Supplemental Digital Content is available in the text.
Keywords: alteplase, elderly, ischemic stroke, recombinant tissue-type plasminogen activator, thrombolysis
Abstract
Background/Purpose:
Expert guidelines specify no upper age limit for alteplase for thrombolysis of acute ischemic stroke (AIS) but, until recently, European regulatory criteria restricted its use to patients aged 18 to 80 years. We performed pooled analyses of randomized controlled trial (RCT) and registry data to evaluate the benefit-risk profile of alteplase for AIS among patients aged >80 years to support a regulatory application to lift the upper age restriction.
Methods:
Individual patient data were evaluated from 7 randomized trials of alteplase (0.9 mg/kg) versus placebo or open control for AIS, and the European SITS-UTMOST registry database. Clinical outcomes, including good functional outcome (score 0–1, modified Rankin Scale day 90 or Oxford Handicap Score day 180), were evaluated in the full RCT and registry populations, and specified age-based subgroups (≤80 or >80 years) who met existing European regulatory criteria for alteplase, excluding upper age restriction.
Results:
Regardless of treatment allocation, 90-day mortality was lower among RCT patients aged ≤80 versus >80 years who otherwise met existing European regulatory criteria (246/2405 [10.2%] versus 307/1028 [29.9%], respectively). Among patients aged >80 years, alteplase versus placebo was associated with a higher proportion of good stroke outcome (modified Rankin Scale score 0–1; 99/518 [19.1%] versus 67/510 [13.1%]; P=0.0109) and similar 90-day mortality (153/518 [29.5%] versus 154/510 [30.2%]; P=0.8382). The odds of a good stroke outcome following alteplase allocation in the full RCT population were independent of age (P=0.7383). Good stroke outcome was reported for almost half (4821/11 169 [43.2%]) of the patients who received alteplase in routine practice. Outcomes in routine practice supported those achieved in RCTs.
Conclusions:
Alteplase for AIS has a positive benefit-risk profile among patients aged >80 years when administered according to other regulatory criteria. Alteplase for AIS should be evaluated on an individual benefit-risk basis.
See related article, p 2279
The clinical benefits of intravenous alteplase (recombinant tissue-type plasminogen activator) for thrombolytic treatment of acute ischemic stroke (AIS) are well established. However, key aspects of its regulatory approval differ by region.1 For example, no upper age limit is stated in the US product label, but treatment must be initiated within 3 hours of stroke onset.2 Alteplase is approved for use in Europe within 4.5 hours of stroke onset but, until recently, regulatory criteria restricted its use to patients aged 18–80 years.1,3
Approximately one-third of acute strokes occur among people aged ≥80 years.4,5 However, elderly patients were represented poorly in early clinical trials of thrombolysis for AIS. Subsequent initiatives have provided additional data on this population, including the randomized IST-3 (Third International Stroke Trial),6 controlled comparison analyses,5,7 and several large observational studies, including the SITS-UTMOST (Safe Implementation of Thrombolysis in Upper Time Window Monitoring Study).8–14 Clinical trial and observational data consistently support the use of alteplase for AIS among patients aged >80 years.6,13,15
Expert management guidelines issued by the European Stroke Organisation and American Stroke Association/American Heart Association recommend that alteplase is administered within 4.5 hours of stroke symptom onset and no longer specify an upper age limit.16–19 Closer alignment of regulatory criteria with clinical guidelines could allow more patients to receive a potentially beneficial therapy for AIS.1,5,20
We performed pooled analyses of randomized trial and registry data to evaluate the benefit-risk profile of alteplase for AIS among elderly patients who otherwise met existing European regulatory criteria. These data were used to support a regulatory application to lift the upper age restriction for use of alteplase for AIS.
Methods
Anonymized data used in these analyses can be made available in line with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data (https://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/documents/Policy.pdf). Institutional ethics review was not required.
Individual patient data were available from 9 randomized controlled trials (RCTs) of intravenous alteplase versus placebo or open control for AIS (n=6756).6,21–27 Data on all patients (n=721) from 2 studies were excluded from our analysis (as described in the Data Supplement).22,26 All randomized patients from the remaining 7 trials (ATLANTIS A, ATLANTIS B, ECASS II, ECASS III, IST-3, NINDS A, and NINDS B) conducted across Australasia, Europe, Central, and North America were considered for inclusion.6,21,23–25,27 Data were evaluated using statistical methods and outcomes similar to those described previously.1,28,29
Briefly, we used logistic regression, stratified by trial, to model the common linear dependence of the log odds of a good stroke outcome on allocation to alteplase, age, baseline stroke severity (National Institutes of Health Stroke Scale [NIHSS] score, excluding distal motor function [range 0–42]) and treatment delay (all linear variables) and interactions between allocation to alteplase and each of these other baseline covariates. Missing continuous baseline variables (age, NIHSS score, and treatment delay) were imputed using the overall mean for each study. Missing end points related to stroke outcome were imputed by last observation carried forward/worst case imputation. We used likelihood ratio tests to assess the extent to which treatment delay, age, and stroke severity (baseline NIHSS score) affected proportional treatment effects; statistical significance was tested by comparing the change in deviance between 2 nested models that differed only by interaction term.
To assess the treatment effect of alteplase for AIS in elderly patients, 2 subgroups who received treatment in line with European regulatory criteria (excluding upper age restriction) were specified based on age (≤80 or >80 years). Patients were excluded if: symptom onset to treatment was >4.5 hours; the stroke was considered severe (assessed clinically [NIHSS score >25] or by appropriate imaging); there was a history of both stroke and diabetes mellitus; systolic BP >185; diastolic BP >110 mm Hg; or blood glucose <50 or >400 mg/dL.
Key trial outcomes were compared among the full RCT population and subgroups who met European regulatory criteria (excluding upper age restriction) aged ≤80 or >80 years. Prespecified end points were good stroke outcome at day 90/180; death ≤7 days; death ≤90 days; symptomatic intracranial hemorrhage (sICH); fatal ICH (parenchymal hemorrhage type 2 and death) ≤7 days; and death ≤7 days not related to parenchymal hemorrhage type 2 bleeding. Data are presented with 95% CI.
sICH was defined in the current analyses, among IST-3 patients, as symptomatic neurological deterioration or death with clear evidence of significant ICH on post-randomization scan (or autopsy) ≤2 days after treatment. In all other trials evaluated, sICH was defined as per SITS-MOST (SITS-Monitoring Study) criteria (parenchymal hemorrhage type 2 with NIHSS score deterioration ≥4 from baseline or death ≤36 hours after treatment).30
Functional stroke outcome was assessed using the Oxford Handicap Score at 6 months in IST-3, and modified Rankin Scale (mRS) score at 3 months in all other studies.6 The Oxford Handicap Score and mRS are very similar assessment tools.31 For patients who died 92 to 180 days poststroke in IST-3, an Oxford Handicap Score of 6 at day 90 was changed to mRS score 5 to align with evaluations in other studies. A good stroke outcome was defined as a score of 0–1 on mRS at day 90 or Oxford Handicap Score at day 180 (and reported herein as mRS score at day 90/180).
The distribution of functional stroke outcome among those who received alteplase or placebo in the full RCT population and age-defined subgroups was visualized. Shift analyses of mRS scores at day 90/180 were conducted and unadjusted Cochran-Mantel-Haenszel test used to compare outcomes with alteplase versus placebo in age-defined subgroups.
To provide a real-life perspective, we also analyzed data from the European SITS-UTMOST registry.8 Data were collected prospectively from May 2012, at centers across Europe, on consecutive patients who received alteplase regardless of whether they met approved treatment criteria.
Similar to the evaluation of individual patient data from RCTs described above, analyses were conducted on data on a patient subset from the European SITS-UTMOST registry who met existing European regulatory criteria (excluding upper age restriction) for use of alteplase in AIS; 2 subgroups were defined based on patient age (≤80 or >80 years). No imputation of missing values was applied; available data were reported using the number of patients with a recorded observation as the denominator for each end point. The distribution of mRS scores at day 90/180 among patients who received alteplase in the full RCT and SITS-UTMOST registry populations and age-defined subgroups were also visualized. No comparative statistical analyses were conducted on SITS-UTMOST data.
Estimated differences between groups are reported with 95% CI. P values <0.05 were considered statistically significant.
Results
Pooled Analysis of Randomized Trial Data
The full RCT population comprised 6035 patients from 7 randomized trials of alteplase (0.9 mg/kg) versus placebo or open control for AIS.6,21,23–25,27 Of these, 3026 patients received alteplase and 3009 received placebo or open control. More than a quarter of the full RCT population (1699/6035) were aged >80 years (Table I in the Data Supplement).
Logistic Regression Analyses of the Effects of Alteplase on Stroke Outcomes
In the full RCT population, alteplase administration within 4.5 hours after symptom onset had a beneficial effect on good stroke outcome (Figure 1A). Earlier administration of alteplase was associated with increased odds of good stroke outcome and greater proportional benefit (P=0.0203 for interaction).
Figure 1.
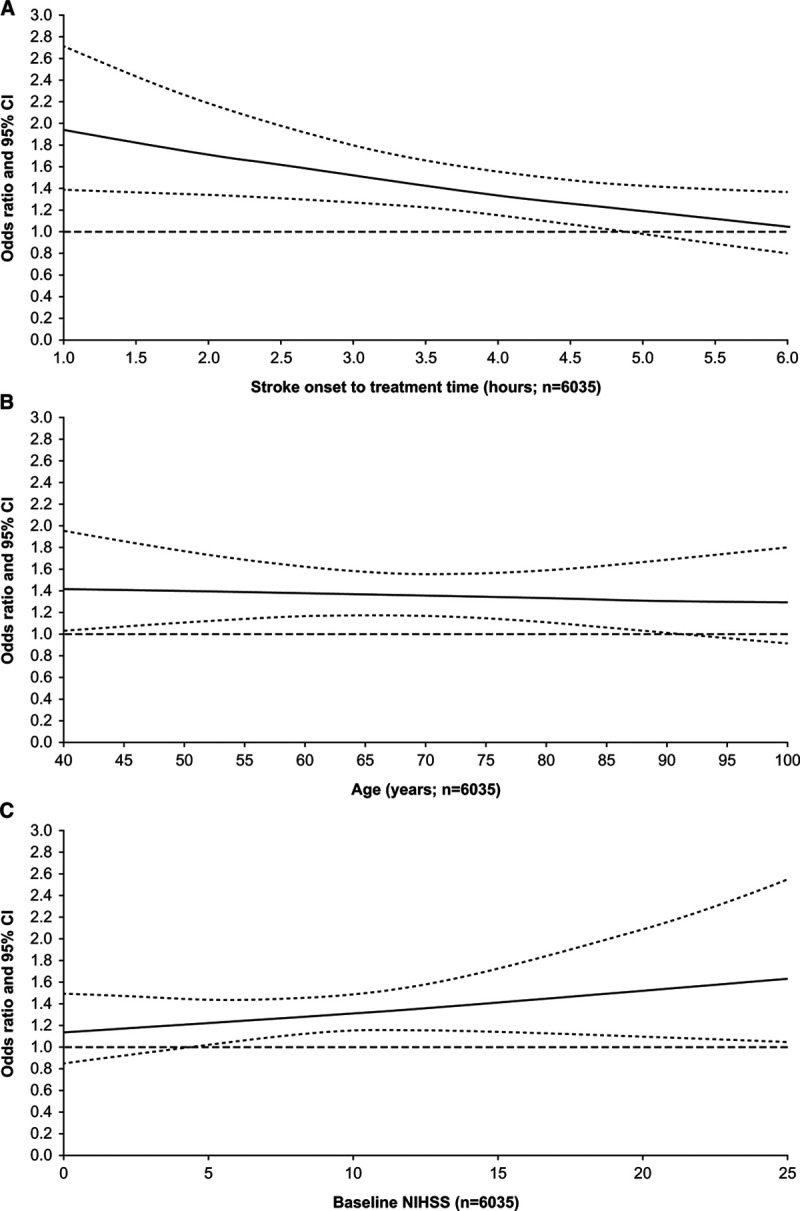
Estimated effect of alteplase vs placebo on the odds of good stroke outcome by time to treatment, patient age, and stroke severity. A, Time to treatment (P=0.0203 for interaction). B, Patient age (P=0.7383 for interaction). C, Stroke severity (baseline National Institutes of Health Stroke Scale [NIHSS] score; P=0.2898 for interaction). Good stroke outcome was defined as a modified Rankin Scale score of 0 to 1 at day 90/180. Each plot is adjusted for treatment delay, age, NIHSS score, and interactions between allocation to alteplase and each of these other covariates, and stratified by study; solid line, estimated odds ratio; dashed lines, 95% CIs.
The odds of a good stroke outcome following alteplase allocation were independent of age (P=0.7383 for interaction; Figure 1B). The 95% CIs widen at extremes of age and cross the odds ratio=1 reference line beyond 90 years.
A significant interaction between baseline NIHSS score and alteplase allocation on stroke outcome was not detected (P=0.2898; Figure 1C). However, CIs support a beneficial effect of alteplase on stroke outcome among patients with a baseline NIHSS score ≥5.
Subgroup Analyses of the Effect of Alteplase on Stroke Outcomes
Two subgroups who met European regulatory criteria (excluding upper age restriction) were specified based on age: ≤80 years (alteplase n=1182; placebo n=1223); >80 years (alteplase n=518; placebo n=510). The most common reason for exclusion was administration of alteplase >4.5 hours after stroke onset (n=2030).
Baseline demographic and disease characteristics of the full RCT population and age-based subgroups who met European regulatory criteria (excluding upper age restriction) are presented in Table I in the Data Supplement. Patients aged >80 years had more severe strokes (mean [SD] baseline NIHSS score 13.2 [6.3] versus 11.2 [5.7]) and higher rates of comorbidities than did younger patients. Baseline characteristics are described in the Data Supplement.
Data on key clinical trial end points are presented in Table 1. Regardless of treatment allocation, overall (90-day) mortality was substantially lower among patients aged ≤80 years versus >80 years who otherwise met existing European regulatory criteria (10.2% [95% CI, 9.0–11.4] versus 29.9% [95% CI, 27.1–32.7]).
Table 1.
Key Clinical Trial End Points for Patients Who Received Alteplase or Placebo for AIS in the Full RCT Population and Specified Age-Defined Subgroups Who Met European Regulatory Criteria
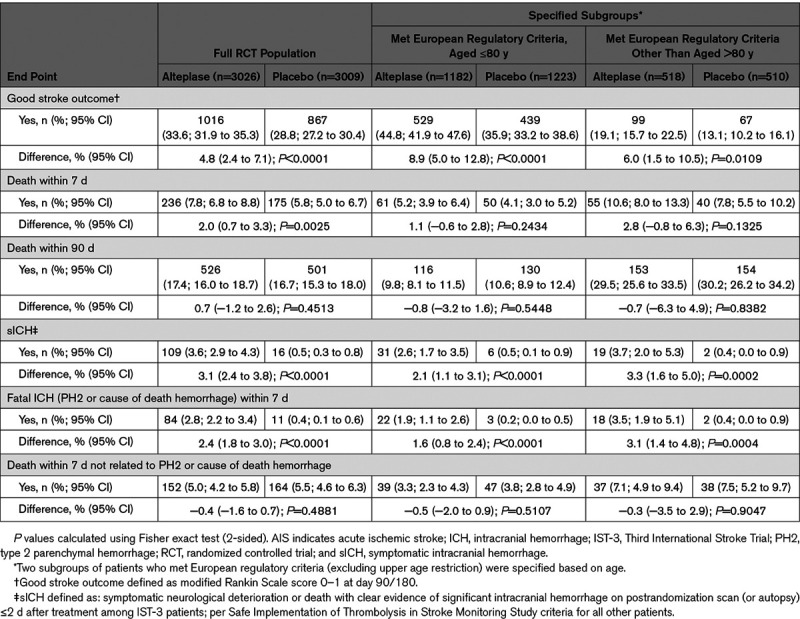
Among patients aged ≤80 years, alteplase versus placebo was associated with a higher proportion of good stroke outcomes (44.8% [95% CI, 41.9–47.6] versus 35.9% [95% CI, 33.2–38.6]; P<0.0001), higher rates of sICH (2.6% [95% CI, 1.7–3.5] versus 0.5% [95% CI, 0.1–0.9]; P<0.0001), and comparable 90-day mortality (9.8% [95% CI, 8.1–11.5] versus 10.6% [95% CI, 8.9–12.4]; P=0.5448).
Likewise, among patients aged >80 years, alteplase versus placebo was associated with a higher proportion of good stroke outcomes (19.1% [95% CI, 15.7–22.5] versus 13.1% [95% CI, 10.2–16.1]; P=0.0109), higher rates of sICH (3.7% [95% CI, 2.0–5.3] versus 0.4% [95% CI, 0.0–0.9]; P=0.0002) and similar 90-day mortality (29.5% [95% CI, 25.6–33.5] versus 30.2% [95% CI, 26.2–34.2]; P=0.8382).
Distribution of mRS Scores at Day 90/180
Distributions of mRS scores at day 90/180 for patients who received alteplase or placebo in the full RCT population and age-defined subgroups are shown in Figure 2. Patients aged ≤80 years had better outcomes than did older patients, regardless of treatment allocation. A positive shift toward better outcomes with alteplase versus placebo can be seen in the full RCT population and both age-defined subgroups (and both time-to-treatment windows [0–3 and 3–4.5 hours]). Shift analyses of mRS scores at day 90/180 showed significantly better outcomes with alteplase (0–4.5 hours) versus placebo among patients aged ≤80 years (P=0.0001) and >80 years (P=0.0106) who otherwise met existing European regulatory criteria for alteplase for AIS.
Figure 2.
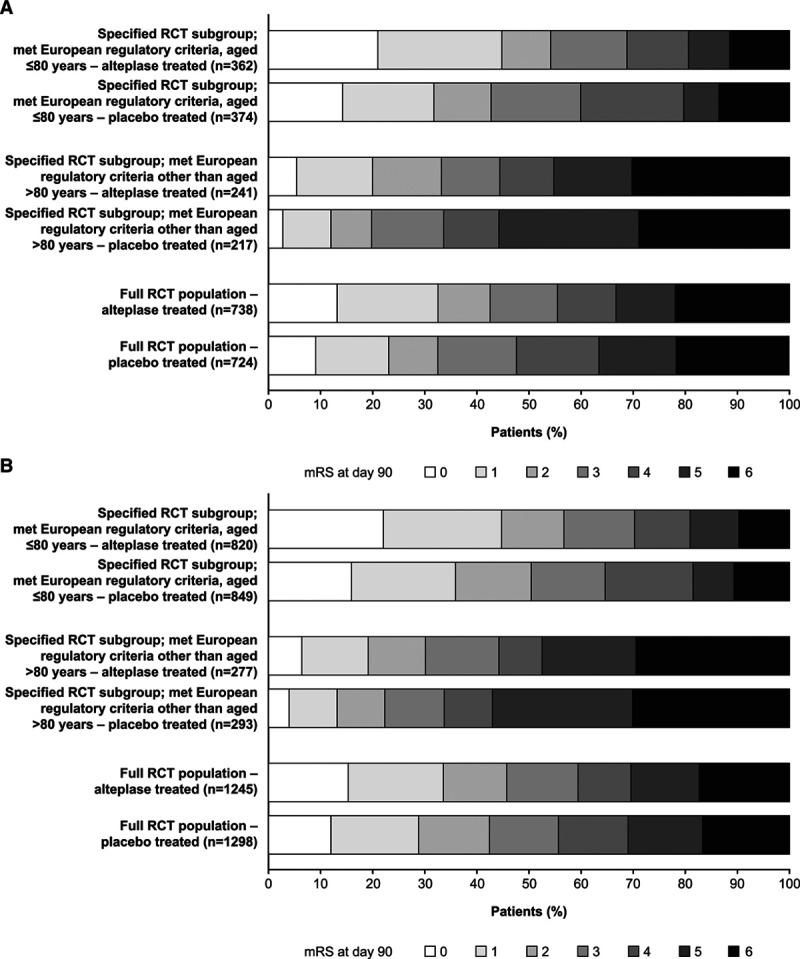
Distribution of modified Rankin Scale (mRS) scores at day 90/180 after acute ischemic stroke (AIS) in the full randomized controlled trial (RCT) population and specified age-defined subgroups (aged ≤80 y or >80 y who otherwise met existing European regulatory criteria for alteplase for AIS). A, Time to treatment 0 to 3 h. B, Time to treatment 3 to 4.5 h.
Analysis of SITS-UTMOST Registry Data
Data were available on 14 086 patients (the full registry population) who received alteplase for AIS at 94 centers. Most patients (11 911/14 086) met existing European regulatory criteria, excluding upper age restriction. From these, 2 subgroups were identified based on patient age: 9491 were aged ≤80 years; 2420 were aged >80 years.
Patients aged >80 years, who otherwise met existing European regulatory criteria had more severe strokes (mean [SD] baseline NIHSS scores 12.9 [6.3] versus 11.0 [6.1], respectively) and higher rates of comorbidities than did younger patients. Baseline data are described in the Data Supplement.
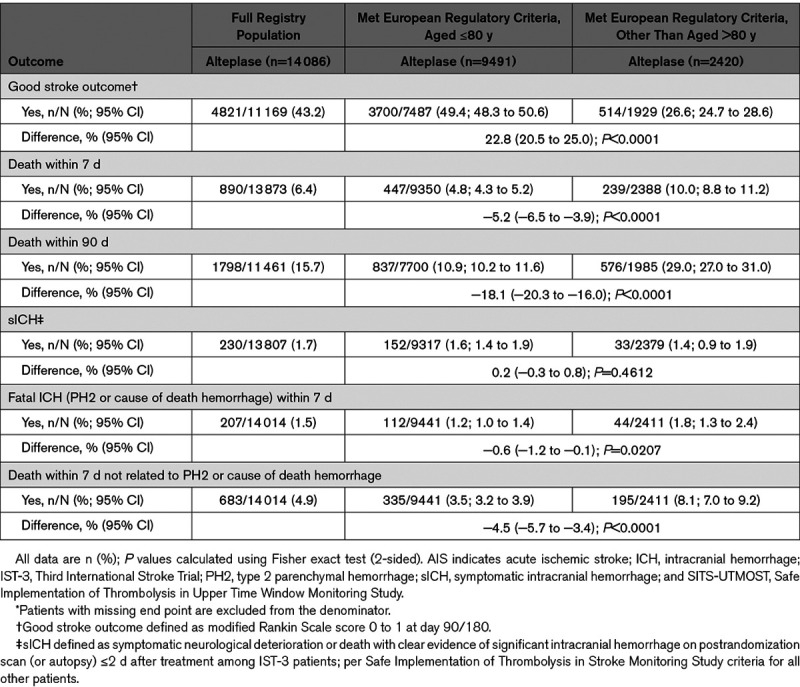
Outcomes among the full registry population and defined subgroups are shown in Table 2. Overall, almost half (4821/11 169; 43.2%) of patients who received alteplase in routine practice (and had a recorded outcome at day 90) had a good stroke outcome. Patients aged ≤80 years had a higher proportion of good stroke outcomes (3700/7487 [49.4%; 95% CI, 48.3–50.6] versus 514/1929 [26.6%; 95% CI, 24.7–28.6]), and lower 90-day mortality (837/7700 [10.9%; 95% CI, 10.2–11.6] versus 576/1985 [29.0%; 95% CI, 27.0–31.0]) versus older patients who otherwise met existing European regulatory criteria. Similar rates of sICH were reported in both subgroups (152/9317 [1.6%; 95% CI, 1.4–1.9] aged ≤80 years versus 33/2379 [1.4%; 95% CI, 0.9–1.9] aged >80 years).
Table 2.
Outcomes Among Patients Who Received Alteplase for AIS in the Full SITS-UTMOST Registry Population and Age-Defined Subgroups Who Met European Regulatory Criteria*
Distribution of mRS Scores at Day 90/180 Among Alteplase-Treated Patients in the RCT and the SITS-UTMOST Registry Populations
Raw mRS score distributions at day 90/180 for alteplase-treated patients in the RCT and the SITS-UTMOST registry populations, and age-defined subgroups are shown in Figure 3 (for 0–3 hour and 3–4.5 hour time-to-treatment periods). Patients aged ≤80 years tended to have better outcomes versus older patients in both time-to-treatment periods. Reported outcomes appeared slightly better among patients in the registry versus RCT populations (in both time-to-treatment periods). For example, a smaller proportion of patients aged >80 years had high mRS scores (5–6) at day 90/180 in the registry versus clinical trial setting.
Figure 3.
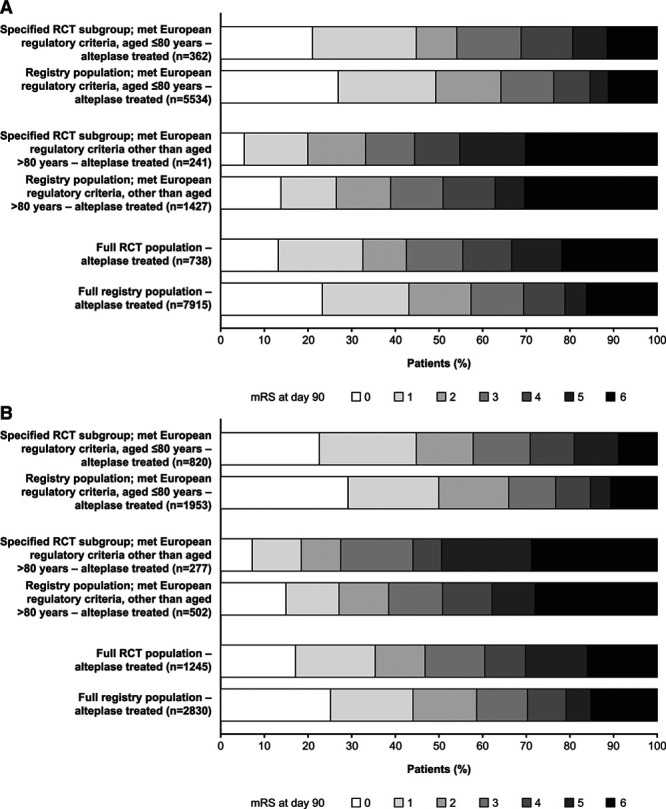
Distribution of mRS scores at day 90/180 after AIS among patients who received alteplase in the full RCT and SITS-UTMOST registry populations, and specified age-defined subgroups (aged ≤80 y or >80 y who otherwise met existing European regulatory criteria for alteplase for AIS). A. Time to treatment 0–3 h. B. Time to treatment 3–4.5 h. AIS indicates acute ischemic stroke; mRS, modified Rankin Scale; RCT, randomized controlled trial; and SITS-UTMOST, Safe Implementation of Thrombolysis in Upper Time Window Monitoring Study.
Discussion
Our pooled analyses of individual patient data from randomized trials and registry studies support a positive benefit–risk profile of intravenous alteplase for AIS, among patients aged >80 years, when administered according to other European regulatory criteria.
Treatment with alteplase in placebo-controlled trials was associated with a greater proportion of good stroke outcome and significantly better functional outcomes overall. The benefits of alteplase were consistent among patient populations aged ≤ or >80 years and were achieved with comparable rates of 90-day mortality versus placebo.
Similarly, the odds of a good outcome of AIS following alteplase treatment versus control were independent of age. Although, the wide CIs (because of restricted patient numbers) at extremes of age limit assessment of any interaction.
Patients aged >80 years, regardless of treatment allocation, had worse functional outcomes and higher mortality rates following AIS compared with younger patients. This is likely to be an effect of increasing age and comorbidity and greater stroke severity. Nonetheless, the positive benefit-risk profile of thrombolysis using alteplase for AIS was consistent across the patient age range.
Our registry data provide reassurance that outcomes among patients aged >80 years who received alteplase for AIS in routine clinical practice correspond well with those in RCTs. The incidence of sICH (1.4% versus 3.7%) and overall mortality (29.0% versus 29.5%) following thrombolysis of patients aged >80 years who otherwise met European regulatory criteria was not increased in routine practice versus clinical trials.
Registry data echo the functional outcomes achieved with alteplase for AIS in randomized trials. Moreover, our data suggest a positive shift toward better outcomes among patients treated in routine practice versus a clinical trial setting although no statistical analyses were performed.
In total, we evaluated outcomes of almost 3000 patients aged >80 years who otherwise met European regulatory criteria and received alteplase for AIS in randomized trials or in clinical practice. This is the largest pooled analysis of alteplase for AIS in this elderly population to date. As for any pooled analysis, our results are dependent on the quality of the primary research. Therefore, we selected datasets for inclusion in our analysis based on strict criteria.
Other key strengths of the current analyses include our access to individual patient data, the use of common definitions for most outcomes, and stratification of logistic regression analyses to retain the influence of individual trials on outcomes. As such, we think that these analyses provide an accurate estimate of the effect size of alteplase for AIS in patients aged >80 years.
Our findings are in line with previously published analyses. The Stroke Thrombolysis Trialists’ Collaborators Group have reported numerous meta-analyses of data on up to 6756 patients who received alteplase or placebo/open control in RCTs.1,15,28,32 Thrombolysis for AIS was associated with an increased risk of sICH soon after treatment.28,32 Nonetheless, alteplase had a positive effect on stroke outcomes regardless of patient age.1,15,28 A beneficial effect of thrombolysis on functional stroke outcome was demonstrated among patients aged >80 years in 2 large nonrandomized, controlled comparison analyses.5,7 Similar benefits of alteplase for AIS were reported among patients aged ≤80 or >80 years in a subsequent Cochrane review (odds ratio of death/dependence 0.85 [95% CI, 0.76–0.95]; P=0.004 versus odds ratio 0.80 [95% CI, 0.64–0.99]; P=0.04, respectively).33
Inclusion of patients in the current analyses followed strict regulatory criteria and, therefore, builds on existing understanding of the benefits of alteplase for AIS in elderly patients. As intended, our data were submitted for regulatory consideration.
A combined body of evidence led to agreement by European regulatory authorities in 2018 to rescind the upper age restriction on the use of alteplase in AIS.3 As such, alteplase is now approved for use in Europe to treat patients aged >80 years.3 Elderly patients should be selected carefully based on their general health and baseline neurological status.3 Regulatory criteria for the use of alteplase for AIS are now better aligned with clinical guidelines.
Conclusions
These pooled analyses of individual patient data from randomized trials and registry studies indicate that intravenous alteplase for AIS has a positive benefit-risk profile in patients aged >80 years, when administered according to other European regulatory criteria. These data are in line with the findings of numerous previous reports. Thrombolysis treatment for patients presenting with AIS should be evaluated on an individual benefit-risk basis. Importantly, age alone is no longer a barrier to alteplase treatment for AIS.
Acknowledgments
Under the direction of the authors, Hannah Wills of 7.4 Limited provided writing and editorial assistance. Boehringer Ingelheim provided funding to 7.4 Limited for writing and editing support and reviewed the manuscript for scientific accuracy. The content of the manuscript and the decision to submit for publication in Stroke was made by the authors independently.
Sources of Funding
Funding for these pooled analyses was provided by Boehringer Ingelheim.
Disclosures
Drs Bluhmki, Danays, and Biegert are employees of Boehringer Ingelheim. Dr Hacke has received a grant from Boehringer Ingelheim and acted as a consultant for Boehringer Ingelheim and Neuravi. Dr Lees has received fees/expenses from data monitoring committee work for Boehringer Ingelheim.
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- AIS
- acute ischemic stroke
- IST-3
- Third International Stroke Trial
- mRS
- modified Rankin Scale
- NIHSS
- National Institutes of Health Stroke Scale
- RCT
- randomized controlled trial
- sICH
- symptomatic intracranial hemorrhage
- SITS-MOST
- SITS-Monitoring Study
- SITS-UTMOST
- Safe Implementation of Thrombolysis in Upper Time Window Monitoring Study
This manuscript was sent to Gregory W. Albers, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 2330.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.028396.
References
- 1.Hacke W, Lyden P, Emberson J, Baigent C, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis SM, et al. ; Stroke Thrombolysis Trialists’ Collaborators Group Effects of alteplase for acute stroke according to criteria defining the European Union and United States marketing authorizations: individual-patient-data meta-analysis of randomized trials. Int J Stroke 201813175–189doi: 10.1177/1747493017744464 [DOI] [PubMed] [Google Scholar]
- 2.Genentech. Activase. Highlights of prescribing information. 2018 https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103172s5259lbl.pdf. Accessed October 10, 2019.
- 3.Boehringer Ingelheim Ltd. Actilyse summary of product characteristics. 2018 https://www.medicines.org.uk/emc/product/898/smpc. Accessed October 10, 2019.
- 4.Marini C, Baldassarre M, Russo T, De Santis F, Sacco S, Ciancarelli I, Carolei A. Burden of first-ever ischemic stroke in the oldest old: evidence from a population-based study. Neurology 20046277–81doi: 10.1212/01.wnl.0000101461.61501.65 [DOI] [PubMed] [Google Scholar]
- 5.Mishra NK, Ahmed N, Andersen G, Egido JA, Lindsberg PJ, Ringleb PA, Wahlgren NG, Lees KR; VISTA Collaborators; SITS Collaborators Thrombolysis in very elderly people: controlled comparison of SITS International Stroke Thrombolysis Registry and Virtual International Stroke Trials Archive. BMJ 2010341c6046.doi: 10.1136/bmj.c6046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, Innes K, Venables G, Czlonkowska A, Kobayashi A, et al. ; IST-3 Collaborative group The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 20123792352–2363doi: 10.1016/S0140-6736(12)60768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra NK, Diener HC, Lyden PD, Bluhmki E, Lees KR; VISTA Collaborators Influence of age on outcome from thrombolysis in acute stroke: a controlled comparison in patients from the Virtual International Stroke Trials Archive (VISTA). Stroke 2010412840–2848doi: 10.1161/STROKEAHA.110.586206 [DOI] [PubMed] [Google Scholar]
- 8.Ahmed N, Hermansson K, Bluhmki E, Danays T, Paiva Nunes A, Kenton A, Lakshmanan S, Toni D, Mikulik R, Ford GA, et al. The SITS-UTMOST: a registry-based prospective study in Europe investigating the impact of regulatory approval of intravenous actilyse in the extended time window (3-4.5 h) in acute ischaemic stroke. Eur Stroke J 20161213–221doi: 10.1177/2396987316661890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wahlgren N, Ahmed N, Dávalos A, Hacke W, Millán M, Muir K, Roine RO, Toni D, Lees KR; SITS Investigators Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 20083721303–1309doi: 10.1016/S0140-6736(08)61339-2 [DOI] [PubMed] [Google Scholar]
- 10.Ahmed N, Lees KR, Ringleb PA, Bladin C, Collas D, Toni D, Ford GA; The SITS Investigators Outcome after stroke thrombolysis in patients >80 years treated within 3 hours vs >3-4.5 hours. Neurology 2017891561–1568doi: 10.1212/WNL.0000000000004499 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed N, Wahlgren N, Grond M, Hennerici M, Lees KR, Mikulik R, Parsons M, Roine RO, Toni D, Ringleb P; SITS Investigators Implementation and outcome of thrombolysis with alteplase 3-4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol 20109866–874doi: 10.1016/S1474-4422(10)70165-4 [DOI] [PubMed] [Google Scholar]
- 12.Cronin CA, Sheth KN, Zhao X, Messé SR, Olson DM, Hernandez AF, Bhatt DL, Schwamm LH, Smith EE. Adherence to Third European Cooperative Acute Stroke Study 3- to 4.5-hour exclusions and association with outcome: data from get with the guidelines-stroke. Stroke 2014452745–2749doi: 10.1161/STROKEAHA.114.005443 [DOI] [PubMed] [Google Scholar]
- 13.Reuter B, Gumbinger C, Sauer T, Wiethölter H, Bruder I, Rode S, Ringleb PA, Kern R, Hacke W, Hennerici MG; Stroke Working Group of Baden-Wuerttemberg Intravenous thrombolysis for acute ischaemic stroke in the elderly: data from the Baden-Wuerttemberg stroke registry. Eur J Neurol 20162313–20doi: 10.1111/ene.12829 [DOI] [PubMed] [Google Scholar]
- 14.Weber R, Eyding J, Kitzrow M, Bartig D, Weimar C, Hacke W, et al. Distribution and evolution of acute interventional ischemic stroke treatment in Germany from 2010 to 2016. Neurol Res Pract. 2019;1:4. doi: 10.1186/s42466-019-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lees KR, Emberson J, Blackwell L, Bluhmki E, Davis SM, Donnan GA, Grotta JC, Kaste M, von Kummer R, Lansberg MG, et al. ; Stroke Thrombolysis Trialists’ Collaborators Group Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke 2016472373–2379doi: 10.1161/STROKEAHA.116.013644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Stroke Organisation Executive Committee ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 200825457–507doi: 10.1159/000131083 [DOI] [PubMed] [Google Scholar]
- 17.Ahmed N, Audebert H, Turc G, Cordonnier C, Christensen H, Sacco S, Sandset EC, Ntaios G, Charidimou A, Toni D, et al. Consensus statements and recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 11-13 November 2018. Eur Stroke J 20194307–317doi: 10.1177/2396987319863606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karolinska Stroke Update: Consensus Statements 2012. 2012 http://www.strokeupdate.org/Cons_Reperf_IVT_2012.aspx. Accessed October 10, 2019.
- 19.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. ; American Heart Association Stroke Council 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 201849e46–e110doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 20.Cameron AC, Bogie J, Abdul-Rahim AH, Ahmed N, Mazya M, Mikulik R, Hacke W, Lees KR; Safe Implementation of Treatments in Stroke (SITS) Investigators Professional guideline versus product label selection for treatment with IV thrombolysis: an analysis from SITS registry. Eur Stroke J 2018339–46doi: 10.1177/2396987317747737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute of Neurological Disorders Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 19953331581–1587doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 22.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Höxter G, Mahagne MH. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 19952741017–1025 [PubMed] [Google Scholar]
- 23.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA 19992822019–2026doi: 10.1001/jama.282.21.2019 [DOI] [PubMed] [Google Scholar]
- 24.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 19983521245–1251doi: 10.1016/s0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 25.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. ; ECASS Investigators Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 20083591317–1329doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 26.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, et al. ; EPITHET Investigators Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 20087299–309doi: 10.1016/S1474-4422(08)70044-9 [DOI] [PubMed] [Google Scholar]
- 27.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke 200031811–816doi: 10.1161/01.str.31.4.811 [DOI] [PubMed] [Google Scholar]
- 28.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 20143841929–1935doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroke Thrombolysis Trialists’ Collaborative Group Details of a prospective protocol for a collaborative meta-analysis of individual participant data from all randomized trials of intravenous rt-PA vs. control: statistical analysis plan for the stroke thrombolysis trialists’ collaborative meta-analysis. Int J Stroke 20138278–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, et al. ; SITS-MOST Investigators Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): an observational study. Lancet 2007369275–282doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 31.Nunn A, Bath PM, Gray LJ. Analysis of the modified Rankin scale in randomised controlled trials of acute ischaemic stroke: a systematic review. Stroke Res Treat. 2016;2016:9482876. doi: 10.1155/2016/9482876. doi: 10.1155/2016/9482876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, et al. ; Stroke Thrombolysis Trialists’ Collaboration Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol 201615925–933doi: 10.1016/S1474-4422(16)30076-X [DOI] [PubMed] [Google Scholar]
- 33.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014:CD000213. doi: 10.1002/14651858.CD000213.pub3. doi: 10.1002/14651858.CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


