Abstract
Purpose
[18F]-2-Fluoro-2-deoxy-D-glucose PET/CT (FDG PET/CT) is a sensitive and quantitative technic for detecting inflammatory process. Glucose uptake is correlated with an increased anaerobic glycolysis seen in activated inflammatory cells such as monocytes, lymphocytes, and granulocytes. The aim of the study was to assess the inflammatory status at the presumed peak of the inflammatory phase in non-critically ill patients requiring admission for COVID-19.
Methods
Patients admitted with COVID-19 were prospectively enrolled. FDG PET/CT was performed from day 6 to day 14 of the onset of symptoms. Depending on FDG PET/CT findings, patients’ profiles were classified as “inflammatory” or “low inflammatory.” FDG PET/CT data were compared with chest CT evolution and short-term clinical outcome. All inflammatory sites were reported to screen potential extra-pulmonary tropism.
Results
Thirteen patients were included. Maximum standardized uptake values ranged from 4.7 to 16.3 in lungs. All patients demonstrated increased mediastinal lymph nodes glucose uptake. Three patients (23%) presented mild nasopharyngeal, two patients (15%) bone marrow, and five patients (38%) splenic mild increase in glucose uptake. No patient had significant digestive focal or segmental glucose uptake. There was no significant physiological myocardial glucose uptake in all patients except one. There was no correlation between PET lung inflammatory status and chest CT evolution or short-term clinical outcome.
Conclusion
Inflammatory process at the presumed peak of the inflammatory phase in COVID-19 patients is obvious in FDG PET/CT scans. Glucose uptake is heterogeneous and typically focused on lungs.
Trial registration
NCT04441489. Registered 22 June 2020 (retrospectively registered).
Electronic supplementary material
The online version of this article (10.1007/s00259-020-04968-8) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, FDG PET/CT, Inflammation
Introduction
In the history of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a minority of patients develop symptoms of acute respiratory distress syndrome (ARDS) that may require invasive mechanical ventilation and threaten short-term outcome. The mean delay from the onset of symptoms to ARDS is 10 days [1–3] and can occur even with very low viral load [4]. Since the beginning of the outbreak, chest computed tomography (CT) scan has been reported as the cornerstone to assist diagnosis and early screening [5, 6]. Repeated CT may also explain some of the secondary clinical worsening by revealing secondary infections or pulmonary embolism [7].
However, unexpected respiratory distress may occur without any significant changes on CT images, once pulmonary embolism or secondary infection is discarded. One possible reason could be lung inflammation, involving the cell-mediated immunity and cytokine storms [1]. This inflammatory cascade sequence can lead to fatal immune-mediated injuries . CT scan displays suggestive images of alveolar and interstitial edema and its extent. Those might be part of inflammation consequences [8] but cannot explain the underlying mechanism (active inflammatory cell infiltration or not) or quantify the intensity of the inflammatory process. Furthermore, CT images abnormalities may persist a long time after clinical symptom resolution and do not reflect current active inflammation [9]. Imaging the active inflammation could be very useful for a better understanding of this particular phase of the infection. Combined [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomographic imaging with fused CT (FDG PET/CT) is fitted for this purpose. FDG uptake is correlated with an increased anaerobic glycolysis seen in activated inflammatory cells such as monocytes, lymphocytes, and granulocytes [10]. Chefer et al. used FDG PET/CT to investigate the host lymphoid tissue response developing in nonhuman primates after exposure to Middle East respiratory syndrome (MERS) caused by a coronavirus, MERS-CoV [11]. FDG PET/CT has also been used to evaluate anti-inflammatory treatments by measuring changes in pulmonary inflammation [12]. FDG PET/CT is able to quantitatively investigate the entire body at the cellular level because FDG uptake is cell-mediated.
The aim of the study was to evaluate the inflammatory status at the presumed peak of the inflammatory phase in non-critically ill patients requiring an hospitalization for COVID-19.
Methods
Patients
Patients presenting with COVID-19 were prospectively enrolled in this study carried out at the Centre Hospitalier Princesse Grace of Monaco from March 27th to May 3rd, 2020. Patients were included if COVID-19 was confirmed according to the WHO guidance [13] by a positive result of RT-PCR assay of nasal and pharyngeal swabs, if they were hospitalized during the time period from day 6 to day 14 of the onset of symptoms, and if their initial (on admission) chest CT scan presented ground-glass opacities (GGO) or consolidation. Patients could not be included if their medical condition was unstable or precluded a safe transfer to the nuclear medicine department, if they were under mechanical ventilation (either non-invasive or invasive), if they were initially referred to the critical care unit, or in case of a pregnancy.
The institutional review board for human studies approved the protocol and a written consent was obtained from the subjects. Personal protective equipment (PPE) was available for the staff, and all measures to ensure strict infection prevention were observed according to established guidance [14].
Chest CT scan and FDG PET/CT
All patients had an initial chest CT scan for diagnosis on admission and a second chest CT scan for follow-up concomitant with a FDG PET/CT between day 6 and day 14 after the onset of symptoms. A full description of the technical considerations and dosimetry is provided in Table E1 in the supplementary data online.
FDG PET/CT and chest CT images were independently analyzed by 2 nuclear medicine physicians (MF and MD) with several years of experience in reading cardiothoracic imaging, on commercially available software (Syngo.via, Siemens version VB30). These physicians were blinded to any biological or clinical data. In case of disagreement, a consensus reading was performed.
Chest CT images interpretation
The degree of involvement of GGO and consolidation, expressed as a percentage of the total pulmonary volume according to previous studies [9, 15], was visually evaluated on the initial chest CT scan (on admission) and on the second chest CT scan concomitant with the FDG PET/CT. Based on the changes between the two chest CTs, patients were classified as follows:
CT worsening: increase of CT abnormalities extent > 20% or additional GGO or consolidation. Conversion of GGO to consolidation was not considered as an additional finding.
CT stability: changes of CT abnormalities extent ≤ 20% without additional GGO or consolidation.
CT improvement: decrease of CT abnormalities extent > 20% without additional GGO or consolidation.
FDG PET/CT images’ interpretation
The amount of FDG pathological uptake was expressed by maximum signal intensity standardized uptake values (SUVmax). Hypermetabolic volumes (defined as the volume of pixels in the region of interest with SUVmax value above 4) were computerized. Based on FDG PET/CT imaging, patients’ profiles were classified as follows:
Inflammatory: lungs hypermetabolic volume ≥ 50 ml and/or SUVmax ≥ 7
Low inflammatory: lungs hypermetabolic volume < 50 ml and/or SUVmax < 7
The rationale for the selection of SUVmax and hypermetabolic volume values is explained in the supplementary data online and in Table E2.
All inflammatory sites in the acquisition field from skull top to mid-thigh were also reported to screen SARS-CoV-2 potential extra-pulmonary tropism.
Adjudication committee classification for patients’ short-term clinical outcome
An expert panel was launched to adjudicate patients’ short-term clinical outcome. The expert panel consisted of 3 physicians experienced in intensive care, pulmonology, and emergency medicine and involved in the management of COVID-19 patients (GC, YEC, and CP). To adjudicate each case, the expert panel was blinded to the results of FDG PET/CT but had full access to medical records, focusing on the kinetics of monitoring, treatment (notably oxygen delivery), and biology from 3 days before to 3 days after FDG PET/CT. Patients’ short-term clinical outcome was classified as follows: worsening, stability, improvement. Further details are provided in the supplementary data online.
Statistical analysis
Categorical variables were collected as numbers (n) and percentages (%). Continuous variables were described as median and interquartile range (IQR). Correlation was computed using Spearman correlation analysis. Data were computed using Statistica for Windows (version 15.3). Wilcoxon signed-rank test were performed. A p value < 0.05 was considered significant.
Results
Fourteen patients were initially included in this study. One patient was secondarily excluded, as it retrospectively appeared that his FDG PET/CT has been performed out-of-time (day 22). The flowchart for patients’ selection is presented in Fig. 1.
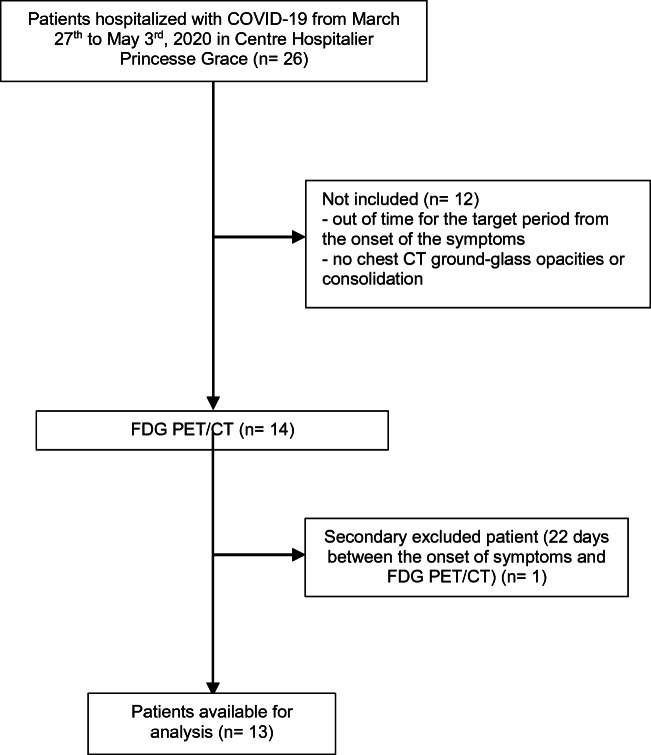
Fig. 1.
Study flow chart. Twenty-six patients with laboratory-confirmed COVID-19 were hospitalized at the Centre Hospitalier Princesse Grace of Monaco from March 27th to May 3rd, 2020. Twelve patients were excluded because they were admitted out of the selected time period from the onset of the symptoms or without chest CT ground-glass opacities or consolidation. One patient was secondary excluded because further elicitation retrospectively revealed the onset of symptoms occurred 22 days before FDG PET/CT study
Patients characteristics
Clinical characteristics of patients on admission are presented in Table 1. Median age was 68 years (IQR 57–80), with a balanced proportion between men and women. Most patients had no underlying disorders, and the most common comorbidity was hypertension in six of thirteen patients (46%), followed by prior cerebrovascular diseases in two of thirteen patients (15%). Fever, irritating cough, and fatigue were the most common symptoms on admission, followed by dyspnea. Three of thirteen patients (23%) had diarrhea, and rhinorrhea was the rarest symptom on admission. Laboratory findings on admission are summarized in Table 2. Eight of thirteen patients (61%) showed lymphopenia (defined as lymphocyte count < 1.0 × 109/L). All patients had a C-reactive protein level above the normal range (defined as > 5 mg/L).
Table 1.
Patients’ clinical characteristics on admission
| n = 13, n (%) | |
|---|---|
| Age, groups | |
| < 65 | 5 (38) |
| ≥ 65 | 8 (61) |
| Gender | |
| Women | 6 (46) |
| Men | 7 (53) |
| Comorbidities | |
| Any | 7 (53) |
| Hypertension | 6 (46) |
| Prior cerebrovascular disease | 2 (15) |
| Chronic kidney disease | 1 (8) |
| Former or current smoking | 1 (8) |
| Diabetes | 1 (8) |
| Chronic obstructive pulmonary disease | 0 |
| Symptoms on admission | |
| Fatigue | 12 (92) |
| Irritative cough | 12 (92) |
| Fever | 12 (92) |
| Dyspnea | 9 (69) |
| Aguesia | 5 (38) |
| Anosmia | 3 (23) |
| Diarrhea | 3 (23) |
| Rhinorrhea | 1 (8) |
Table 2.
Selected biological results on admission
| n = 13, median (IQR) | |
|---|---|
| C-reactive protein, mg/L | 69 (35.8–105) |
| ≥ 5 mg/L, n (%) | 13 (100) |
| White blood cell count, × 109/L | 5.83 (4.56–7.73) |
| > 10 × 109/L, n (%) | 1 (8) |
| < 4 × 109/L, n (%) | 2 (15) |
| Neutrophil count, × 109/L | 5.25 (4.11–6.62) |
| Lymphocyte count, × 109/L | 0.91 (0.77–1.20) |
| < 1.0 × 109/L, n (%) | 8 (61) |
| Eosinophil count, × 109/L | 0 (0–0.012) |
| Basophil count, × 109/L | 0.012 (0–0.016) |
| Hemoglobin, g/dL | 13.6 (12.6–15.5) |
| Platelet count, × 109/L | 201 (168–285) |
| AST, U/L | 36 (30–46) |
| ALT, U/L | 23 (18–40) |
| Urea, mmol/L | 6 (5.2–8.3) |
| Creatinine, μmol/L | 82 (61–99) |
| PCT, ng/ml | 0.12 (0.08–0.13) |
| NT-proBNP, pg/mL | 124 (55–307) |
| Fibrinogen, g/L | 4.54 (4.15–6.32) |
| > 4 g/l, n (%) | 11 (85) |
| D-dimer, μg/L | 1.069 (0.899–3.346) |
| High-sensitivity cardiac troponin I, ng/mL | 0.01 (0.009–0.015) |
AST aspartate aminotransferase, ALT alanine transaminase, PCT procalcitonin, NT-proBNP N terminal pro B type natriuretic peptide, LDH lactate dehydrogenase
The median duration from the onset of symptoms to hospital admission was 5 days (IQR 4–7). The median duration from the onset of symptoms to FDG PET/CT was 10 days (IQR 9–12). During the time period including 3 days before and 3 days after the PET study, five patients (38%) were classified as clinical worsening, one patient (8%) as clinical stability, and seven patients (54%) as clinical improvement; eleven patients (85%) required oxygen therapy through nasal canula at low flow rates (1–9 L/min), none needed non-invasive mechanical ventilation, no patient experienced a secondary bacterial infection, none developed any acute cardiac or kidney injuries, none was coinfected with influenza A and B viruses, Mycoplasma pneumoniae, Streptococcus pneumoniae, and Legionella pneumophila, and none received corticosteroids. None of our patients had any clinical evidence for myocardial ischemia, neurological, or gastrointestinal involvement during this time period.
In the hospitalization phase up to the end of 3rd of May, none of our patients died, eleven patients (85%) were discharged, five patients (38%) required intensive care unit (ICU) care, and one patient (8%) required invasive mechanical ventilation. Discharged patients had experienced a median of 8 days (IQR 7–13) of hospital stay.
Chest CT findings (Table 3)
Table 3.
Chest CT findings
| Number of patients (%) | Initial chest CT scan on admission | Second chest CT scan concomitant with FDG PET/CT |
|---|---|---|
| Ground-glass opacities | 13 (100) | 13 (100) |
| Consolidation | 7 (54) | 13 (100) |
| Subclavicular adenopathy* | 0 | 0 |
| Mediastinal adenopathy* | 0 | 0 |
| Pleural effusion | 0 | 0 |
| Linear opacities | 6 (46) | 9 (69) |
| Opacities with a cravy-paving pattern | 3 (23) | 4 (31) |
| Emphysema or fibrosis | 0 | 0 |
| Number of lobes affected, median (IQR) | 5 (5–5) | 5 (5–5) |
| Overall extent of lung involvement in %, median (IQR) | 50 (20–60) | 60 (40–60) |
*Defined as lymph node with short-axis dimension ≥ 10 mm
Seven of thirteen patients (54%) were classified as CT worsening, five of thirteen patients (38%) as CT stability, and one of thirteen patients (8%) as CT improvement. The median duration from the initial chest CT scan to the FDG PET/CT was 5 days (IQR 2–5). There was a tendency for the CT abnormalities extension to increase between the two chest CT scans (median 50%; IQR 20–60 versus median 60%; IQR 40–60, p = 0.11, Table E3 in the supplementary data online). GGO appeared to be the first step, then focal consolidation occurred. Lower lobes were chronologically the first areas to exhibit lung consolidation. Only one patient had a unique lobe involvement (right lower lobe, Fig. E1 in the supplementary data online). No patient demonstrated any adenopathy on CT (defined as lymph node with short-axis dimension ≥ 10 mm).
FDG PET/CT findings (Table 4)
Table 4.
FDG PET/CT findings
| Increased FDG uptake, CT localization | Number of patients (%) |
|---|---|
| Subclavicular lymph node | 5 (38) |
| Mediastinal lymph node, | 13 (100) |
| Gallbladder | 1 (8) |
| Nasopharynx | 3 (23) |
| Bone marrow | 2 (15) |
| Spleen | 5 (38) |
| Brain focal abnormalities | 0 |
| Digestive tube, focal or segmental | 0 |
| Myocardium, pathological | 0 |
| Myocardium, physiological | 1 (8) |
FDG uptake for lungs GGO and consolidation areas was increased and heterogeneous, with SUVmax ranging from 4.7 to 16.3 and hypermetabolic volumes ranging from 3.3 to 990 mL. Three of thirteen patients (23%) presented mild nasopharyngeal FDG uptake. Two of thirteen patients (15%) had mild diffuse digestive FDG uptake and one of thirteen patients (8%) had mild incidental diffuse FDG uptake in the gallbladder, without CT abnormalities. No patient had significant digestive focal or segmental FDG uptake abnormality. Two of thirteen patients (15%) and five of thirteen patients (38%) presented bone marrow or splenic FDG mild increased uptake respectively. All patients showed increased FDG uptake in mediastinal lymph nodes, and five of thirteen patients (38%) showed increased FDG uptake in subclavicular lymph nodes.
We did not find any evidence for myocardial inflammation according to clinical symptoms, cardiac biomarkers, electrocardiography, or echocardiography. There was no significant physiological myocardial FDG uptake in any of the patients except one, despite the absence of a dedicated regimen designed for inducing myocardial extinction. Myocardial FDG uptake was sometimes below the circulating blood activity and appeared as a negative halo (see Fig. E2 in the supplementary data).
Relationship between FDG PET/CT inflammatory status, chest CT evolution, and short-term clinical outcome is summarized in Table 5. Overall, two of thirteen patients (15%) were classified as having a low inflammatory profile and eleven of thirteen patients (85%) as having an inflammatory profile. Figure 2 illustrates an inflammatory profile patient and Fig. 3 a low inflammatory profile patient. The two low inflammatory profile patients demonstrated clinical improvement. However, five of the eleven inflammatory profile patients (45%) also clinically improved. All patients with worsening CTs were inflammatory patients. No significant correlation was found between either overall extent of lung abnormalities on chest CT images or blood levels of C-reactive protein on one hand, and lung SUVmax, lung hypermetabolic volumes, and mediastinal lymph nodes SUVmax on the other hand.
Table 5.
Relationship between FDG PET/CT inflammatory status, chest CT evolution and short-term clinical outcome
| Short-term clinical outcome | ||||
|---|---|---|---|---|
| Worsening | Stability | Improvement | ||
| Chest CT evolution | Worsening (n = 7) | 4 | 0 | 3 |
| Stability (n = 5) | 1 | 1 | 3 | |
| Improvement (n = 1) | 0 | 0 | 1 | |
| Inflammatory status FDG PET/CT | Inflammatory profile (n = 11) | 5 | 1 | 5 |
| Low inflammatory profile (n = 2) | 0 | 0 | 2 | |
| Inflammatory profile FDG PET/CT and CT worsening (n = 7) | 4 | 0 | 3 | |
| Low inflammatory profile FDG PET/CT and CT improvement/stability (n = 2) | 0 | 0 | 2 | |
| Inflammatory profile FDG PET/CT and CT improvement/stability (n = 4) | 1 | 0 | 3 | |
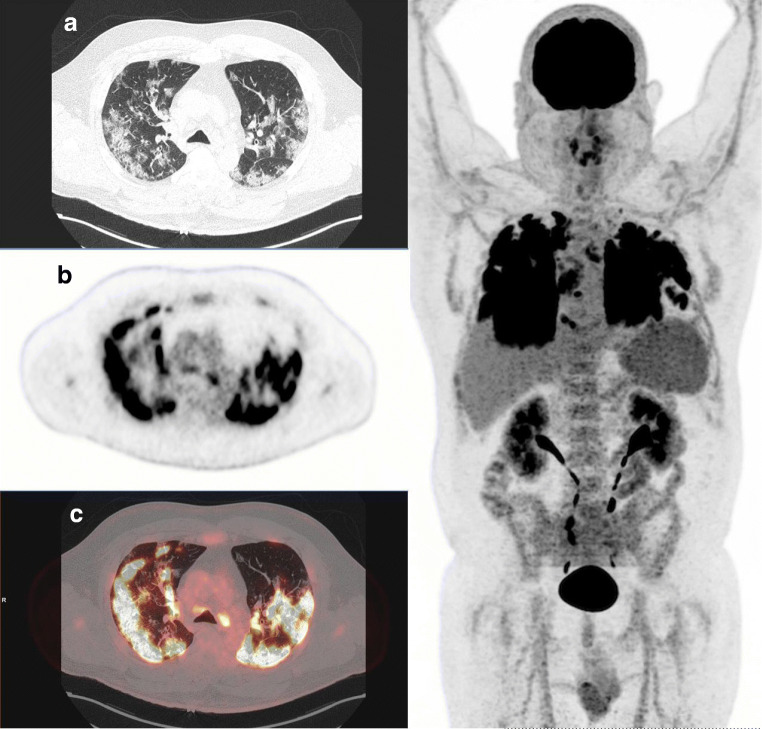
Fig. 2.
A 53-year-old man patient with moderate respiratory symptoms referred for FDG PET/CT at day 14 after the onset of symptoms. Images showed multiple peripheral and bilateral ground-glass opacities with intense increased FDG uptake (SUVmax 16.3). The following day, respiratory status dramatically improved without need of supplemental oxygen, and patient was discharged from hospital 3 days later. Left: A CT transverse slice, B FDG PET slice, and C FDG PET and CT-fused images. Right: whole-body maximal intensity projection (MIP) image, displaying also mediastinal lymph nodes FDG uptake
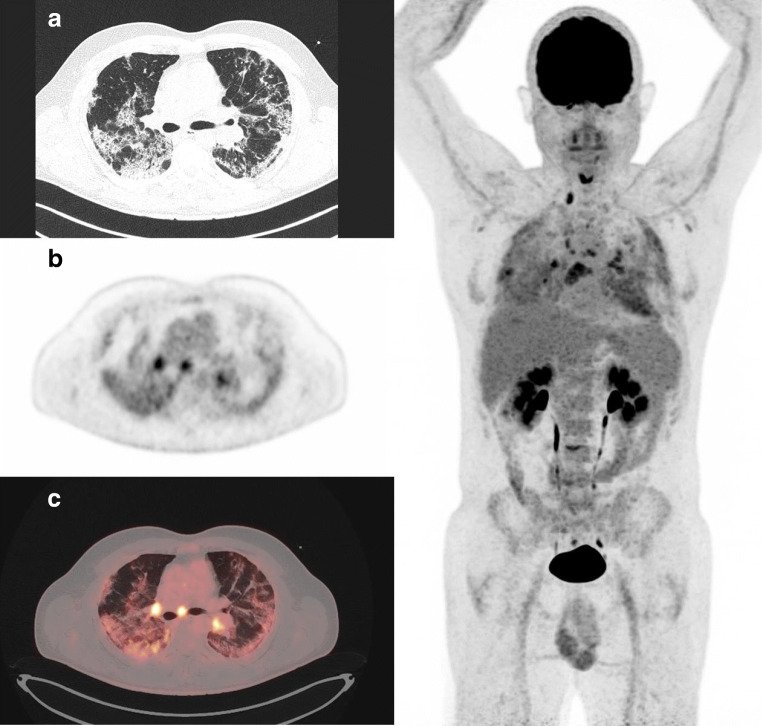
Fig. 3.
A 55-year-old man patient who presented severe respiratory symptoms in the days before FDG PET/CT. Images showed bilateral consolidation with mild increased FDG uptake (SUVmax 5). Despite this mild inflammation, the patient remained symptomatic for days requiring oxygen therapy through nasal canula at low flow rates and was discharged from hospital only 13 days later. Left: A CT transverse slice, B FDG PET slice, and C FDG PET and CT-fused images. Right: whole-body MIP image demonstrating that FDG uptake in mediastinal lymph nodes is above lung FDG uptake (respectively, SUVmax 6.9 versus 5)
Among the two patients with a mild, although abnormal, FDG uptake on bone marrow, one showed a short-term clinical improvement, while the other one showed a clinical worsening. Moreover, among the five patients with splenic FDG mild increased uptake, two were classified as having a short-term clinical improvement and three showed a short-term clinical worsening. Patients with bone marrow or splenic FDG mild increased uptake did not have a higher body temperature than the other patients (bone marrow FDG uptake group median 38 °C; IQR 37–39 versus median 37.3 °C; IQR 37–38, p = 0.66; splenic FDG uptake group median 37.3 °C; IQR 37–38.5 versus median 37; IQR 37–37.75, p = 0.76).
Discussion
Imaging of inflammatory process in patients with COVID-19 at the presumed peak of the disease using FDG PET/CT has not been previously performed. Our main result is that COVID-19 patients presenting GGO or consolidation on initial chest CT demonstrate a lung inflammation at the presumed peak of the inflammatory phase that varies widely from minor to extensive involvement. Our results suggest that tropism of this inflammatory process is mostly focused on the lower respiratory tract. However, we were unable to correlate this inflammation to chest CT evolution or short-term clinical outcome.
The increased and heterogeneous FDG uptake we observed in lungs probably results from an intense activation of inflammatory cells. Glycolytic activity in human lymphocytes has been known for decades and FDG PET/CT has a high sensitivity to evidence lymphocyte activation [10, 16, 17]. The exact contribution of specific immune cells activated in lung parenchyma during COVID-19 cannot be deducted from FDG PET/CT data alone as increasing anaerobic glycolysis is also described in activate inflammatory cells such as monocytes and granulocytes [10]. However, intra-alveolar neutrophilic infiltration found in medical autopsies is rather reported during secondary bacterial infection [18]. Intra-alveolar infiltration by mononuclear inflammatory cells is therefore the most likely phenomenon explaining FDG pathological uptake. The good clinical tolerance to this infiltration, at least at the beginning of the inflammatory phase, has been observed in other disorders such as lymphoma or sarcoidosis [19]. In COVID 19, Gattinoni et al. [20] reported two groups of patients at the critical phase: those with a preserved pulmonary compliance (type L) who do not behave as classical ARDS, and those with an impaired pulmonary compliance (type H) with typical ARDS behavior and prognosis. It is likely that our patients belonged to the first group, with a better prognosis.
Lung changes on chest CT scan were consistent with other studies [8, 9, 15]. As previously reported, consolidation occurred after GGO and lung abnormality involvement reached a maximum on the second chest CT scan for follow-up concomitant with the FDG PET/CT scan. We found neither pleural effusion nor thoracic lymphadenopathy, which are known as uncommon findings in COVID-19 [8]. Despite the absence of abnormal CT criteria for thoracic lymphadenopathy, FDG PET/CT demonstrated mediastinal and subclavicular lymph node activation which was consistent with the few other reported cases [21–24]. Angiotensin-converting enzyme 2 is a functional receptor for SARS-Cov [25]. Lymph nodes FDG uptake is probably linked to cytokine-mediated inflammation rather than to direct viral infection, considering the absence of angiotensin-converting enzyme 2 receptors (ACE2) on hemato-lymphoid cells [26].
The mildly increased FDG uptake observed in the spleen and bone marrow in some patients might be in response to an inflammatory condition [27]. Clinical short-term outcome of these patients was similar to that of other patients. However, larger series will be required to elucidate if an increased spleen or bone marrow FDG uptake represents an early sign of severe systemic inflammatory response and could be associated with clinical outcome in COVID-19.
Surprisingly, in all patients except one, no significant physiological myocardial FDG uptake was observed, despite the lack of any specific preparation aiming to suppress physiologic myocardial glucose metabolism. We did not find any explanation for this, suggesting that during COVID-19 infection, the myocardial metabolic pathway toward glycolysis is not (or weakly) activated. It also suggests that catecholaminergic stimulation increasing myocardial glucose uptake [28] was not present in almost all our patients.
Only few extra-pulmonary hotspots were found among our population although FDG PET/CT should be sensitive enough for detecting extra-pulmonary lesions in COVID-19. This is consistent with the fact that none of our patients had clinical or biological manifestations of extra-pulmonary disease at the time of the PET study. This may be explained by our eligibility criteria, especially 1/excluding patients under mechanical ventilation, therefore discarding the most severe patients and 2/ with a time period from day 6 to day 14 of the onset of symptoms, when some minor non-pulmonary manifestations could have already disappeared.
Despite close geographical vicinity to Northern Italy, which was the second most affected country in the world at the beginning of the study in March 27th, 2020, Monaco has been poorly affected by COVID-19 during the study period. For evident safety reasons, we did not include patients with mechanical-assisted ventilation. Therefore, our population is a subset of non-critically ill patients. All patients were nevertheless consecutively enrolled, and their images are probably representative of the rate and the extent of lung inflammation. The limited statistical power may explain some of our results, especially the absence of significant relationship between biological markers of inflammation and lung FDG uptake.
A prolonged follow-up is required to determine if a relationship exists between initial lung inflammation and long-term sequelae such as residual lung fibrosis and respiratory failure. Therefore, further studies comprising late CT images and functional respiratory examination will be required. However, thanks to a high sensitivity, FDG PET/CT may be a useful imaging method for a comprehensive understanding of the pathogenesis underlying COVID-19 inflammatory process.
Electronic supplementary material
(DOCX 3.12 mb)
Acknowledgments
We would like to thank all staff at the dedicated COVID unit and the Nuclear Medicine Department staff of Centre Hospitalier Princesse Grace for their efforts to make this study possible.
We acknowledge all healthcare staff who are on the frontline of caring for patients.
We thank Dr. Jérémie Tordo for his contributions to the revision.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
MF, GC, YEC, and CP designed the study and enrolled the patients. MF supervised the study. MD, GC, YEC, RF, IR, BS, VN, FH, BP, FB, OKP, FG, CP, and MF contributed to acquisition of clinical, biological, and imaging data. MF, MD, and FB performed statistical analysis. MD and MF wrote the manuscript. GC, YEC, RF, OKP, FG, and CP contributed to critical revision of the report. All authors approved the final draft of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. “Comité de Pilotage de la recherche du Centre Hospitalier Princesse Grace” (Pilot committee for research of Centre Hospitalier Princesse Grace) was the institutional review board that approved the study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
This article is part of the Topical Collection on Infection and inflammation
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marc Faraggi, Email: marc.faraggi@chpg.mc.
for the MONACOVID Group:
C. Fissore-Magdelein, S. Gabriel-Solean, B. Dahmani, I. Riss, P. Sorlin, V. Dupasquier, A. Pathak, C. Dugourd, M. Nicolai, N. Rijo, N. Beau, X. Magdelein, F. Olyve, S. Chaillou-Orpitz, J. Lépine, M. Lorillou, C. Maincent, S. Bertrand, P. Brunner, C. Dittlot, F. Fuerxer, M. Liberatore, G. Armando, M. Bouregba, J. Catineau, P. Garcia, and D. Tran
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020. 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed]
- 4.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee E, Ng MY, Khong PL. COVID-19 pneumonia: what has CT taught us? Lancet Infect Dis. 2020;20(4):384–385. doi: 10.1016/S1473-3099(20)30134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Yao L, Li J, Chen L, Song Y, Cai Z, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020. 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed]
- 7.Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020:201561. 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed]
- 8.Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, Chen B, Zhang Z, Guan W, Ling Z, Jiang R, Hu T, Ding Y, Lin L, Gan Q, Luo L, Tang X, Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding X, Xu J, Zhou J, Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur J Radiol. 2020;127:109009. doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Prost N, Tucci MR, Melo MF. Assessment of lung inflammation with 18F-FDG PET during acute lung injury. AJR Am J Roentgenol. 2010;195(2):292–300. doi: 10.2214/AJR.10.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chefer S, Thomasson D, Seidel J, Reba RC, Bohannon JK, Lackemeyer MG, Bartos C, Sayre PJ, Bollinger L, Hensley LE, Jahrling PB, Johnson RF. Modeling [(18)F]-FDG lymphoid tissue kinetics to characterize nonhuman primate immune response to Middle East respiratory syndrome-coronavirus aerosol challenge. EJNMMI Res. 2015;5(1):65. doi: 10.1186/s13550-015-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen DL, Bedient TJ, Kozlowski J, Rosenbluth DB, Isakow W, Ferkol TW, Thomas B, Mintun MA, Schuster DP, Walter MJ. [18F] fluorodeoxyglucose positron emission tomography for lung antiinflammatory response evaluation. Am J Respir Crit Care Med. 2009;180(6):533–539. doi: 10.1164/rccm.200904-0501OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance, 2020. Published January 25, 2020. Accessed Mar 20, 2020. https://apps.who.int/iris/handle/10665/330854
- 14.Paez D, Gnanasegaran G, Fanti S, Bomanji J, Hacker M, Sathkge M, et al. COVID-19 pandemic: guidance for nuclear medicine departments. Eur J Nucl Med Mol Imaging. 2020. 10.1007/s00259-020-04825-8. [DOI] [PMC free article] [PubMed]
- 15.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020:200463. 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed]
- 16.Marjanovic S, Skog S, Heiden T, Tribukait B, Nelson BD. Expression of glycolytic isoenzymes in activated human peripheral lymphocytes: cell cycle analysis using flow cytometry. Exp Cell Res. 1991;193:425–431. doi: 10.1016/0014-4827(91)90116-C. [DOI] [PubMed] [Google Scholar]
- 17.Ishimori T, Saga T, Mamede M, Kobayashi H, Higashi T, Nakamoto Y, Sato N, Konishi J. Increased 18F-FDG uptake in a model of inflammation: concanavalin A-mediated lymphocyte activation. J Nucl Med. 2002;43:658–663. [PubMed] [Google Scholar]
- 18.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020:1–8. [DOI] [PMC free article] [PubMed]
- 19.Spagnolo P, Rossi G, Trisolini R, Sverzellati N, Baughman RP, Wells AU. Pulmonary sarcoidosis. Lancet Respir Med. 2018;6(5):389–402. doi: 10.1016/S2213-2600(18)30064-X. [DOI] [PubMed] [Google Scholar]
- 20.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020:1–4. [DOI] [PMC free article] [PubMed]
- 21.Setti L, Kirienko M, Dalto SC, Bonacina M, Bombardieri E. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur J Nucl Med Mol Imaging. 2020. 10.1007/s00259-020-04819-6. [DOI] [PMC free article] [PubMed]
- 22.Zou S, Zhu X. FDG PET/CT of COVID-19. Radiology. 2020:200770. 10.1148/radiol.2020200770.
- 23.Qin C, Liu F, Yen TC, Lan X. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47:1281–1286. doi: 10.1007/s00259-020-04734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polverari G, Arena V, Ceci F, Pelosi E, Ianniello A, Poli E, et al. 18F-fluorodeoxyglucose uptake in patient with asymptomatic severe acute respiratory syndrome coronavirus 2 (coronavirus disease 2019) Referred to positron emission tomography/computed tomography for NSCLC restaging. J Thorac Oncol. 2020;0. 10.1016/j.jtho.2020.03.022. [DOI] [PMC free article] [PubMed]
- 25.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn SS, Hwang SH, Jung SM, Lee SW, Park YB, Yun M, Song JJ. Evaluation of spleen glucose metabolism using 18F-FDG PET/CT in patients with febrile autoimmune disease. J Nucl Med. 2017;58(3):507–513. doi: 10.2967/jnumed.116.180729. [DOI] [PubMed] [Google Scholar]
- 28.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–588. doi: 10.1161/01.CIR.99.4.578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 3.12 mb)
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.