Abstract
Background.
Triple-knockout (TKO) pigs (in which expression of the 3 known pig carbohydrate xenoantigens has been deleted) are likely to be an optimal source of organs for transplantation into human recipients, many of whom do not have natural antibodies against TKO pig cells. However, old world monkeys, for example, baboons, have natural antibodies directed to TKO cells (to a “fourth” xenoantigen that is exposed after TKO).
Methods.
We measured (1) anti-pig IgM/IgG binding, and (2) complement-dependent cytotoxicity (CDC), by flow cytometry to α1,3-galactosyltransfearse gene-knockout (GTKO), GTKO/β4GalNT2KO (that do not express the “fourth” xenoantigen), and TKO pig peripheral blood mononuclear cells (PBMCs) using 72 baboon sera (30 specific pathogen-free [SPF], and 42 non-SPF baboons).
Results.
Mean IgM antibody binding to GTKO/β4GalNT2KO pig PBMCs was significantly lower than to GTKO or TKO pig PBMCs (P < 0.01). Mean IgG antibody binding to GTKO/β4GalNT2KO pig PBMCs was significantly lower than to TKO PBMCs (P < 0.01). Mean CDC of GTKO/β4GalNT2KO pig PBMCs was significantly lower than of GTKO or TKO pig PBMCs (P < 0.01). SPF baboon serum IgM and IgG binding to, and CDC of, GTKO/β4GalNT2KO or TKO PBMCs were significantly lower than non-SPF baboon sera (P < 0.01).
Conclusions.
Although TKO pigs form the basis for proposed clinical trials of xenotransplantation, it is difficult to identify baboons with a low or negative CDC to TKO pigs. For pig-to-baboon organ transplantation, the use of GTKO/β4GalNT2KO pigs would be preferable. The use of SPF baboons as recipients might be a minor advantage.
INTRODUCTION
The shortage of organs available for clinical transplantation is a worldwide problem.1 Xenotransplantation using pig organs can possibly provide a solution. Clinical trials of pig kidney or heart xenotransplantation are anticipated within the next few years.2 Triple-knockout (TKO) pigs (that do not express any of the 3 known carbohydrate xenoantigens) (Table 1) are likely to be an optimal source of organs for transplantation into human recipients, many of whom do not have preformed antibodies against TKO pig cells.3
TABLE 1.
Three known carbohydrate xenoantigens expressed on pig cells

In terms of modeling the human immune response, the field has historically used Old World nonhuman primates (NHPs) for preclinical pig-to-NHP studies.4–6 However, like pigs, Old World NHPs express N-glycolylneuraminic acid (Neu5Gc), and therefore do not develop natural anti-Neu5Gc antibodies.7 If the CMP-N-acetylneuraminic acid hydroxylase (CMAH) gene is knocked out (resulting in deletion of expression of Neu5Gc), as in TKO pigs, this appears to expose 1 or more new xenoantigens on the pig cells (the so-called “fourth xenoantigen”).8 In contrast, if CMAH is not knocked out (eg, in α1,3-galactosyltransferase gene-knockout [GTKO] and GTKO/β-1,4 N-acetylgalactosaminyltransferase gene-knockout [β4GalNT2KO] pigs), the pig appears not to express the “fourth antigen” (or expresses it at a lower level).9,10
We have studied serum antibody binding in several different Old World NHPs (including 6 baboons) to various genetically engineered pig cells (eg, GTKO and TKO).10 TKO pigs are not an ideal source of organs for Old World NHPs.10 Therefore, a pig of a different genotype is required that more closely mimics the TKO pig-to-human model.
In previous studies to investigate this topic, Estrada et al tested sera from 34 rhesus monkeys and 10 baboons,8 and Adams et al tested sera from 43 rhesus monkeys.11 Hence, we have attempted to confirm their findings by testing sera from 72 baboons against various genetically engineered pig cells, and also by testing serum cytotoxicity against these cells, which to our knowledge has not been carried out previously.
The aims of the present study, therefore, were to investigate (1) anti-pig IgM/IgG binding, and (2) complement-dependent cytotoxicity (CDC) to GTKO, GTKO/β4GalNT2KO, and TKO pig peripheral blood mononuclear cells (PBMCs) using 72 baboon sera. Furthermore, as our previous studies indicated that specific pathogen-free (SPF) baboons have lower anti-nonGal IgM (though not IgG) levels,12 we have investigated (3) whether they would be preferable recipients of GTKO, TKO, or GTKO/β4GalNT2KO pig organ grafts. Sera were therefore obtained from 42 baboons that were bred and housed under standard conditions and from 30 bred and housed under SPF conditions.
MATERIALS AND METHODS
Sources of Pig Cells
PBMCs were obtained from (1) GTKO, (2) GTKO/β4GalNT2KO, and (3) TKO pigs (Revivicor, Blacksburg, VA). All pigs were of blood type O (nonA). PBMCs were isolated as previously described.13 In order to reduce variability, only 1 pig of each phenotype was used as the source of the PBMCs for all of the studies. The pigs expressed no human transgenes, and so the results were not influenced by expression of human “protective” proteins.
Sources of Baboon Sera
Sera was obtained from 72 baboons (30 SPF baboons from the Michale E Keeling Center, MD Anderson Cancer Center, Bastrop, TX, and 42 non-SPF baboons from the Mannheimer Foundation, Homestead, FL) of all AB blood types. Sera were stored at −80°C. When required, decomplementation was carried out by heat-inactivation for 30 minutes at 56°C.
Protocols for pig and baboon study are approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham (UAB) (#20673).
Sources of Human Sera
Serum was drawn from 24 human volunteers (adults of all ABO blood groups) after informed consent per the guidelines of the Institutional Review Board (IRB) of the UAB (#300001924). As the volumes of human sera were limited, these sera were only used for testing against TKO pig cells. Two different lot numbers of pooled human serum (pooled from 50 to 150 donors) were purchased from Innovative Research, Novi, MI. The sera were stored at −80°C. When required, decomplementation was carried out by heat-inactivation for 30 minutes at 56°C.
Detection of Expression of Xenoantigens on Pig Cells by Flow Cytometry
PBMCs from pigs were stained for expression of Gal (by isolectin BSI-B4), Neu5Gc(chicken anti-Neu5Gc mAb), and Sda (Dolichos biflorus agglutinin, DBA) as previously described.10
Binding of Baboon or Human of Serum IgM and IgG to Pig PBMCs by Flow Cytometry
IgM and IgG binding to pig PBMCs was measured after exposure to baboon or human serum, as previously described.10,13 Data acquisition was performed with a flow cytometer (Becton Dickinson, San Jose, CA). Binding of IgM and IgG was assessed as the relative geometric mean (rGM), calculated by dividing the geometric mean fluorescence for each sample by that of the negative control, as previously described.7,13
Baboon or Human Serum Complement-dependent Cytotoxicity (CDC) of Pig PBMCs by Flow Cytometry
CDC to pig PBMCs was evaluated in a final concentration of 50%, as previously described.10 Briefly, PBMCs (5 × 104 cells in 50 µL medium; Roswell Park Memorial Institute Medium, fetal bovine serum [10%], antibiotics [1%] and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [1%]) were incubated with 50 µL titrated decomplemented serum at 37°C for 30 minutes. After washing with PBS, medium and rabbit complement (Sigma-Aldrich, St. Louis, MO) (final concentration 10%) were added, and incubation was carried out at 37°C for 1 hour. After washing with PBS, 0.5 µL of fluorescent-reactive dye solution (LIVE/DEAD Fixable Dead Cell Stain Kits [Invitrogen by Thermo Fisher Scientific, Eugene, OR]) was added to 1 mL of the cell suspension with PBS, and incubation was performed for 30 minutes in the dark at 4°C. After washing with FACS buffer, 200 µL diluted (×3) Fixation Buffer (Becton Dickinson) was added, and incubation carried out for 20 minutes at 4°C.
Data acquisition was performed with a flow cytometer (BD LSR II, BD Biosciences). Cytotoxicity was calculated, as follows:
where A represented the percentage of dead cells, B was the maximal percentage of dead cells (PBMCs treated with 70% ethanol), and C was the minimal percentage of dead cells (PBMCs incubated with medium and rabbit complement only [final concentration 10%]).
Statistical Analysis
Continuous variables were expressed as mean ± SD. Comparisons among multiple groups were performed using an ANOVA test, and comparisons between 2 groups were performed using paired t-test for continuous variables. Correlations between IgM and CDC were analyzed by calculating a Pearson correlation coefficient. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using social sciences software GraphPad Prism 8 (GraphPad Software, San Diego, CA).
RESULTS
Expression of Galactose-α1,3-galactose (Gal), Neu5Gc, and Sda on Pig PBMCs
PBMCs from GTKO pigs expressed Neu5Gc and Sda, but not Gal, while PBMCs from GTKO/β4GalNT2KO pigs did not express Gal or Sda (Table 1) (Figure S1, SDC, http://links.lww.com/TXD/A267). TKO pig PBMCs did not express any of the 3 carbohydrate xenoantigens, as previously described.10
Baboon Serum IgM and IgG Binding to GTKO, GTKO/β4GalNT2KO, and TKO pig PBMCs by Flow Cytometry
Mean baboon serum IgM antibody binding to GTKO/β4GalNT2KO pig PBMCs (rGM 2.2) was significantly lower than to GTKO (rGM 3.2) or TKO (rGM 4.3) pig PBMCs (P < 0.01) (Figure 1). Binding to TKO PBMCs was significantly higher than to GTKO PBMCs (P < 0.01).
FIGURE 1.
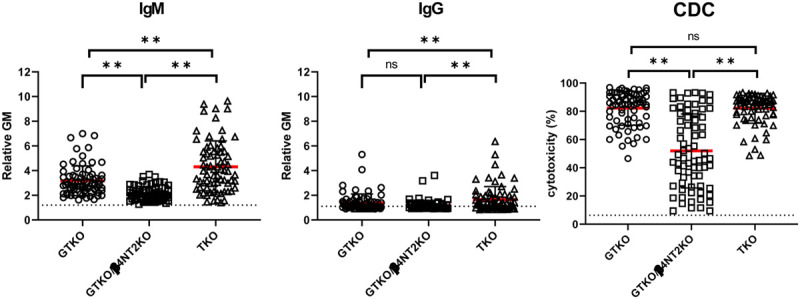
Comparison of mean IgM (left)/IgG (middle) binding and CDC (right) of baboons (n = 72) to GTKO, GTKO/β4GalNT2KO, and TKO pig PBMCs. On the y axis, the dotted line represents the cut-off values (IgM [rGM] 1.2, IgG [rGM] 1.1, CDC 6.4%) below which there is no binding or cytotoxicity, as previously described.10 The red lines indicate the mean values. (*P < 0.05, **P < 0.01; ns = not significant). Most baboons have low levels of IgM and IgG binding to GTKO/β4GalNT2KO PBMCs, and approximately 50% have a low levels of CDC to these cells. CDC, complement-dependent cytotoxicity; GTKO, α1,3-galactosyltransfearse gene-knockout; TKO, triple-knockout.
Mean baboon serum IgG antibody binding to GTKO/β4GalNT2KO pig PBMCs (rGM 1.2) was significantly lower than to TKO PBMCs (rGM 1.7) (P < 0.01), but not significantly different to that to GTKO PBMCs (Figure 1). IgG binding to TKO PBMCs was significantly higher than to GTKO (rGM 1.4) PBMCs (P < 0.01).
These results suggest that, after transplantation in baboons, serum IgM and IgG binding to TKO pig organs or cells will be greater than to GTKO or GTKO/β4GalNT2KO pig organs or cells. IgM binding to GTKO/β4GalNT2KO pig cells will be the lowest among the 3 groups.
Baboon Serum CDC to GTKO, GTKO/β4GalNT2KO, and TKO Pig PBMCs by Flow Cytometry
Mean baboon serum cytotoxicity of GTKO/β4GalNT2KO pig PBMCs (52%) was significantly lower than of GTKO (82%) or TKO (82%) pig PBMCs (both P < 0.01) (Figure 1). There was no significant difference in baboon serum CDC to GTKO and TKO PBMCs.
These results indicate that baboon serum CDC is weakest to GTKO/β4GalNT2KO pig cells.
Correlation of Baboon Anti-pig IgM or IgG Binding and CDC to Pig PBMCs
To evaluate the extent to which IgM or IgG antibody correlates with the results of the CDC assay, the correlation between CDC (at 50% serum concentration) and binding (rGM) of IgM or IgG antibody was assessed. Significant correlations were found between baboon serum IgM/IgG binding with the CDC of GTKO and TKO cells (P < 0.01) (Figure 2). A significant correlation was found between IgM binding and CDC (P < 0.01), but not between IgG binding and CDC, to GTKO/β4GalNT2KO pig cells (Figure 2).
FIGURE 2.
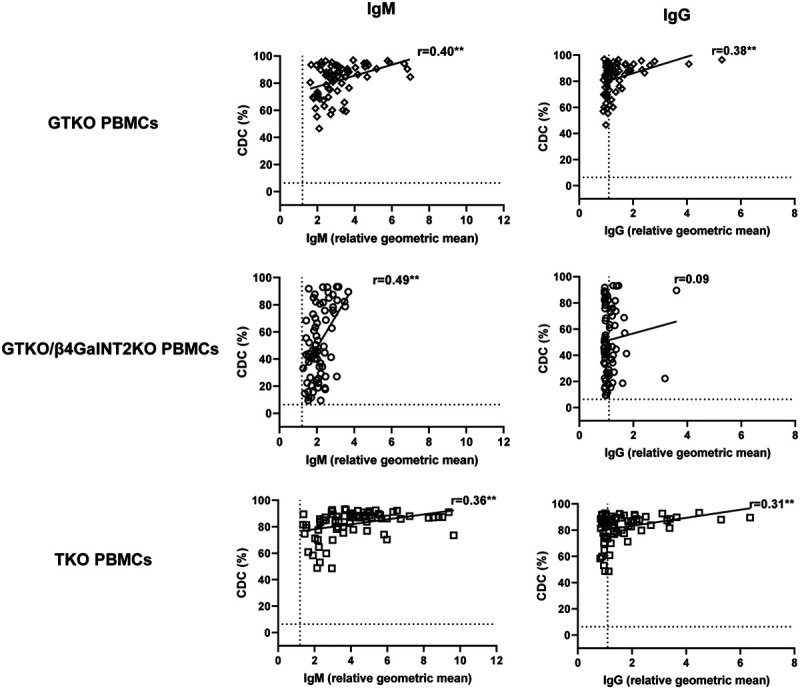
Correlation of baboon serum IgM (left) and IgG (right) antibody binding with CDC (at 50% serum concentration) to GTKO (top), GTKO/β4GalNT2KO (middle), and TKO (bottom) pig PBMCs. On the y axis, the dotted line represents the cut-off value (6.4%) below which there is no cytotoxicity. On the x axis, the dotted line represents the cut-off values (IgM 1.2, IgG 1.1) below which there is no binding. (**P < 0.01). Approximately half of the baboons demonstrated low levels of IgM and IgG binding to GTKO/β4GalNT2KO pig PBMCs, and these correlated with a low CDC of these cells. Low levels of antibody binding or CDC were not documented to GTKO or TKO pig PBMCs. Baboons with low serum antibody binding and low CDC to pig PBMCs (Figures 1 and 2) were not necessarily SPF baboons (see Figure 3). CDC, complement-dependent cytotoxicity; GTKO, α1,3-galactosyltransfearse gene-knockout; PBMCs, peripheral blood mononuclear cells; TKO, triple-knockout.
It is important to note that no baboon with low IgM or IgG binding to GTKO or TKO pig PBMCs had a low level of CDC, whereas approximately 50% of the baboons had a low level of CDC to GTKO/β4GalNT2KO PBMCs (Figure 2). This indicates that, in future organ transplantation studies, no baboons could be selected with a low CDC to GTKO or TKO pig organs, but many could be selected with a low CDC to GTKO/β4GalNT2KO pig organs.
Correlation of Human (n = 26) Anti-pig IgM or IgG Binding and CDC to TKO Pig PBMCs
Human and baboon serum IgM or IgG binding (rGM) and CDC (at 50% serum concentration) to TKO pig PBMCs were compared (Figure S2, SDC, http://links.lww.com/TXD/A267). In both humans and baboons, there was a significant increase in cytotoxicity as IgM and IgG antibody binding to TKO pig PBMCs increased. In baboons, however, cytotoxicity was high whether IgM or IgG binding was high (eg, 80% cytotoxicity at a rGM of 8 [IgM]) or low (eg, 75% cytotoxicity at a rGM of 2 [IgM]).
Comparison of Antibody Binding and CDC to GTKO, GTKO/β4GalNT2KO, and TKO Pig PBMCs Between SPF and Non-SPF Baboons
There was no significant difference of IgM/IgG binding or CDC to GTKO PBMCs between SPF and non-SPF baboons (Figure 3). In contrast, SPF baboon serum IgM and IgG binding to GTKO/β4GalNT2KO PBMCs were significantly lower than in non-SPF baboon sera (P < 0.01), but CDC was not significantly different (Figure 3). SPF baboon serum IgM (but not IgG) binding and CDC were significantly lower to TKO PBMCs than in non-SPF baboons (P < 0.01) (Figure 3). Baboons with low serum antibody binding and low CDC to pig PBMCs (Figures 1 and 2) were not necessarily SPF baboons (Figure 3).
FIGURE 3.
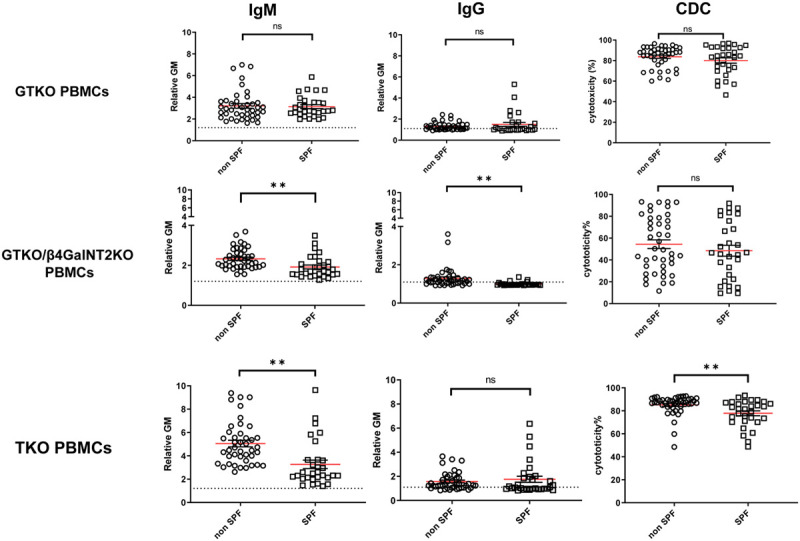
Comparison of SPF and non-SPF baboon serum IgM/IgG antibody binding and CDC to PBMCs from GTKO, GTKO/β4GalNT2KO, and TKO pigs. On the y axis, the dotted line represents the cut-off values (IgM 1.2, IgG 1.1, CDC 6.4%) below which there is no binding or CDC. The red lines indicate the mean values (**P < 0.01; ns = not significant). CDC, complement-dependent cytotoxicity; GTKO, α1,3-galactosyltransfearse gene-knockout; PBMCs, peripheral blood mononuclear cells; SPF, specific pathogen-free; TKO, triple-knockout.
These results indicate that pig organ transplantation in baboons bred and maintained under SPF conditions might be advantageous when GTKO/β4GalNT2KO or TKO pigs are the sources of organs.
DISCUSSION
Previously, we reported that Old World NHPs (eg, baboons and rhesus monkeys) have higher levels of anti-TKO pig antibodies (and CDC) than of anti-GTKO pig antibodies.10 This is associated with the exposure of the so-called “fourth antigen” in these pigs. In contrast, humans have low levels or no anti-TKO pig antibodies (and CDC).9,10 Therefore, if a TKO pig is used as the organ-source in baboon recipients, this will not mimic the TKO pig-to-human organ transplantation model.
Li et al demonstrated that, using New World NHPs (eg, capuchin monkeys) as recipients would be preferable.9 However, pig kidney or heart transplantation would be difficult because of the small size of these monkeys.9 Moreover, some important immunosuppressive agents, eg, Rituximab, are not functional in a New World NHPs.14 Therefore, Old World NHPs remain the standard preclinical large animal model.
Previous reports have indicated that baboons8,10 or rhesus monkeys8,11 have less IgM/IgG antibody binding to GTKO/β4GalNT2KO pig cells compared with TKO pig cells, and we have confirmed their findings and have additionally demonstrated that serum antibody binding is less if the baboons are from a SPF colony. These observations are based on a larger number of baboons (n = 72), including 30 SPF baboons.
We conclude that baboons have significantly lower levels of anti-pig IgM/IgG binding and of CDC to GTKO/β4GalNT2KO pig cells compared with GTKO and TKO pig cells.
Almost half of the baboons tested had much less anti-pig IgM binding and CDC to GTKO/β4GalNT2KO pig cells than to GTKO or TKO pig cells. However, not all of the baboons had low CDC against GTKO/β4GalNT2KO pig cells, even though they had low anti-IgM/IgG binding. In the present study, we chose 50% serum concentration to increase the sensitivity of cytotoxicity based on a previous study10 when we used 25% serum concentration. This underestimated the CDC of baboons against GTKO/β4GalNT2KO pig cells or that of human CDC against TKO pig cells. Even for human samples, we now use 50% serum concentration to exclude any humans who have cytotoxicity to TKO pig PBMCs (Figure S2, SDC, http://links.lww.com/TXD/A267).
Almost half of the baboons had a serum CDC level of <40% against GTKO/β4GalNT2KO pig cells, whereas there was no baboon that had a CDC of <40% against GTKO or TKO pig cells (Figure 1). Selecting baboons that have a low or negative CDC to pig cells will be important in mimicking the pig-to-human model (as many humans have no or low antibody binding or CDC to TKO pig cells) (Figure S2, SDC, http://links.lww.com/TXD/A267).15
By comparison, human sera demonstrate similar antibody binding, but greatly reduced cytotoxicity, to TKO pig cells when compared with baboon sera (Figure S2, SDC, http://links.lww.com/TXD/A267), which we have discussed fully elsewhere.10 The Old World monkey, therefore, presents a much greater challenge in demonstrating potential success of pig organ transplantation in human patients. At present, however, it is our understanding that the regulatory authorities would ideally like evidence in a pig-to-NHP model using the identical genetically engineered pig, if possible. Our data suggest that this may not be possible, and a different genetically engineered pig, for example, based on GTKO/β4GalNT2KO, may be necessary as the source of the organs in the NHP model.
In our previous study16 and our current study,10 pigs that express human complement-regulatory proteins (eg, human CD46, CD55) were used for kidney xenotransplantation in baboons. In vitro, the CDC of baboon sera to GTKO pig PBMCs expressing CD46/CD55 was significantly less than to GTKO pig PBMCs not expressing CD46/CD55 (Figure S3, SDC, http://links.lww.com/TXD/A267). Even so, in an in vivo study, pretransplant anti-pig IgM/IgG levels and CDC to pig cells not expressing CD46 or CD55 seemed to affect graft survival.10
Previously, we reported that IgM antibody levels in SPF baboons (n = 8) to GTKO pig aortic endothelial cells (ECs) were significantly lower than those of non-SPF baboons (n = 32).12 In the present study, we used PBMCs instead of pig aortic ECs, and the number of baboon sera tested was considerably increased. Although we did not document any difference in IgM/IgG binding and CDC to GTKO PBMCs between non-SPF and SPF baboons, IgM and IgG binding to GTKO/β4GalNT2KO PBMCs, and IgM binding and CDC to TKO PBMCs, were significantly lower in SPF baboons than in non-SPF baboons. Whether these marginal decreases would be of any biological significance in vivo remains unknown.
These results suggest that baboons bred and housed under SPF conditions may be marginally advantageous in preclinical studies of pig-to-baboon organ transplantation. It is possible that the slightly lower levels of antibody documented may be related to a reduced number of gastrointestinal flora,17 but we have no evidence for this.
Which pig cells are the best to evaluate antibody binding and CDC remains controversial. In clinical transplantation, donor lymphocytes are the targets of crossmatch tests and CDC assays. However, pig ECs may be potentially good targets. In the present study, TKO pig ECs not expressing CD46/CD55 were not available to us. Moreover, the volumes of serum available to us from the 72 baboons were limited. Therefore, we were not able to evaluate antibody binding or CDC to pig ECs in the present study.
In conclusion, although TKO pigs form the basis for the proposed first clinical trials of xenotransplantation, it is almost impossible to identify baboons with a low or negative CDC to TKO pigs. For pig-to-baboon organ transplantation, the use of GTKO/β4GalNT2KO pigs would be preferable. The use of SPF baboons as recipients might be a minor advantage.
ACKNOWLEDGMENTS
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959 and in part by a grant to UAB from United Therapeutics, Silver Spring, MD.
Footnotes
Published online 24 July, 2020.
Y.C. and T.Y. contributed equally to the writing of this article.
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959, and in part by a grant to UAB from United Therapeutics, Silver Springs, MD.
David Ayares is an employee of Revivicor, Blacksburg, VA. The other authors declare no conflicts of interest.
Protocols for pig and baboon study are approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham (#20673). The protocol for blood draws from human volunteers was approved by the Institutional Review Board of the University of Alabama at Birmingham (#300001924).
Y.C., T.Y., H.H. and D.K.C.C. designed the research. Y.C., T.Y., S.S.R and D.K.C.C. wrote the article. Y.C., T.Y., S.S.R, M.M, H.Q.N., D.A., D.K.C.C. and H.H. performed the research. Y.C. and T.Y. participated in the data analysis.
REFERENCES
- 1.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014; 25:1842–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper DK. Is successful orthotopic heart transplantation in the pig-to-non-human primate model required before proceeding to a clinical trial? Xenotransplantation. 2016; 23:328–329 [DOI] [PubMed] [Google Scholar]
- 3.Smood B, Hara H, Schoel LJ, et al. Genetically-engineered pigs as sources for clinical red blood cell transfusion: what pathobiological barriers need to be overcome? Blood Rev. 2019; 35:7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijkstrom M, Iwase H, Paris W, et al. Renal xenotransplantation: experimental progress and clinical prospects. Kidney Int. 2017; 91:790–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murthy R, Lamberti J. Invited commentary. Ann Thorac Surg. 2016; 101:1841. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DK, Matsumoto S, Abalovich A, et al. Progress in clinical encapsulated islet xenotransplantation. Transplantation. 2016; 100:2301–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao B, Shen X, Shiroishi MS, et al. A pilot study of pre-operative motor dysfunction from gliomas in the region of corticospinal tract: evaluation with diffusion tensor imaging. PLoS One. 2017; 12:e0182795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation. 2015; 22:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Shaikh S, Iwase H, et al. Carbohydrate antigen expression and anti-pig antibodies in New World capuchin monkeys: relevance to studies of xenotransplantation. Xenotransplantation. 2019; 26:e12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto T, Iwase H, Patel D, et al. Old world monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout). Sci Rep. 2020; 10:9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams AB, Kim SC, Martens GR, et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann Surg. 2018; 268:564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Iwase H, Wolf RF, et al. Are there advantages in the use of specific pathogen-free baboons in pig organ xenotransplantation models? Xenotransplantation. 2014; 21:287–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara H, Ezzelarab M, Rood PP, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006; 13:357–365 [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Ladowski JM, Bikhet M, et al. Efficacy of ATG and rituximab in capuchin monkeys (a new world monkey)—an in vitro study relevant to xenotransplantation. Xenotransplantation. 2020;e12627. [DOI] [PubMed] [Google Scholar]
- 15.Cooper DKC, Hara H, Iwase H, et al. Clinical pig kidney xenotransplantation: how close are we? J Am Soc Nephrol. 2020; 31:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto T, Hara H, Foote J, et al. Life-supporting kidney xenotransplantation from genetically engineered pigs in baboons: a comparison of two immunosuppressive regimens. Transplantation. 2019; 103:2090–2104 [DOI] [PubMed] [Google Scholar]
- 17.Galili U, Mandrell RE, Hamadeh RM, et al. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988; 56:1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]


