Abstract
Five years after the last prostatic carcinoma grading consensus conference of the International Society of Urological Pathology (ISUP), accrual of new data and modification of clinical practice require an update of current pathologic grading guidelines. This manuscript summarizes the proceedings of the ISUP consensus meeting for grading of prostatic carcinoma held in September 2019, in Nice, France. Topics brought to consensus included the following: (1) approaches to reporting of Gleason patterns 4 and 5 quantities, and minor/tertiary patterns, (2) an agreement to report the presence of invasive cribriform carcinoma, (3) an agreement to incorporate intraductal carcinoma into grading, and (4) individual versus aggregate grading of systematic and multiparametric magnetic resonance imaging–targeted biopsies. Finally, developments in the field of artificial intelligence in the grading of prostatic carcinoma and future research perspectives were discussed.
Key Words: prostate cancer, grading, ISUP grade group, consensus, minor grades, intraductal carcinoma, targeted biopsies
The International Society of Urological Pathology (ISUP) last held a prostate cancer grading consensus conference in 2014 in Chicago,1 and the modifications from that conference (summarized in Table 1) were incorporated into the 2016 World Health Organization (WHO) Classification of Tumours of the Urinary System and Male Genital Organs blue book.2 In the past 5 years, further data in the field of Gleason pattern quantities, tumor growth patterns, and clinical practice advancements such as widespread introduction of multiparametric magnetic resonance imaging (mpMRI)-targeted biopsies or fusion ultrasound/magnetic resonance imaging (MRI) biopsies have added to challenges in reporting and grading for pathologists. Furthermore, rapid developments in the field of image analysis might influence daily pathology practice in the near future. This accrual of new data has generated a need to resolve several crucial matters in prostate cancer grading3 and served as the impetus for a follow-up consensus conference. This meeting was held on September 12, 2019, in Nice, France, and the resulting recommendations are summarized in the current manuscript (summarized in Table 2).
TABLE 1.
ISUP 2014 Modifications to Growth Patterns and Grade Grouping of Prostatic Carcinoma1
TABLE 2.
Summary of ISUP 2019 Modifications to Prostate Cancer Grading

ORGANIZATION OF CONSENSUS CONFERENCE
The conference was initiated and prepared by ISUP council members (K.A.I., G.J.L.H.v.L., T.H.v.d.K., D.J.G.). Topics were delegated to 4 working groups, who reviewed the relevant literature and evidence on the following: (1) quantitative grading, including assignment of Gleason patterns 4 and 5 percentages to biopsy and prostatectomy specimens, and tertiary/minor patterns; (2) grading, significance, and reporting of invasive cribriform and intraductal carcinoma (IDC); (3) grade heterogeneity including grading based on the level of the whole case, biopsy sites, and individual cores, and reporting of targeted biopsies; and (4) the future of grading including incorporation of artificial intelligence (AI) and potential future grading scheme improvements. In total, 16 international prostate cancer experts from 4 continents participated in the working groups, including 13 genitourinary pathologists, 1 urologist (N.M.), 1 radiologist (I.G.S.), and 1 image analysis expert (G.L.).
A premeeting online survey with 31 questions was held open to the ISUP membership for 34 days and generated 252 complete responses; by continent: North America 39%, Europe 34%, South America 11%, Asia 10%, Australia 5%, and Africa 1%. The survey results informed the working groups with data on current clinical practice and identified controversial topics to be clarified in the consensus meeting, or in future meetings in case reasonable scientific evidence was still lacking.
The conference meeting was attended by 93 participants (Appendix). Each working group presented a detailed literature review of its topics, provided an overview of the relevant survey outcome, and put proposal statements to the meeting. Twenty-three statements were voted upon in an agree/disagree manner using a VoxVote.com application. Consensus on a proposal statement was considered to be met when at least two thirds (67%) of the voters agreed.
QUANTITATIVE GRADING, WORKING GROUP 1
Percentage of High-grade Gleason Patterns
The premeeting survey indicated that 93% of respondents reported both the Gleason score (GS) and the ISUP 5-tier grading system as recommended at the 2014 ISUP consensus meeting1 further labeled here as (ISUP) grade group (GG). Although GS (GG) is a standard prognostic measure, the extents of higher grade patterns 4 and 5 affect patient outcomes and could influence management decisions.4 By separating GS 7 cancers into 3+4=7 (GG2) and 4+3=7 (GG3), the proportion of pattern 4 is at least partly incorporated into patient management algorithms.5–7 However, a number of studies have shown that further quantification of pattern 4 has clinical significance.8–13 For instance, biopsy specimens with GS 3+4=7 in which the greatest amount of pattern 4 was <5% of the tumor volume in any one core had similar prostatectomy findings and biochemical recurrence-free survival to GS 3+3=6 cases in a recent study.7 If other clinical variables are favorable, some GS 3+4=7 (GG2) patients with limited amounts of Gleason pattern 4 may qualify for active surveillance.14–16 In radical prostatectomy specimens, a higher percentage of Gleason pattern 4 and even limited amounts of pattern 5 have been associated with increased biochemical relapse.17,18 The 2016 WHO Classification, College of American Pathologists, and the International Collaboration of Cancer Reporting (ICCR) dataset recommend reporting of pattern 4 percentage for GS 7 in biopsies and radical prostatectomies, although no precise methodology is specified.2,19 Reporting the percentage of pattern 5 is currently not a requirement.
The premeeting survey showed that 49% of the respondents reported percentage Gleason patterns 4 and 5 in radical prostatectomies, irrespective of GS. Overall, 33% reported a percentage only for GS 7, either 3+4=7, 4+3=7, or both (9%, 3%, and 21%, respectively). For biopsies, 44% gave the percentage for all carcinomas, whereas a similar number did so only for GS 7, either for 3+4=7, 4+3=7, or both (13%, 2%, and 29%, respectively). Also, grading of minute cancer foci in biopsies as, for example, GS 4+4=8 may result in overgrading and overtreatment. Grading of limited foci does not correlate well with pathologic stage and has been associated with downgrading in radical prostatectomy specimens.20–22 Moreover, assessment of percentage pattern 4 in minute cancer foci has poor reproducibility, particularly for poorly formed glands.23–27 Although this topic was not discussed in depth at the meeting, the literature suggests restraint in grading minute (<1 mm) foci of Gleason patterns 4 or 5 cancer, possibly by adding a comment that the specimen vial grade may overestimate the grade of the entirety of the cancer.
Minor/Tertiary Patterns
The presence of minor/tertiary patterns was considered separately for biopsy and radical prostatectomy specimens. For biopsies, the ISUP has recommended the inclusion of tertiary higher grade patterns in the GS, irrespective of extent, since the 2005 consensus meeting.28 Thus, a needle biopsy with 60% Gleason pattern 4, 36% pattern 3, and 4% pattern 5 would be reported as GS 4+5=9 (GG5). It has since been suggested that use of the term “minor” rather than “tertiary” is preferable because the primary and secondary grades may be identical, with a very small second higher grade cancer component.29 The presence of a minor component of Gleason pattern 5 in GS 4+3=7 cancer at biopsy predicted higher tumor volume at prostatectomy.30 Sauter et al18 showed that incorporation of minor patterns into a quantitative grading model at biopsy improved the prediction of prostatectomy pathology. Incorporation of minor high-grade patterns in the biopsy GS ensures that they are accounted for when considering patients for active surveillance.
In radical prostatectomy specimens, the preferred approach to reporting minor patterns has remained uncertain. It was agreed in 2005 to assign GS based only on the primary and secondary patterns with a comment on the presence of any minor pattern.28 However, neither the 2005 nor 2014 meetings recommended a percent cutoff for a minor pattern. A minor pattern that can technically range from 1% to 32% is excluded from current prognostic tools; therefore, a significant volume of Gleason pattern 5 may be ignored clinically. Although 5% is a somewhat arbitrary cutoff, several studies suggest that <5% Gleason pattern 4 is not associated with increased risk of biochemical recurrence or adverse pathology at prostatectomy,31–33 although a few others contradict this.34–37 The situation for Gleason pattern 5 is different, making it more discriminatory than Gleason pattern 4.38 Multiple studies have shown that any minor component of Gleason pattern 5 worsens biochemical recurrence-free survival.4,18,31–33,38–42 Small amounts (mostly <5%) of minor Gleason pattern 5 generally worsen the prognosis of the baseline GS,43 but reports disagree on whether a minor component warrants promotion to the next higher GS.18,39–42
In the premeeting survey, for carcinomas with 60% primary Gleason pattern 3, 36% secondary pattern 4, and 4% pattern 5, 86% of the respondents would report a GS 3+5=8 on biopsy, whereas only 7% would do this for prostatectomy. In prostatectomies with 60% pattern 3, 30% pattern 4 and 10% pattern 5, 66% would report a GS 3+5=8 and 30% would report as 3+4=7, with or without a comment on the presence of minor pattern 5. Discussion revealed a concern that inclusion of <5% pattern 5 in the GS could blur the definition of high-grade cancers because the finding of focal single cells carries poor reproducibility,44 a point that significantly influenced the proposal and subsequent vote.
Proposals and Voting
At the meeting, a consensus emerged in favor of reporting percentage of Gleason pattern 4 for all GS 7 (GG 2, 3) biopsies, but there was no consensus to do so for radical prostatectomies (Table 3). Subsequent votes affirmed the current use29 of the “<5% rule” for reporting minor patterns in prostatectomy specimens, which is different from its use in biopsies. Similar to previous policy, any highest Gleason patterns 4 or 5 present in a radical prostatectomy must account for at least 5% of the tumor to be incorporated as a secondary pattern of the GS. Hence, in radical prostatectomy specimens, 60% Gleason pattern 3, 37% pattern 4, and 3% pattern 5 would be reported as GS 3+4=7 (GG2) with minor/tertiary pattern 5, whereas 60% pattern 3, 30% pattern 4, and 10% pattern 5 would be reported as GS 3+5=8 (GG4). The same rule also applies to cases with predominant Gleason pattern 3 and minor high-grade foci.
TABLE 3.
Grade Quantification Voting Results
IDC AND TUMOR GROWTH PATTERNS, WORKING GROUP 2
Intraductal Carcinoma
IDC is characterized by extension of cancer cells into preexisting prostatic ducts and acini, distending them, with preservation of basal cells. At least 3 conflicting definitions of IDC have been given.45–47 Cohen et al46 state that duct diameter must exceed 2 times that of benign peripheral zone glands, and add minor criteria of right-angle branching, smooth contours, and dimorphic cell population with only central cells being prostate-specific antigen–positive. All definitions include trabecular, cribriform, and solid/comedo patterns, but Guo et al47 add a papillary pattern without fibrovascular cores. Guo and colleagues, overlooking the above additions of Cohen and colleagues, also stipulate that if growth is papillary or loose cribriform, there must be nuclear size at least 6× normal, or comedonecrosis. Two recent studies have shown that in many instances where cribriform or solid fields with comedonecrosis were morphologically considered invasive Gleason pattern 5, there were in fact surrounding basal cells by immunostaining, suggesting that comedonecrosis is often a manifestation of IDC.48,49 Although the definition of Guo and colleagues is part of the 2016 WHO blue book, it was agreed that a separate meeting is needed to resolve definitional ambiguities.50,51 Many groups have reported an independent adverse prognostic value of IDC in biochemical recurrence-free survival and cancer-specific survival of prostate cancer patients on the basis of biopsy and radical prostatectomy specimens.52,53
IDC typically occurs adjacent to high-grade invasive carcinoma and only rarely is unaccompanied by invasion. The invasive component might be GS 6 to 10 (GG1 to 5). IDC has been reported without an invasive component in 0.06% to 0.26% of prostate biopsies.53–55 The 2014 ISUP Gleason grading consensus conference1 recommended that IDC without invasive carcinoma should not be assigned a GG and this proposal was endorsed in the WHO 2016.53 This endorsement was done without voting separately on the very different scenarios of IDC encountered with and without invasive carcinoma at the 2014 ISUP consensus conference.1 The current consensus conference considered these scenarios separately because different rules may apply to both scenarios.
The premeeting survey disclosed that 90% of the respondents would not assign a Gleason grade to pure IDC in the absence of an invasive component, and 76% to 81% would not grade IDC if present adjacent to GS 3+3=6 (GG1) cancer, in accordance with the 2014 ISUP and 2016 WHO guidelines. However, 65% of the respondents felt that IDC could not be reliably diagnosed without immunohistochemistry and 67% indicated that they would consider a scheme by which IDC associated with invasive cancer can be incorporated into the GS instead of exclusively mentioning it separately. IDC without invasive carcinoma in biopsies, conversely, usually is an epiphenomenon of an unsampled, high-grade invasive component.53 There is currently no clinical consensus that patients with biopsies showing only IDC should be managed with radical therapy as opposed to urgent rebiopsy.
IDC With Invasive Carcinoma
It may be argued that incorporating IDC adjacent to invasive cancer into the GS could result in overgrading since Khani and Epstein55 found that 3 (21%) of 14 patients with the unusual scenario of IDC and GS 6 on biopsy had only GS 6 cancer in their radical prostatectomy specimens. These 3 patients’ prostatectomy specimens were, however, only partially submitted for histologic examination, precluding exclusion of unsampled higher grade cancer.55 Among 62 patients with biopsies showing GS 6 associated with IDC, 7% had metastasis at presentation, 13% of men who received radical therapy ultimately progressed to metastatic cancer, and 55% of the 11 (18%) men initially put on surveillance were actively treated because of progressive cancer,55 which is clearly different from patients with biopsy GS 6 only.3
Several compelling arguments support incorporating IDC associated with invasive cancer into the GS. First, all historical and contemporary GS outcome data, including those used in multiple clinical phase 3 trials, are based on morphology without application of routine immunohistochemical basal cell staining, setting a precedent for incorporating IDC into the GS. Although a majority of urologic pathologists surveyed (62% to 78%) would not include IDC in the overall GG assessment in diagnostic biopsies, 59% do include intermingled IDC in the quantification of percentage/linear core involvement; 59% to 88% do rarely if ever perform basal cell immunohistochemistry for distinguishing IDC from invasive carcinoma; and 95% believe that GG1 cancer with IDC should be a contraindication to active surveillance.56
Second, there is general agreement that basal cells are not always identifiable by hematoxylin-eosin stain alone. In fact, distinction of IDC from invasive carcinoma by basal cell immunostaining might even be impossible. It is well known that some high-grade prostate intraepithelial neoplasia (PIN) glands lack a basal cell layer on routine tissue sections probably due to sampling artifact; the same may be true for IDC, which can have an even more dispersed basal cell layer owing to gland distention. It is also uncertain how to interpret morphologically irregularly invasive cribriform structures with sporadic basal cells (Fig. 1). A survey in which 38 photomicrographs were circulated to 39 genitourinary pathologists disclosed only 43% consensus for an unequivocal diagnosis of IDC.57 Varma et al50 surveyed 23 genitourinary pathologists and found considerable variation in the diagnostic criteria and rules used to report IDC. Thus, excluding IDC from grading carries the risk of undergrading a cancer equivalent to 4+3 as 3+4 or 3+3. If IDC also has comedonecrosis, grading disparities might become even greater. Rates of immunostaining use vary among different laboratories, but incorporating IDC into the GS obviates the requirement to perform immunostaining in most cases. The number of additional immunostain procedures to discriminate IDC from invasive cancer could be limited if applied only for those cases in which immunostaining outcome would affect the GS. However, recommendations of the 2016 WHO, ICCR, and the current consensus meeting to report percentage Gleason pattern 4 in all heterogenous GS 7 tumors would in fact require performing immunostaining in significantly more cases, as IDC would have to be excluded from Gleason pattern quantification because it would be contradictory not to incorporate IDC into tumor grading, but to include it in Gleason pattern quantification.3 Older studies (before 2014) did not use immunostaining to distinguish IDC because IDC was not recognized broadly as an independent prognosticator. Instead, comedonecrosis was just graded as Gleason pattern 5 cancer, when much of it was IDC. Also, requiring immunohistochemistry to avoid overgrading cancer may result in Gleason scoring not being feasible in countries having no or limited access to this technique, and in interpretive problems: are rare basal cells permitted in invasive cancer? Third, recent evidence has shown that incorporation of IDC and invasive cribriform cancer into the GG improved the predictive value of the system for cancer-specific survival and metastasis-free survival.58 It is noteworthy that IDC carries an association with germline mutations in genes mediating DNA repair.59 The latest National Comprehensive Cancer Network guideline recommends taking IDC detected on biopsy into account for the genetic testing of germline variants including BRCA1 and BRCA2, and this may influence our practice.60 Fourth, many clinicians might disregard any comment on IDC presence in considering therapy. In the series reported by Khani and colleagues, 11 (18%) of the 62 patients were inappropriately placed on active surveillance despite the pathology reports’ inclusion of a note highlighting that IDC is a high-grade cancer. Thus, presenting IDC outside the GS may result in using only the score for management and make some patients inappropriately eligible for active surveillance. Cancer registries around the world do not record the presence of IDC; thus, the important information inherent in its presence would be lost by not incorporating the finding into the GS. It is noteworthy that this was the rationale for incorporating a minor component of a higher grade pattern into the biopsy GS rather than conveying this information in an accompanying note.1
FIGURE 1.
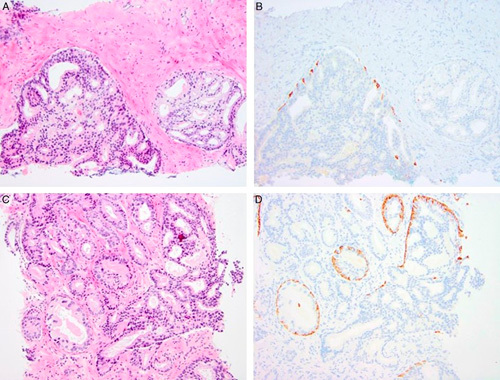
Cribriform epithelial proliferations showing overlap between IDC and invasive cribriform Gleason pattern 4. A and B, Two adjacent cribriform proliferations, one (left) having scattered basal cells, the other (right) without basal cell layer. The left structure fulfills the criteria of IDC. The right structure may represent either invasive cribriform carcinoma, or IDC without basal cells due to sampling artifact in this section. C and D, Cribriform proliferation with a basal cell layer compatible with IDC with irregular tubular outgrowths. Classification of outgrowth is unclear as either IDC, IDC transitioning to Gleason pattern 3, or IDC and invasive cribriform carcinoma. A and C, hematoxylin-eosin; B and D, high molecular weight cytokeratin.
Proposals and Voting
There was 91% consensus that IDC without invasive carcinoma should not be graded (Table 4). It was proposed that IDC associated with invasive carcinoma should be incorporated into the GS. Overall, 76% voted in favor of this proposal. Thus, without having to perform immunohistochemistry, cribriform IDC with invasive carcinoma should be graded as a Gleason pattern 4 component, and solid pattern IDC or IDC with comedonecrosis should be assigned Gleason pattern 5. Furthermore, assignment of a grade to IDC implies that IDC can be included in Gleason pattern 4 or 5 quantification and tumor volume assessment. Because IDC is likely to have prognostic significance independent of the GS, 83% of the participants agreed that the presence and significance of IDC should be commented on, despite incorporating IDC into the GS.
TABLE 4.
IDC and Tumor Growth Pattern Voting Results
Tumor Growth Patterns
Gleason pattern 4 has been expanded from its 1966 definition61 and now comprises a heterogenous group of tumors with poorly formed, fused, cribriform, and glomeruloid glandular structures.1,28 Starting with Iczkowski et al,62 numerous studies have shown that patients with cribriform pattern 4 in both biopsies and radical prostatectomies have worse biochemical recurrence-free survival, metastasis-free survival, and cancer-specific survival than those without.63,64 Although the value of cribriform growth has mostly been studied for GS 7 patients,65 it also has independent prognostic value in men with GS 8 (GG4) prostate cancer.27,65 Its role in GS 9 prostate cancer is uncertain, although cribriform growth holds a strong independent association with the presence of GG5.62 Relevant molecular differences, such as significantly more frequent PTEN and p27 loss at the RNA and protein levels, have been found in cribriform as opposed to noncribriform Gleason pattern 4 cancer.66 However, most studies on cribriform carcinoma do not explicitly state whether and how invasive cribriform carcinoma was distinguished from IDC. In a series of 1031 biopsies with additional immunohistochemical staining, Kweldam et al65 found that both IDC and invasive cribriform carcinoma had added predictive value for cancer-specific survival. In 2017, Roobol et al67 published a study on including cribriform pattern 4 in the ERSPC risk calculator. In this study, it was shown that by inclusion of the cribriform pattern, the definition of clinically significant cancer improved. Given its independent adverse prognostic value, invasive cribriform carcinoma is considered in therapeutic decision-making together with IDC in an increasing number of institutions. In the premeeting survey, 52% of the respondents stated that they recorded the presence of cribriform cancer in biopsy and 44% in radical prostatectomy reports.
Proposals and Voting
Overall, 93% of the participants agreed that cribriform Gleason pattern 4 had worse prognosis than poorly formed or fused Gleason pattern 4 (Table 4). Furthermore, 97% agreed on commenting on the presence of cribriform pattern 4 in GS 7 and 84% in GS 8 prostate cancer cases at biopsy and radical prostatectomy.
GRADE HETEROGENEITY, WORKING GROUP 3
Grading of Multifocal Prostate Cancer at Radical Prostatectomy
Detailed morphometric studies have found multifocality in 68% to 87% of radical prostatectomy specimens.68–70 Wise et al69 measured separate tumor volumes and found that the vast majority of additional tumors had a tumor volume of <0.5 mL. In a comparison of progression rates, they noted that the presence of additional tumors did not worsen outcome. Also, index tumor size was inversely correlated to the number of additional tumors, which might be explained by the fact that smaller tumors are readily separable. In conclusion, the authors recommended use of the largest tumor to estimate prognosis.
The definition of the index tumor was debated at the 2009 ISUP consensus conference on handling and reporting of prostatectomy specimens, in Boston. At that meeting, there was no consensus on whether index tumor was defined by size, size and grade, or size and stage.71 Huang et al10 analyzed in detail the relationship of the index tumor with GS and pT stage in a series of 201 prostatectomies, and confirmed that the largest nodule defined the behavior of the tumor. In 89% of multifocal cases, the highest GS, tumor volume, and stage were seen in the index tumor. In the premeeting survey, 60% of the respondents stated that they graded spatially distinct tumors separately, and only 38% would recommend using one global GS, merging the grades of multifocal tumors, as standard.
Proposal and Voting
In grading radical prostatectomy specimens with multifocal tumors, 67% consensus was reached to report the GS of the (a) largest, (b) highest stage, and (c) highest grade tumor if these are not one and the same. There was further consensus (67%) that a global GS would, in most instances, be sufficient for patient management (Table 5).
TABLE 5.
Grade Heterogeneity Voting Results
Heterogenous Grades of Prostate Cancer in Biopsy Specimens
Whenever carcinoma is present in multiple cores of one biopsy session, dissimilarities of GS are frequently encountered among biopsies. Risk stratification and patient management might subsequently be based either (1) on the biopsy with the highest/worst GS or (2) on a global/overall GS taking into account Gleason pattern quantities of all positive biopsies together. A consensus was reached at the 2005 ISUP consensus conference on a proposal to assign separate scores for each container of specimens (unless different color inks were used), but there was no consensus on whether to score multiple cores per single container separately.28 The use of the highest score may cause grade inflation in 14% to 51% of cases, but 78% of urologists prefer to rely on the highest score,72 believing that when tumor is multifocal, behavior is driven by the highest score. The premeeting survey showed that 85% of the respondents reported that the GS per individual biopsy in case systematic biopsies revealed dissimilar grade on the right and left sides, and 27% would additionally report a global GS. Several studies have analyzed the prognostic value of global versus highest GS in biopsies. Earlier studies found the highest grade to be more predictive of tumor stage and grade in prostatectomy specimens.73,74 Tolonen et al75 reported that both approaches were equal predictors of biochemical recurrence in patients treated by hormone ablation. Some contemporary studies showed no statistically significant differences between global and highest biopsy GS, although some suggest a slight superiority of global grade75–80 (but they tend to be identical in >90% of cases81). However, because little information is provided about potential multifocality and about the exact grading methodology in reporting prostatectomies in these studies, it is unclear what GS was used for comparison. This could explain the increased rates of concordance of globally graded biopsies and globally graded prostatectomy specimens.
The critical problem of current biopsy grading practice is the lack of integration of imaging information that would allow determination of whether topographically different biopsies represent different ends of the same tumor focus and may thus be lumped into a global grade. With the wider use of mpMRI, clinical practice is changing rapidly. The PRECISION trial has demonstrated that mpMRI-based biopsies outperform classic systematic transrectal ultrasound–guided biopsies in cancer detection rate.82 Patients increasingly ask for MRI before having a classic transrectal ultrasound–guided biopsy and clinicians are more open to defer or omit a biopsy, if the mpMRI yields PIRADS scores ≤2. Comparison of sensitivity for GG ≥2 cancer detection in biopsy-naive men and men with a previous negative biopsy shows that targeted biopsies are superior to systematic biopsies.83,84 Targeted biopsies usually provide a higher core length of cancer than standard biopsies, which better predicts radical prostatectomy findings.85 Furthermore, extraprostatic extension is better predicted by GS of targeted biopsies than of systematic biopsies.86 Gordetsky et al87 showed that aggregate GS (and cancer extent) for targeted biopsies better predicted tumor volume and extraprostatic extension than grading (and extent) in the worst single core. Zhao et al88 found that targeted biopsy GS’s were less likely to be upgraded at prostatectomy.
False-positive mpMRI findings remain problematic, especially in younger patients, because they might lead to patient anxiety, and raise the question of potentially false-negative biopsies missing a high-grade tumor.89 Inflammation and granulomas are sometimes confused with prostatic carcinoma on mpMRI, depending on clinician inexperience. Reporting of these non-neoplastic findings can help explain false-positive mpMRI findings and assist patient management.
Proposal and Voting
At discussion, it appeared that systematic and/or targeted biopsy grading practices significantly varied among laboratories and were incited by the number of biopsies that were submitted in one container, local clinical demands, and topographical differences. At the conference, 80% of the participants agreed that in systematic biopsies, a GS should be assigned to individual biopsy sites when multiple cores are submitted together in one vial. There was no consensus (54%) on reporting one global GS for systematic biopsies. Figure 2 summarizes the special recommendations applying to MRI-targeted biopsies. Overall, 78% voted for providing a global GS for each suspicious MRI lesion, whereas 81% voted against grading each targeted biopsy core separately. Participants did not agree (41%) to provide a global GS in case the systematic and targeted scores were unequal. A majority of 78% agreed on reporting of benign histologic findings when targeted biopsies of suspicious MRI lesions (PIRADS 4 to 5) are nonmalignant (Table 5).
FIGURE 2.
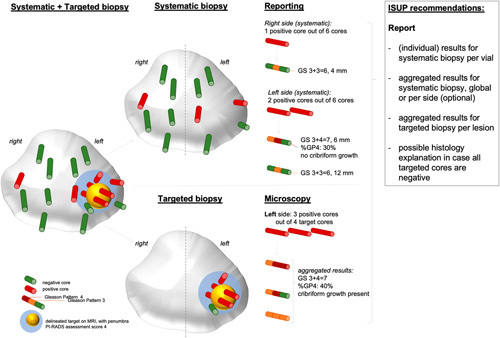
Schematic overview of reporting systematic and mpMRI-targeted biopsies. GP indicates Gleason pattern.
FUTURE OF PROSTATE CANCER PATHOLOGY, WORKING GROUP 4
AI in Prostate Pathology
The emergence of whole-slide scanners and AI-based tools in prostatic pathology has several benefits. Fully digital workflows combined with AI bring the capability to store, manage, and analyze digital data in a high-throughput manner. Pathologists are facing an increasing number of prostate biopsies with an expectation of rapid turnaround and highly nuanced reporting. These problems are coupled with widely appreciated interobserver variability in Gleason grading of prostate cancer, particularly at key clinical decision points such as GG1 versus GG2, and lack of subspecialty expertise required to achieve optimal grading precision makes machine learning (ML)-based tools an obvious option to optimize clinical decision-making. The preconference survey indicated that ISUP members have a generally positive view of AI as applied in prostatic pathology. Specifically, 31% of the respondents indicated that they had participated in ML projects focused on prostate cancer detection and/or grading, and 71% believed that there was a role for ML in screening, decision support, and improving efficiency over the next decade. Finally, 74% would use ML tools to screen prostatic biopsies, provided there was no cost barrier to implement validated algorithms that function at the same level as an experienced genitourinary pathologist, whereas only 2% believed that ML would replace most of a pathologist’s work. ML models for prostatic pathology published to date include those designed to detect carcinoma, measure the extent of tissue involvement by tumor, and perform Gleason grading at the level of expert genitourinary pathologists.90–92
Prostate Cancer Detection
In 2016, Litjens et al90 devised the first published algorithm designed to detect prostatic carcinoma in whole-slide images of core biopsies resulting in a receiver operator characteristic area under the curve (ROC-AUC) of 0.99 for distinguishing malignant from benign in an independent set of 270 biopsy slides. This algorithm could confidently screen 30% to 40% of prostate biopsies as benign without additional human intervention. A subsequent algorithm based on over 24,000 prostate biopsy slides developed by Campanella et al91 using a weakly supervised approach without laborious annotation by pathologists obtained a ROC-AUC of 0.991. They concluded that their algorithm could remove 65% to 75% of biopsy slides from pathologist review with 100% sensitivity for case-level cancer detection. Importantly, these investigators demonstrated the generalizability of their algorithm on an external slide set. Another group92 reported a ROC-AUC of 0.997 on digitized slides of 8313 biopsies from 1222 patients in the Swedish 2012-2015 STHLM3 study with no false positives. Their algorithm missed 3 small foci of carcinoma in 721 cores, all cancer foci under 0.5 mm in size. They also reported a correlation of 0.96 between their algorithm and the study pathologist with respect to linear measurement of the carcinoma in cores.
Gleason Grading
AI can create algorithms allowing generalists to function at the subspecialist level. Nagpal et al93 used a classic supervised learning approach to develop a deep learning system (DLS) for Gleason grading on radical prostatectomy specimens from 3 different sources. Accuracy was assessed for the assignment of GG by 29 generalist pathologists relative to a panel of 3 genitourinary subspecialists. The DLS outperformed the generalists with a mean accuracy of 0.70 versus 0.64. Although on prostatectomy slides of The Cancer Genome Atlas (TCGA) grading performance in relation to clinical outcome of a DLS algorithm was inferior to a panel of 3 subspecialists, it was superior to the 29 generalists for this task.93 Nordström et al94 assessed the grading performance of their algorithm compared with cases scored by a panel of 23 international ISUP subspecialists in the Imagebase project.95 The AI model performed within the range of the subspecialists, with an average κ value of 0.62. Bulten et al96 used 5759 digitized biopsies from 1243 patients to develop a GS algorithm based on a semiautomated approach using cores with pure Gleason patterns 3, 4, or 5, and the resulting model was adapted for reviewing biopsies with mixed patterns like 3+4. On a test set of 550 biopsies, their system achieved high agreement with consensus review by 3 subspecialists (Cohen κ=0.918). These examples clearly demonstrate the potential of AI to improve efficiency through prescreening of prostate biopsies to filter out benign biopsies and to improve quality by providing expert-level decision support for Gleason grading, particularly at critical clinical thresholds. Despite the promising results of AI in diagnosing, quantifying, and grading of prostate cancer, several major challenges must be addressed before above AI tools can assume routine clinical use. These include demonstrating generalizability, obtaining regulatory approval, validating against clinical outcome, and being able to handle biopsies that contain nonprostatic neoplasms such as urothelial or rectal carcinoma, or lymphoma.
FUTURE PERSPECTIVES
Many studies support the added predictive and prognostic value for percentage Gleason pattern 4, minor high-grade patterns, IDC, and invasive cribriform carcinoma, as reflected by the current ISUP consensus conference. Reporting of these histopathologic findings is therefore recommended. Clinicians, however, may be less cognizant of the practical impact of these morphologic findings on individual patients’ risk stratification. For instance, many studies have shown that GG2 prostatic carcinoma patients without invasive cribriform carcinoma and/or IDC have less aggressive cancer than those with these features, but it is not yet clear whether their clinical outcome approaches that of men with GG1 cancer. Kweldam and colleagues65,97 found that men with biopsy GG2 without cribriform architecture had similar cancer-specific and postoperative biochemical recurrence-free survival to those with GG1. If the excellent outcome of patients with GG2 without cribriform can be validated in cohorts that have not undergone subsequent radical therapy, it might have a major impact for active surveillance eligibility. More studies comparing clinical and pathologic outcomes between newly defined subgroups are necessary to improve individual risk stratification.
Comprehensive modification of the current grading system incorporating these pathologic features might have significant clinical impact, if its predictive value and reproducibility can outperform current Gleason grading. In the premeeting survey, the majority of respondents were open to altering the current GS/GG system incorporating the recently recognized prognostic factors, but 63% of the respondents considered that more validation was needed before actually changing the grading system. A few grading system modifications have been proposed in recent years. Iczkowski et al64 proposed adding the suffix “C” to define GG 2C, 3C, and 4C signifying that cribriform cancer is present; GG 2, 3, and 4 without C would signify absence of cribriform cancer. While being comprehensive and easy to implement, this would change the current 5-tier to an 8-tier grading system, and as stated previously, it is not clear whether a difference in clinical outcome exists for instance between GG 2C and 3. In a large radical prostatectomy cohort, Sauter et al38 showed that a continuous scale quantifying percent pattern 4 and 5 outperformed Gleason grading. An important advantage of this model is that interobserver variability in grading has less impact than with current Gleason grading/Grade grouping. A disadvantage of this system is the complexity of the grading scheme leading to a continuous risk scale from 0 to 117.5, which would require additional cutoff definitions for practical clinical decision-making. van Leenders et al58 showed that reducing the GG by one point if no invasive cribriform or IDC were present at biopsy led to better discriminative value of the GG for cancer-specific mortality. This improvement was attributable mainly to the overall good outcome of GG2 patients without cribriform architecture, whereas its value in higher GG is less pronounced.
A caveat in most of the studies to date is that they investigate only one pathologic feature, such as percent Gleason pattern 4, minor patterns, invasive cribriform, and/or IDC. It is not clear yet to what extent each of these features holds independent predictive value if analyzed together with the other features as covariates. In GG2 biopsies, the presence of invasive cribriform and/or IDC correlated with incremental percent Gleason pattern 4; it occurred in 6% of men with 1% to 10% Gleason pattern 4, in 22% with 10% to 25% pattern 4, and in 44% of patients with 25% to 50% pattern 4.98 In multivariate analysis, cribriform architecture was an independent predictor for postoperative biochemical recurrence-free survival, whereas percent Gleason pattern 4 as a continuous variable was not. Studies on the interaction of these pathologic variables and their independent predictive values are warranted to identify the most important features, and incorporate those into an improved and reproducible grading system.
Because invasive cribriform and IDC have independent prognostic value and can support individual therapeutic decision-making, it is important that their diagnostic characteristics and distinguishing features are well defined. Among Gleason grade 4 growth patterns, interobserver agreement is best for glomeruloid and cribriform architecture, but is significantly worse for poorly formed and fused glands.99,100 It is, however, not defined yet as to what are the exact distinguishing features of cribriform growth pattern and (1) distended glomeruloid glands with large intraluminal protrusions, and (2) large complex fused glands.100 The premeeting survey revealed that a large range of pathologic features were used for differentiating large glomeruloid, fused, and cribriform glands. Similarly, differentiating features of IDC and high-grade PIN need a clearer distinction.50,57 A subgroup of intraglandular lesions falling short of IDC, but exceeding high-grade PIN, has been labeled as atypical intraductal proliferation, atypical cribriform proliferation, or atypical proliferation suspicious for IDC. A few studies suggest that lesions currently considered suspicious, but not definitive for IDC, are associated with more aggressive cancer than high-grade PIN and are more reminiscent of IDC, but more investigations are needed to eventually expand the current IDC criteria.101 Apart from the common Gleason growth patterns as previously mentioned, certain patterns morphologically overlap or merge with cribriform, including papillary and complex fused glands. Although these are currently assigned Gleason pattern 4, insufficient data exist on their prognostic impact compared with the acknowledged cribriform, small fused, and poorly formed patterns.
Whereas invasive cribriform and IDC are used as dichotomized criteria either being present or absent, percent Gleason patterns 4 and 5 represent continuous variables. As extensively discussed for global and highest GS, it is not yet clear whether percent Gleason patterns 4 and 5 have similar impact as global measures for the entire case or should be reported per biopsy site. Radiopathologic correlation of mpMRI and biopsy sites might facilitate estimation of percentages within one tumor, and differentiate multifocal tumors in the future. Another key development will be developing and evaluating ML-based systems with patient outcome as the standard of reference in contrast to the GS or GG. Several techniques exist to inspect the visual cues used by such systems, which can lead to the identification of prognostic factors such as novel growth patterns or stromal features. These could help drive future revisions of the ISUP grading scheme.
CONCLUSIONS
The 2019 ISUP consensus conference on prostatic carcinoma grading acknowledges the important added value of Gleason pattern quantity, minor Gleason patterns, invasive cribriform carcinoma, and IDC, and provides recommendations for their reporting. Furthermore, it summarizes the grading challenges for the pathologist in the current era of increasing mpMRI-targeted biopsies, and identifies unresolved issues for further research. There is strong belief that novel ML will support prostatic carcinoma grading in the near future. The next steps should be integration of cribriform and IDC and percent high-grade patterns in AI tools. The 2019 consensus conference has not only updated reporting recommendations with the latest state-of-the-art scientific evidence but also identified important objectives for future research.
APPENDIX
TABLE A1.
Participants at the 2019 ISUP Consensus Conference on Grading of Prostatic Carcinoma

Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. [DOI] [PubMed] [Google Scholar]
- 2.Moch H, Humphrey PA, Ulbright TM, Reuter VE, eds. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed Lyon, France: IARC; 2016. [DOI] [PubMed] [Google Scholar]
- 3.Srigley JR, Delahunt B, Samartunga H, et al. Controversial issues in Gleason and International Society of Urological Pathology (ISUP) prostate cancer grading: proposed recommendations for international implementation. Pathology. 2019;51:463–473. [DOI] [PubMed] [Google Scholar]
- 4.Cheng L, Davidson DD, Lin H, et al. Percentage of Gleason pattern 4 and 5 predicts survival after radical prostatectomy. Cancer. 2007;110:1967–1972. [DOI] [PubMed] [Google Scholar]
- 5.Sakr WA, Tefilli MV, Grignon DJ, et al. Gleason score 7 prostate cancer: a heterogeneous entity? Correlation with pathologic parameters and disease-free survival. Urology. 2000;56:730–734. [DOI] [PubMed] [Google Scholar]
- 6.Chan TY, Partin AW, Walsh PC, et al. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823–827. [DOI] [PubMed] [Google Scholar]
- 7.Pierorazio PM, Walsh PC, Partin AW, et al. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int. 2013;111:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole AI, Morgan TM, Spratt DE, et al. Prognostic value of percent Gleason Grade 4 at prostate biopsy in predicting prostatectomy pathology and recurrence. J Urol. 2016;196:405–411. [DOI] [PubMed] [Google Scholar]
- 9.Dean LW, Assel M, Sjoberg DD, et al. Clinical usefulness of total length of Gleason pattern 4 on biopsy in men with Grade Group 2 prostate cancer. J Urol. 2019;20:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CC, Deng FM, Kong MX, et al. Re-evaluating the concept of “dominant/index tumor nodule” in multifocal prostate cancer. Virchows Arch. 2014;464:589–594. [DOI] [PubMed] [Google Scholar]
- 11.Kir G, Seneldir H, Gumus E. Outcomes of Gleason score 3+4=7 prostate cancer with minimal amounts (<6%) vs ≥6% of Gleason pattern 4 tissue in needle biopsy specimens. Ann Diagn Pathol. 2016;20:48–51. [DOI] [PubMed] [Google Scholar]
- 12.Perlis N, Sayyid R, Evans A, et al. Limitations in predicting organ confined prostate cancer in patients with Gleason pattern 4 on biopsy: implications for active surveillance. J Urol. 2017;197:75–83. [DOI] [PubMed] [Google Scholar]
- 13.Iczkowski KA, Lucia MS. Current perspectives on Gleason grading of prostate cancer. Curr Urol Rep. 2011;12:216–222. [DOI] [PubMed] [Google Scholar]
- 14.Morash C, Tey R, Agbassi C, et al. Active surveillance for the management of localized prostate cancer: guideline recommendations. Can Urol Assoc J. 2015;9:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin MB, Lin DW, Gore JL, et al. The critical role of the pathologist in determining eligibility for active surveillance as a management option in patients with prostate cancer: consensus statement with recommendations supported by the College of American Pathologists, International Society of Urological Pathology, Association of Directors of Anatomic and Surgical Pathology, the New Zealand Society of Pathologists, and the Prostate Cancer Foundation. Arch Pathol Lab Med. 2014;138:1387–1405. [DOI] [PubMed] [Google Scholar]
- 16.Sato S, Kimura T, Yorozu T, et al. Cases having a Gleason Score 3+4=7 with 5% of Gleason pattern 4 in prostate needle biopsy show similar failure-free survival and adverse pathology prevalence to Gleason Score 6 cases in a radical prostatectomy cohort. Am J Surg Pathol. 2019;43:1560–1565. [DOI] [PubMed] [Google Scholar]
- 17.Choy B, Pearce SM, Anderson BB, et al. Prognostic significance of percentage and architectural types of contemporary Gleason pattern 4 prostate cancer in radical prostatectomy. Am J Surg Pathol. 2016;40:1400–1406. [DOI] [PubMed] [Google Scholar]
- 18.Sauter G, Pearce SM, Anderson BB, et al. Prognostic significance of percentage and architectural types of contemporary Gleason pattern 4 prostate cancer in radical prostatectomy. Eur Urol. 2018;73:674–683. [DOI] [PubMed] [Google Scholar]
- 19.Egevad L, Kench JG, Delahunt B, et al. Prostate Core Needle Biopsy Histopathology Reporting Guide, 1st ed Sydney, QLD, Australia: International Collaboration on Cancer Reporting; 2017. [Google Scholar]
- 20.Yang XJ, Lecksell K, Potter SR, et al. Significance of small foci of Gleason score 7 or greater prostate cancer on needle biopsy. Urology. 1999;54:528–532. [DOI] [PubMed] [Google Scholar]
- 21.Rubin MA, Dunn R, Kambham N, et al. Should a Gleason score be assigned to a minute focus of carcinoma on prostate biopsy? Am J Surg Pathol. 2000;24:1634–1640. [DOI] [PubMed] [Google Scholar]
- 22.Treurniet KM, Trudel D, Sykes J. Downgrading of biopsy based Gleason score in prostatectomy specimens. J Clin Pathol. 2014;67:313–318. [DOI] [PubMed] [Google Scholar]
- 23.McKenney JK, Simko J, Bonham M, et al. The potential impact of reproducibility of Gleason grading in men with early stage prostate cancer managed by active surveillance: a multi-institutional study. J Urol. 2011;186:465–469. [DOI] [PubMed] [Google Scholar]
- 24.Egevad L, Algaba F, Berney DM, et al. Interactive digital slides with heat maps: a novel method to improve the reproducibility of Gleason grading. Virchows Arch. 2011;459:175–182. [DOI] [PubMed] [Google Scholar]
- 25.Sadimin ET, Khani F, Diolombi M, et al. Interobserver reproducibility of percent Gleason pattern 4 in prostatic adenocarcinoma on prostate biopsies. Am J Surg Pathol. 2016;40:1686–1692. [DOI] [PubMed] [Google Scholar]
- 26.Zhou M, Li J, Cheng L, et al. Diagnosis of “poorly formed glands” Gleason pattern 4 prostatic adenocarcinoma on needle biopsy: an interobserver reproducibility study among urologic pathologists with recommendations. Am J Surg Pathol. 2015;39:1331–1339. [DOI] [PubMed] [Google Scholar]
- 27.Harding-Jackson N, Kryvenko ON, Whittington EE, et al. Outcome of Gleason 3+5=8 prostate cancer diagnosed on needle biopsy: prognostic comparison with Gleason 4+4=8. J Urol. 2016;196:1076–1081. [DOI] [PubMed] [Google Scholar]
- 28.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol, 2005:1228–1242. [DOI] [PubMed] [Google Scholar]
- 29.Epstein JI, Amin MB, Reuter VE, et al. Contemporary Gleason Grading of Prostatic Carcinoma: an update with discussion on practical issues to implement the 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2017;41:e1–e7. [DOI] [PubMed] [Google Scholar]
- 30.Ranaweera M, Samaratunga H, Duffy D, et al. Tertiary Gleason pattern 5 on needle biopsy predicts greater tumour volume on radical prostatectomy. Pathology. 2011;43:693–696. [DOI] [PubMed] [Google Scholar]
- 31.Isbarn H, Ahyai SA, Chun FK, et al. Prevalence of a tertiary Gleason grade and its impact on adverse histopathologic parameters in a contemporary radical prostatectomy series. Eur Urol. 2009;55:394–493. [DOI] [PubMed] [Google Scholar]
- 32.Servoll E, Saeter T, Vlatkovic L, et al. Impact of a tertiary Gleason pattern 4 or 5 on clinical failure and mortality after radical prostatectomy for clinically localised prostate cancer. BJU Int. 2011;109:1489–1494. [DOI] [PubMed] [Google Scholar]
- 33.Adam M, Hannah A, Budäus L, et al. A tertiary Gleason pattern in the prostatectomy specimen and its association with adverse outcome after radical prostatectomy. J Urol. 2014;192:97–102. [DOI] [PubMed] [Google Scholar]
- 34.van Oort IM, Schout BM, Kiemeney LA, et al. Does the tertiary Gleason pattern influence the PSA progression-free interval after retropubic radical prostatectomy for organ-confined prostate cancer? Eur Urol. 2005;49:572–576. [DOI] [PubMed] [Google Scholar]
- 35.Pan CC, Potter SR, Partin AW, et al. The prognostic significance of tertiary Gleason patterns of higher grade in radical prostatectomy specimens: a proposal to modify the Gleason grading system. Am J Surg Pathol. 2000;24:563–569. [DOI] [PubMed] [Google Scholar]
- 36.Trock BJ, Guo CC, Gonzalgo ML, et al. Tertiary Gleason patterns and biochemical recurrence after prostatectomy: proposal for a modified Gleason scoring system. J Urol. 2009;182:1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turker P, Bas E, Bozkurt S, et al. Presence of high grade tertiary Gleason pattern upgrades the Gleason sum score and is inversely associated with biochemical recurrence-free survival. Urol Oncol. 2013;31:93–98. [DOI] [PubMed] [Google Scholar]
- 38.Sauter G, Clauditz T, Steurer S, et al. Integrating tertiary Gleason 5 patterns into quantitative grading in prostate biopsies and prostatectomy specimens. Eur Urol. 2018;71:674–683. [DOI] [PubMed] [Google Scholar]
- 39.Lucca I, Shariat SF, Briganti A, et al. Validation of tertiary Gleason pattern 5 in Gleason score 7 prostate cancer as an independent predictor of biochemical recurrence and development of a prognostic model. Urol Oncol. 2015;33:71.e21–71.e26. [DOI] [PubMed] [Google Scholar]
- 40.Baras AS, Nelson JB, Han M, et al. The effect of limited (tertiary) Gleason pattern 5 on the new prostate cancer grade groups. Hum Pathol. 2017;63:27–32. [DOI] [PubMed] [Google Scholar]
- 41.Borhan W, Epstein JI. Significance of Gleason Score 7 with tertiary pattern 5 at radical prostatectomy. Urology. 2017;100:175–179. [DOI] [PubMed] [Google Scholar]
- 42.Jang WS, Yoon CY, Kim MS, et al. The prognostic role of tertiary Gleason pattern 5 in a contemporary grading system for prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:93–98. [DOI] [PubMed] [Google Scholar]
- 43.Kato M, Hirakawa A, Kobayashi Y, et al. Integrating tertiary Gleason pattern 5 into the ISUP grading system improves prediction of biochemical recurrence in radical prostatectomy patients. Mod Pathol. 2019;32:122–127. [DOI] [PubMed] [Google Scholar]
- 44.Shah RB, Li J, Cheng L, et al. Diagnosis of Gleason pattern 5 prostate adenocarcinoma on core needle biopsy: an interobserver reproducibility study among urologic pathologists. Am J Surg Pathol. 2015;39:1242–1249. [DOI] [PubMed] [Google Scholar]
- 45.McNeal JE, Yemoto CE. Significance of demonstrable vascular space invasion for the progression of prostatic adenocarcinoma. Am J Surg Pathol. 1996;20:1351–1360. [DOI] [PubMed] [Google Scholar]
- 46.Cohen RJ, Chan WC, Edgar SG, et al. Prediction of pathological stage and clinical outcome in prostate cancer: an improved pre-operative model incorporating biopsy-determined intraductal carcinoma. Br J Urol. 1998;81:413–418. [DOI] [PubMed] [Google Scholar]
- 47.Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: histologic features and clinical significance. Mod Pathol. 2006;19:1528–1535. [DOI] [PubMed] [Google Scholar]
- 48.Fine SW, Al-Ahmadie HA, Chen YB, et al. Comedonecrosis revisited: strong association with intraductal carcinoma of the prostate. Am J Surg Pathol. 2018;42:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madan R, Deebajah M, Alanee S, et al. Prostate cancer with comedonecrosis is frequently, but not exclusively, intraductal carcinoma: a need for reappraisal of grading criteria. Histopathology. 2019;74:1081–1087. [DOI] [PubMed] [Google Scholar]
- 50.Varma M, Egevad L, Algaba F, et al. Intraductal carcinoma of prostate reporting practice: a survey of expert European uropathologists. J Clin Pathol. 2016;69:852–857. [DOI] [PubMed] [Google Scholar]
- 51.Varma M, Egevad L, Delahunt B, et al. Reporting intraductal carcinoma of the prostate: a plea for greater standardization. Histopathology. 2017;70:504–507. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto A, Kato M, Hattori K, et al. Propensity score-matched comparison of docetaxel and androgen receptor axis-targeted agents in patients with castration-resistant intraductal carcinoma of the prostate. BJU Int. 2020;125:702–708. [DOI] [PubMed] [Google Scholar]
- 53.Epstein JI, Oxley J, Ro JY, et al. Moch H, Humphrey PA, Ulbright TM, Reuter V. Tumours of the prostate: intraductal carcinoma. WHO Classification of Tumours of the Urinary System and Male Genital Organs. Lyon, France: International Agency for Research on Cancer; 2016:164–165. [Google Scholar]
- 54.Robinson BD, Epstein JI. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings. J Urol. 2010;184:1328–1333. [DOI] [PubMed] [Google Scholar]
- 55.Khani F, Epstein JI. Prostate biopsy specimens with Gleason 3+3=6 and intraductal carcinoma: radical prostatectomy findings and clinical outcomes. Am J Surg Pathol. 2015;39:1383–1389. [DOI] [PubMed] [Google Scholar]
- 56.Gandhi JS, Smith SC, Paner GP, et al. Reporting practices and resource utilization in the era of intraductal carcinoma of the prostate: a survey of genitourinary subspecialists. Am J Surg Pathol. 2020;44:673–680. [DOI] [PubMed] [Google Scholar]
- 57.Iczkowski KA, Egevad L, Ma J, et al. Intraductal carcinoma of the prostate: interobserver reproducibility survey of 39 urologic pathologists. Ann Diagn Pathol. 2014;18:333–342. [DOI] [PubMed] [Google Scholar]
- 58.van Leenders GJLH, Kweldam CF, Hollemans E, et al. Improved prostate cancer biopsy grading by incorporation of invasive cribriform and intraductal carcinoma in the 2014 grade groups. Eur Urol. 2020;77:191–198. [DOI] [PubMed] [Google Scholar]
- 59.Isaacsson Velho P, Silberstein JL, Markowski MC, et al. Intraductal/ductal histology and lymphovascular invasion are associated with germline DNA-repair gene mutations in prostate cancer. Prostate. 2018;78:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caffrey M. NCCN prostate cancer update emphasizes germline testing; 2019. Available at: www.ajmc.com/conferences/nccn-2019/nccn-prostate-cancer-update-emphasizes-germline-testing Accessed January 11, 2020.
- 61.Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50:125–128. [PubMed] [Google Scholar]
- 62.Iczkowski KA, Torkko KC, Kotnis GR, et al. Digital quantification of five high-grade prostate cancer patterns, including the cribriform pattern, and their association with adverse outcome. Am J Clin Pathol. 2011;136:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kweldam CF, Wildhagen MF, Steyerberg EW, et al. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod Pathol. 2015;28:457–464. [DOI] [PubMed] [Google Scholar]
- 64.Iczkowski KA, van der Kwast TA, Paner G. The new realization about cribriform prostate cancer. Adv Anat Pathol. 2018;25:31–37. [DOI] [PubMed] [Google Scholar]
- 65.Kweldam CF, Kümmerlin IP, Nieboer D, et al. Disease-specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Mod Pathol. 2016;29:630–636. [DOI] [PubMed] [Google Scholar]
- 66.Ronen S, Abbott DW, Kravtsov O, et al. PTEN loss and p27 loss differ among morphologic patterns of prostate cancer, including cribriform. Hum Pathol. 2017;65:85–91. [DOI] [PubMed] [Google Scholar]
- 67.Roobol MJ, Verbeek JFM, van der Kwast T, et al. Improving the Rotterdam European Randomized Study of Screening for Prostate Cancer Risk Calculator for Initial Prostate Biopsy by Incorporating the 2014 International Society of Urological Pathology Gleason Grading and Cribriform growth. Eur Urol. 2017;72:45–51. [DOI] [PubMed] [Google Scholar]
- 68.Häggman M, Nordin B, Mattson S, et al. Morphometric studies of intra-prostatic volume relationships in localized prostatic cancer. Br J Urol. 1997;80:612–617. [DOI] [PubMed] [Google Scholar]
- 69.Wise AM, Stamey TA, McNeal JE, et al. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60:264–269. [DOI] [PubMed] [Google Scholar]
- 70.Arora R, Koch MO, Eble JN, et al. Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer. 2004;100:2362–2366. [DOI] [PubMed] [Google Scholar]
- 71.van der Kwast TH, Amin MB, Billis A, et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 2: T2 substaging and prostate cancer volume. Mod Pathol. 2011;24:16–25. [DOI] [PubMed] [Google Scholar]
- 72.Varma M, Narahari K, Mason M, et al. Contemporary prostate biopsy reporting: insights from a survey of clinicians’ use of pathology data. J Clin Pathol. 2018;71:874–878. [DOI] [PubMed] [Google Scholar]
- 73.Kunz GM, Epstein JI. Should each core with prostate cancer be assigned a separate Gleason score? Hum Pathol. 2003;34:911–914. [DOI] [PubMed] [Google Scholar]
- 74.Kunju LP, Daignault S, Wei JT, et al. Multiple prostate cancer cores with different Gleason grades submitted in the same specimen container without specific site designation: should each core be assigned an individual Gleason score? Hum Pathol. 2009;40:558–564. [DOI] [PubMed] [Google Scholar]
- 75.Tolonen TT, Kujala PM, Tammela TL, et al. Overall and worst Gleason scores are equally good predictors of prostate cancer progression. BMC Urol. 2011;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berney DM, Beltran L, Fisher G, et al. Validation of a contemporary prostate cancer grading system using prostate cancer death as outcome. Br J Cancer. 2016;114:1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verhoef EI, Kweldam CF, Kümmerlin IP, et al. Characteristics and outcome of prostate cancer patients with overall biopsy Gleason score 3+4=7 and highest Gleason score 3+4=7 or >3+4=7. Histopathology. 2018;72:760–765. [DOI] [PubMed] [Google Scholar]
- 78.Sauter G, Steurer S, Clauditz TS, et al. Clinical utility of quantitative Gleason grading in prostate biopsies and prostatectomy specimens. Eur Urol. 2016;69:592–598. [DOI] [PubMed] [Google Scholar]
- 79.Arias-Stella JA, 3rd, Shah AB, Montoya-Cerrillo D, et al. Prostate biopsy and radical prostatectomy Gleason score correlation in heterogenous tumors: proposal for a composite Gleason score. Am J Surg Pathol. 2015;39:1213–1218. [DOI] [PubMed] [Google Scholar]
- 80.Athanazio D, Gotto G, Shea-Budgell M, et al. Global Gleason grade groups in prostate cancer: concordance of biopsy and radical prostatectomy grades and predictors of upgrade and downgrade. Histopathology. 2017;70:1098–1106. [DOI] [PubMed] [Google Scholar]
- 81.Trpkov K, Sangkhamanon S, Yilmaz A, et al. Concordance of “case level” global, highest, and largest volume cancer grade group on needle biopsy versus grade group on radical prostatectomy. Am J Surg Pathol. 2018;42:1522–1529. [DOI] [PubMed] [Google Scholar]
- 82.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goel S, Shoag JE, Gross MD, et al. Concordance between biopsy and radical prostatectomy pathology in the era of targeted biopsy: a systematic review and meta-analysis. Eur Urol Oncol. 2020;3:10–20. [DOI] [PubMed] [Google Scholar]
- 84.van der Leest M, Cornel E, Israël B, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol. 2019;75:570–578. [DOI] [PubMed] [Google Scholar]
- 85.Baboudjian M, Bandelier Q, Gondran-Tellier B, et al. MRI-targeted biopsy for detecting prostate cancer: have the guidelines changed our practices and our prostate cancer detection rate? Int Urol Nephrol. 2020;52:611–618. [DOI] [PubMed] [Google Scholar]
- 86.Raskolnikov D, Rais-Bahrami S, Turkbey B, et al. Current ability of multiparametric prostate magnetic resonance imaging and targeted biopsy to improve the detection of prostate cancer. Urol Pract. 2014;1:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gordetsky JB, Schultz L, Porter KK, et al. Defining the optimal method for reporting prostate cancer grade and tumor extent on magnetic resonance/ultrasound fusion-targeted biopsies. Hum Pathol. 2018;76:68–75. [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y, Deng FM, Huang H, et al. Prostate cancers detected by magnetic resonance imaging-targeted biopsies have a higher percentage of gleason pattern 4 component and are less likely to be upgraded in radical prostatectomies. Arch Pathol Lab Med. 2019;143:86–91. [DOI] [PubMed] [Google Scholar]
- 89.Rouvière O, Schoots IG, Mottet N. EAU-EANM-ESTRO-ESUR-SIOG Prostate Cancer Guidelines Panel. Multiparametric magnetic resonance imaging before prostate biopsy: a chain is only as strong as its weakest link. Eur Urol. 2019;75:889–890. [DOI] [PubMed] [Google Scholar]
- 90.Litjens G, Sánchez CI, Timofeeva N, et al. Deep learning as a tool for increased accuracy and efficiency of histopathological diagnosis. Sci Rep. 2016;6:26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Campanella G, Hanna MG, Geneslaw L, et al. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat Med. 2019;25:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ström P, Kartasalo K, Olsson KH, et al. Pathologist-level grading of prostate biopsies with artificial intelligence. Lancet Oncol. 2020;21:222–232. [DOI] [PubMed] [Google Scholar]
- 93.Nagpal K, Foote D, Liu Y, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer. NPJ Digit Med. 2019;2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nordström T, Picker W, Aly M, et al. Detection of prostate cancer using a multistep approach with prostate-specific antigen, the Stockholm 3 Test, and Targeted Biopsies: the STHLM3 MRI Project. Eur Urol Focus. 2017;3:526–528. [DOI] [PubMed] [Google Scholar]
- 95.Egevad L, Delahunt B, Berney DM, et al. Utility of Pathology Imagebase for standardisation of prostate cancer grading. Histopathology. 2018;73:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bulten W, Bándi P, Hoven J, et al. Epithelium segmentation using deep learning in H&E-stained prostate specimens with immunohistochemistry as reference standard. Sci Rep. 2019;9:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kweldam CF, Kümmerlin IP, Nieboer D, et al. Prostate cancer outcomes of men with biopsy Gleason score 6 and 7 without cribriform or intraductal carcinoma. Eur J Cancer. 2016;66:26–33. [DOI] [PubMed] [Google Scholar]
- 98.Kweldam CF, Kümmerlin IP, Nieboer D, et al. Presence of invasive cribriform or intraductal growth at biopsy outperforms percentage grade 4 in predicting outcome of Gleason score 3+4=7 prostate cancer. Mod Pathol. 2017;30:1126–1132. [DOI] [PubMed] [Google Scholar]
- 99.Berney DM, Algaba F, Camparo P, et al. The reasons behind variation in Gleason grading of prostatic biopsies: areas of agreement and misconception among 266 European pathologists. Histopathology. 2014;64:405–411. [DOI] [PubMed] [Google Scholar]
- 100.Kweldam CF, Nieboer D, Algaba F, et al. Gleason grade 4 prostate adenocarcinoma patterns: an interobserver agreement study among genitourinary pathologists. Histopathology. 2016;69:441–449. [DOI] [PubMed] [Google Scholar]
- 101.Shah RB, Nguyen JK, Przybycin CG, et al. Atypical intraductal proliferation detected in prostate needle biopsy is a marker of unsampled intraductal carcinoma and other adverse pathological features: a prospective clinicopathological study of 62 cases with emphasis on pathological outcomes. Histopathology. 2019;75:346–353. [DOI] [PubMed] [Google Scholar]



