Supplemental Digital Content is available in the text.
Keywords: artificial intelligence; outcomes assessment, health care; radiology; stroke; treatment outcome
Abstract
Background and Purpose:
Mechanical thrombectomy (MTB) is a reference treatment for acute ischemic stroke, with several endovascular strategies currently available. However, no quantitative methods are available for the selection of the best endovascular strategy or to predict the difficulty of clot removal. We aimed to investigate the predictive value of an endovascular strategy based on radiomic features extracted from the clot on preinterventional, noncontrast computed tomography to identify patients with first-attempt recanalization with thromboaspiration and to predict the overall number of passages needed with an MTB device for successful recanalization.
Methods:
We performed a study including 2 cohorts of patients admitted to our hospital: a retrospective training cohort (n=109) and a prospective validation cohort (n=47). Thrombi were segmented on noncontrast computed tomography, followed by the automatic computation of 1485 thrombus-related radiomic features. After selection of the relevant features, 2 machine learning models were developed on the training cohort to predict (1) first-attempt recanalization with thromboaspiration and (2) the overall number of passages with MTB devices for successful recanalization. The performance of the models was evaluated on the prospective validation cohort.
Results:
A small subset of radiomic features (n=9) was predictive of first-attempt recanalization with thromboaspiration (receiver operating characteristic curve–area under the curve, 0.88). The same subset also predicted the overall number of passages required for successful recanalization (explained variance, 0.70; mean squared error, 0.76; Pearson correlation coefficient, 0.73; P<0.05).
Conclusions:
Clot-based radiomics have the ability to predict an MTB strategy for successful recanalization in acute ischemic stroke, thus allowing a potentially better selection of the MTB strategy, as well as patients who are most likely to benefit from the intervention.
Mechanical thrombectomy (MTB) is a reference treatment for acute ischemic stroke (AIS) due to large vessel occlusion.1–5 While rapid recanalization of the occluded vessel correlates with a better clinical outcome6 and a lower rate of periprocedural complications,7 the rate of recanalization decreases with an increasing number of thrombectomy attempts.8,9 The selection of patients eligible for endovascular treatment is based on pretherapy brain imaging, and some causes of recanalization failure following MTB have been linked to clinical, demographic, and procedural factors.10,11 However, it is currently not possible to predict the success of recanalization following endovascular treatment based on pretherapy images alone.
Several strategies for MTB have been suggested12 by using different medical devices and relying mainly on stent retriever or direct thromboaspiration,13 such as a direct aspiration first pass technique (ADAPT) approach. Although both are comparable in terms of overall effectiveness,14,15 there is no method to determine which one will be the most effective for any specific patient. In addition, there is no quantitative method based on the clot, the treatment target, to predict the difficulty of endovascular treatment. It has been shown recently that vessel architecture at the occlusion site in the form of the angle of interaction between the aspiration catheter and the clot was associated with successful recanalization.16 Another recent study also reported that the texture of the clot, or radiomic features (RFs), is predictive of recanalization following treatment with intravenous alteplase for AIS.17 This suggests that the extraction of pretherapeutic RF from radiological imaging may contain valuable information related to the composition of the clot, with an impact on the future success of MTB.
In the current study, we used pretherapeutic computed tomography (CT) to extract RF from the clot to investigate their capacity to predict the success of the MTB strategy, with the intention to develop and validate 2 models: (1) to identify patients with first-attempt recanalization with thromboaspiration and (2) to predict the overall number of passages with an MTB device required for successful recanalization.
Methods
Patient Selection
The study included 2 cohorts of patients who underwent brain CT for suspected AIS. These comprised (1) a training cohort of 109 patients admitted between January 2017 and December 2018 that was built retrospectively to select relevant RF and train our 2 predictive machine learning (ML) models and (2) a prospective validation cohort of 47 patients admitted between January and September 2019 that was used to assess the accuracy of our predictive models against an external dataset (Figure 1). All patients in both cohorts underwent a whole-brain stroke CT protocol using a SOMATOM Force scanner (Siemens Healthcare), including thin-slice noncontrast CT (NCCT; ≤2 mm), CT angiography (≤0.625 mm), and CT perfusion. All patients were then treated by MTB with first-attempt recanalization using thromboaspiration according to the ADAPT technique, followed by additional devices at the operator’s discretion, such as stent retrievers, if first-attempt recanalization with the ADAPT technique failed. The study was approved by the local ethics committee for research on human subjects (CCER 2017-00922), which waived the need for written informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Figure 1.

Flowchart of the validation cohort. AIS indicates acute ischemic stroke; CT, computed tomography; CTA, computed tomography angiography; MRI, magnetic resonance imaging; MTB, mechanical thrombectomy; and NCCT, noncontrast computed tomography.
Thrombus Segmentation
NCCT and CT angiography images were automatically coregistered for each patient in both cohorts using a rigid registration algorithm provided in 3D Slicer (version 4.10.2).18 A neuroradiologist fellow (J.H.) manually segmented all thrombi on NCCT in 3D Slicer while viewing the corresponding CT angiography image for guidance, similar to Qui et al.17
RF Extraction
A large number of RFs were automatically extracted from the segmented thrombi using pyradiomics (version 2.2.0).19 This allowed to compute first-order statistics related to thrombus intensity, shape, and size features, including higher order textural features using gray level co-occurrence matrix features, gray level size zone matrix features, gray level run length matrix, neighboring gray tone difference matrix, and gray level dependence matrix features. Further higher order features were added by applying filters to the native NCCT images (see Table 1 for details on image filters used). Overall, a total of 1485 RFs were extracted from each thrombus segmented on NCCT.
Table 1.
Radiomics Features and Filters

RF Selection
Before developing the 2 models to predict first-attempt recanalization and the overall number of passages, we selected the most relevant subset of RF on the training cohort using univariate feature selection. We used the χ2 test to identify individual RF significantly associated with first-attempt recanalization following thromboaspiration with the ADAPT strategy (P<0.05) in the training cohort among all 1485 RFs extracted previously. These RFs were first normalized to scale all individual features to have unit norm and then used to develop models to predict both success using the ADAPT strategy and the overall number of passages with MTB for successful recanalization.
MTB Strategy Prediction
We developed 2 ML models to predict (1) first-attempt recanalization with thromboaspiration with ADAPT and (2) the number of passages required for successful recanalization. In both cases, successful recanalization was defined as a modified Thrombolysis in Cerebral Infarction score ≥2b.20 The 2 ML models were trained on the RF selected previously. The models were also retrained and assessed individually for each RF selected, following the same procedure. In all cases, ML models were developed and trained on the training cohort and then prospectively assessed on the validation cohort. The selection of RF and the development of the 2 ML models were performed using scikit-learning (version 0.21.3), an open-source Python library.21
The first ML model was based on a support vector machine classifier, and its best parameters were estimated during a 5×2 nested cross-validation procedure (inner, outer folds) on the training cohort. The support vector machine model with the best receiver operating characteristic curve–area under the curve in the training cohort was assessed on the prospective validation cohort. Similarly, the second ML regression model was based on a support vector regression, and its best parameters were also estimated during a 5×2 nested cross-validation procedure on the training cohort. The support vector machine model with the best mean squared error score in the training cohort was assessed on the validation cohort. Results of explained variance, mean squared error, and the Pearson correlation coefficient are reported in the independent validation cohort.
Radiomics Quality Score
A radiomics quality score has been developed to ensure scientific rigor and a high level of reporting in radiomics studies.21 The score of this study was 17 of 36; further details are provided in Table I in the Data Supplement. Our study also meets the criteria laid out in the Transparent Reporting of Multivariable Prediction Model for Individual Prognosis or Diagnosis statement (Table II in the Data Supplement).
Results
Patient Characteristics
The baseline demographic and clinical characteristics of the 47 patients included in the validation cohort are summarized in Table 2. The difference regarding clinical and demographic variables was assessed using t test for parametric variables, Fisher exact test of proportion for categorical variables, and the Wilcoxon rank-sum test for nonparametric variables to compare patients with and without first-attempt recanalization with ADAPT. None of the variables assessed was significantly different between the 2 groups (all P>0.05).
Table 2.
Demographic and Clinical Characteristics of the Validation Cohort

Selection of the Best RF
The most relevant subset of RF was identified using univariate feature selection on the training cohort. Four of the 9 RFs selected were positively associated with first-attempt recanalization following thromboaspiration (P<0.05): large area low gray level emphasis, gray level variance, large dependence emphasis, and short run emphasis. Thus, a good response to thromboaspiration was positively associated with lower Hounsfield unit (HU) values, more variance in the clot HU values, and a more homogenous and finer clot texture. Five of the 9 RFs selected were negatively associated with first-attempt recanalization following thromboaspiration (P<0.05): entropy, maximum, run percentage, coarseness, and gray level nonuniformity normalized. Hence, rapid recanalization was negatively associated with higher HU values, as well as texture randomness, coarseness, and heterogeneity of the clot.
Predicting First-Attempt Recanalization With ADAPT
The first ML model based on a support vector machine classifier accurately predicted successful recanalization after first-attempt recanalization with ADAPT on the independent validation cohort. It performed with an overall accuracy of 85.1% (95% CI, 71.7–93.8), a sensitivity of 50.0% (95% CI, 21.1–78.9), a specificity of 97.1% (95% CI, 85.1–99.9), a positive predictive value of 85.7% (95% CI, 44.5–97.8), and a negative predictive value of 85.0% (95% CI, 76.2–90.9). Figure 2 shows the receiver operating characteristic curve of this predictive model based on the 9 selected RFs on the validation cohort, with a value of 0.88.
Figure 2.
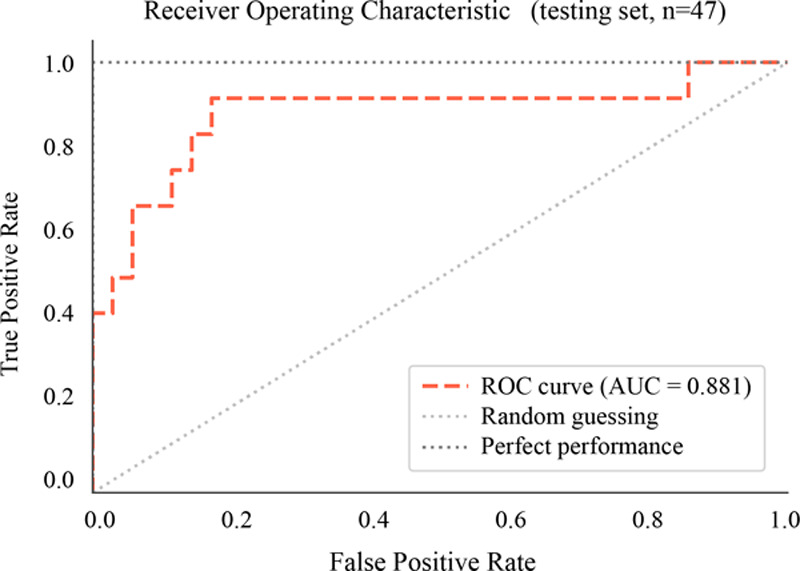
Receiver operating characteristic curve (ROC)–area under the curve (AUC) for the identification of patients with first-attempt recanalization with the direct aspiration first pass technique strategy in the validation cohort. The first machine learning model showed good classification accuracy, with an ROC-AUC of 0.88 on the independent validation cohort.
Predicting the Number of Passages With an MTB Device for Successful Recanalization
The second ML model was based on a support vector regression and accurately predicted the number of passes with MTB devices for obtaining successful recanalization, with an explained variance of 0.70, an mean squared error of 0.76, and a Pearson correlation coefficient of 0.73 (P<0.05) on the independent validation cohort. The predicted number of passages for all individual patients of the validation cohort is shown in Figure 3, together with the observed number of passages performed to achieve successful recanalization (modified Thrombolysis in Cerebral Infarction score, ≥2b).
Figure 3.

Number of passages with a mechanical thrombectomy (MTB) device predicted and observed. Overall number of predicted and observed passages with an MTB device for successful recanalization (modified Thrombolysis in Cerebral Infarction score, ≥2b) in the independent validation cohort. The second machine learning model achieved a good prediction based on clot-derived radiomic features, with an explained variance of 0.70, an mean squared error of 0.76 and a Pearson correlation coefficient of 0.73 (P<0.05).
Discussion
We aimed to predict the optimal MTB strategy in patients with AIS, based on RF derived from clots identified on pretherapeutic NCCTs. By calculating the texture, size, shape, and higher order parameters of these clots and then developing a predictive model on a first training cohort, our study showed that we were able to identify patients with first-attempt recanalization following thromboaspiration in a second validation cohort. We were also able to predict the overall number of passages with an MTB device required for successful recanalization in this same validation cohort. Overall, this may allow a selection of the first-line endovascular strategy to be tailored to each patient to reduce the number of passages performed. The reduction of MTB passes could allow a prompt cerebral reperfusion,6 the reduction of potential complications related to several passes,7 and, finally, to an improvement of the clinical outcome.8,9
Recent research has shown that it is possible to predict the clinical outcome of patients undergoing MTB in the context of AIS using ML models based on clinical variables,22 and this may provide better decision support to perform MTB in some patients. In a complementary manner, our study showed that it is possible to guide the endovascular procedure based on imaging biomarkers contained in the pretherapeutic imaging of the clot. A few studies have recently focused on the role of thrombus in the response to medical or endovascular treatment of AIS.23 For example, it has been shown that the vascular architecture adjacent to the clot (ie, the angle of interaction between the thrombectomy material and the clot) is associated with successful thromboaspiration.16 In our study, we showed that some thrombus parameters are predictive of first-attempt recanalization following thromboaspiration, as well as the overall difficulty of thrombus removal. As the RFs include information on the shape of the clot, these may have contributed to the good prediction observed in the validation cohort. Previous studies did not find a relationship between clot size, volume or density, and the efficacy of endovascular treatment.24 Similarly, these parameters were not associated with rapid recanalization in our study or in a previous trial predicting a response to recombinant tPA (tissue-type plasminogen activator) therapy17 and thus were not selected in our final models. However, due to the automatic calculation of numerous RF, we were able to identify several other imaging biomarkers of the clot associated with a good response to endovascular treatment. We found that rapid recanalization was positively associated with thrombi of a lower HU value, being more homogeneous with a finer texture. Conversely, recanalization was slower when the thrombi were of a higher HU value and more heterogeneous and coarser.
In a recent study, Qiu et al17 evaluated the value of radiomics in predicting the efficacy of intravenous alteplase in the treatment of patients with AIS. They found that radiomics analysis of heterogeneous thrombi texture was able to predict alteplase efficacy. Although our study design is similar to that of Qiu et al, we focused on the evaluation of the efficacy of radiomics in predicting results obtained by endovascular MTB, instead of intravenous alteplase. However, the results of the two studies can be considered to be complementary as Qiu et al demonstrated the efficacy of radiomics based on the identification of heterogeneous thrombi texture, while we demonstrated their efficacy based on the identification of homogeneous thrombi. Such findings might have a clinical impact since the treatment offered to patients with AIS could be tapered according to thrombus texture identified by radiomics on the admitting cerebral imaging.
Intracranial thrombi have different biochemical compositions, and it has been previously shown that this composition appears to affect their treatment response. Indeed, thrombi with a high red blood cell content are removed more rapidly25 and with significantly less MTB passages than thrombi with a low content.26 These insights into clot composition appear to be useful for the selection of the endovascular treatment strategy, as well as for the development of new treatment methods. The different compositions of these intracranial clots also seem to be reflected in their CT or magnetic resonance imaging characteristics where red blood cell and fibrin levels influence the appearance of the clot.27 It also appears possible to quantify the red blood cell content of the clot based on T2*-weighted magnetic resonance imaging. Since RFs are based, in part, on clot texture information, it is, therefore, possible that they reflect, at least in part, the biochemical characteristics of the clot. However, studies are needed to directly relate the different RFs to the biochemical characteristics of clots in AIS.
Limitations
Our study has some limitations. First, it is a single-center, nonrandomized study. Patients were included after MTB, and the effect of our predictive models on endovascular treatment and clinical outcome was not evaluated. Second, our sample size is limited, due, in part, to some patients who did not have a CT before MTB as they were sent from a primary hospital to our stroke referral center. Third, we were unable to directly link the RFs that were useful in our prediction models to the biology of thrombi as thrombi composition was not systematically analyzed. Fourth, RFs can sometimes suffer from poor reproducibility, particularly due to changes in image acquisition parameters. Finally, the manual segmentation of clots on NCCT is inefficient in an emergency clinical setting, and future studies will have to show whether this segmentation can be performed semiautomatically.
Our study had an exploratory function regarding the potentialities of radiomics analysis. At present, the tools and the time needed to perform the analysis are not adapted for clinical practice. In the present setup, radiomics analysis takes ≈20 minutes for each patient, with the most time-consuming step being the manual segmentation of the clot. Nevertheless, different research groups are currently developing automatic segmentation algorithms that will hopefully reduce the analysis timing.28 A prompt, up-front radiomics analysis of the thrombi of patients presenting with an AIS would help to predict the most appropriate treatment for each person. For example, a combined approach of MTB/intravenous alteplase could be offered to patients having heterogeneous thrombi, while a stand-alone MTB could be offered to those with homogenous thrombi. However, such clinical benefits related to the radiomics approach need to be investigated by further studies.
Conclusions
Extraction of RF from the clot visualized on the pretherapeutic NCCT provides information on the success and difficulty of different MTB strategies in patients with AIS. By characterizing the clot, the target of MTB, radiomics, might be a promising technology to personalize the endovascular management of patients with AIS.
Acknowledgments
Drs Hofmeister, Bernava, Vargas, Poletti, Platon, Lovblad, and Machi contributed to the study concept and design. Drs Bernava, Rosi, Vargas, and E. Carrera are neuroradiologists and neurologists who performed patient inclusion and data retrieval. Drs Burgermeister and Montet helped with radiomic feature extraction. Drs Hofmeister and Machi performed the development and validation of models and wrote the first draft of the manuscript. All authors revised and approved the final draft.
Sources of Funding
This work has been supported by a Swiss National Science Foundation grant No. 320030_188942 and 32003B_182382. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
None.
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- ADAPT
- a direct aspiration first pass technique
- AIS
- acute ischemic stroke
- CT
- computed tomography
- HU
- Hounsfield unit
- ML
- machine learning
- MTB
- mechanical thrombectomy
- NCCT
- noncontrast computed tomography
- RF
- radiomic feature
- tPA
- tissue-type plasminogen activator
For Sources of Funding and Disclosures, see page 2493.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.030334.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. ; MR CLEAN Investigators A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 201537211–20doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. ; EXTEND-IA Investigators Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 20153721009–1018doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. ; ESCAPE Trial Investigators Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 20153721019–1030doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, et al. ; REVASCAT Trial Investigators Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 20153722296–2306doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et al. ; SWIFT PRIME Investigators Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 20153722285–2295doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 6.Linfante I, Starosciak AK, Walker GR, Dabus G, Castonguay AC, Gupta R, Sun CH, Martin C, Holloway WE, Mueller-Kronast N, et al. Predictors of poor outcome despite recanalization: a multiple regression analysis of the NASA registry. J Neurointerv Surg 20168224–229doi: 10.1136/neurintsurg-2014-011525 [DOI] [PubMed] [Google Scholar]
- 7.Bourcier R, Saleme S, Labreuche J, Mazighi M, Fahed R, Blanc R, Gory B, Kyheng M, Marnat G, Bracard S, et al. ; ASTER Trial Investigators More than three passes of stent retriever is an independent predictor of parenchymal hematoma in acute ischemic stroke. J Neurointerv Surg 201911625–629doi: 10.1136/neurintsurg-2018-014380 [DOI] [PubMed] [Google Scholar]
- 8.Loh Y, Jahan R, McArthur DL, Shi ZS, Gonzalez NR, Duckwiler GR, Vespa PM, Starkman S, Saver JL, Tateshima S, et al. Recanalization rates decrease with increasing thrombectomy attempts. AJNR Am J Neuroradiol 201031935–939doi: 10.3174/ajnr.A1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baek JH, Kim BM, Heo JH, Nam HS, Kim YD, Park H, Bang OY, Yoo J, Kim DJ, Jeon P, et al. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke 2018492088–2095doi: 10.1161/STROKEAHA.118.021320 [DOI] [PubMed] [Google Scholar]
- 10.Goda T, Oyama N, Kitano T, Iwamoto T, Yamashita S, Takai H, Matsubara S, Uno M, Yagita Y. Factors associated with unsuccessful recanalization in mechanical thrombectomy for acute ischemic stroke. Cerebrovasc Dis Extra 20199107–113doi: 10.1159/000503001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaesmacher J, Gralla J, Mosimann PJ, Zibold F, Heldner MR, Piechowiak E, Dobrocky T, Arnold M, Fischer U, Mordasini P. Reasons for reperfusion failures in stent-retriever-based thrombectomy: registry analysis and proposal of a classification system. AJNR Am J Neuroradiol 2018391848–1853doi: 10.3174/ajnr.A5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munich SA, Vakharia K, Levy EI. Overview of mechanical thrombectomy techniques. Neurosurgery 201985suppl_1S60–S67doi: 10.1093/neuros/nyz071 [DOI] [PubMed] [Google Scholar]
- 13.Turk AS, Spiotta A, Frei D, Mocco J, Baxter B, Fiorella D, Siddiqui A, Mokin M, Dewan M, Woo H, et al. Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J Neurointerv Surg 20146231–237doi: 10.1136/neurintsurg-2013-010713 [DOI] [PubMed] [Google Scholar]
- 14.Tsang COA, Cheung IHW, Lau KK, Brinjikji W, Kallmes DF, Krings T. Outcomes of stent retriever versus aspiration-first thrombectomy in ischemic stroke: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2018392070–2076doi: 10.3174/ajnr.A5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turk AS, 3rd, Siddiqui A, Fifi JT, De Leacy RA, Fiorella DJ, Gu E, Levy EI, Snyder KV, Hanel RA, Aghaebrahim A, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet 2019393998–1008doi: 10.1016/S0140-6736(19)30297-1 [DOI] [PubMed] [Google Scholar]
- 16.Bernava G, Rosi A, Boto J, Brina O, Kulcsar Z, Czarnetzki C, Carrera E, Schaller K, Lovblad KO, Machi P. Direct thromboaspiration efficacy for mechanical thrombectomy is related to the angle of interaction between the aspiration catheter and the clot. J Neurointerv Surg 202012396–400doi: 10.1136/neurintsurg-2019-015113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu W, Kuang H, Nair J, Assis Z, Najm M, McDougall C, McDougall B, Chung K, Wilson AT, Goyal M, et al. Radiomics-based intracranial thrombus features on CT and CTA predict recanalization with intravenous alteplase in patients with acute ischemic stroke. AJNR Am J Neuroradiol 20194039–44doi: 10.3174/ajnr.A5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, et al. 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 2012301323–1341doi: 10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational radiomics system to decode the radiographic phenotype. Cancer Res 201777e104–e107doi: 10.1158/0008-5472.CAN-17-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization Working Group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013442650–2663doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedregosa F, Michel V, Grisel O, Blondel M, Prettenhofer P, Weiss R, Varoquaux G, Gramfort A, Thirion B, Dubourg V, et al. Scikit-learn: Machine Learning in Python [Internet]. 2011 http://scikit-learn.sourceforge.net. Accessed February 21, 2020.
- 22.Nishi H, Oishi N, Ishii A, Ono I, Ogura T, Sunohara T, Chihara H, Fukumitsu R, Okawa M, Yamana N, et al. Predicting clinical outcomes of large vessel occlusion before mechanical thrombectomy using machine learning. Stroke 2019502379–2388doi: 10.1161/STROKEAHA.119.025411 [DOI] [PubMed] [Google Scholar]
- 23.Luthman AS, Bouchez L, Botta D, Vargas MI, Machi P, Lövblad KO. Imaging clot characteristics in stroke and its possible implication on treatment. Clin Neuroradiol 20203027–35doi: 10.1007/s00062-019-00841-w [DOI] [PubMed] [Google Scholar]
- 24.Borst J, Berkhemer OA, Santos EMM, Yoo AJ, den Blanken M, Roos YBWEM, van Bavel E, van Zwam WH, van Oostenbrugge RJ, Lingsma HF, et al. ; MR CLEAN Investigators Value of thrombus CT characteristics in patients with acute ischemic stroke. AJNR Am J Neuroradiol 2017381758–1764doi: 10.3174/ajnr.A5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, Yamasaki M, Kobayashi K, Sano T, Mori G, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra 2018839–49doi: 10.1159/000486042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy S, McCarthy R, Farrell M, Thomas S, Brennan P, Power S, O’Hare A, Morris L, Rainsford E, MacCarthy E, et al. Per-pass analysis of thrombus composition in patients with acute ischemic stroke undergoing mechanical thrombectomy. Stroke 2019501156–1163doi: 10.1161/STROKEAHA.118.023419 [DOI] [PubMed] [Google Scholar]
- 27.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011421237–1243doi: 10.1161/STROKEAHA.110.605576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You J, Yu PLH, Tsang ACO, Tsui ELH, Woo PPS, Leung GKK. Automated segmentation for hyperdense middle cerebral artery sign of acute ischemic stroke on non-contrast CT images. 2019 [cited May 12, 2020]. http://arxiv.org/abs/1905.09049.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


