey’s multiple comparison using with Prism 7.0 software (GraphPad Software, CA). p ≤ 0.05 was considered statistically significant. To assess if percent recovery of ticks and engorgement weights were significantly different between control and experimental groups ordinary ANOVA or two-way ANOVA with Tukey’s or Holms-Sidak’s multiple comparison or Mann-Whitney test was done using Prism 7.0 software. p ≤ 0.05 was considered statistically significant.
Results
Immunization of guinea pigs against tick saliva provokes robust tick-resistance
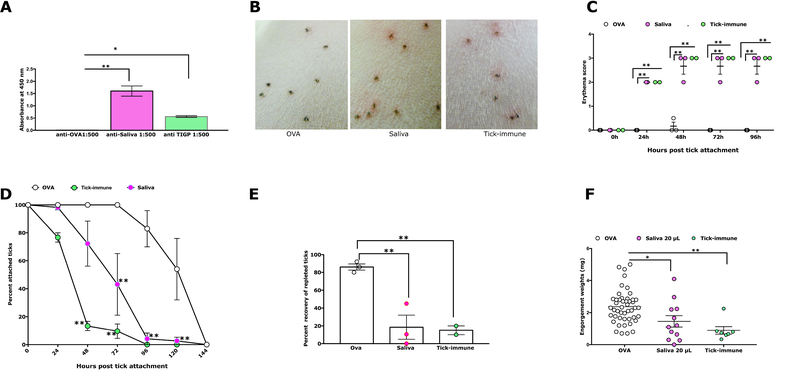
Guinea pigs were immunized with 20μl(~4 μg) of adult tick saliva in the absence of any adjuvant and control animals were immunized in parallel with Ovalbumin (Ova) as described in the Materials and Methods. After the last boost, blood was drawn from each animal and serum levels of antibody specific to tick saliva was confirmed by ELISA prior to challenge of each animal with ~ 30 I. scapularis nymphs (Fig. 1A). Within 24 h of tick attachment, we observed the hallmark redness at the tick bite sites (Fig. 1B) that significantly increased in intensity by 48 h as judged by visible erythema at all tick bite-sites (Fig. 1C) and was comparable to that seen on tick-resistant guinea pigs. Little or no redness was observed in control animals (Fig. 1B–C). Ticks also detached significantly more rapidly on saliva-immunized animals (Fig. 1D) when compared to that on Ova-immunized animals. Although, tick rejection on tick-resistant guinea pigs was significantly more rapid than that on saliva-immunized animals (Fig. 1D), the recovery of engorged ticks from saliva-immunized animals was comparable to that on tick-resistant animals and was significantly less than that obtained from control animals (Fig. 1E). The engorgement weights of the small number of ticks that fed to repletion on saliva-immunized animals was decreased compared to that on Ova-immunized animals (Fig. 1F).
Figure 1. Immunity elicited by tick saliva recapitulates tick-resistance phenotype on guinea pigs.
A. Sera fromguinea pigs immunized with 20 μl of adult saliva withnoadjuvant(Saliva)or Ovalbumin (OVA) or from tick-immune guinea pigs (TIGP) were assessed by ELISA for specific antibodies to tick saliva. About 30 clean I. scapularis nymphs were allowed to engorge on each of 3 Hartley female guinea pigs immunized with 20 μl of adult saliva (Saliva) or Ovalbumin (OVA) or on tick-resistant (Tick-immune) guinea pigs and the following parameters assessed: B. Visualization of redness at the tick bite sites 24 h post-tick attachment; C. Erythema over the course of feeding; D. Rate of tick detachment; E. Percent recovery of repleted ticks; and F. Engorgement weights of individual nymphs. Error bars in A and C through F represent means ± SEM. Significance of differences assessed in: C, and D by 2-way ANOVA with Tukey’s multiple comparison test; A, E and F by one-way ANOVA with Tukey’s multiple comparison test (*p< 0.05; **p<0.005).
To determine the minimum concentration of saliva that would provide tick resistance, we immunized guinea pigs with decreasing amounts of saliva as described in the Materials and Methods. We observed that immunization of guinea pigs with as low as 1 and 0.1 μL (~20 ng) of saliva elicited visible redness at tick bite-sites, and 0.01 μL did not provide any redness at the bite-site (Suppl Fig. 1B–C). Although, tick recovery was comparable to that observed in control animals (Suppl Fig. 1C), engorgement weights of ticks were significantly reduced on 1, 0.1 and 0.01 μL saliva-immunized compared to ticks that fed control animals. Tick resistance was however significantly reduced when animals were immunized with 0.01 μl (~ 2 ng) of tick saliva when compared to that on animals immunized with 1 μL saliva (Suppl Fig. 1).
While elicitation of tick-resistance phenotype was achieved without any adjuvant (Fig. 1), we examined if immunization in presence of Incomplete Freund’s (IFA) would enhance the phenotype. Although not a classic adjuvant, by virtue of the oil-water emulsion to form an antigen depot at the injection site and enhance the immune responses, we reasoned that IFA could boost immune responses to saliva. Animals were immunized with 10 μl (~2 μg) in presence of IFA and boosted twice as described in the Materials and Methods. After the last boost, antibodies specific to tick saliva on the serum was assessed by ELISA (Fig. 2A) and shown to be comparable to that observed in animals immunized with saliva alone (Fig. 2A). Further, upon tick challenge of animals immunized with saliva and IFA we observed the hallmark redness at tick bite-sites, tick rejection and tick recovery that was comparable to that observed in animals immunized with saliva alone (Fig. 2B–E). The engorgement weights of ticks that repleted on the immunized animals were also comparably decreased when compared to that on control animals immunized with Ova and IFA (Fig. 2E).
Figure 2. Tick saliva elicits protective immunity in the absence of adjuvant.
A. Sera from guinea pigs immunized with 10 μl of adult saliva with no adjuvant (Saliva) or with adjuvant (Saliva+IFA) or Ovalbumin (OVA) were assessed by ELISA for specific antibodies to tick saliva. About 30 clean I. scapularis nymphs were allowed to engorge on each of 2 Saliva, Saliva+IFA or OVA-immunized female guinea pigs and the following parameters assessed: B. Visualization of redness at the tick bite sites 24 h post-tick attachment; C. Erythema over the course of feeding; D. Rate of tick detachment; E. Percent recovery of repleted ticks; and F. Engorgement weights of individual nymphs. Error bars in A, and B through D represent means ± SEM. Significance of differences assessed in: A by one-way ANOVA with Holm-Sidak test; C, and D by 2-way ANOVA with Tukey’s multiple comparison test; E and F by one-way ANOVA with Tukey’s multiple comparison test. (*p< 0.05; **p<0.005).
Salivary proteins and glycosylations are critical for eliciting tick-resistance
In an effort to determine the components of saliva that play a critical role in eliciting tick-resistance we focused on the salivary proteins, and their post-translational modifications including glycosylations, phosphorylations and lipidations. Saliva 15 –20μl (~3–4 μg) was treated with protease to enzymatically digest proteins in saliva, with a cocktail of glycosidases to enzymatically deglycosylate salivary proteins, with lipases to remove lipid moieties, or with phosphatase to remove phosphorylations as described in the Materials and Methods. Treated or untreated saliva was used to immunize guinea pigs and 10 days after the final boost challenged with ticks as described in the Materials and Methods and the development of tick-resistance monitored. ELISA assessment of IgG antibodies specific to saliva showed that glycosidase or protease treatment significantly diminished the reactivity to saliva (Fig. 3A). Protease treatment significantly decreased the development of erythema at the tick bite-sites (Fig. 3B–C) and abolished the development of tick resistance as seen by tick detachment rate (Fig. 3D), percent recovery of ticks (Fig. 3E) and tick engorgement weights that were comparable to that on control animals (Fig. 3F). Although, tick bite-sites on glycosidase treated saliva-immunized animals showed the hallmark redness that was significantly greater than that on untreated saliva-immunized animals (Fig. 3C), deglycosylation significantly diminished the development of tick resistance as seen by a slower tick detachment rate and higher percent recovery of ticks compared to untreated saliva-immunized animals (Fig. 3D–E). Engorgement weights of ticks that repleted on untreated saliva were significantly decreased compared to ticks that repeleted on protease- or glycosidase-treated saliva-immunized animals (Fig. 3F).
Figure 3. Proteins and glycosylations are critical elicitors of tick-resistance.
A. Serafromguinea pigs immunized with 20 μl of adult saliva (Saliva) or Ovalbumin (OVA) or saliva treated with a cocktail of glycosidases (Saliva-deglycosylated) or saliva treated with proteinase K (Saliva-protease) were assessed by ELISA for specific antibodies to tick saliva. About 30 clean I. scapularis nymphs were allowed to engorge on each of 3 Hartley female guinea pigs immunized with Saliva or OVA or Saliva-deglycosylated or Saliva-protease and the following parameters assessed: B. Visualization of redness at the tick bite sites 24 h post-tick attachment; C. Erythema over the course of feeding; D. Rate of tick detachment; E. Percent recovery of repleted ticks; and F. Engorgement weights of individual nymphs. Error bars in A, and C through F represent means ± SEM. Significance of differences assessed in: C, and D by 2-way ANOVA with Tukey’s multiple comparison test; A and E by one-way ANOVA with Tukey’s multiple comparison test; F by one-way ANOVA with Dunn’s multiple comparison test. (*p< 0.05; **p<0.005).
ELISA assessment of IgG antibodies specific to saliva showed that lipase treatment, but not phosphatase treatment, significantly diminished the reactivity to saliva (Suppl Fig. 2A). Phosphatase-treated saliva-immunized animals showed all the parameters of tick-resistance including redness at the bite-sites (Suppl Fig. 2B–C), rapid tick detachment and decreased tick recovery and decreased engorgement weights (Suppl Fig. 2D–F) that was comparable to that on untreated saliva-immunized animals. Lipase treatment prevented the development of erythema at the tick bite-sites (Suppl Fig. 2B–C) but did not significantly impact the elicitation of other parameters of tick-resistance including tick detachment, tick recovery and engorgement weights (Suppl Fig. 2D–F).
Saliva-immunized animal sera elaborate robust humoral responses to Salp14 and TSLPI
Using various screening approaches we have earlier identified several salivary proteins (Salps) that avidly react with tick-resistant animal sera (Das et al., 2001). Since saliva-immunized animals were significantly protected from tick infestation, seroreactivity of these Salps to saliva-immunized guinea pig sera was assessed by ELISA and western blot using recombinant proteins of these Salps generated in the Drosophila expression system. Recombinant (r) Salp14 (Narasimhan et al., 2002) and rTSLPI (Schuijt et al., 2011a) showed strong reactivity to anti-saliva sera when compared to all other recombinant Salps (Fig. 4A). Therefore, we immunized guinea pigs with a cocktail of rSalp14 and TSLPI as described in the Materials and Methods and challenged the animals with I. scapularis nymphs to examine if immunity to rSalp14 and rTSLPI was sufficient to elicit tick-resistance. After the last boost, blood was drawn from each animal and presence of antibodies specific to rSalp14 and rTSLPI in the sera was confirmed by ELISA prior to challenge of each animal with ~ 30 I. scapularis nymphs (Fig. 4B). The tick bite-sites on rSalp14/TSLPI-immunized animals showed erythema by about 24 h of tick attachment and significantly increased by 48 h when compared to that on control animals (Fig. 4C–D). Tick attachment was reduced significantly by day 4 (Fig 4E), and tick recovery was diminished (Fig. 4F). The engorgement weights of the recovered ticks were comparable to that on Ova-immunized animals (Fig. 4G).
Fig 4. Salp14 and TSLPI are predominant immunogens in saliva.
A. Sera fromguineapigs immunized with 20 μl of adult saliva (Anti-saliva) or Ovalbumin (Anti-OVA) or saliva with IFA (Anti saliva+IFA) or Ovalbumin with IFA (OVA+IFA) were assessed by ELISA for specific antibodies to tick saliva or to individual recombinant secreted salivary protein antigens listed in Supplemental Table 1. B. Sera from each of 3 guinea pigs immunized with a cocktail of 20 μg each of recombinant Salp14 and TSLPI (Anti-Salp14/TSLPI) or Ovalbumin (Anti-OVA) were assessed by ELISA for specific antibodies to Salp14 or TSLPI. About 30 clean I. scapularis nymphs were allowed to engorge on each of 3 Hartley female guinea pigs immunized with Salp14/TSLPI or OVA and the following parameters assessed: C. Visualization of redness at the tick bite sites 24 h post-tick attachment; D. Erythema over the course of feeding; E. Rate of tick detachment; F. Percent recovery of repleted ticks; and G. Engorgement weights of individual nymphs. Error bars in A, B and D through G represent means ± SEM. Significance of differences assessed in: D, and E by 2-way ANOVA and Sidak’s multiple comparison test; F and G by Mann-Whitney non-parametric test. (*p< 0.05; **p<0.005).
Given that glycosylations on proteins played a significant role in tick rejection, we also examined if immunization of guinea pigs with Salp14 and TSLPI generated in a mammalian system (rSalp14-m and rTSLPI-m) would impact tick resistance. Guinea pigs were immunized with rSalp14-m/rTSLPI-m and challenged with nymphal ticks as described in the Materials and Methods. Presence of antibodies specific to rSalp14m and rTSLPIm in the sera was confirmed by ELISA prior to challenge of each animal with ~ 30 I. scapularis nymphs (Suppl Fig. 3A). In contrast to the results using rSalp14 and rTSLPI made in Drosophila expression system, no significant redness was observed at tick attachment sites in the first 3–4 days (Suppl Fig. 3B–C). However, consistent with the previous results, tick attachment was reduced by day 4 (Suppl Fig. 3D) when compared to Ova-immunized animals and the recovery of repleted ticks from rSalp14-m/rTSLPI-m was reduced compared to Ova-immunized animals (Suppl Fig. 3E). Engorgement weights of the recovered ticks were comparable in both groups (Suppl Fig. 3F).
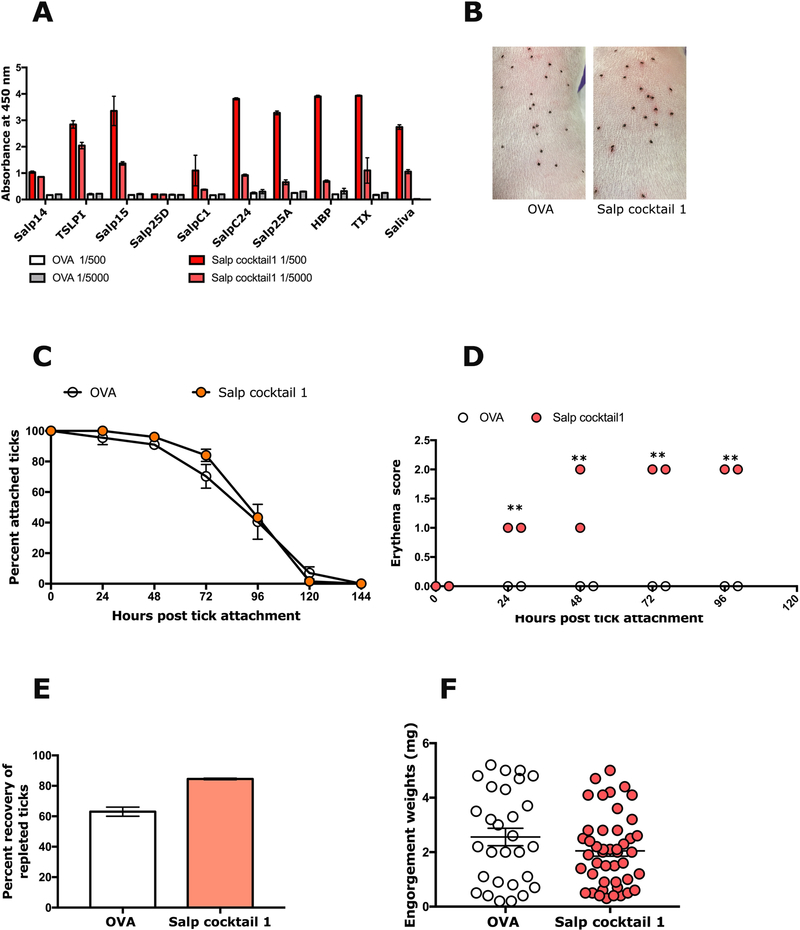
To determine if inflammation at the tick bite-sites on rSalp14/TSLPI immunized animals was unique to Salp14 and TSLPI or if it simply represented reactivity to the respective Salps in tick saliva, we also immunized animals with a cocktail of salivary antigens that did not show reactivity to anti-saliva sera (Supplemental Tables. 1–2). Indeed, only cocktails that contained rSalp14 and rTSLPI showed redness at the tick bite-sites (Fig. 5 and Supplemental Fig. 4) and none of the cocktails tested provided tick-resistance phenotype as seen by tick detachment rate, tick recovery and engorgement weights that were comparable to that on Ova-immunized animals (Fig. 5 and Supplemental Fig. 4).
Fig 5. Immunity elicited by a cocktail of recombinant salivary proteins including Salp14 and TSLPI elicits erythema at tick bite sites.
A. Sera from each of 2 guinea pigs immunized with a cocktail of 20 μg each of recombinant salivary proteins (Anti-Salp cocktail 1) or Ovalbumin (Anti-OVA) were assessed by ELISA for specific antibodies to each of the salivary proteins. About 30 clean I. scapularis nymphs were allowed to engorge on each of 2 Hartley female guinea pigs immunized with Salp cocktail 1 or OVA and the following parameters assessed: B. Visualization of redness at the tick bite sites 24 h post-tick attachment; D. Erythema over the course of feeding; E. Rate of tick detachment; F. Percent recovery of repleted ticks; and G. Engorgement weights of individual nymphs. Error bars represent means ± SEM. Significance of differences assessed in: C, and D by 2-way ANOVA with Sidak’s multiple comparison test; E and F by Mann-Whitney test. (*p< 0.05; **p<0.005).
Discussion
While several proteomic, transcriptomic and functional genomic strategies to develop anti-tick vaccines continue to emerge (Artigas-Jeronimo et al., 2018; Contreras et al., 2016; de la Fuente et al., 2010; Nuttall et al., 2006), Trager’s observations (Trager, 1939) that selected non-permissive hosts reject ticks upon multiple infestations remains a robust paradigm to define potential tick vaccine targets to control ticks and prevent tick-transmitted diseases. Over the last several decades, research aimed at understanding the molecular and mechanistic basis of acquired tick-resistance has revealed insights into various host immune components that drive this phenomenon (Tabakawa et al., 2018; Wada et al., 2010; Wikel, 1984; Wikel, 1996; Willadsen, 1980) and also invoked several salivary antigens that likely play a role in eliciting tick-resistance (Das et al., 2001; Mulenga et al., 2000a; Mulenga et al., 2000b; Schuijt et al., 2011b). However, the paramount goal of exploiting this phenomenon to develop anti-tick vaccines has not been achieved (Mulenga et al., 2000b; Willadsen, 2004). Salivary antigens invoked in acquired tick-resistance when tested in vaccine-challenge experiments provided partial protection from tick infestations and pathogen transmission (Brown and Askenase, 1986; Dai et al., 2009; Das et al., 2001; Mulenga et al., 2000a; Mulenga et al., 1999; Schuijt et al., 2011b). It was suggested that salivary antigens “exposed” to the host immune responses have likely evolved to counter the immune pressures of the mammalian host (Kotsyfakis et al., 2008; Nuttall et al., 2006) and dampened enthusiasm for the search for tick salivary antigen-based vaccine targets. Given the complexity of the functional genome of ticks (Gulia-Nuss et al., 2016; Pagel Van Zee et al., 2007; Ribeiro et al., 2006), it is likely that multiple factors need to be taken into consideration to fully harness the vaccine potential of tick salivary antigens. In this study, we utilized the guinea pig model of acquired tick-resistance (Allen, 1973) to examine whether immunity directed against I. scapularis tick saliva elicits robust tick-resistance and to determine salivary components that are critical for eliciting this phenotype.
I. scapularis ticks remain attached to the host for several days and feeding progresses in phases of slow to rapid as feeding culminates in repletion (Anderson and Magnarelli, 2008). It is now recognized that the tick salivary proteome is dynamic, shifting in composition during the different phases of feeding to counter the defense responses of the host and successfully feed to repletion (Kim et al., 2016; Narasimhan et al., 2007). While targeting salivary antigens expressed early in feeding is presumed critical to interrupt tick feeding early (Lewis et al., 2015; Narasimhan et al., 2007), it also suffers from the potential disadvantage of a short window of time for a robust anamnestic response to develop. We reasoned that salivary proteins secreted into the host throughout the process of feeding are likely to elicit a robust host response. Therefore, we utilized tick saliva collected from repleted adults that is expected to include secreted salivary antigens expressed throughout the course of tick feeding. Tick salivary and gut proteins have been reported to contain diverse post-translational modifications including glycosylations, phosphorylations and lipidations (de la Fuente et al., 2006; McKenna et al., 1998; Sauer et al., 1989; Sonenshine, 1991) and these modifications could provide an adjuvant effect. Given that repeated tick infestations deposit natural saliva into the host and elicit a robust immune response that rejects tick feeding, we examined whether immunization of animals with saliva without added adjuvant was sufficient to provoke host immune responses critical for tick rejection. Indeed, when animals immunized with tick saliva were challenged with I. scapularis nymphs we observed the hallmarks of acquired tick-resistance (Fig. 1) including significant erythema at the tick bite-sites, impaired tick feeding, and diminished tick recovery when compared to control animals. Animals immunized with as low as 20 ng of tick saliva provided partial tick-resistance phenotype as seen by erythema at the bite site, but not tick rejection (Suppl Fig. 1), attesting to the potency of tick saliva. The phenotype was not significantly enhanced when animals were immunized with saliva in presence of adjuvant such as IFA (Fig. 2). Histologic examination of the tick bite-sites on saliva-immunized animals demonstrated increased inflammation characterized predominantly by neutrophils and mononuclear cells and scattered basophils and mast cells. These observations were consistent with that observed by Anderson et al (2017) on repeatedly tick-infested guinea pigs. OVA-immunized animals did not show significant inflammation at the tick bite-site.
To determine the role of different components of tick saliva in eliciting tick-resistance we enzymatically depleted the saliva of proteins, glycan moieties, phosophorylations or lipid moieties and immunized animals with specific enzyme-treated saliva. The abrogation of the tick resistance phenotype upon depletion of proteins and glycosylations, but not phosphorylations or lipidations suggested that proteins and glycosylations are critical players in eliciting the tick-resistance phenotype (Fig. 3, and Suppl Fig. 3). These findings, especially the role of glycosylations, emphasize earlier observations that recombinant salivary antigens generated in eukaryotic expression systems were more effective antigens than those made in bacterial expression systems (de la Fuente et al., 2006). There is currently no robust tick cell-line-based protein expression system and most studies utilize insect expression systems such as Drosophila (Anguita et al., 2002) or yeast expression systems such as Pichia pastoris (Kumar et al., 2016). Characterization of tick glycosylation patterns and development of tick-expression systems would help refine tick vaccine antigen and adjuvant development.
Both humoral and cellular immunity is invoked in the elicitation of acquired tick-resistance (Wikel and Allen, 1976; Willadsen, 1980) and transfer of serum from tick-resistant guinea pigs to naïve guinea pigs was shown to confer partial yet significant tick-resistance phenotype (Askenase et al., 1982; Brossard and Girardin, 1979; Brown, 1982). Degranulation of basophils at the tick bite-site, a critical prelude to tick rejection (Brown and Askenase, 1985) is initiated when specific salivary antigens engage with antigen-specific IgG bound to cognate receptors on basophils (Brown and Askenase, 1983), emphasizing the role of humoral immunity in acquired tick-resistance. Our earlier studies aimed at defining tick salivary antigens that react with tick-resistant animal sera had identified several antigens (Das et al., 2001; Schuijt et al., 2011b). Of these antigens, we observed that Salp14, a putative anticoagulant, and TSLPI, an inhibitor of the lectin pathway of the complement system, reacted avidly with anti-saliva sera from saliva-immunized guinea pigs (Fig. 4). The observation that saliva immunization with IFA increased sero-reactivity to several other antigens in addition to Salp14 and TSLPI (Fig. 4A), but did not enhance the tick-resistance phenotype (Fig. 2) suggests that Salp14 and TSLPI are likely among the critical elicitors of tick-resistance.
Salp14 and TSLPI are glycosylated proteins and share 93% identity in the N-terminal region (Narasimhan et al., 2002) and belong to a family of structurally related proteins (Valenzuela et al., 2002). Guinea pigs immunized with a cocktail of recombinant Salp14 and TSLPI (rSalp14/rTSLPI) generated in the Drosophila expression system and challenged with I. scapularis nymphs provided significant erythema at the tick bite-sites, a notable hall mark of tick-resistance, about 24 h post tick attachment (Fig. 4). Despite the significant erythema at the tick bite-sites reminiscent of acquired tick-resistance, immunity against rSalp14-rTSLPI provided modest tick-rejection only around 72–96 hours post tick attachment, and showed a trend towards decreased tick repletion. It is likely that antigens in addition to Salp14 and TSLPI might be required to achieve a more robust tick-resistance phenotype.
Interestingly, when we immunized guinea pigs with rSalp14-m/rTSLPI-m generated in a mammalian expression system, we did not observe erythema at the bite site (Suppl Fig. 3), although tick rejection was comparable to that seen on animals immunized with rSalp14/rTSLPI generated in the Drosophila expression system. It is likely that glycosylations on recombinant proteins generated using the mammalian expression system might be less immunogenic in the mammalian host. It is important to note that insect-cell-generated glycosylations by themselves are not contributing to the erythema and that it is a combination of the antigen-glycan epitope. When guinea pigs immunized with cocktails of different subsets of recombinant salivary antigens generated in the Drosophila expression system were challenged with ticks, only cocktails that included rSalp14/rTSLPI provided erythema at the tick bite-site (Fig. 5 and Supplemental Figs. 4).
While immunization against rSalp14/rTSLPI did not provide optimal tick-rejection, it did provide significant erythema at the tick bite-site. Erythema at the tick bite-site is a result of the congregation of immune cells at the bite site that are thought to initiate responses detrimental to tick feeding, including release of histamines from platelets, mast cells and basophils (Wikel, 1996). This would potentially initiate itching of the skin, alert the host to the presence of the tick and result in removal of the tick. We reasoned that a vaccine formulation that would alert the host of tick presence would result in rapid tick detection and tick removal that could potentially interrupt tick-transmission of pathogens. We recognize that ticks often attach on parts of the body that are not readily visible, but itching and accompanying redness would promote a more rapid surveillance for tick attachment and removal.
We must bear in mind that tick-resistance phenotype observed upon multiple tick infestations was more effective at rejecting ticks (Fig. 1) compared to that observed on saliva immunized animals. Natural tick infestations might boost the host immune responses additionally by components including the cement cone, and mouth parts directly or indirectly and accelerate tick rejection earlier. Therefore, it is likely that saliva immunizations using higher doses of saliva and using adjuvants might provide more potent tick rejection. Further, saliva obtained from adult ticks is likely not fully reflective of nymphal saliva and could also account, in part, for the differences in the tick-resistance phenotype between saliva-immunized and tick-immune animals.
The demonstration that immunity against tick saliva is sufficient to elicit the hall marks of acquired tick resistance narrows the search to salivary proteins represented in tick saliva and advances in proteomic strategies (Villar et al., 2017) make this a tractable proposition. Our observation, overall, indicate a correlation between humoral responses to specific salivary components, as measured by total IgG, and the elicitation of tick rejection. Erythema, a hall mark of tick resistance, appears to be less critical for tick rejection. It is also evident that the immunogenicity of saliva must be assessed in conjunction with adjuvants to further improve the efficiency of tick-rejection. These observations renew our focus on tick saliva and demonstrate that salivary antigens are key players in eliciting tick resistance, and expand our understanding of the biochemical coordinates on the salivary antigens to enable a viable vaccine design and development.
CONCLUSIONS
Trager’s observations (Trager, 1939) that non-permissive hosts reject ticks upon multiple tick infestations remains a robust paradigm to define potential tick vaccine targets to control ticks and prevent tick-transmitted diseases. In this report we provide evidence that immunity elicited by tick saliva in the absence of added adjuvant is sufficient to recapitulate the parameters of tick-resistance including erythema at the tick bite-sites and tick rejection. We also demonstrate that protein components of tick saliva in conjunction with glycan moieties on these proteins are key elicitors of tick-resistance. These observations redirect our focus on tick salivary proteins as potential anti-tick vaccine targets and emphasize the need to select appropriate recombinant protein expression systems to achieve optimal vaccine formulations.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank Dr. Michael Tiemeyer, University of Georgia, for his input on saliva deglycosylation experiments. This work was conducted by funding support from the Steven and Alexandra Cohen Foundation to EF. This work was also supported in part by R21 AI128182 award from NIAID/NIH to SN, and by the John Monsky and Jennifer Weiss Monsky Lyme Disease Research Fund to EF. CIC bioGUNE thanks the Spanish Ministry of Economy and Competitiveness for the Severo Ochoa Center of Excellence Award (SEV2016-0644). EF is an HHMI investigator.
Literature Cited
- Allen JR 1973. Tick resistance: basophils in skin reactions of resistant guinea pigs. International journal for parasitology 3:195–200. [DOI] [PubMed] [Google Scholar]
- Anderson JF, and Magnarelli LA. 2008. Biology of ticks. Infectious disease clinics of North America 22:195–215,v. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Moore IN, Nagata BM, Ribeiro JMC, Valenzuela JG, and Sonenshine DE. 2017. Ticks, Ixodes scapularis, Feed Repeatedly on White-Footed Mice despite Strong Inflammatory Response: An Expanding Paradigm for Understanding Tick-Host Interactions. Frontiers in immunology 8:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, and Fikrig E. 2002. Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity 16:849–859. [DOI] [PubMed] [Google Scholar]
- Artigas-Jeronimo S, De La Fuente J, and Villar M. 2018. Interactomics and tick vaccine development: new directions for the control of tick-borne diseases. Expert Rev Proteomics 15:627–635. [DOI] [PubMed] [Google Scholar]
- Askenase PW 1977. Role of basophils, mast cells, and vasoamines in hypersensitivity reactions with a delayed time course. Prog Allergy 23:199–320. [PubMed] [Google Scholar]
- Askenase PW, Bagnall BG, and Worms MJ. 1982. Cutaneous basophil-associated resistance to ectoparasites (ticks). I. Transfer with immune serum or immune cells. Immunology 45:501–511. [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, and Fish D. 1993. The biological and social phenomenon of Lyme disease. Science 260:1610–1616. [DOI] [PubMed] [Google Scholar]
- Brossard M, and Girardin P. 1979. Passive transfer of resistance in rabbits infested with adult Ixodes ricinus L: humoral factors influence feeding and egg laying. Experientia 35:1395–1397. [DOI] [PubMed] [Google Scholar]
- Brossard M, and Wikel SK. 2004. Tick immunobiology. Parasitology 129 Suppl:S161–176. [DOI] [PubMed] [Google Scholar]
- Brown SJ 1982. Antibody- and cell-mediated immune resistance by guinea pigs to adult Amblyomma americanum ticks. The American journal of tropical medicine and hygiene 31:1285–1290. [DOI] [PubMed] [Google Scholar]
- Brown SJ, and Askenase PW. 1981. Cutaneous basophil responses and immune resistance of guinea pigs to ticks: passive transfer with peritoneal exudate cells or serum. Journal of immunology 127:2163–2167. [PubMed] [Google Scholar]
- Brown SJ, and Askenase PW. 1983. Immune rejection of ectoparasites (ticks) by T cell and IgG1 antibody recruitment of basophils and eosinophils. Fed Proc 42:1744–1749. [PubMed] [Google Scholar]
- Brown SJ, and Askenase PW. 1985. Rejection of ticks from guinea pigs by anti-hapten-antibody-mediated degranulation of basophils at cutaneous basophil hypersensitivity sites: role of mediators other than histamine. Journal of immunology 134:1160–1165. [PubMed] [Google Scholar]
- Brown SJ, and Askenase PW. 1986. Amblyomma americanum: physiochemical isolation of a protein derived from the tick salivary gland that is capable of inducing immune resistance in guinea pigs. Experimental parasitology 62:40–50. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Barker RW, and Askenase PW. 1984. Bovine resistance to Amblyomma americanum ticks: an acquired immune response characterized by cutaneous basophil infiltrates. Veterinary parasitology 16:147–165. [DOI] [PubMed] [Google Scholar]
- Contreras M, Villar M, Alberdi P, and de la Fuente J. 2016. Vaccinomics Approach to Tick Vaccine Development. Methods in molecular biology 1404:275–286. [DOI] [PubMed] [Google Scholar]
- Dai J, Wang P, Adusumilli S, Booth CJ, Narasimhan S, Anguita J, and Fikrig E. 2009. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell host & microbe 6:482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, and Fikrig E. 2001. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. The Journal of infectious diseases 184:1056–1064. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Canales M, and Kocan KM. 2006. The importance of protein glycosylation in development of novel tick vaccine strategies. Parasite immunology 28:687–688. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Manzano-Roman R, Naranjo V, Kocan KM, Zivkovic Z, Blouin EF, Canales M, Almazan C, Galindo RC, Step DL, and Villar M. 2010. Identification of protective antigens by RNA interference for control of the lone star tick, Amblyomma americanum. Vaccine 28:1786–1795. [DOI] [PubMed] [Google Scholar]
- Embers ME, and Narasimhan S. 2013. Vaccination against Lyme disease: past, present, and future. Frontiers in cellular and infection microbiology 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, and Ribeiro JM. 2009. The role of saliva in tick feeding. Frontiers in bioscience: a journal and virtual library 14:2051–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M, Nuss AB, Meyer JM, Sonenshine DE, Roe RM, Waterhouse RM, Sattelle DB, de la Fuente J, Ribeiro JM, Megy K, Thimmapuram J, Miller JR, Walenz BP, Koren S, Hostetler JB, Thiagarajan M, Joardar VS, Hannick LI, Bidwell S, Hammond MP, Young S, Zeng Q, Abrudan JL, Almeida FC, Ayllon N, Bhide K, Bissinger BW, Bonzon-Kulichenko E, Buckingham SD, Caffrey DR, Caimano MJ, Croset V, Driscoll T, Gilbert D, Gillespie JJ, Giraldo-Calderon GI, Grabowski JM, Jiang D, Khalil SM, Kim D, Kocan KM, Koci J, Kuhn RJ, Kurtti TJ, Lees K, Lang EG, Kennedy RC, Kwon H, Perera R, Qi Y, Radolf JD, Sakamoto JM, Sanchez-Gracia A, Severo MS, Silverman N, Simo L, Tojo M, Tornador C, Van Zee JP, Vazquez J, Vieira FG, Villar M, Wespiser AR, Yang Y, Zhu J, Arensburger P, Pietrantonio PV, Barker SC, Shao R, Zdobnov EM, Hauser F, Grimmelikhuijzen CJ, Park Y, Rozas J, Benton R, Pedra JH, Nelson DR, Unger MF, Tubio JM, Tu Z, Robertson HM, Shumway M, Sutton G, Wortman JR, Lawson D, Wikel SK, Nene VM, Fraser CM, Collins FH, Birren B, Nelson KE, Caler E, and Hill CA. 2016. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nature communications 7:10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius JW, Levi M, and Fikrig E. 2008. Salivating for knowledge: potential pharmacological agents in tick saliva. PLoS Med 5:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazimirova M, and Stibraniova I. 2013. Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Frontiers in cellular and infection microbiology 3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Tirloni L, Pinto AF, Moresco J, Yates JR, 3rd, da Silva Vaz I, and Mulenga A. 2016. Ixodes scapularis Tick Saliva Proteins Sequentially Secreted Every 24 h during Blood Feeding. PLoS neglected tropical diseases 10:e0004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotal J, Langhansova H, Lieskovska J, Andersen JF, Francischetti IM, Chavakis T, Kopecky J, Pedra JH, Kotsyfakis M, and Chmelar J. 2015. Modulation of host immunity by tick saliva. Journal of proteomics 128:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsyfakis M, Anderson JM, Andersen JF, Calvo E, Francischetti IM, Mather TN, Valenzuela JG, and Ribeiro JM. 2008. Cutting edge: Immunity against a "silent" salivary antigen of the Lyme vector Ixodes scapularis impairs its ability to feed. Journal of immunology 181:5209–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, P A., and Ghosh S. 2016. Laboratory Scale Production of Recombinant Haa86 Tick Protein in Pichia pastoris and in Escherichia coli System. Methods in molecular biology 1404:459–482. [DOI] [PubMed] [Google Scholar]
- Lewis LA, Radulovic ZM, Kim TK, Porter LM, and Mulenga A. 2015. Identification of 24h Ixodes scapularis immunogenic tick saliva proteins. Ticks and tick-borne diseases 6:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna RV, Riding GA, Jarmey JM, Pearson RD, and Willadsen P. 1998. Vaccination of cattle against the Boophilus microplus using a mucin-like membrane glycoprotein. Parasite immunology 20:325–336. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Sugimoto C, Ohashi K, and Onuma M. 2000a. Characterization of an 84 kDa protein inducing an immediate hypersensitivity reaction in rabbits sensitized to Haemaphysalis longicornis ticks. Biochimica et biophysica acta 1501:219–226. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Sugimoto C, and Onuma M. 2000b. Issues in tick vaccine development: identification and characterization of potential candidate vaccine antigens. Microbes and infection / Institut Pasteur 2:1353–1361. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Sugimoto C, Sako Y, Ohashi K, Musoke A, Shubash M, and Onuma M. 1999. Molecular characterization of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infection and immunity 67:1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murfin KE, and Fikrig E. 2017. Tick Bioactive Molecules as Novel Therapeutics: Beyond Vaccine Targets. Frontiers in cellular and infection microbiology 7:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Booth CJ, DePonte K, Wu MJ, Liang X, Mohanty S, Kantor F, and Fikrig E. 2019. Host-specific expression of Ixodes scapularis salivary genes. Ticks and tick-borne diseases 10:386–397. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Deponte K, Marcantonio N, Liang X, Royce TE, Nelson KF, Booth CJ, Koski B, Anderson JF, Kantor F, and Fikrig E. 2007. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PloS one 2:e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Koski RA, Beaulieu B, Anderson JF, Ramamoorthi N, Kantor F, Cappello M, and Fikrig E. 2002. A novel family of anticoagulants from the saliva of Ixodes scapularis. Insect molecular biology 11:641–650. [DOI] [PubMed] [Google Scholar]
- Nazario S, Das S, de Silva AM, Deponte K, Marcantonio N, Anderson JF, Fish D, Fikrig E, and Kantor FS. 1998. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. The American journal of tropical medicine and hygiene 58:780–785. [DOI] [PubMed] [Google Scholar]
- Nuttall PA, and Labuda M. 2004. Tick-host interactions: saliva-activated transmission. Parasitology 129 Suppl:S177–189. [DOI] [PubMed] [Google Scholar]
- Nuttall PA, Trimnell AR, Kazimirova M, and Labuda M. 2006. Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite immunology 28:155–163. [DOI] [PubMed] [Google Scholar]
- Pagel Van Zee J, Geraci NS, Guerrero FD, Wikel SK, Stuart JJ, Nene VM, and Hill CA. 2007. Tick genomics: the Ixodes genome project and beyond. International journal for parasitology 37:1297–1305. [DOI] [PubMed] [Google Scholar]
- Patton TG, Dietrich G, Brandt K, Dolan MC, Piesman J, and Gilmore RD 2012. Saliva, salivary gland, and hemolymph collection from Ixodes scapularis ticks. Journal of visualized experiments: JoVE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules CI, Marston HD, Bloom ME, and Fauci AS. 2018. Tickborne Diseases - Confronting a Growing Threat. The New England journal of medicine 379:701–703. [DOI] [PubMed] [Google Scholar]
- Perner J, Kropackova S, Kopacek P, and Ribeiro JMC. 2018. Sialome diversity of ticks revealed by RNAseq of single tick salivary glands. PLoS neglected tropical diseases 12:e0006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA, and Plotkin SL. 2011. The development of vaccines: how the past led to the future. Nature reviews. Microbiology 9:889–893. [DOI] [PubMed] [Google Scholar]
- Radulovic ZM, Kim TK, Porter LM, Sze SH, Lewis L, and Mulenga A. 2014. A 24–48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC genomics 15:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, and Fikrig E. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436:573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, and Wikel SK. 2006. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect biochemistry and molecular biology 36:111–129. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, and Francischetti IM. 2003. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annual review of entomology 48:73–88. [DOI] [PubMed] [Google Scholar]
- Sauer JR, McSwain JL, Tucker JS, Shelby KS, Williams JP, and Essenberg RC. 1989. Protein phosphorylation and control of tick salivary gland function. Experimental & applied acarology 7:81–94. [DOI] [PubMed] [Google Scholar]
- Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, Brouwer M, Oei A, Roelofs JJ, van Dam AP, van der Poll T, Vanť Veer C, Hovius JW, and Fikrig E. 2011a. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell host & microbe 10:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijt TJ, Narasimhan S, Daffre S, DePonte K, Hovius JW, Vanť Veer C, van der Poll T, Bakhtiari K, Meijers JC, Boder ET, van Dam AP, and Fikrig E. 2011b. Identification and characterization of Ixodes scapularis antigens that elicit tick immunity using yeast surface display. PloS one 6:e15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo L, Kazimirova M, Richardson J, and Bonnet SI. 2017. The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Frontiers in cellular and infection microbiology 7:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE 1991. Biology of ticks Oxford University Press, New York. v. pp. [Google Scholar]
- Tabakawa Y, Ohta T, Yoshikawa S, Robinson EJ, Yamaji K, Ishiwata K, Kawano Y, Miyake K, Yamanishi Y, Ohtsu H, Adachi T, Watanabe N, Kanuka H, and Karasuyama H. 2018. Histamine Released From Skin-Infiltrating Basophils but Not Mast Cells Is Crucial for Acquired Tick Resistance in Mice. Frontiers in immunology 9:1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W 1939. Accquired immunity to ticks. J. Parasitology 25:57–81. [Google Scholar]
- Valenzuela JG, Francischetti IM, Pham VM, Garfield MK, Mather TN, and Ribeiro JM. 2002. Exploring the sialome of the tick Ixodes scapularis. J Exp Biol 205:2843–2864. [DOI] [PubMed] [Google Scholar]
- Villar M, Marina A, and de la Fuente J. 2017. Applying proteomics to tick vaccine development: where are we? Expert Rev Proteomics 14:211–221. [DOI] [PubMed] [Google Scholar]
- Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, Kawano Y, Minegishi Y, Yokozeki H, Watanabe N, and Karasuyama H. 2010. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. The Journal of clinical investigation 120:2867–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel S 2013. Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Frontiers in microbiology 4:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK 1984. Immunomodulation of host responses to ectoparasite infestation--an overview. Veterinary parasitology 14:321–339. [DOI] [PubMed] [Google Scholar]
- Wikel SK 1996. Host immunity to ticks. Annual review of entomology 41:1–22. [DOI] [PubMed] [Google Scholar]
- Wikel SK, and Allen JR. 1976. Acquired resistance to ticks. I. Passive transfer of resistance. Immunology 30:311–316. [PMC free article] [PubMed] [Google Scholar]
- Willadsen P 1980. Immunity to ticks. Adv Parasitol 18:293–311. [DOI] [PubMed] [Google Scholar]
- Willadsen P. Anti-tick vaccines. Parasitology. 2004;129(Suppl:S367–387.) doi: 10.1017/s0031182003004657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.