Screening and monitoring for QT prolongation when certain medications are initiated are routinely performed to avoid arrhythmic complications. However, the novel coronavirus disease 2019 (COVID-19) pandemic and its proposed treatments—including hydroxychloroquine and azithromycin, which are known to prolong the QT interval1—raise logistical and safety concerns with established QT monitoring strategies. Recently, the American College of Cardiology made recommendations for QT monitoring in outpatients with COVID-19 on hydroxychloroquine/azithromycin, suggesting that the use of direct-to-consumer mobile devices such as the Apple Watch 1-lead ECG could be considered in cases of resource constraints or quarantines.2 The Apple Watch ECG is cleared by the US Food and Drug Administration for detecting atrial fibrillation but has not been studied for QT monitoring. Lead I (the lead recorded by the Apple Watch) may be suboptimal for measuring this interval; however, other leads can be reproduced by placing the smartwatch on the left ankle or chest.3 We therefore sought to validate the use of the Apple Watch for QT measurement, including using nonstandard smartwatch positions, in an unselected outpatient population.
Between December 2019 and January 2020, 100 consecutive patients in sinus rhythm were enrolled from outpatient or emergency departments. The study was approved by our institutional review committee, and the subjects gave informed consent. Standard 12-lead ECGs were performed, followed by smartwatch electrocardiographic recordings using the Apple Watch Series 4 (Apple Inc, Cupertino, CA). After a brief demonstration, patients recorded 30-second Apple Watch electrocardiographic equivalents of lead I (AW-I; watch on left wrist), lead II (AW-II; watch on left ankle), and a simulated lead V6 (AW-LAT; watch on left lateral chest; Figure [A]). Using commercially available software (EP Calipers, EP studios Inc, Louisville, KY), a cardiologist measured 3 RR and QT intervals to calculate the corrected QT interval (QTc) using the Bazett formula (Figure [B]). A QTc >480 milliseconds was considered high risk. Agreement between the 12-lead and Apple Watch QTc measurements was calculated with the use of the median absolute error and Bland-Altman analyses. To measure interobserver variability, all AW-I QTc measurements were repeated by a second blinded cardiologist, and the intraclass correlation coefficient was calculated on the basis of a 2-way random absolute agreement model with single measurements. Agreement on whether a tracing was interpretable was assessed with the Cohen κ statistic. T-wave amplitude was measured to evaluate its association with QTc measurement accuracy.
Figure.
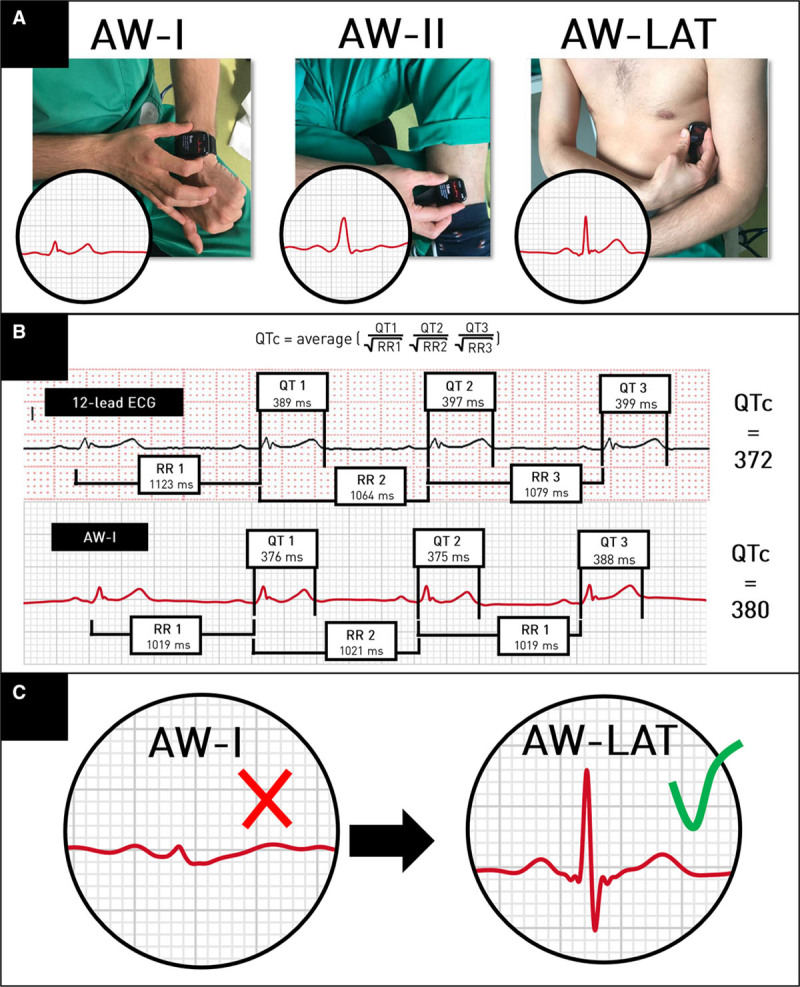
Apple Watch electrocardiographic recordings, QT interval measurements, and impact of recording tracings from nonstandard smartwatch positions. A, Recording Apple Watch electrocardiographic equivalents of lead I (AW-I; watch on left wrist), lead II (AW-II; watch on left ankle), and a simulated lead V6 (AW-LAT; watch on left lateral chest). B, Corrected QT interval (QTc) measurements from a 12-lead ECG and an Apple Watch lead I ECG (AW-I). QTc using the 12-lead ECG is 372 milliseconds; QTc using AW-I is 380 milliseconds (absolute difference, 8 milliseconds; bias, −8 milliseconds). C, Repositioning the Apple Watch may improve signal quality and measurement of the QT interval. Example of 2 smartwatch electrocardiographic recordings from the same patient. Measurement of the QT interval was deemed impossible in lead II (AW-II) because of low T-wave amplitude (≤0.1 mV). After the smartwatch was moved to the left lateral chest wall (AW-LAT), the T-wave amplitude increased to 2 mV, markedly facilitating the measurement of the QT interval. Note the similar increase in QRS complex amplitudes.
The mean age was 67±7 years; 59% were male; and 35% had diagnosed cardiac disease. Heart rates were similar on 12-lead and Apple Watch recordings (69±11 bpm versus 70±13 bpm, respectively; P=0.2, paired Student t test). QTc intervals ranged from 336 to 530 milliseconds on 12-lead ECGs. Eight patients were identified as high risk, all of whom were similarly identified by the smartwatch. QTc measurements could not be performed in 15 patients (15%) with AW-I, in 31 patients (31%) with AW-II, and in 15 patients (15%) with AW-LAT because of low T-wave amplitude, baseline artifact, or both. Repositioning the smartwatch to a different location often yielded an adequate tracing (Figure [C]); however, in 6 patients (6%), none of the 3 smartwatch positions allowed an adequate measurement.
There was excellent agreement between cardiologists on whether recordings were acceptable for QTc measurements (κ=0.92). Interobserver agreement for QTc measurements was similarly high with a median absolute error of 9 milliseconds (interquartile range, 5–15 milliseconds) and an intraclass correlation coefficient of 0.92 (95% CI, 0.85–0.95). Compared with the 12-lead ECG, the median absolute error in QTc was 18 milliseconds for AW-I (interquartile range, 9–35 milliseconds), 20 milliseconds for AW-II (interquartile range, 8–33 milliseconds), and 16 milliseconds for AW-LAT (interquartile range, 8–27 milliseconds). There were no significant differences in absolute offset between smartwatch positions. Bland-Altman analyses revealed a bias of −5 milliseconds (95% limits of agreement, −65 to 54) with AW-I, −9 milliseconds with AW-II (95% limits of agreement, −67 to 50), and −11 milliseconds (95% limits of agreement, −60 to 37) with AW-LAT. The negative bias suggests that using the smartwatch slightly overestimated the QTc interval. T waves were significantly higher in AW-LAT than in AW-I and AW-II (0.28 mV versus 0.20 mV and 0.19 mV, respectively; P<0.001, ANOVA). Tracings with T-wave amplitudes >1 mV demonstrated lower absolute errors in AW-I (21±16 milliseconds versus 30±25 milliseconds; P=0.03, unpaired t test).
In our study, Apple Watch electrocardiographic tracings allowed adequate QT measurements when the smartwatch was worn on the left wrist in 85% of patients. This figure increased to 94% when the smartwatch was moved to alternative positions. Performance depends on factors such as electrocardiographic tracing quality and T-wave amplitude. Therefore, identifying the best smartwatch position (ie, T-wave mapping) at baseline may improve accuracy. Other electrocardiographic systems were not evaluated but may be similarly valuable4,5 such as the Kardia 6 L system (AliveCor, Mountain View, CA), which has received US Food and Drug Administration clearance for QT measurement. Overall, this technology has the potential to facilitate remote QT monitoring, including among quarantined outpatients on QT-prolonging treatments.
Sources of Funding
This work received financial support from the French government as part of the “Investments of the Future” program managed by the National Research Agency, grant reference ANR-10-IAHU-04. Dr Ramirez is supported by a Canadian Institutes of Health Research Banting Postdoctoral Fellowship and a Royal College of Physicians and Surgeons of Canada Detweiler Travelling Fellowship.
Disclosures
None.
Footnotes
The data from this study are available from the corresponding author on reasonable request.
References
- 1.Chorin E, Dai M, Shulman E, Wadhwani L, Bar-Cohen R, Barbhaiya C, Aizer A, Holmes D, Bernstein S, Spinelli M, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin [published online April 24, 2020]. Nat Med. doi: 10.1038/s41591-020-0888-2. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 2.Simpson TF, Kovacs RJ, Stecker EC. Ventricular arrhythmia risk due to hydroxychloroquine-azithromycin treatment for COVID-19March 29, 2020American College of Cardiology; https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 [Google Scholar]
- 3.Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S. Single-lead ECG recordings including Einthoven and Wilson leads by a smartwatch: a new era of patient directed early ECG differential diagnosis of cardiac diseases? Sensors. 2019;19:4377. doi: 10.3390/s19204377. doi: 10.3390/s19204377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung EH, Guise KD. QTC intervals can be assessed with the AliveCor heart monitor in patients on dofetilide for atrial fibrillation. J Electrocardiol 2015488–9doi: 10.1016/j.jelectrocard.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 5.Castelletti S, Dagradi F, Goulene K, Danza AI, Baldi E, Stramba-Badiale M, Schwartz PJ. A wearable remote monitoring system for the identification of subjects with a prolonged QT interval or at risk for drug-induced long QT syndrome. Int J Cardiol 201826689–94doi: 10.1016/j.ijcard.2018.03.097 [DOI] [PubMed] [Google Scholar]


