Abstract
Purpose
During the COVID-19 pandemic, most breast surgery for benign and malignant conditions has been postponed, creating a backlog of patients who will need surgery. A fair and transparent system for assessing the risk of further delaying surgery for individual patients to prioritize surgical scheduling is needed.
Methods
Factors related to risk of delaying surgery for breast patients were identified. Scores were assigned to each factor, with higher scores indicating a greater risk from delaying surgery. REDCap and Microsoft Excel tools were designed to track and score delayed patients.
Results
Published data and multidisciplinary clinical judgement were used to assign risk scores based on patient and tumor factors, length of delay, and tumor response to preoperative therapy. Patients completing neoadjuvant chemotherapy were assigned the highest scores as their options for delaying surgery are most limited. Among patients receiving neoadjuvant endocrine therapy or no medical therapy, higher scores were assigned for low-estrogen receptor or high-genomic risk scores, higher grade, larger tumors, younger age and longer delay. High priority scores were assigned for progression during preoperative therapy. Low scores were assigned for re-excisions, atypical lesions and other benign indications. There was good agreement of the tool’s ranking of sample patients with rankings by experienced clinicians. The tool generates risk-stratified patient lists by surgeon or institution to facilitate assignment of surgery dates.
Conclusions
This tool generates a clinically consistent, risk-stratified priority list of breast surgical procedures delayed by the COVID-19 pandemic. This systematic approach may facilitate surgical scheduling as conditions normalize.
Keywords: Breast surgery, Clinical decision-making, COVID-19, Surgical priority, Scoring system, Surgical scheduling
Introduction
During the COVID-19 pandemic, surgery for breast cancer and other breast conditions is severely restricted as operating rooms become ICU’s, OR staff are deployed for urgent COVID-19 care, and preservation of personal protection equipment and critical care medications is necessary. Many breast cancer patients are being managed with neoadjuvant endocrine therapy or chemotherapy to delay surgery. There are inflexible limits to the duration of most neoadjuvant chemotherapy regimens, and surgery will become urgent for these patients. Some patients receiving neoadjuvant endocrine therapy may develop progressive disease, increasing the urgency for their surgery.
It is expected that OR availability for breast surgery will increase only gradually. When access improves, the significant backlog of delayed patients will compete with newly diagnosed patients for operating room time. Although broad guidelines have been issued for initial prioritization of surgery for breast conditions [1, 2] and for other surgical patients [3], there is no clear guidance for determining order of surgery among a large number of delayed breast surgery patients. A system for assessing the risk of further delaying surgery and prioritizing access to the OR is needed. Fairness and transparency must be central features of such a system.
To address this need, we created a system for scoring risk of delaying breast surgery to help prioritize assignment of surgical dates. Using published data and multidisciplinary clinical judgement, scores were assigned to patient and tumor risk factors and to length of delay. Patients were assessed at diagnosis and for response during treatment to determine the safety of additional delay versus the urgency of proceeding with surgery.
Tools were created to allow rapid entry of patient data, with automatic calculation and updating of risk scores, to generate risk-stratified lists of breast patients whose surgery has been delayed.
Methods
Factors related to risk of delaying surgery for breast surgery were identified by a multidisciplinary breast team and scores assigned based on the estimated contribution of each factor to risk. Factors used for T1-2N0, estrogen-receptor positive (ER+) patients included:
Endocrine responsiveness estimated by genomic risk testing [4–6] if performed, or by percentage of cells staining ER+ and/or progesterone receptor positive (PR+), and intensity of immunohistochemistry (IHC) staining
Tumor grade
Tumor size
Axillary node status
Patient age
Time since biopsy
Evidence of progression on follow up imaging and/or physical examination
For patients receiving neoadjuvant chemotherapy, chemotherapy regimen received, date of final chemotherapy dose and ER status were recorded.
Positive lumpectomy margins and non-malignant conditions were assigned low scores but included to create a comprehensive list of delayed surgeries. Factors scored for patients without cancer included presence of atypical ductal hyperplasia (ADH) or other atypia.
Additional data collected included surgeon name and institution to create risk stratified lists by surgeon or by institution. Tools were prepared in both REDCap [7, 8] and Microsoft Excel formats.
To compare the priority rankings generated by the scoring tool with ranking by clinical judgement, we created 10 hypothetical patients with newly diagnosed ER+ breast cancers. A second scenario included updated information on the initial 10 patients’ responses to treatment and 5 newly diagnosed patients. Priority ranking for surgery calculated by the tool was compared with priority rankings by 3 breast surgeons who had not participated in creating the scoring system.
Results
Assignment and justification of risk scores
Patient and tumor factors thought to impact risk of delaying breast surgery were identified by a multidisciplinary breast team and each assigned a score, with higher scores indicating greater potential risk from delaying surgery. Scores were assigned using published data when available (Table 1), and using multidisciplinary clinical judgement where data was lacking. Score assignments assumed that patients completing neoadjuvant chemotherapy should proceed with surgery on schedule except under the most extreme resource limitations. ER- tumors received priority over ER+ and human epidermal growth factor 2 positive (HER2+) tumors, since endocrine therapy or anti-HER2 therapy might be used to delay surgery for these patients. Assignments assumed that most ER+ , T1-2N0-1 patients can defer surgery for several months with neoadjuvant endocrine therapy, but must be monitored and proceed urgently to surgery or other systemic therapy for progression. It was assumed that most surgery for positive lumpectomy margins, risk reduction, benign lesions and non-reconstruction cosmetic reasons can be deferred until COVID-19 delayed cancers have been treated.
Table 1.
Response rates to neoadjuvant endocrine therapy regimens in early stage ER+ breast cancer
| Author year | # pts | Tumor stage | Menopause status | Regimen | N | Therapy duration | Responding (%) |
Stable (%) |
Progressing (%) |
|---|---|---|---|---|---|---|---|---|---|
| Ellis (2011) | 349 | T2-4c, N0-3, M0 | Post | Anastrozole | 114 | 16–18w | 74.6 | 17.5 | 7.9 |
| Exemestane | 114 | 68.4 | 24.6 | 7 | |||||
| Letrozole | 121 | 78.5 | 16.5 | 5 | |||||
| Iwata (2019) | 295 | T1c-T2, cN0, M0 | Post | Letrozole | 295 | 24–28w | 45 | 51 | 4 |
| Smith (2005) | 282 | T1-4, N0-3, M0 | Pre and Post | Anastrozole | 94 | 3mo | 40.4 | 50 | 9.6 |
| Tamoxifen | 96 | 37.5 | 57.3 | 5.2 | |||||
| Combination | 92 | 43.5 | 51.1 | 5.4 | |||||
| Johnston (2019) | 279 | T2-T4, M0 | Post | Letrozole | 93 | 14–16w | 50 | 45 | 5 |
| Palbo + Let | 186 | 54 | 43 | 3 | |||||
| Allevi (2013) | 118 | T2-4, N0-1, M0 | Post | Letrozole | 40 | 4mo | 45 | 45 | 10 |
| 38 | 8mo | 87 | 10 | 3 | |||||
| 40 | 12mo | 95 | 5 | 0 | |||||
| Toi (2011) | 104 | T2-3, N0-1, M0 | Post | Exemestane | 104 | 6mo | 57 | 39 | 4 |
| Olson (2009) | 100 | T2-4, N0-2, M0 | Post | Letrozole | 100 | 16–24w | 62 | 26 | 12 |
| Akashi-Tanaka (2009) | 87 | T2-T4 | Post | Anastrozole | 48 | 4mo | 42.5 | 50.5 | 7 |
| Tamoxifen | 39 | ||||||||
| Fontein (2014) | 79 | T2-T4, N0-3, M0 | Post | Exemestane | 77 | 6mo | 65 | 30 | 5 |
| Ueno (2014) | 61 | T2-3, N0-2, M0 | Post | Exemestane | 61 | 6mo | 52.5 | 39.3 | 8.2 |
| Barnadas (2009) | 54 | T2-4c, N1-2, M0 | Post | Exemestane | 54 | 6mo | 61 | 35 | 4 |
Progression defined as 20–25% increase in tumor area or appearance of new lesions
Palbo Palbociclib, Let letrozole
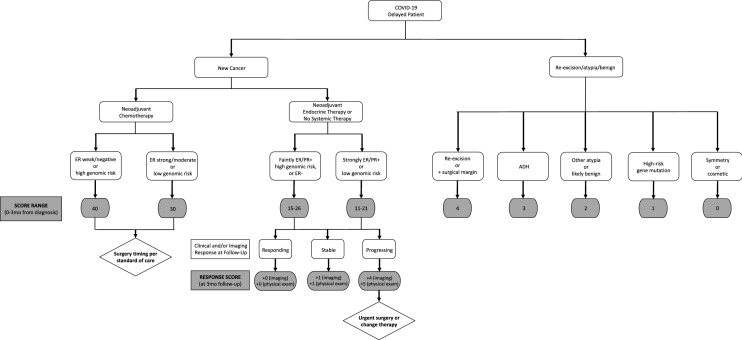
Scores were assigned to separate patients into 3 groups and create a range of scores within each group for eventual assignment of surgical dates: (1) Very urgent (score ≥ 30), narrow window for safe surgery, for example, patients completing neoadjuvant chemotherapy; (2) Limited delay acceptable (score 10–29), but may become urgent, for example, patients receiving neoadjuvant endocrine therapy and (3) Lowest priority (score < 10), likely safe to wait, for example, re-excisions, atypical lesions, prophylactic mastectomies, benign lesions and cosmetic procedures (Fig. 1). Very urgent patients will generally have surgery within 2–4 weeks of completion of chemotherapy as was done prior to the pandemic. Limited delay acceptable patients will generally wait 2–4 months, or longer if they continue to respond to neoadjuvant endocrine therapy. Lowest priority patients will wait until conditions allow elective surgery to resume. The scores assigned to each patient, tumor, delay and response measure are shown in Table 2.
Fig. 1.
Impact of histology and treatment factors on risk score assignments
Table 2.
Score assignments for factors related to risk of delaying breast surgery
| Risk factor | Risk score |
|---|---|
| Indication priority score—all patients | |
| Indication score | |
| Cancer—neoadjuvant chemotherapy | 30 |
| Cancer—neoadjuvant endocrine therapy or ER- DCIS or ER-, no chemotherapy | 10 |
| Re-excision, positive lumpectomy margin | 4 |
| ADH | 3 |
| Other atypia/probably benign | 2 |
| High-risk gene mutation | 1 |
| Symmetry/cosmetic | 0 |
| Scored only for cancer patients receiving neoadjuvant endocrine therapy | |
| Endocrine sensitivity score | |
| If genomic risk testing done | |
| Genomic risk test score—Oncotype DX | |
| < 18 | 0 |
| ≥ 18, < 31 | 1 |
| ≥ 31 | 5 |
| Genomic risk test score—MammaPrint, EndoPredict, or other | |
| Low risk | 0 |
| High risk | 5 |
| If no genomic risk testing done | |
| ER strength score | |
| ≥ 50% strong/moderate | 0 |
| 11–49% strong/moderate | 1 |
| Any % faint or 1–10% strong/moderate or ER- | 4 |
| PR strength score | |
| Strong/moderate | 0 |
| Weak/negative | 1 |
| Tumor grade score | |
| 1 | 1 |
| 2 | 2 |
| 3 | 3 |
| Tumor size (cm) score | |
| DCIS | 0 |
| Microinvasion (≤ 0.1) | 1 |
| > 0.1, ≤ 1.0 | 1 |
| > 1.0, ≤ 2.0 | 2 |
| > 2.0, ≤ 3.0 | 3 |
| > 3.0 | 4 |
| Patient age score | |
| ≥ 70 | 0 |
| ≥ 50, < 70 | 1 |
| ≥ 35, < 50 | 3 |
| < 35 | 4 |
| Delay score | |
| Time since biopsy | |
| ≥ 0, < 3 months | 0 |
| ≥ 3, < 4 months | 1 |
| ≥ 4, < 6 months | 2 |
| ≥ 6 months | 3 |
| Imaging response score | |
| Responding | 0 |
| Stable | 1 |
| Progressing any site | 4 |
| Physical exam response score | |
| Not palpable and not palpable at diagnosis | 0 |
| Responding | 0 |
| Stable | 1 |
| Progressing any site | 5 |
| Scored only for cancer patients receiving neoadjuvant chemotherapy | |
| ER score—neoadjuvant chemotherapy patients | |
| ER strong/moderate or low genomic risk | 0 |
| ER weak/negative or high genomic risk | 10 |
| Total risk score | |
| Total score | |
ER estrogen receptor, PR progesterone receptor
ER+ tumors receiving neoadjuvant endocrine therapy or no neoadjuvant therapy
The majority of patients whose surgery is being delayed have T1-2N0-1, ER+ , HER2- breast cancers. Most are receiving neoadjuvant endocrine therapy to allow postponement of surgery for several months. Preoperative endocrine therapy is well tolerated and increases rates of breast conservation [9, 10]. While pathologic complete response rates are low, few tumors will progress [9, 11–22]. Although the duration of neoadjuvant endocrine therapy was 4–6 months in most trials [23], preoperative treatment for 12 months or more is possible, often with continued response. Overall response rates to letrozole in postmenopausal women rose from 49.6% at 4 months to 95% at 12 months, with pCR rates of 2.5% at 4 months and 17.5% at 12 months [15].
Scores for the following factors were assigned to T1-2N0, ER + , HER2- patients to predict risk of delaying surgery.
Endocrine responsiveness score
Oncotype score has been validated as a predictor of benefit of endocrine therapy in node-negative [4, 24–27] and node-positive [26, 28] ER+ , HER2- early-stage breast cancers, with correlation [18, 20] and validation [12] of Oncotype score as a predictor of response to neoadjuvant endocrine therapy. Rates of progression in the TransNEOS study were 1% with Oncotype scores of 0–18, 4% with scores of 19–30, but 17% with scores ≥ 31 [12]. MammaPrint [5], or EndoPredict [6] genomic risk tests have also been shown to predict outcomes with endocrine therapy.
Endocrine responsiveness was scored by genomic risk testing when performed, or by strength of ER and PR expression by IHC. The tool assigned a significantly higher risk score for Oncotype scores ≥ 31 or high risk on MammaPrint or EndoPredict testing. For patients without genomic testing, percentage of cells staining ER+ and intensity of IHC staining was used. A high score was assigned for only faint/weak ER-staining at any percentage, for moderate or strong staining in only 1–10% of cells, or for ER- tumors. As PR expression is dependent on ER expression, weak or absent PR expression was assigned a higher score.
Tumor grade score
Higher breast tumor grade is associated with a poorer prognosis [29], and higher scores were assigned for higher grade. For tumors containing a mixture of grades or reported as a range of grades (eg grade 2–3), score was assigned using the highest grade present.
Tumor size score
Higher scores were assigned to larger invasive tumors. While risk of finding invasive tumor for DCIS on core biopsy increases with longer surgical delay [30], trials of active surveillance without immediate surgery for DCIS are underway [31, 32]. Prognosis for DCIS or DCIS with microinvasion is excellent and they are assigned lower scores than invasive cancers, DCIS with microinvasion higher than DCIS alone.
Patient age score
As there is limited data on the efficacy of prolonged neoadjuvant endocrine therapy in premenopausal women, higher priority scores were assigned to younger women. In one study, response rates at 24 weeks in premenopausal women were 70.4% with anastrozole + goserelin and 50.5% with tamoxifen + goserelin [33]. Response rates increased with longer duration of endocrine therapy for both anastrozole and tamoxifen.
Duration of delay score
A higher scores were assigned to patients with longer times between diagnostic biopsy and evaluation, increasing with each 3-month increment of delay.
Progression/response score
Rates of tumor progression in studies of neoadjuvant endocrine therapy range from 3–12% (Table 1) [9, 11–22], but are as high as 17% in a subset of patients with high Oncotype scores [12]. Patients whose tumors are progressing should undergo surgery as soon as possible. To identify progression, we recommend reassessment with physical exam 2–3 months after initiation of neoadjuvant endocrine therapy, potentially with repeat imaging. The score assigned at diagnosis is adjusted significantly upward for progression on physical examination and/or imaging, with progression detectable on physical examination given a higher score. Both imaging and examination scores are added to the total score, creating a high priority for progressing tumors. Stable tumors are given a low progression score and no points are added for responding tumors. Patients unable to tolerate neoadjuvant endocrine therapy should be followed closely and undergo surgery for progression.
Other patient and tumor factors
Node status at diagnosis is recorded but does not contribute to the score assigned at diagnosis. Although node status may be used in deciding between neoadjuvant chemotherapy or neoadjuvant endocrine therapy, once neoadjuvant endocrine therapy is selected, we believe the scoring of factors described above and scoring of response versus progression appropriately prioritizes patients for surgery.
ER-, HER2+ , and higher stage patients receiving neoadjuvant chemotherapy
Patients receiving neoadjuvant chemotherapy generally have the highest risk breast cancers. In contrast to patients receiving neoadjuvant endocrine therapy, the toxicity of neoadjuvant chemotherapy precludes prolonged treatment. After completing the standard course of chemotherapy, patients should ideally have surgery within a narrow time frame that allows enough time for resolution of neutropenia and other toxicities, but not so long that tumor progression could occur.
Key data recorded for neoadjuvant chemotherapy patients are chemotherapy regimen and date of last treatment, as these define the safe window for surgery. A very high score was assigned to all neoadjuvant chemotherapy patients, which always puts them at the top of the priority list for surgery. ER- tumors were given higher priority scores than ER+ tumors, where endocrine therapy might safely allow further delay of surgery. This system does not provide detailed prioritization among patients receiving neoadjuvant chemotherapy, as complex individual factors determine risk of delaying surgery in these patients. We recommend multidisciplinary review on a case-by-case basis.
Positive lumpectomy margins, atypical lesions, benign and prophylactic surgery patients
The tool creates a comprehensive prioritized list of all patients whose breast surgery has been delayed. Postponing surgery for patients with positive lumpectomy margins has been deemed reasonable [1] as extent of residual disease is likely low and other tumor properties are known from the initial surgery. A score was assigned placing their priority below that of other cancers but above that of non-malignant conditions.
Patients without malignancy were assigned scores reflecting their lower risk. Atypical ductal hyperplasia (ADH) on core biopsy is scored highest, as the upgrade rate to DCIS or invasive carcinoma on excisional biopsy is approximately 20% [34, 35]. Other atypical lesions (LCIS, ALH, FEA, radial scars) with lower upgrade rates [34, 36] receive the next highest score. Prophylactic mastectomies were scored below atypical lesions but before surgery for other benign conditions.
Validation
To determine whether this tool produced priority scores consistent with clinical judgement, we created 10 hypothetical patients with a variety of newly diagnosed ER+ breast cancers. Three breast surgeons who had not participated in creating the scoring system reviewed patient data and ranked patients from lowest to highest priority for surgery. The rankings produced by the clinicians were nearly identical to the ranking produced by the tool (Table 3).
Table 3.
Priority ranks generated by the scoring system compared with priority ranks generated by experienced breast surgeons
| Priority rank | Patient (system score) | Scoring system | Test patient and tumor characteristics | |||
|---|---|---|---|---|---|---|
| Surgeon 1 | Surgeon 2 | Surgeon 3 | Patient | Score | ||
| 1 | F (18) | H (20) | H (20) | H | 20 | H: 45 yo, 2.5 cm grade 2 IDC, moderate ER+ , PR-, N0 |
| 2 | H (20) | F (18) | F (18) | F | 18 | F: 49 yo, 1.8 cm grade 2 IDC, ER, Oncotype = 25, N0 |
| 3 | G (16) | A (17) | G (16) | A | 17 | A: 49 yo, 1.2 cm grade 2 IDC, strongly ER/PR+ , N0 |
| 4 | I (14) | G (16) | A (17) | G | 16 | G: 68 yo, 1.3 cm grade 3 IDC, strongly ER/PR+ , N0 |
| 5 | A (17) | I (14) | D (13) | I | 14 | I: 55 yo, 0.9 cm grade 2 IDC, ER+ Oncotype = 16, N0 |
| 6 | C (14) | C (14) | I (14) | C | 14 | C: 63 yo, 0.9 cm grade 2 ILC, ER+ Oncotype = 11, N0 |
| 7 | B (12) | D (13) | C (14) | D | 13 | D: 60 yo, 1.4 cm grade 1 DCIS+ mi, strongly ER/PR+ , N0 |
| 8 | D (13) | E (13) | E (13) | E | 13 | E: 62 yo, 1.0 cm grade 2 DCIS, strongly ER + , N0 |
| 9 | E (13) | B (12) | B (12) | B | 12 | B: 90 yo, 1.0 cm grade 1 IDC, strongly ER/PR+ , N0 |
| 10 | J (11) | J (11) | J (11) | J | 11 | J: 79 yo, grade 1 DCIS, strongly ER/PR+ , N0 |
Scores of 18 or higher were considered highest risk, 15–17 considered medium risk, and 14 or lower considered lowest risk
yo years old, ER estrogen receptor, PR progesterone receptor, IDC invasive ductal cancer, DCIS ductal carcinoma in situ; mi: microinvasion, ILC invasive lobular cancer
To simulate the mix of delayed and newly diagnosed patients expected when screening resumes, each surgeon reviewed updated records of the initial 10 patients that included examination and imaging findings 3 months after initiation of neoadjuvant endocrine therapy. Intermingled with these delayed patients were 5 newly diagnosed patients. Clinicians were not given rules for prioritizing delayed versus new patients. They again ranked priority for surgery. There was less consensus among surgeons in ranking this group, but assignments to low, medium and high priority groups were very similar to assignments generated by the tool; surgeons 1, 2 and 3 agreed with the scoring system in 13/15, 14/15 and 11/15 patients, respectively.
Discussion
Many patients with breast cancer, atypical lesions and other breast conditions have had their surgery postponed during the COVID-19 pandemic. A systematic approach is needed for surgeons and institutions to track their delayed and newly diagnosed breast patients, with a fair and transparent process to prioritize patients for surgery. Assignment of OR priority must consider nuances of each patient’s circumstances.
We describe a tool that generates an objective, risk-stratified list of all types of benign and malignant breast surgical cases delayed during the COVID-19 pandemic. It provides a detailed initial stratification of a patient’s risk based on familiar clinical factors, and updates priority based on delay time and any progression of disease. It produces priority rankings consistent with the priority categories of the Consortium recommendations [1]. Importantly, it generates a range of scores within the large cohort of ER+ /HER2- cancers (Consortium Priorities B3, C1 and C2) to help prioritize assignment of surgery dates. Priority ranking of test ER+ cancer patients by the tool agreed well with rankings by experienced breast surgeons.
The tool was prepared in REDCap and Microsoft Excel formats to accommodate surgeon preference and different practice settings. Both options have a user-friendly interface suitable for use in small or large practices. Priority lists can be generated by surgeon or by institution. Data entry for an ER+ patient generally takes less than a minute. Even less data entry is required for neoadjuvant chemotherapy patients and for patients with benign conditions.
A limitation of this tool is that assignment of risk scores was based largely on clinical judgement and multidisciplinary discussion. Unfortunately, there is little data on outcomes of prolonged neoadjuvant endocrine therapy in early stage breast cancer, particularly in premenopausal patients. Risks of delaying DCIS surgery, re-excision procedures or diagnostic excisional biopsies for atypia are largely unknown. However, precedent exists for using clinical judgement to create breast cancer decision aids, for example the Adjuvant! tool for estimating chemotherapy benefit for individual breast cancer patients [37].
A standardized scoring system will provide needed data on the effects of delaying breast surgery. New information about outcomes of neoadjuvant endocrine therapy in early stage and premenopausal patients can be gained, including how patient and tumor factors impact rates of response or progression. With data collected now, scoring systems can be refined for use in future situations where surgery must be delayed.
It is important to note that this tool is not a substitute for clinical judgment or multidisciplinary consultation in managing breast patients during the COVID-19 pandemic. While this tool can help manage and prioritize large numbers of delayed breast surgery patients, it remains essential to consider individual patient factors and conditions at individual practice locations. Resumption of breast surgery will be concurrent with resumption of many other hospital activities. Use of hospital resources will need to be balanced across specialties and with consideration of personnel and resource constraints in the operating room, hospital and community [38]. For each surgical procedure, the patient’s health status, including risk of having or acquiring a SARS-CoV-2 infection must be considered. Prioritization of breast surgery in this environment will require ongoing multidisciplinary discussion.
This risk stratification tool is undergoing additional testing at other sites, and was included in the COVID-19 Pandemic Breast Cancer Consortium’s Considerations for Re-entry [39] issued 5/15/2020 by the Breast Cancer Consortium (American College of Radiology, American College of Surgeons Commission on Cancer, National Accreditation Program for Breast Centers, American Society of Breast Surgeons, American Society of Clinical Oncology and the National Comprehensive Cancer Network and the Society of Surgical Oncology).
Data availaibility
Copies of the REDCap and Excel databases are available to individuals or institutions without charge via the Mass General Division of Surgical Oncology website. https://www.massgeneral.org/surgical-oncology/about/news-and-events/re-entry-tool-for-breast-surgeons/.
Compliance with ethical standards
Conflict of interest
The authors report no conflict of interest relevant to this publication. Drs. Smith, Nguyen, Specht, Gadd, Moy and Ms. Korotkin and Ms. Kelly declare they have no conflict of interest. Dr. Spring reports consulting (Novartis), travel (Tesaro), and research funding to the institution (Tesaro, Merck). Dr. Isakoff reports consulting (Abbvie, OncoPrep) and research funding to institution (Abbvie, AstraZeneca, Genetech, Merck, OncoPrep, Pharmamar).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic the COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020 doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daraï E, Mathelin C, Gligorov J. Breast cancer management during the COVID 19 pandemic: french guidelines. Eur J Breast Health. 2020 doi: 10.5152/ejbh.2020.200420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prachand VN, Milner R, Angelos P, Posner MC, Fung JJ, Agrawal N, Jeevanandam V, Matthews JB. Medically-necessary, time-sensitive procedures: a scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic. J Am Coll Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Kaehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryan J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 5.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 6.Dubsky P, Filipits M, Jakesz R, et al. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013;24(3):640–647. doi: 10.1093/annonc/mds334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Minor BL, Elliot V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, REDCap Consortium The REDCap consortium: building an international community of software partners. J. Biomed Inform. 2019 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eiermann W, Paepke S, Llombart-Cussac A, Eremin J, Vinholes J, Mauriac L, Ellis M, Lassus M, Chaudri-Ross HA, Dugan M, Borgs M, Semiglazov V; Letrozole Neo-Adjuvant Breast Cancer Study Group Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol. 2001;12:1527–1532. doi: 10.1023/a:1013128213451. [DOI] [PubMed] [Google Scholar]
- 10.Cataliotti L, Buzdar AU, Noguchi S, Bines J, Takatsuka Y, Petrakova K, Dube P, de Oliveira CT. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the pre-operative “Arimidex” compared to tamoxifen (PROACT) trial. Cancer. 2006;106(10):2095–2103. doi: 10.1002/cncr.21872. [DOI] [PubMed] [Google Scholar]
- 11.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol. 2011;29(17):2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwata H, Masuda N, Yamamoto Y, Fujisawa T, Toyama T, Kashiwaba M, Ohtani S, Taira N, Sakai T, Hasegawa Y, Nakamura R, Akabane H, Shibahara Y, Sasano H, Yamaguchi T, Sakamaki K, Bailey H, Cherbavas DB, Jakubowski DM, Sugiyama N, Chao C, Ohashi Y. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. J Breast Cancer Res Treat. 2019;173:123–133. doi: 10.1007/s10549-018-4964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, Ashley SE, Francis S, Boeddinghaus I, Walsh G, Group IT Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–5116. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Johnston S, Puhalla S, Wheatley D, et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor–positive early breast cancer: PALLET trial. J Clin Oncol. 2019;37:178–189. doi: 10.1200/JCO.18.01624. [DOI] [PubMed] [Google Scholar]
- 15.Allevi G, Strina C, Andreis D, Zanoni V, Bazzola L, Bonardi S, Foroni C, Milani M, Cappelleti MR, Gussago F, Aguggini S, Giardini R, Martinotti M, Fox SB, Harris AL, Bottini A, Berruti A, Generali D. Increased pathological complete response rate after long-term neoadjuvant letrozole treatment in postmenopausal oestrogen and/or progesterone receptor-positive breast cancer. Br J Cancer. 2013;108:1587–1592. doi: 10.1038/bjc.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toi M, Saji S, Masuda N, Kuroi K, Sato N, Takei H, Yamamoto Y, Ohno S, Yamashita H, Hisamatsu K, Aogi K, Iwata H, Takada M, Ueno T, Saji S, Chanplakorn N, Suzuki T, Sasano H. Ki67 index changes, pathological response and clinical benefits in primary breast cancer patients treated with 24 weeks of aromatase inhibition. Cancer Sci. 2011;102(4):858–865. doi: 10.1111/j.1349-7006.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- 17.Olson JA, Jr, Budd GT, Carey LA, Harris LA, Esserman LJ, Fleming GF, Marcom PK, Leight GS, Jr, Giuntoli T, Commean P, Bae K, Luo J, Ellis MJ. Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: results from a multicenter phase II trial. J Am Coll Surg. 2009;208(5):906–914. doi: 10.1016/j.jamcollsurg.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akashi-Tanaka S, Shimizu C, Ando M, Shibata T, Katsumata N, Kouno T, Terada K, Shien T, Yoshida M, Hojo T, Kinoshita T, Fujiwara Y, Yoshimura K. 21-gene expression profile assay on core needle biopsies predicts responses to neoadjuvant endocrine therapy in breast cancer patients. Breast. 2009;18(3):171–174. doi: 10.1016/j.breast.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Fontein DB, Charehbili A, Nortier JW, et al. Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients—a phase II trial. Eur J Cancer. 2014;50:2190–2200. doi: 10.1016/j.ejca.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Ueno T, Masuda N, Yamanaka T, Saji S, Kuroi K, Sato N, Takei H, Yamamoto Y, Ohno S, Yamashita H, Hisamatsu K, Aogi K, Iwata H, Sasano H, Toi M. Evaluating the 21-gene assay recurrence score as a predictor of clinical response to 24 weeks of neoadjuvant exemestane in estrogen receptor-positive breast cancer. Int J Clin Oncol. 2014;19(4):607–613. doi: 10.1007/s10147-013-0614-x. [DOI] [PubMed] [Google Scholar]
- 21.Barnadas A, Gil M, González S, et al. Exemestane as primary treatment of oestrogen receptor-positive breast cancer in postmenopausal women: a phase II trial. Br J Cancer. 2009;100(3):442–449. doi: 10.1038/sj.bjc.6604868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krainick-Strobel UE, Lichtenegger W, Wallwiener D, Tulusan AH, Janicke F, Bastert G, Kiesel L, Wackwitz B, Paepke S. Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer. 2008;8:62. doi: 10.1186/1471-2407-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, Moy B, Bardia A. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1477–1486. doi: 10.1001/jamaoncol.2016.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryan J, Costantino JP, Geyer CE, Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 25.Habel LA, Shak S, Jacobs MK, Capra A, Alexander C, Pho M, Baker J, Walker M, Watson D, Hackett J, Blick NT, Greenberg D, Fehrenbacher L, Langholz B, Quesenberry CP. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8(3):R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, BugariniR BFL, Shak S. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 27.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE, Jr, Dees EC, Goetz MP, Olson JA, Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW., Jr Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018 doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF, Breast Cancer Intergroup of North A Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomized trial. Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakha EA, Reis-Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12:207. doi: 10.1186/bcr2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward WH, DeMora L, Handord E, Sigurdson ER, Ross EA, Daly JM, Aggon AA, Bleicher RJ. Preoperative delays in the treatment of DCIS and the associated incidence of invasive breast cancer. Ann Surg Oncol. 2020;27:386–396. doi: 10.1245/s10434-019-07844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang ES, Hyslop T, Lynch T, et al. The COMET (comparison of operative versus monitoring and endocrine therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS) BMJ Open. 2019;9(3):e026797. doi: 10.1136/bmjopen-2018-026797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanbayashi C, Thompson AM, Hwang ES, et al. The international collaboration of active surveillance trials for low-risk DCIS (LORIS, LORD, COMET, LORETTA) J Clin Oncol. 2019 doi: 10.1200/JCO.2019.37.15_suppl.TPS603. [DOI] [Google Scholar]
- 33.Masuda N, Sagara Y, Kinoshita T, Iwata H, Nakamura S, Yanagita Y, Nishimura R, Iwase H, Kamigaki S, Takei H, Noguchi S. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomized phase 3 trial. Lancet Oncol. 2012;13(4):345–352. doi: 10.1016/S1470-2045(11)70373-4. [DOI] [PubMed] [Google Scholar]
- 34.Linsk A, Mehta TS, Dialani V, Brook A, Chadashvili T, Houlihan M, Sharma R. Surgical upgrade rate of breast atypia to malignancy: an academic center’s experience and validation of a predictive model. Breast J. 2017;24:115–119. doi: 10.1111/tbj.12885. [DOI] [PubMed] [Google Scholar]
- 35.Eby PR, Ochsner JE, DeMartini WB, Allison KH, Peacock S, Lehman CD. Frequency and upgrade rates of atypical ductal hyperplasia diagnosed at stereotactic vacuum-assisted breast biopsy: 9- Versus 11-Gauge. AJR Am J Roentgenol. 2008;192:229–234. doi: 10.2214/AJR.08.1342. [DOI] [PubMed] [Google Scholar]
- 36.Lamb LR, Bahl M, Hughes KS, Lehman CD. Pathologic upgrade rate of high-risk breast lesions on digital two-dimentional vs tomosynthesis mammography. J Am Coll Surg. 2018;226:858–867. doi: 10.1016/j.jamcollsurg.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 37.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 38.American College of Surgeons, American Society of Anesthesiologists, Association of Perioperative Registered Nurses, American Hospital Association. (2020) Joint Statement: Roadmap for Resuming Elective Surgery after COVID-19 Pandemic https://www.aha.org/system/files/media/file/2020/04/roadmap-from-aha-others-for-safely-resuming-elective-surgery-as-covid-19-curve-flattens.pdf
- 39.COVID-19 Pandemic Breast Cancer Consortium’s Considerations for Re-entryhttps://www.surgonc.org/wp-content/uploads/2020/05/COVID-BC-Reentry-Paper-May15_0100P_Clean.pdf?_zs=r7ljB1&_zl=PP5m5
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Copies of the REDCap and Excel databases are available to individuals or institutions without charge via the Mass General Division of Surgical Oncology website. https://www.massgeneral.org/surgical-oncology/about/news-and-events/re-entry-tool-for-breast-surgeons/.