Abstract
Aim
Survival outcomes in elderly patients with pathological stages (pStages) II and III gastric cancer remain inadequately elucidated. We retrospectively analyzed outcomes of elderly and nonelderly patients who underwent curative gastrectomy for this cancer and considered clinical results of the Estimation of Physiologic Ability and Surgical Stress (E‐PASS) scoring system for prediction.
Methods
Among 1041 patients who underwent gastrectomy for pStages II and III gastric cancer between 2008 and 2013 consecutively, 898 patients were enrolled. Of these, 158 patients (17.6%) were elderly and 740 patients (82.4%) were nonelderly.
Results
Disease‐specific survival (DSS) in the elderly group with pStage III cancer was significantly worse than that in the same stage nonelderly group (P = .001), while there was no difference in DSS for pStage II cancer between the groups (P = .45). Overall survival (OS) was significantly worse in elderly patients for both pStages II and III. Elderly patients with pStage II cancer had larger survival gaps between OS and DSS compared with those with pStage III cancer. OS for elderly patients with comprehensive risk score (CRS) > 0.159 was significantly worse than that for elderly patients with CRS ≤ 0.159 in pStage II cancer.
Conclusions
Compared with nonelderly patients, different characteristics were observed in the survival outcomes of elderly patients between pStages II and III gastric cancer. The survival gap between OS and DSS of elderly patients was larger in pStage II cancer than in pStage III cancer. The E‐PASS scoring system could be a relatively useful predictor in elderly patients.
Keywords: elderly, stage II/III gastric cancer, predictor, survival
Compared with nonelderly patients, different characteristics were observed in the survival outcomes of elderly patients between pStages II and III gastric cancer. The survival gap between OS and DSS of elderly patients was larger in pStage II cancer than in pStage III cancer. The E‐PASS scoring system could be a relatively useful predictor in elderly patients.

1. INTRODUCTION
The proportion of elderly individuals among the general population has been exponentially increasing in Japan; the country now has the highest aged population worldwide. 1 In 2017, the life expectancy of 75 years in Japan increased by an additional 12.18 years for males and 15.79 years for females. 2 In turn, the proportion of elderly patients with gastric cancer undergoing surgery also has been rising, not only due to population aging but also due to high infection rates of Helicobacter pylori among elderly patients. 3 The proportion of these patients will further increase, making it one of the most serious problems in Japan.
Gastric cancer is the fifth most common cancer diagnosed worldwide and the third leading cause of cancer‐related death, affecting approximately 1 million new individuals each year and causing at least 700 000 deaths. 4 Although early‐stage gastric cancer is typically curable, locally advanced disease is more likely to relapse as the cancer stage advances. 5 Multimodal treatment combined with radical surgery and perioperative chemotherapy is a developing approach for further improving survival outcomes. However, prospective trials have included only limited numbers of elderly patients 6 , 7 or even excluded them entirely, 8 as they often have unique comorbidities, age‐related physiological problems, and higher risks of death from other diseases. Thus, survival outcomes in elderly patients with pathological stages (pStages) II and III gastric cancer remain to be better elucidated on a large scale. Few reports focusing on the survival outcomes of elderly patients with gastric cancer, particularly focusing on each stage, have been published. 9 , 10
As mentioned above, elderly patients often have age‐related perioperative risks. Accordingly, higher rates of postoperative morbidity and mortality have been noted in this population. 9 , 11 Therefore, surgeons should carefully consider when to perform radical surgery in elderly patients with gastric cancer. They often require a prediction system of the clinical outcomes after surgery. Some scoring systems have been reported as useful predictors of survival in patients with gastric cancer 12 , 13 , 14 , 15 ; the Estimation of Physiologic Ability and Surgical Stress (E‐PASS) scoring system is one example. 12 , 16 , 17 E‐PASS includes both a preoperative risk score (PRS) and a surgical stress score (SSS), which together may reflect a patient's condition more precisely. However, there have been no reports regarding the clinical results of the E‐PASS scoring system according to each gastric cancer stage in a large case series.
Here, we conducted a retrospective analysis of the survival outcomes of elderly patients who underwent curative gastrectomy for pStages II and III gastric cancer in comparison with those of nonelderly patients and evaluated the clinical results of the E‐PASS scoring system among elderly patients. This information is important, especially for aging countries, and will provide support better comprehension of the appropriateness of surgery in elderly gastric cancer patients.
2. MATERIALS AND METHODS
2.1. Patients
We reviewed the clinical records of patients with pStages II and III gastric cancer who underwent gastrectomy at the Cancer Institute Hospital in Tokyo, Japan, between 2008 and 2013 (Figure 1). We excluded patients who received preoperative chemotherapy or underwent R1/R2 resection and those with gastric cancer in remnant stomach or with special histology types. We classified patients aged 75 years or older as elderly and those younger than 75 years as nonelderly.
FIGURE 1.
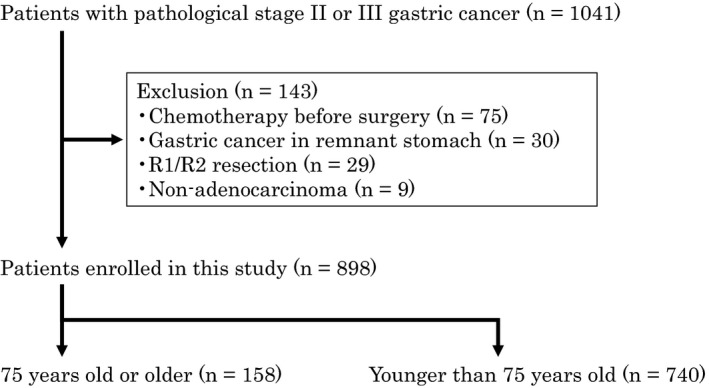
Patient selection
2.2. Surgical procedure and adjuvant chemotherapy
We enrolled patients with any extent of lymphadenectomy if they underwent radical surgery (R0 resection). According to the Japanese Gastric Cancer Treatment Guidelines, 18 pStages II and III gastric cancer excluding pT1N2‐3 or pT3N0 were indicated for adjuvant chemotherapy. Attending physicians determined on an individual basis whether to administer adjuvant chemotherapy, especially in elderly patients.
2.3. Survival
Overall survival (OS) and disease‐specific survival (DSS) were evaluated to determine survival outcomes. OS was defined as the time from radical surgery until death from any cause, whereas DSS was defined as the time from surgery until death due to a recurrence of gastric cancer. We compared the OS and DSS between elderly patients and nonelderly patients according to cancer stage.
Furthermore, we evaluated whether the E‐PASS scoring system could be a predictor of survival in elderly gastric cancer patients. 16 E‐PASS scores were calculated based on PRS (including age, severe heart disease, severe pulmonary disease, diabetes mellitus, performance status index, and American Society of Anesthesiologists physiological status classification), SSS (including the ratio of blood loss to body weight, operation time, and extent of skin incision), and comprehensive risk score (CRS) determined by both PRS and SSS (Table 1). We divided elderly patients into those with CRS > 0.159 and with CRS ≤ 0.159 and compared the findings between the two groups.
TABLE 1.
Equations for Estimation of Physiologic Ability and Surgical Stress (E‐PASS) scores: preoperative risk score (PRS), surgical stress score (SSS), and comprehensive risk score (CRS)
| 1. PRS = −0.0686 + 0.00345X1 + 0.323X2 + 0.205X3 + 0.153X4 + 0.148X5 + 0.0666X6 |
| X1, age; X2, presence (1) or absence (0) of severe heart disease; X3, presence (1) or absence (0) of severe pulmonary disease; X4, presence (1) or absence (0) of diabetes mellitus; X5, performance statusindex (0‐4); X6, American Society of Anesthesiologists physiological status classification (1‐5) |
| Severe heart disease was defined as heart failure of New York Heart Association Class III or IV, or severe arrhythmia requiring mechanical support. Severe pulmonary disease was defined as any condition with a %VC of less than 60% and/or a FEV1.0% of less than 50%. Performance status index was based on the definition by Eastern Cooperative Oncology Group. |
| 2. SSS = −0.342 + 0.0139X1 + 0.0392X2 + 0.352X3 |
| X1, blood loss/body weight (g/kg); X2, operation time (h); X3, extent of skin incision (0: minor incisions for laparoscopic or thoracoscopic surgery[including scope‐assisted surgery]; 1: laparotomy or thoracotomy alone; 2: both laparotomy and thoracotomy) |
| 3. CRS = −0.328 + 0.936 (PRS) + 0.976 (SSS) |
FEV, forced expiratory volume; VC, vital capacity.
2.4. Statistical analysis
All descriptions of gastric cancer were based on the Japanese classification of gastric carcinoma, third English edition. 19 Statistical calculations were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). 20 Background factors were evaluated by univariate analyses using the Mann–Whitney U test or Fisher's exact test. Survival was calculated with the Kaplan–Meier method. A log‐rank test was used to compare survival between groups. The optimal cut‐off levels of CRS of the E‐PASS scoring system were calculated using the receiver operating characteristic curve (Figure S1). P values of less than .05 were considered to indicate statistical significance.
3. RESULTS
3.1. Patient characteristics
Among 1041 patients with pStages II or III gastric cancer seen between 2008 and 2013 consecutively, 898 patients were enrolled in this study (Figure 1). Of these, 158 patients (17.6%) were elderly and 740 patients (82.4%) were nonelderly. Regarding clinical background, female gender (P = .036) and histologically differentiated type (P < .001) were significantly higher and rates of ≥D2 gastrectomy (P = .031) and adjuvant chemotherapy (P < .001) were significantly lower in the elderly group (Table 2). There were no differences in the proportions of substages or complication rates. Table 3 shows the details of patients regarding adjuvant chemotherapy. There were significant differences between the proportion of elderly and nonelderly patients undergoing adjuvant chemotherapy for both pStage II cancer excluding pT1N2‐3 or pT3N0 and pStage III, which were indicated for adjuvant chemotherapy. Among 158 elderly patients, there were 24 patients with diabetes mellitus, 21 patients with cardiovascular diseases (myocardial infarction, angina pectoris, aortic dissection, and carotid artery stenosis), and eight patients with strokes. According to the E‐PASS scoring system, one patient showed severe arrhythmia and three patients showed severe pulmonary disease.
TABLE 2.
Baseline patient characteristics
| Characteristic | Elderly | Nonelderly | P |
|---|---|---|---|
| n = 158 | n = 740 | ||
| Age, median (range) | 79 (75‐92) | 63 (22‐74) | <.001 |
| Sex | |||
| Male | 89 | 484 | .036 |
| Female | 69 | 256 | |
| Histological type | |||
| Differentiated | 80 | 259 | <.001 |
| Undifferentiated | 78 | 481 | |
| Procedure | |||
| Total gastrectomy | 56 | 278 | .23 |
| Distal gastrectomy | 100 | 427 | |
| Proximal gastrectomy | 0 | 7 | |
| Pylorus‐preserving gastrectomy | 2 | 28 | |
| Approach | |||
| Open | 140 | 620 | .15 |
| Laparoscopic | 18 | 120 | |
| D‐number | |||
| ≥D2 | 127 | 646 | .031 |
| <D2 | 31 | 94 | |
| pT factor | |||
| T1/T2/T3/T4a/T4b | 5/25/59/63/6 | 44/123/252/289/32 | .83 |
| pN factor | |||
| N0/N1/N2/N3 | 38/43/42/35 | 182/179/199/180 | .91 |
| pStage | |||
| IIA/IIB/IIIA/IIIB/IIIC | 35/46/29/25/23 | 195/189120/113/123 | .69 |
| Postoperative complication | |||
| Clavien‐Dindo grade 2 or higher | 22 | 148 | .093 |
| Adjuvant chemotherapy | |||
| + | 42 | 484 | <.001 |
| − | 116 | 256 | |
TABLE 3.
Details of patients regarding adjuvant chemotherapy
| Characteristic | Elderly | Nonelderly | P |
|---|---|---|---|
| n = 158 | n = 740 | ||
| pStage II cancer including pT1N2‐3 and pT3N0 | 21 | 148 | |
| With adjuvant chemotherapy | 0 (0%) | 22 (14.9%) | .079 |
| Without adjuvant chemotherapy | 21 (100%) | 126 (85.1%) | |
| pStage II cancer excluding pT1N2‐3 or pT3N0 | 60 | 236 | |
| With adjuvant chemotherapy | 11 (18.3%) | 168 (71.2%) | <.001 |
| Without adjuvant chemotherapy | 49 (81.7%) | 68 (28.8%) | |
| pStage III | 77 | 356 | |
| With adjuvant chemotherapy | 31 (40.3%) | 293 (82.3%) | <.001 |
| Without adjuvant chemotherapy | 46 (59.7%) | 63 (17.7%) |
3.2. Survival outcomes
Kaplan–Meier survival curves of OS and DSS according to cancer stage are shown in Figures 2 and 3. OS was significantly worse in elderly patients with both pStages II and III gastric cancer (P < .001). The five‐year OS rates were 72.4% (95% confidence interval [CI]: 61.1%‐80.9%) in elderly patients and 89.2% (95% CI: 85.7%‐92.0%) in nonelderly patients with pStage II gastric cancer, respectively. Similarly, the same rates were 43.9% (95% CI: 32.6%‐54.6%) in elderly patients and 65.3% (95% CI: 60.0%‐70.0%) in nonelderly patients with pStage III gastric cancer. Besides, when the elderly patients were divided as patients aged 75‐79 years and those aged ≥ 80 years, the latter significantly had worse OS than the former in pStage II cancer (Figures S2 and S3). On the other hand, although there was no difference in DSS among patients with pStage II gastric cancer (P = .45) for the two groups, DSS in elderly patients with pStage III gastric cancer was significantly worse than that in the nonelderly group (P = .001). The five‐year DSS rates were 52.4% (95% CI: 40.1%‐63.3%) in elderly patients and 68.6% (95% CI: 63.3%‐73.2%) in nonelderly patients with pStage III gastric cancer, respectively.
FIGURE 2.
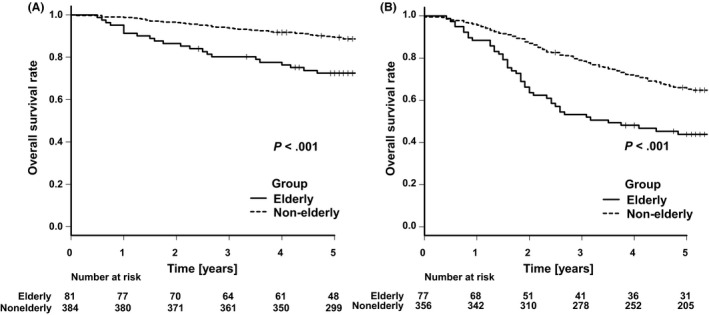
Kaplan–Meier analyses of overall survival comparing elderly and nonelderly patients with pStage II gastric cancer (A) and those with pStage III gastric cancer (B)
FIGURE 3.
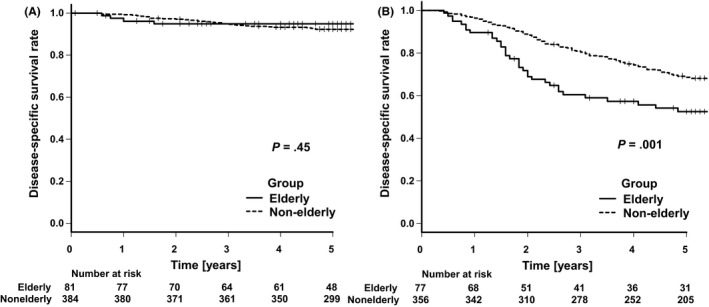
Kaplan–Meier analyses of disease‐specific survival comparing elderly and nonelderly patients with pStage II gastric cancer (A) and those with pStage III gastric cancer (B)
Among the 158 elderly patients, 39 (24.7%) experienced a recurrence of gastric cancer; however, 23.4% of nonelderly patients experienced a recurrence. Table 4 shows the comparison of recurrence sites in elderly and nonelderly patients. The sites of relapse could be divided into the four groups of peritoneal dissemination, hematogenous metastasis, lymph node metastasis, and local recurrence, affecting 19, 14, 10, and three elderly patients, respectively. Six patients experienced overlapping sites. On the other hand, in the present study, 66 elderly patients died during follow‐up, with the recurrence of gastric cancer being the most common cause of death (n = 38). Others less frequently observed causes including: other malignant diseases (n = 7); infectious diseases (n = 4); heart disease (n = 2); renal failure (n = 1); senility (n = 1); and unknown causes (n = 13).
TABLE 4.
Details of recurrence sites. Some patients experienced recurrences at two sites
| Characteristic | Elderly | Nonelderly |
|---|---|---|
| n = 158 | n = 740 | |
| Patients with recurrence | 39 (24.7%) | 173 (23.4%) |
| Recurrence sites | ||
| Peritoneal dissemination | 19 (48.7%) | 80 (46.2%) |
| Hematogenous metastasis | 14 (35.9%) | 60 (34.7%) |
| Lymph node metastasis | 10 (25.6%) | 30 (17.3%) |
| Local recurrence | 3 (7.7%) | 9 (5.2%) |
| Unknown | 0 (0%) | 1 (0.6%) |
3.3. Predictors
Figure 4 displays Kaplan–Meier survival curves, comparing elderly patients with CRS ≤ 0.159 and those with CRS > 0.159 using the E‐PASS scoring system according to cancer stage. Hazard ratios for death in patients with CRS > 0.159 were 4.41 (95% CI: 1.31‐14.84) for pStage II and 1.61 (95% CI: 0.72‐3.62) for pStage III. The OS for patients with CRS > 0.159 was significantly worse only in pStage II gastric cancer (P = .009). There were significant differences in surgical procedure (P = .013), approach (P < .001), D‐number (P = .041), and adjuvant chemotherapy (P < .001) between patients with CRS > 0.159 and those with CRS ≤ 0.159 in pStage II cancer (Table S1). However, there were no differences pT factor, pN factor, pStage, and postoperative complication between the two groups.
FIGURE 4.
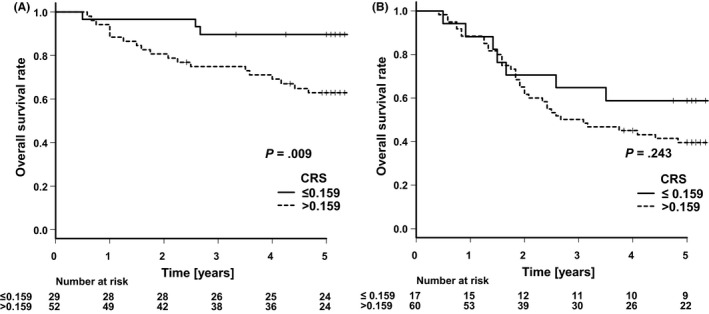
Kaplan–Meier analyses of overall survival comparing the elderly patients with CRS ≤ 0.159 and those with CRS > 0.159 in pStage II gastric cancer (A) and pStage III gastric cancer (B)
4. DISCUSSION
Here, we present two key findings regarding elderly patients with pStages II and III gastric cancer. First, the DSS for elderly patients with pStage III gastric cancer was worse than that in nonelderly patients, while there was no difference in DSS between elderly and nonelderly patients with pStage II gastric cancer. The survival gap between the OS and DSS of elderly patients was larger in pStage II cancer than in pStage III cancer. Second, the E‐PASS scoring system could be a useful predictor of OS among elderly patients with pStage II gastric cancer.
In the present study, we newly found different characteristics in terms of DSS for elderly patients between pStages II and III gastric cancer. The DSS for elderly patients with pStage II gastric cancer was comparable with that for nonelderly patients. The oncological outcomes of the elderly patients with pStage II cancer in the present study seemed to be similar to those of pStage I cancer reported by Nunobe et al. 11 This thought was reasonable because pStage II gastric cancer includes some substages which were less likely to recur and were not indicated for adjuvant chemotherapy. Besides, disease‐specific mortality in patients with pStage II cancer which was indicated for adjuvant chemotherapy also seemed to be very low. We concluded that the DSS of pStage II disease would be more similar to that of pStage I disease than pStage III disease. On the contrary, Sakurai et al reported that DSS for elderly patients with pStage II gastric cancer was significantly worse than that of nonelderly patients. 9 However, there were some problems in their research. For example, the total size of the elderly group was small and the DSS for nonelderly patients was 10% worse than our data in a high‐volume center specializing in cancer treatment, suggesting that the outcomes might have been influenced by surgical quality.
On the other hand, the DSS for elderly patients with pStage III gastric cancer was worse than that for nonelderly patients, as Sakurai et al also reported in a smaller case series. 9 We suggested some causes, as follows. First, although some types of locally advanced gastric cancer were indicated for neoadjuvant chemotherapy, 18 , 21 elderly patients tended to undergo upfront surgery as they were not supposed to receive high‐dose intensity of chemotherapy. In this study, five elderly patients with bulky lymph node metastasis (bulky N2) underwent upfront surgery (data not shown). Thus, patients with unfavorable cancer might be enrolled in the elderly group while we excluded similar patients who received neoadjuvant chemotherapy in the nonelderly group. Second, the number of elderly patients who received adjuvant chemotherapy was significantly less than nonelderly patients and could not gain a benefit from such treatment. Patients older than 80 years of age were excluded in the Adjuvant Chemotherapy Trial of S‐1 for Gastric Cancer (ACTS‐GC), 8 and adjuvant S‐1 monotherapy did not significantly improve survival outcomes in patients aged between 70 and 80 years in a subgroup analysis of ACTS‐GC data. Taking these findings into consideration, we did not often deliver chemotherapy in elderly patients. Besides, they might receive lower dose intensities for adjuvant chemotherapy than nonelderly patients if they started chemotherapy, as reported previously, 22 which indicated a reduced effectiveness of treatment. Although elderly patients with adjuvant chemotherapy had better OS than those without adjuvant chemotherapy according to our data, there was no significant difference in DSS (Figure S4). We suppose that this may be attributed to selection bias. However, the number of patients was too few to analyze the relationship. Third, aging itself would be one of the risk factors for gastric cancer recurrence. Age is closely related with immune function, and immunosenescence is recognized to occur with increasing age. 23 We suspected that micrometastasis could not be eliminated by innate immunity in elderly patients with advanced immunosenescence. In breast cancer, increasing age was reported to be associated with higher disease‐specific mortality, 24 which supported our assumption.
OS for elderly patients with pStages II and III gastric cancer was worse when compared with that of nonelderly patients in the present study as well as of patients with pStage I gastric cancer. 11 The differences of 5‐year OS rates and DSS for elderly patients were revealed to be 22.5% in pStage II and 8.5% in pStage III. These survival gaps between OS and DSS meant that patients died from other causes than recurrent gastric cancer. Elderly patients often died from not only recurrent gastric cancer but also comorbidity and secondary malignant disease. The survival gap was larger in elderly patients with pStage II cancer than in those with pStage III cancer. The disease‐specific mortality of pStage II gastric cancer was very low, and these had a higher risk of suffering from other diseases. Therefore, we should consider different strategies for elderly patients with pStage II or III cancer. The prediction system of clinical outcomes after surgery will be helpful for surgeons to consider when to perform radical surgery in elderly patients with gastric cancer. It might be a good idea to establish alternative strategies rather than deploying the standard treatment for elderly patients, particularly those with stage II gastric cancer, because they had poor survival outcomes due to comorbidity and secondary malignant disease. Accordingly, we focused on the E‐PASS scoring system, which could be a useful predictor for OS after surgery. In this study, we identified some differences in clinical characteristics between patients with CRS > 0.159 and those with CRS ≤ 0.159 in pStage II cancer. We supposed that less invasive surgeries had been indicated for patients with perioperative risks such as comorbidities and poor performance status. We suspected that perioperative risks and surgical stress were important and reflected survival outcomes. However, the E‐PASS scoring system contains some intraoperative findings, which could not be evaluated preoperatively. We also evaluated other scoring systems reported as useful predictors of survival in gastric cancer patients as follows: the modified Glasgow Prognostic Score, 13 the Prognostic Nutritional Index, 15 and the Neutrophil–Lymphocyte Ratio 14 (data not shown). However, the results obtained with these approaches were not significantly related to survival outcomes in our data. We analyzed the data of blood tests collected just before surgery, which changed when compared with those at first visit. The data from elderly patients might have been especially influenced by starvation for examinations like endoscopy or computed tomography. Thus, these scoring systems could not accurately predict survival outcomes. More accurate preoperative predictors of survival outcomes are expected to determine the most appropriate treatment strategy. To predict survival outcomes, we may be able to use PRS in the E‐PASS scoring system or predictive CRS prior to surgery based on the planned procedure, although CRS includes intraoperative data. Furthermore, for elderly patients with stage II gastric cancer with a poor prognosis prediction, we could suggest performing reduced surgery, such as partial resection with sentinel lymph node sampling as well as for patients with early gastric cancer, 25 because they have less meaningful reasons for standard lymphadenectomy. On the other hand, elderly patients with pStage III cancer have a much higher incidence of recurrence. Therefore, we should develop better treatment strategies, such as postoperative adjuvant chemotherapy to improve survival outcomes of elderly patients.
Our study had certain limitations. First, the number of study subjects was 158. Although the total size of this group was relatively large for a study such as this, the number in each stage was small. Besides, some of these patients were lost to follow‐up. Thus, the results of the present study are not definitive, though it is the largest such study conducted in Japan at a high‐volume center specializing in cancer treatment to date. Also, elderly patients without severe comorbidity tended to visit our institution to want higher‐quality treatment, as we did not provide medical care for cardiovascular disease or neurological disease. Thus, patients enrolled in the present study might not reflect the real world.
In conclusion, different characteristics were observed in terms of survival outcomes for elderly patients between pStages II and III gastric cancer as compared with nonelderly patients. The survival gap between OS and DSS of elderly patients was larger in patients with pStage II cancer than in those with pStage III cancer. The E‐PASS scoring system could be a useful predictor for elderly patients with pStage II gastric cancer. More accurate preoperative predictors of survival outcomes are expected to determine the most appropriate treatment strategy when a modified surgical strategy including reduced surgery is considered.
DISCLOSURE
Funding: Authors have no financial relationships to disclose.
Conflict of Interest: Authors declare no conflicts of interest for this article.
Approval of the Research Protocol: The Institutional Review Boards approved this study (2019‐1058).
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Table S1
ACKNOWLEDGEMENTS
Dr Kensei Yamaguchi, Dr Daisuke Takahari, Dr Mariko Ogura, and Dr Takeru Wakatsuki of the Department of Gastroenterological Chemotherapy, Cancer Institute Hospital, Japanese Foundation for Cancer Research treated the patients with adjuvant chemotherapy, and we thank them for collecting data.
Takahashi R, Nunobe S, Makuuchi R, et al. Survival outcomes of elderly patients with pathological stages II and III gastric cancer following curative gastrectomy. Ann Gastroenterol Surg. 2020;4:433–440. 10.1002/ags3.12339
REFERENCES
- 1. World Population Ageing . 2017 Highlights DoEaSA, United Nations. 2017.
- 2. Minister of Health, Labour and Welfare . Japan: Abridged Life Tables for Japan 2017 [cited 20 Apr 2020]. Available from https://www.mhlw.go.jp/english/database/db‐hw/lifetb17/dl/lifetb17‐06.pdf
- 3. Wang C, Nishiyama T, Kikuchi S, Inoue M, Sawada N, Tsugane S, et al. Changing trends in the prevalence of H. pylori infection in Japan (1908–2003): a systematic review and meta‐regression analysis of 170,752 individuals. Sci Rep. 2017;7:15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 5. Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bang Y‐J, Kim Y‐W, Yang H‐K, Chung HC, Park Y‐K, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open‐label, randomised controlled trial. Lancet. 2012;379(9813):315–21. [DOI] [PubMed] [Google Scholar]
- 7. Al‐Batran S‐E, Hofheinz RD, Pauligk C, Kopp H‐G, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4‐AIO): results from the phase 2 part of a multicentre, open‐label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697–708. [DOI] [PubMed] [Google Scholar]
- 8. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20. [DOI] [PubMed] [Google Scholar]
- 9. Sakurai K, Muguruma K, Nagahara H, Kimura K, Toyokawa T, Amano R, et al. The outcome of surgical treatment for elderly patients with gastric carcinoma. J Surg Oncol. 2015;111:848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wakahara T, Ueno N, Maeda T, Kanemitsu K, Yoshikawa T, Tsuchida S, et al. Impact of gastric cancer surgery in elderly patients. Oncology. 2018;94:79–84. [DOI] [PubMed] [Google Scholar]
- 11. Nunobe S, Oda I, Ishikawa T, Akazawa K, Katai H, Isobe Y, et al. Surgical outcomes of elderly patients with Stage I gastric cancer from the nationwide registry of the Japanese Gastric Cancer Association. Gastric Cancer. 2020;23(2):328–38. [DOI] [PubMed] [Google Scholar]
- 12. Haga Y, Yagi Y, Ogawa M. Less‐invasive surgery for gastric cancer prolongs survival in patients over 80 years of age. Surg Today. 1999;29:842–8. [DOI] [PubMed] [Google Scholar]
- 13. Hirashima K, Watanabe M, Shigaki H, Imamura YU, Ida S, Iwatsuki M, et al. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49:1040–6. [DOI] [PubMed] [Google Scholar]
- 14. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M, et al. The neutrophil‐to‐lymphocyte ratio (NLR) predicts short‐term and long‐term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44:607–12. [DOI] [PubMed] [Google Scholar]
- 15. Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, et al. The prognostic nutritional index predicts long‐term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647–54. [DOI] [PubMed] [Google Scholar]
- 16. Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E‐PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29:219–25. [DOI] [PubMed] [Google Scholar]
- 17. Ariake K, Ueno T, Takahashi M, Goto S, Sato S, Akada M, et al. E‐PASS comprehensive risk score is a good predictor of postsurgical mortality from comorbid disease in elderly gastric cancer patients. J Surg Oncol. 2014;109:586–92. [DOI] [PubMed] [Google Scholar]
- 18. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma. Gastric Cancer. 2011;14:101–12. [DOI] [PubMed] [Google Scholar]
- 20. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M, et al. Neoadjuvant chemotherapy with S‐1 and cisplatin followed by D2 gastrectomy with para‐aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–60. [DOI] [PubMed] [Google Scholar]
- 22. Tanahashi T, Yoshida K, Yamaguchi K, Okumura N, Takeno A, Fujitani K, et al. Questionnaire survey on adjuvant chemotherapy for elderly patients after gastrectomy indicates their vulnelabilities. Gastric Cancer. 2019;22:130–7. [DOI] [PubMed] [Google Scholar]
- 23. Malaguarnera L, Cristaldi E, Malaguarnera M. The role of immunity in elderly cancer. Crit Rev Oncol Hematol. 2010;74:40–60. [DOI] [PubMed] [Google Scholar]
- 24. van de Water W, Markopoulos C, van de Velde CJH, Seynaeve C, Hasenburg A, Rea D, et al. Association between age at diagnosis and disease‐specific mortality among postmenopausal women with hormone receptor‐positive breast cancer. JAMA. 2012;307:590–7. [DOI] [PubMed] [Google Scholar]
- 25. Aoyama J, Kawakubo H, Goto O, Nakahara T, Mayanagi S, Fukuda K, et al. Potential for local resection with sentinel node basin dissection for early gastric cancer based on the distribution of primary sites. Gastric Cancer. 2019;22:386–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Table S1


