Abstract
Models of Care (MoCs), and their local Models of Service Delivery, for people with musculoskeletal conditions are becoming an acceptable way of supporting effective implementation of value-based care. MoCs can support the quadruple aim of value-based care through providing people with musculoskeletal disease improved access to health services, better health outcomes and satisfactory experience of their healthcare; ensure the health professionals involved are experiencing satisfaction in delivering such care and health system resources are better utilised. Implementation of MoCs is relevant at the levels of clinical practice (micro), service delivery organisations (meso) and health system (macro) levels. The development, implementation and evaluation of MoCs has evolved over the last decade to more purposively engage people with lived experience of their condition, to operationalise the Chronic Care Model and to employ innovative solutions. This paper explores how MoCs have evolved and are supporting the delivery of value-based care in health systems.
Keywords: Models of care, Musculoskeletal, Patient-centred, Implementation, Evaluation, Value-based care, Health system strengthening
Introduction
Musculoskeletal conditions are the leading cause of disability, loss of healthy life years and loss of productive life years across the globe [1,2]. In responding to these data, health systems and their healthcare practitioners worldwide have sought to understand how to reduce this burden. Despite many disease-specific and more general clinical guidelines for musculoskeletal conditions [3,4], delivery of high-value care to people living with long-term musculoskeletal conditions is not commonplace. There is repeated evidence over decades revealing poor alignment between clinical guidelines and clinical practice [[5], [6], [7], [8], [9], [10]]. Many barriers to the uptake of clinical guidelines have been reported, including the need to show clinical teams how to implement guidelines, time factors in clinical practice, inflexible fiscal arrangements at the system level, lack of multidisciplinary team members required to effectively implement evidence-informed care strategies, perceived complicated treatment regimens and community perceptions that are not founded on evidence [[11], [12], [13]].
An evolving web of evidence points to Models of Care (MoCs) as one potential solution for health systems to close evidence–practice gaps and support delivery of high-value care to people with musculoskeletal conditions [14,15]. MoCs are generally jurisdiction-specific frameworks that articulate what care people with a particular health condition should receive and how the local health system should deliver that care [16]. MoCs are grounded in best evidence with appropriate contextual consideration around what is achievable in the jurisdiction where it is to be applied. Relevant considerations include the fiscal environment, existing health policy and health governance, local clinical expertise and the lived experience of local communities across the jurisdiction.
When implemented locally within a jurisdiction, an MoC will be adapted and operationalised for local service delivery considering these elements, usually with ‘must have’ elements identified in the MoC as well as adaptable components that are flexible to meet local needs. Adaption and operationalising the MoC from a system-level framework to a local model is sometimes referred to as the ‘Model of Service Delivery’ (MoSD) [14].
Over the past decade, there has been a growing evolution towards more collaboration in the development, implementation and evaluation of MoCs and MoSDs. Historically, approaches to development of clinical guidelines have been the role of expert clinicians and researchers, although more contemporary guidelines are starting to involve patient partners in development processes [17]. Development of MoCs has definitively transitioned towards this co-design approach to be more collaborative with all those involved and innovative co-design methods have been applied to achieve this. These partnerships are relevant not only to MoC development, but also to care delivery in communities, evaluation and determination of any changes required to achieve better health outcomes and use of healthcare resources [18].
Value-based care is a concept that was first described by Porter in 2010 [19]:
‘Achieving high value for patients must become the overarching goal of health care delivery, with value defined as the health outcomes achieved per dollar spent’. (p. 2477)
The premise is that value relies on health outcomes and not the volume of service provided. This is a shift in health system thinking that many jurisdictions globally are now aiming to achieve; that is, a move from ‘volume-based’ to ‘value-based’ care. Value-based care is touted to achieve the quadruple aim of better health outcomes, better patient and health professional experiences and subsequently improved use of health service resources (Fig. 1 ) [20].
Fig. 1.
Working towards value-based care across a health system. Developed by Speerin R, Chua J and Briggs AM.
Value-based care was first discussed by Berwick in 2008 as a triple aim of measuring health outcomes, improved patient experience and better use of health resources [21]. Porter added to the concept by including health professionals' experiences, seen as an important factor in achieving value-based care. Value-based care can be viewed as a continuum from high to low. ‘High-value’ care refers to care for which evidence suggests it confers benefit on patients, or probability of benefit exceeds possible harm, or, more broadly, the added costs of the intervention provides proportional added benefits relative to alternatives [22].
Are MoCs supporting the shift to value-based care across the quadruple aim? Does the evolution of MoCs for people living with musculoskeletal conditions support the achievement of the quadruple aim? The paper will reflect on the changing attitudes towards healthcare by all stakeholders in the health system. Consideration of some of the workforce requirements to deliver the health outcomes will be discussed, along with progress of consumer and clinical experiences. Are better health outcomes being achieved?
Evolution of contemporary models of care for musculoskeletal conditions
MoCs were borne from frustration that traditional clinical pathways developed from evidence-informed healthcare were not being implemented for individuals, let alone across health settings and systems, resulting in unacceptable care variation and inequity in access to care [[23], [24], [25]]. Evolving population health states, changing health requirements and expectations of the patient and their families, and changing approaches to care, mandated that a different approach to care planning, delivery and participation be taken. MoCs were conceived to combine best practice healthcare and other services that are important to the person, population group or patient cohort across the continuum of a condition, injury or health event. MoCs aim to ensure people get the right care, at the right time, by the right team, in the right place, with the right resources [[26], [27], [28]]. MoCs for long-term conditions represent a shift from episodic provision of care to meet crisis situations, to care delivery across the disease continuum and life course [29,30].
The chronic care approach and the biopsychosocial model
While the Chronic Care Model proposed by Wagner et al. in the 1990s [31] and the biopsychosocial model proposed by Engel in 1977 [32] are not new, the acceptance and integration of these person-centred and multidimensional approaches into care planning and delivery continues to evolve beyond the historic reductionist biomedical model. For the majority of long-term musculoskeletal conditions, application of the reductionist biomedical model has not been effective in improving outcomes [[33], [34], [35]]. Thus, the Chronic Care approach and the biopsychosocial model are now central foci for MoCs for long-term conditions. They are particularly relevant to musculoskeletal MoCs, where target conditions are typically life-long and associated with persistent pain and functional impairment, and require interaction with a range of health services over decades from primary care to specialist medical and surgical services, even if intermittent. In Chronic Care, self-management support remains a fundamental component of effective care. Operationalising these approaches within MoCs requires consideration of the person's lived experience (incorporating biological, psychological and social sequelae), person-centred health outcomes and their experiences of care. Recent debate in chronic pain care, for example, suggests that the biopsychosocial model of pain care is inadequately fit for purpose and needs to evolve further towards a sociopsychobiological MoC [34].
The lived experience of disease and healthcare
The inclusion of the person's lived experience of a condition(s), including the psychosocial sequelae, is now accepted as fundamental to designing, implementing and evaluating MoCs. In order to understand and listen to those who know the condition best – the person living with the condition and their family/carer – several elements have been added to the development, delivery and evaluation of MoCs in recent years [18]. This a clear shift from MoCs developed prior to the past decade or so. This shift has seen health teams, organisations and systems working to incorporate these concepts within musculoskeletal MoCs through the use of multi-stakeholder networks, such as those in Western Australia and New South Wales, Australia (see https://ww2.health.wa.gov.au/Articles/J_M/Musculoskeletal-Health-Network and https://www.aci.health.nsw.gov.au/networks/musculoskeletal).
While efforts were made in the past to include the lived experience, recent thinking and evidence has revealed that an even higher level of partnership between stakeholders is required to develop health services that meet the needs of those who access them as recipients of care [36]. Therefore, methodologies, such as Clinical Redesign [37], Shared Decision-Making [38] and Co-Design [30], have been used in recent years to facilitate this in-depth level of partnership. Incorporating advice borne from experience can richly inform ‘what’ and ‘how’ care should be delivered in a local system. With the voice of the lived experience augmenting clinical experience and healthcare evidence concerning a particular condition, the resulting care is more likely to be acceptable to all involved and better reflect care that is high value [[39], [40], [41], [42]]. Critically, satisfaction of receivers of care is more likely to be associated with effective and active participation in their healthcare requirements [43,44].
Practice point.
Shared decision-making is imperative for those with lived experience to be more inclined than previously to actively participate in their care plan.
Measuring what matters to people: patient-reported measures
Patient-reported measures (PRMs) have evolved as a mechanism to report on and incorporate the patient voice in MoCs. PRMs enable evaluation of healthcare, and can inform higher value-oriented changes to health services and health systems [45,46]. PRMs are commonly classified as two separate outcome measures: patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs). Studies have shown that inclusion of PROMs enhances treatment decisions, patient satisfaction and subsequent adherence to agreed treatments [47,48]. Systematic review evidence points to the likely benefit of using PROMs in healthcare [49]. PREMs indicate the patient's perspective on issues, such as access to care, how they were approached and included in their treatment decisions [[49], [50], [51]]. While satisfaction surveys have long been a part of MoCs, PREMs are relatively new with few tools to support their inclusion in clinical practice with local teams often developing their own.
PROMs have the ability to identify issues that may not be elucidated during clinical assessments. There is appreciation now that background psychological issues, such as depression, anxiety and an individual's health beliefs and expectations, may impact upon recovery and their participation in care. This has led to specific PROMs, such as those that assess important person-centred impacts of living with a condition (e.g. in the management of people with back pain [52]) and others that focus more on generic issues that occur across healthcare needs, inclusive of social requirements [53]. As such, PROMs have the dual capability of informing clinicians as to the whole of health impact of disease while at the same time providing a conduit via which previously unstated patient concerns may be addressed. PROMs and PREMs are important components to the quadruple aim of value-based care.
Research point.
There is an urgent need for a comprehensive research agenda concerning PREMs.
Innovation in development and implementation of models of care and service delivery
As our understanding of the requisite components and practices for developing MoCs and MoSDs evolve, innovation in development practices similarly evolves.
Innovation in development
Box 1 provides a case study on the application of multi-criteria decision analysis (MCDA) to the development of a national-level MoSD.
Box 1. Application of multi-criteria decision analysis to co-design an osteoarthritis Model of Service Delivery in New Zealand.
Policy context
Like many other countries, early and appropriate conservative care for osteoarthritis (OA) remains underutilised in New Zealand (NZ). Currently, NZ has an outcomes framework for service development and commissioning for long-term conditions [54], including specific strategies for diabetes [55] and healthy ageing [56]. However, no frameworks or policies specifically exist for musculoskeletal healthcare. In response to this gap, in 2015, the NZ government allocated NZD 6 million over 3 years to trial and evaluate local healthcare programmes that aimed to improve access to early community-based interventions that provided contemporary clinical interventions and self-management support of people with musculoskeletal conditions – the ‘Mobility Action Programme’ [57]. An additional $44 million (New Zealand $) was allocated to address access issues to joint replacement surgery [58]. The aim of the Mobility Action Programme is to trial, evaluate and commission effective and sustainable programmes for broader dissemination across NZ in the future.
Why a co-design approach was needed in New Zealand for an OA Model of Service Delivery
Despite these investments, a national framework to align the health system with best practice recommendations for musculoskeletal conditions does not exist prompting a call to action for an OA MoC for NZ [59]. Furthermore, there is currently no guidance for health service delivery for decision-makers, such as planners, coordinators or funders, about which interventions for OA offer the greatest perceived value in the NZ health system. For OA, this decision can be particularly challenging given that there are many interventions to choose or recommend in a national-level service model, and because implementing recommended OA care is typically complex and influenced by many interdependent barriers and facilitators across the health system [12,60]. For these reasons, adopting the principle of co-design with stakeholders from across the sector in reform efforts is essential, including the perspectives of people with a lived experience of the condition and vulnerable groups, to ensure appropriate consideration of ‘context’ and ‘fit’ [18,36].
Strong evidence points towards incorporating context, that is, the environmental conditions which influence the barriers and enablers of implementation [61]) into the decision to adopt or commission an intervention for implementation [12,60]. In particular, establishing the ‘fit’ of established or emerging interventions within the NZ context could help to enhance implementation of OA care by more closely aligning intervention performance with what stakeholders want. Alignment of recommendations for OA care will potentially reduce healthcare waste [36]. For example, interventions that align closely with decision-makers’ decision-making criteria, such as intervention cost, accessibility and effectiveness, and evidence about interventions' performances on these criteria could enhance implementation efforts and better align with system policy priorities.
Application of MCDA to informing an osteoarthritis Model of Service Delivery
Developing national recommendations for OA care is a complex endeavour and should ideally represent the views and opinions of the people most relevant to OA care in a particular context. In NZ, this context includes not only people across the community living with OA, but also specifically Māori healthcare advocates. They need to work in tandem with healthcare providers, policy-makers and OA research and clinical experts working in the various care settings. However, these eclectic stakeholders typically make complex decisions involving many considerations, or criteria, which often compete. Weighing up these different criteria to reach a decision should ideally occur through a transparent, trustworthy and fair process. Approaches for reaching consensus include, for example, verbal agreement, Delphi surveys, Nominal Group Technique and consensus development panels (e.g. deliberative dialogues) [36]. Some limitations of these methods include: (1) decision-making criteria may not be explicit and decisions may be made without appropriate time for reflection; (2) decision-makers’ preferences or values are typically implicit; (3) new evidence, ideas or alternative choices may invalidate previously reached consensus and (4) engagement can be limited, particularly among those with a lived experience of a health condition. Multi-criteria decision analysis (MCDA) is a systematic, transparent and fair approach to decision-making that can address these important considerations [62].
MCDA structures decision-making by incorporating subjective and objective data in a systematic and transparent process that identifies and weighs multiple evaluation criteria in order to prioritise different healthcare interventions, policy options or alternatives [63]. This structured, systematic approach is a defining characteristic of MCDA and helps to overcome mental shortcuts (often employed in complex decisions, e.g. ‘gut feeling’) which can lead to systematic mistakes, poor decisions, and ultimately, poor decision-making credibility [64]. The advantages of MCDA include: i) explicit evaluation criteria enforces transparency, accountability and fairness; ii) scalability – the ability to prioritise new or emerging interventions without capturing stakeholders' preferences for these interventions each time and iii) efficiencies in design and deployment streamline decision-making allow for broader and more meaningful engagement with a diverse range of stakeholders.
In recent years, MCDA has become widespread in healthcare research [65,66]. For example, it has been used to explore the preferences of people with OA concerning physical activity [67], patients' preferences for the use of medicines [36], healthcare providers' treatment choices for people with OA [68] and policy-makers’ and clinical decision-makers’ preferences for intervention choice [69]. It has not been applied previously to inform a cross-sector, consensus-based MoSD for people with OA.
How has MCDA been applied in the NZ context for OA care?
-
1.
Identify the most relevant decision-making criteria
Through a qualitative study in 2016–2017, multidisciplinary and cross-sectoral stakeholders in NZ (people with the lived experience of OA, healthcare providers, policymakers and OA clinical and research experts) identified nine criteria for selecting or recommending OA interventions in the NZ public health system: accessibility, active versus passive interventions, appropriateness, cost, duration, effectiveness, quality of evidence, recommendation and risk of harm [70].
-
2.
Select and structure the criteria and weight them according to stakeholders' preferences
Criteria were organised according to the guidance for MCDA [71], for example, selecting non-redundant and non-overlapping criteria. The criteria were then categorised into ‘levels’ describing their performance. For example, the criterion ‘effectiveness’ was categorised into high, moderate and low levels of performance using the standardised mean difference. Choice-based surveys were used to quantify people's preferences for the criteria, that is, their relative weights.
-
3.
Score the performance of interventions on the criteria
Interventions were then rated on the performance levels of the criteria, sourced from clinical guidelines [72], local data and a nationally representative panel of experts in OA management in NZ. The final step involved combining the preference weights and intervention ratings together to calculate a total performance score for each intervention.
-
4.
Outcome
Findings from this activity could be used to inform recommendations in a national MoSD for OA, which outlines those interventions that offer the greatest perceived value to NZ stakeholders. The MCDA process enabled the views and perspectives of all stakeholder groups, particularly people living with OA and Māori advocates, to be respected and equally considered.
Alt-text: Box 1
Digital and electronic health solutions
Digital health systems now offer unprecedented capabilities to health systems and consumers in data collection, delivery of information and access to health services. Access is enhanced through real-time access to care (e.g. through telehealth) and digitally based tailored care (e.g. through adaptive mHealth platforms). The healthcare opportunities offered by digital systems and the ubiquitous use of digital platforms globally, such as mobile phones, rationalise digital health systems as a key strategy for health system strengthening [73]. These technologies have appeal, particularly for younger people [74], and also provide opportunities to close care disparity gaps that exist due to geography and socioeconomic circumstance, and allow for electronic recording of care access and delivery over time.
While there has been an uptake in telehealth and mHealth solutions for some chronic conditions in the past decade, including opportunities for musculoskeletal and pain care [75], system-wide adoption in musculoskeletal care outside hospitals has been slow, despite promising opportunities. Many reasons can be attributable to the slow uptake, in particular the major gap between innovation and testing and appropriate scale-up into systems [76]. There is an opportunity to close innovation-adoption gaps by better integrating digital solutions into the design of MoCs. In the case of telehealth, one reason is the belief that physical clinical assessment is paramount to diagnosing and monitoring a musculoskeletal condition. Perhaps this will change following the COVID-19 pandemic, which forced clinicians to adopt these tools as part of routine care. In the case of electronic medical recording, the required allocation of significant resources in settings (out of hospital acute care) that have traditionally not been seen as important is limiting uptake. However, these systems can add value to better utilisation of clinical time, ease of recording of PRMs and greatly assist in evaluation efforts [77].
Practice, policy and research points.
The development, implementation and evaluation of MoCs for musculoskeletal conditions requires equal partnerships between those with lived experience of the disease/condition and their carers, health professionals across all disciplines, service resource personnel, researchers and policy-makers in jurisdictions.
Enhanced decision-making methods, such as multi-criteria decision analysis, can be used to inform more robust and trustworthy health policy decision-making through broader and more inclusive engagement of people with lived experience; this could help policy-makers identify better value-based care options.
There is an urgent need for further uptake of digital technologies to support implementation of MoCs.
How well are models of care accepted in health systems and their services?
It has long been accepted and expected that a health system uses structures and processes to create an integrated care environment that is appropriate for the population it serves [78,79]. MoCs, when developed as described in this paper, have been a fundamental means of enabling this to happen in some jurisdictions and care settings [80,81]. For example, national health policies for non-communicable diseases refer to the need to develop and implement MoCs to drive health system strengthening [82]. The National Health Service in the United Kingdom is a good example where MoCs have been the main drivers of change including in the development of strategy to turn attention to value-based healthcare [83,84]. Acceptability of MoCs by stakeholders from across the health system in Australia was validated in a large qualitative study [25]. Informants identified the value of MoCs in translating evidence to policy and practice and supporting system-wide health transformation.
Implementing models of care to support value-based care
When MoCs are developed as described in this paper and a quality improvement cycle is used, such as an established framework for evaluation [18], they can provide clear evidence for health systems and policy-makers when making decisions regarding equitable use of resources that will optimise health system outcomes across the quadruple aim for value-based care.
Low back pain, identified as the single greatest contributor to the global burden of disability since 1990 [1] represents an important focus for health systems to realign to deliver value-based care. Currently, health systems deliver too much low-value care and too little high-value care for low back pain. Recent reviews have identified key challenges for health systems in delivery of high-value care for low back pain and pain care in general [33,34,85,86]. These include the financial interests of pharmaceutical and other companies; inflexible payment systems that favour medical care over patients’ self-management; and deep-rooted biomedical traditions and beliefs about pain among physicians and the community at large. Authors have argued for system-level reform strategies, such as the implementation of MoCs, to shift resources from unnecessary, low-to high-value care and that such endeavours could be cost-neutral and have widespread impact.
Evidence is building concerning whether MoCs can support value-based care. Health systems are now using MoCs to inform strategy concerning value-based care [87,88]. For example, musculoskeletal MoCs for people living with osteoarthritis and those experiencing fragility fractures are included in the suite of services for value-based care initiatives in Australia [89], while in Canada, a national approach to hip fracture care has been developed [90]. The New South Wales health system in Australia is now into the fourth year of formally implementing value-based care, with MoCs remaining at the forefront of decision-making on each tranche of implementation [91,92].
Multidisciplinary team-based care as an enabler to implementing musculoskeletal models of care
Healthcare transformation, designed to improve access and quality of care while containing cost, is a worldwide priority [[93], [94], [95]], aligned with value-based care and enabled through MoCs. Many countries have responded by enabling existing healthcare professionals to work to their maximum scope (advanced scope of practice [[96], [97], [98], [99], [100], [101]], which has also been articulated as an enabler to implementing MoCs [102]. Historically, this approach has worked to address shortages in available healthcare practitioners in remote and rural areas and on the front line to triage injured soldiers during wartime. More recently, advanced practice roles have been implemented as part of new inter-professional models of team-based musculoskeletal care. These innovative MoCs require close collaboration and communication among healthcare providers, integration across care sectors (private/public, hospital/primary/community based) and funding models that enable and promote evidence-based care across the full continuum of care [103].
While people with musculoskeletal conditions access a variety of medical and surgical specialties, there is a need for other non-medical health professionals to be involved in order to access high-value care; for example, supporting the person's understanding of and addressing issues, such as psychological needs, co-morbidities, weight loss and physical activity in osteoarthritis care. Nurses and allied health professionals, such as physiotherapists, dieticians and others, are key members of a true multidisciplinary musculoskeletal care team. Not surprisingly, the high cost and volume of people, who require improved access to musculoskeletal care in the primary care sector, has resulted in the emergence of the new inter-professional service models inclusive of health professionals with advanced scope of practice skills such as nurse practitioners and physiotherapists who have had advanced training in musculoskeletal healthcare [98,100,[104], [105], [106], [107], [108]]. As demand for musculoskeletal health services increase, MoCs will increasingly recommend alternate and innovative workforce models to ensure timely access to care and high-value service delivery.
In high-income settings, these examples of new cadres who are educated and authorised to function autonomously and collaboratively in advanced and extended clinical roles may help to achieve some of the quadruple aims of value-based care. These may include reducing wait times in emergency departments, triaging to surgical consult for total knee or hip joint replacement, re-fracture prevention assessments and investigation, and performing as the lead health professional in supporting people presenting with acute low back pain [[109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121]]. Ongoing evaluation of clinical, economic and patient experience outcomes will be important to maintain momentum in optimising workforce configurations. In lower resourced settings, building capacity among appropriately skilled and trained community health workers will be important to implementing MoCs and avoiding catastrophic out of pocket expenditure for citizens, such as integrated care services for older people [122] and community-based spine care [123].
An important aspect of value-based care and involvement of multidisciplinary teams is health professional experience. To understand their experience, there is a real need to develop a standardised way of measuring elements that are meaningful to health professionals in the delivery of care. An example is the National Health Service in the UK that could be developed for use across jurisdictional borders. See at: https://www.nhsstaffsurveys.com/Caches/Files/ST19_Core%20questionnaire_FINAL_20190705.pdf.
Practice, policy and research points.
There is an urgent need to develop a tool that measures health professional experience across the domains of job satisfaction, burnout, how adverse events are handled, how engagement up and down the hierarchy occurs, their ability to provide care that incorporates all the impacting issues that a person presents for care is experiencing across biopsychosocial areas, and their capacity to influence change within the health service.
Funding and implementation of team-based MoCs that allow for local adaptation in rural and remote as well as urban areas that can be scaled and spread are essential.
Ongoing evaluation of advanced practice nursing and allied health professional MoCs will be important to maintain and optimise workforce configurations.
Implementation efforts for models of care internationally
As the acceptance of MoCs as levers for system reform becomes more widespread, there will be an increasing need for implementation support within health systems and sharing of experiences. Implementation support can occur at different levels: from local support for an MoSD, to sub-national, to national support for system wide implementation efforts, through to global-level support for multi-national implementation. Along this continuum, the level and focus of support may vary from comprehensive local support (e.g. organisational site visits) through to principles or guiding actions for countries to work towards and self-evaluate.
Local and sub-national implementation support initiatives typically involve assisting organisations across a health system to implement an MoC through an operational-level MoSD, usually adapted to the local context. For example, in New South Wales, Australia since 2009, there has been increasing support for implementation of several MoCs for the healthcare needs of people with musculoskeletal conditions, including osteoarthritis, secondary fragility fracture prevention, hip fracture care, acute low back pain, and children and adolescents living with rheumatologic conditions. This has been achieved initially through a clinical network working with government in supporting local organisations to develop business cases for implementing the MoCs and the provision of seed funds to enable pilot implementation initiatives with common evaluation frameworks. Implementation scale-up across the system was achieved through integration of the MoCs for osteoarthritis and secondary fragility fracture prevention within a larger whole-of-system reform initiative – the ‘Leading Better Value Care’ programme: https://www.health.nsw.gov.au/Value/lbvc/Pages/default.aspx [89].
The South West England MoCs support network is an example of an online community of practice formed to support implementation of MoCs across primary care in the South West of England and beyond (https://modelsofcare.co.uk/). Multiple other sub-national implementation support programmes also likely exist, although currently no repository exists to share implementation experiences.
International efforts to support the implementation of MoCs and MoSDs across countries focus on specific service models or offer guidance that is transferable across conditions. Examples of specific service models include:
-
•
The Global Fragility Fracture Network (https://www.fragilityfracturenetwork.org/) supports implementation of best practice and localised MoCs for people with fragility fractures across the continuum of care through formal education opportunities and peer mentoring. This includes efforts for health professionals working with the patient cohort at presentation with fracture to peri-operative care, to rehabilitation and to secondary fracture prevention.
-
•
The Good Life with osteoArthritis in Denmark (GLA:D®) (https://www.glaid.dk/english.html), now implemented in Denmark, Canada, China and Australia, with further countries joining (Switzerland, New Zealand and Austria) [124]. The MoSD provides specific implementation guidance for physiotherapist-led exercise and education for people with hip and/or knee osteoarthritis.
-
•
The International Osteoporosis Foundation Capture the Fracture® programme (https://www.capturethefracture.org/) [125] facilitates the implementation of coordinated, multi-disciplinary service models for secondary fragility fracture prevention. The programme offers multiple implementation resources to support countries implement the programme and evaluate the scale of implementation achieved (https://www.capturethefracture.org/resources).
-
•
The Global Spine Care Initiative (https://www.globalspinecareinitiative.org/) [123] supports the implementation of local spine care services in low- and middle-income countries. It is based on eight principles that consider implementation from the micro to macro contexts. The clinical and health economic outcomes of the model are yet to be established.
-
•A framework (the ‘Framework’; Fig. 2 ) to support implementation of any musculoskeletal MoC was developed in 2016, empirically derived with input from 93 stakeholders across 30 countries [126]. The framework guides those tasked with implementation in a health system to consider and evaluate the following domains:
-
-Implementation readiness
-
-Best practice approaches to support implementation
-
-Evaluation.
-
-
Fig. 2.
Summary framework for assessing readiness of an MoC for implementation (domains 1–4), initiating implementation (domain 5) and measuring success of a musculoskeletal MoC (domains 6–9). Reprinted from ‘Arthritis Care and Research, 69(4), Briggs AM, Jordan JE, Jennings M, Speerin R, Bragge P, Chua J, Woolf AD, Slater H: Supporting the evaluation and implementation of musculoskeletal Models of Care: A globally informed framework for judging readiness and success, 567–77, Copyright (2017)’, with permission from John Wiley and Sons, license number 4760990333394 [18]).
The framework is now endorsed by 54 peak international organisations and has been used internationally to support development, implementation and evaluation of MoCs (see Fig. 2).
The majority of public-facing MoCs for musculoskeletal conditions have been developed and supported for implementation in high-income countries. While there are some initiatives occurring in low- and middle-income countries [127,128], there remains a need to support development and implementation of musculoskeletal MoCs in low- and middle-income countries that supports, rather than threatens, fragile health systems [129,130]. Recent research, for example, has confirmed the suitability of the Chronic Care Model for low- and middle-income settings, but identified that some adaptation and expansion is required for low-resource settings [131]. The WHO Integrated Care for Older People (ICOPE) model supports countries at all stages of maturity to consider actions required to realign health systems towards the needs of older people, including their mobility and musculoskeletal health [122]. Implementation tools are now available to support the implementation of ICOPE internationally in health services and health systems [132].
Systematic reviews have reported a range of factors that have been identified as barriers and enablers to implementation of MoCs in health services and health systems [12,[133], [134], [135]]. As outlined in the framework (Fig. 2), consideration of these factors and others that are relevant to the local context are essential in approaching any implementation effort.
Policy and practice point.
It is timely to now consider multi-morbidity in Models of Care (MoCs), given the prevalence of multi-morbidity of non-communicable diseases with musculoskeletal conditions commonly being prevalent. Evolution of future MoCs needs to more explicitly consider multi-morbidity and how condition-specific or multi-morbidity MoCs can support delivery of high-value care.
While there are some examples in recent years to support the development and implementation of some musculoskeletal MoCs in low- and middle-income countries, for example, fragility fracture and spine care, there is a dire need to adapt and support implementation of MoC in a manner that is suitable for fragile health systems.
More research is required concerning health and economic outcomes achievable through implementation of MoCs in order to drive further health system improvements globally. The approach proposed by Jessup and colleagues is a good starting point [136].
Evaluation of models of care to support implementation efforts
Importance of evaluation
System-level MoCs and their operational-level MoSDs are one of many tools that support the translation of evidence into system (macro)-level (e.g. policy), service (meso)-level (e.g. programme implementation) and clinical (micro)-level (e.g. practice) changes [14]. However, all too often evidence translation initiatives end with short-term and non-recurrent resourcing, resulting in multiple short-term trial reforms, programmes or pilots. These then often lead to reform fatigue among those tasked with implementation and monitoring [137]. On this background, appropriate evaluation of MoCs is an essential component of supporting long-term implementation and sustained reform and quality improvements efforts in health systems. Local system evaluation is critical [138], recognising that outcomes between settings may not necessarily be comparable; for example, between high- and low-income economies, or even at the sub-national level between jurisdictions with geographic differences that can impede access to services and/or expert services.
Approaching evaluation of models of care
Evaluation of MoCs must be pragmatic, flexible to a dynamic health system, respectful of local contextual issues and meaningful to intended end users. The parameters of evaluation should be co-designed with decision-makers and end users to ensure the outcomes are meaningful, useable and contemporary [139]. The perspectives of people with lived experience of a health condition are also critical to ensure meaningful concepts are measured [36]. Evaluation approaches need to consider design and methods, target levels of the health system and outcomes.
While ‘effectiveness’ evaluation (research) remains essential to advancing health innovation, identifying high-value care and minimising health waste, this mode of evaluation may be less relevant and feasible at the stage of implementing MoCs in health systems. By definition, the components of an MoC should be evidence based [14]; suggesting that evaluation of effectiveness is less of a priority for the downstream evidence translation phase of implementation. The necessary structured and rigid design of gold standard effectiveness research designs, such as the randomised controlled trial (RCT), may collide with the dynamic nature of health systems and health services and inadequately reflect outcomes of the broader population health state. In particular, RCTs may often exclude people with multi-morbid health states, which now reflect the norm rather than a subgroup [140]. Researchers are starting to apply more innovative trial designs to deal with this challenge and focus specifically on implementation trial designs [141], although more experience and work is required to apply such methods across services or across a system. The incompatibility between RCTs and health systems research is particularly relevant in the context of MoCs with a strong eHealth component [142], where evaluation approaches other than effectiveness designs are recommended and supported by guiding frameworks [76,[143], [144], [145], [146]]. These evolving frameworks could feasibly be applied beyond eHealth, with many of the guiding principles already reflected in a framework developed to specifically guide evaluation of musculoskeletal MoCs [126]. Collectively, the framework advocates for continuous cycles of mixed-methods evaluation (or formative evaluations), aligned with a process evaluation approach to iteratively understand how implementation could be optimised in a given context and be responsive to changing circumstances [147], before initiating a summative (impact) evaluation. The framework also suggests that evaluation should not be based on single outcomes, but consider a multidimensional approach to defining ‘success’, ‘performance’ or ‘benefit’, achieved through mixed-methods approaches that consider outcomes relevant to the consumer (e.g. PROMs and PREMs) and the health system [126,145]. The importance of emphasising qualitative research exploring stakeholders' perceptions and attitudes towards implementation feasibility, acceptability and sustainability is widely proposed [36,148].
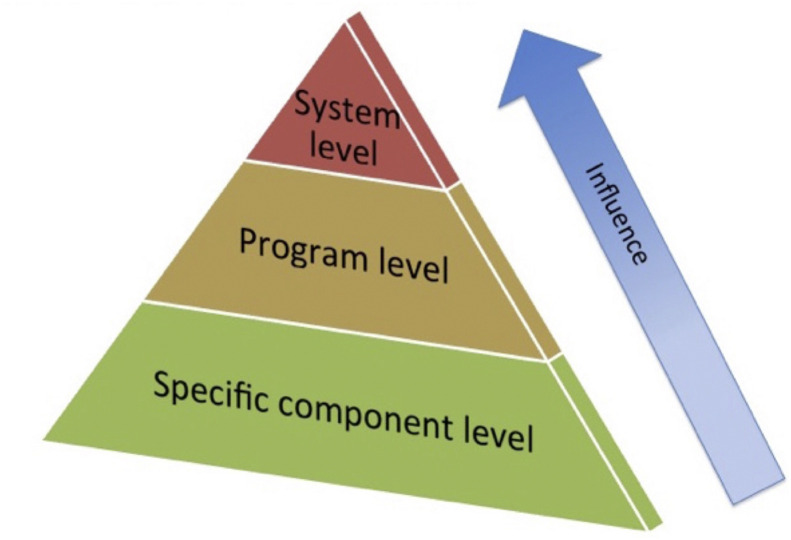
Evaluation of MoCs or downstream MoSDs can be targeted at different levels of the health system. An evaluation hierarchy, or pyramid, has been proposed, which suggests evaluation can be targeted at one or more of three levels (see Fig. 3 ) [14]: 1) specific components of an MoC or MoSD (e.g. a specific clinical behaviour, work cadre or self-management strategy); 2) at the programme level (e.g. where a specific health improvement programme is implemented, such as the GLA:D® programme [124] or Capture the Fracture® initiative [125]) or 3) at the whole-of-system level (e.g. the system wide impact of implementing an MoC or multiple service models across a whole system such as the WHO ICOPE approach [122]).
Fig. 3.
Evaluation hierarchy for Models of Care. Reprinted from ‘Best Pract Res Clin Rheumatol, 30(5), Briggs AM, Chan M, Slater H: Models of Care for musculoskeletal health: Moving towards meaningful implementation and evaluation across conditions and care settings, 359–74, Copyright (2016)’, with permission from Elsevier, license number 4760981373908 [14]).
Most implementation and evaluation efforts occur at the base of the pyramid, while the least occurs at the system level [149,150], likely reflecting the challenges associated with whole-of-system evaluation endeavours. Evaluation at the system level, however, remains of critical importance and underscores the need for appropriate health surveillance infrastructure and systems [33,82,122,151], particularly in low- and middle-income countries [130]. As implementation of MoCs evolve and evaluation systems become more sophisticated, the need for a pyramid inversion will become greater. Indeed, many jurisdictions are now emphasising the need to reorient evaluation towards a whole-of-system lens in order to build a better picture of overall system functioning and performance against reform targets, inclusive of an approach that integrates patient, clinician and system performance outcomes [89,152]. While system- and service-level integration of PROMs and PREMs is challenging, as identified in a recent systematic review of barriers to implementation [153], recent data from New South Wales, Australia, point to the feasibility of measuring PROMS across a system though electronic platforms [154]. In order to achieve integrated measurement of patient and clinical outcomes (micro level), service-level (meso) outcomes and system-level (macro) outcomes, co-design with end users of the evaluation (typically policy-makers, health administrators/mangers and funders) is needed on a background of a lens to healthcare planning and delivery that prioritises ‘value’ over ‘volume’ [89,139]. Infrastructure and governance to enable consistent and systematic measurement is essential and enabled through registries to capture PROMs and PREMS, agreed performance indicators for health services [155,156] and linked data systems [89]. In the context of MoCs specifically, the recent evaluation framework guides measurement of ‘success’ of a musculoskeletal MoC in a health system and recommends a range of outcome domains should be considered (Fig. 2) [18].
Policy points.
-
•
Evaluation of MoCs is a critical enabler to supporting sustainable implementation and resourcing of MoCs.
-
•
Shifting from a reliance on ‘effectiveness’ evaluations to evaluation approaches that better align with multiple domains relevant to implementation is important.
-
•
Outcomes should be meaningful and useable to intended end users and decision-makers, informed through purposive co-design.
-
•
Evaluation at the system level, rather than at the programme level, may better support implementation at scale.
Discussion
This paper has revealed that in the past decade, many positive influences have supported the concept of utilising MoCs to support value-based musculoskeletal care.
The evidence is clear that the development, implementation and evaluation of MoCs must rely more on the involvement of those with the lived experience. Their involvement must be in collaboration with those who provide care, their managers and funders. Collectively, the advice from those intimately involved whether through personal experience of musculoskeletal diseases or conditions, or clinical practice, or research of the conditions, or through management and funding leads to all elements of the quadruple aim being achieved, as described by Porter in 2010 [19].
However, challenges remain that hinder widespread implementation in many areas of the globe, with many being directly attributable to some specific embedded practices [85,[157], [158], [159]]. Fig. 4 summarises challenges commonly experienced at the various levels of the health system.
Fig. 4.
Challenges to implementation of contemporary MSK models of care in health systems. Reprinted with permission by the owner, Briggs AM.
For MoCs to be even more successful in guiding clinical practice global strategies such as Choosing Wisely need to be encouraged and supported by governments internationally [[160], [161], [162]].
In low-to middle-income countries, there is a policy swing that now acknowledges the contribution from non-communicable diseases to the burden on population health [163]. For example, osteoporotic fractures in Asia are predicted to be higher than seen elsewhere in the next decade [164]. Future MoCs for healthcare will need to be incorporated into government policy concerning housing, education and employment. The momentum for such change is evidenced by the recent US document encouraging the addition of social care to all healthcare considerations [165].
Summary
In summary MoCs are ‘adopting evidence-based clinical pathways and protocols, aligning incentives, effectively managing resource, continuously monitoring and improving performance, and investing in supporting information technologies’ [166]. Their use is to encouraged more and more across the globe.
All involved in the development, implementation and evaluation of musculoskeletal MoCs should remain alert to opportunities for whole of government approaches to managing musculoskeletal conditions such as partnering with housing, education and employment sectors.
Funding statement
This paper was developed without specific funding for any of the authors.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Footnotes
This is part of a series prepared on behalf of the Global Alliance for Musculoskeletal Health to provide the background for developing global and national policies and strategies to address the enormous and growing burden of musculoskeletal conditions and to promote musculoskeletal health for all
References
- 1.James S.L., Abate D., Abate K.H., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schofield D.J., Shrestha R.N., Cunich M., et al. Lost productive life years caused by chronic conditions in Australians aged 45-64 years, 2010-2030. Med J Aust. 2015;203:260. doi: 10.5694/mja15.00132. [DOI] [PubMed] [Google Scholar]
- 3.Babatunde O.O., Jordan J.L., Van der Windt D.A., et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PloS One. 2017;12 doi: 10.1371/journal.pone.0178621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin I., Wiles L., Waller R., et al. What does best practice care for musculoskeletal pain look like? Eleven consistent recommendations from high-quality clinical practice guidelines: systematic review. Br J Sports Med. 2020;54:79–86. doi: 10.1136/bjsports-2018-099878. [DOI] [PubMed] [Google Scholar]
- 5.McGlynn E.A., Asch S.M., Adams J., et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 6.Runciman W.B., Hunt T.D., Hannaford N.A., et al. CareTrack: assessing the appropriateness of health care delivery in Australia. Med J Aust. 2012;197:100–105. doi: 10.5694/mja12.10510. [DOI] [PubMed] [Google Scholar]
- 7.Foster N.E., Anema J.R., Cherkin D., et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391:2368–2383. doi: 10.1016/S0140-6736(18)30489-6. [DOI] [PubMed] [Google Scholar]
- 8.Zadro J., O'Keeffe M., Maher C. Do physical therapists follow evidence-based guidelines when managing musculoskeletal conditions? Systematic review. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-032329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzortziou Brown V., Underwood M., Mohamed N., et al. Professional interventions for general practitioners on the management of musculoskeletal conditions. Cochrane Database Syst Rev. 2016;5 doi: 10.1002/14651858.CD007495.pub2. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suman A., Dikkers M.F., Schaafsma F.G., et al. Effectiveness of multifaceted implementation strategies for the implementation of back and neck pain guidelines in health care: a systematic review. [Review] Implement Sci. 2016;11:126. doi: 10.1186/s13012-016-0482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francke A.L., Smit M.C., de Veer A.J.E., et al. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inf Decis Making. 2008;8(38) doi: 10.1186/1472-6947-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau R., Stevenson F., Ong B.N., et al. Achieving change in primary care--causes of the evidence to practice gap: systematic reviews of reviews. Implement Sci. 2016;11:40. doi: 10.1186/s13012-016-0396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall A.M., Scurrey S.R., Pike A.E., et al. Physician-reported barriers to using evidence-based recommendations for low back pain in clinical practice: a systematic review and synthesis of qualitative studies using the Theoretical Domains Framework. Implement Sci. 2019;14 doi: 10.1186/s13012-019-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briggs A.M., Chan M., Slater H. Models of care for musculoskeletal health: moving towards meaningful implementation and evaluation across conditions and care settings. Best Pract Res Clin Rheumatol. 2016;30:359–374. doi: 10.1016/j.berh.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Speerin R., Slater H., Li L., et al. Moving from evidence to practice: models of care for the prevention and management of musculoskeletal conditions. Best Pract Res Clin Rheumatol. 2014;28:479–515. doi: 10.1016/j.berh.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Briggs A.M., Towler S.C., Speerin R., et al. Models of care for musculoskeletal health in Australia: now more than ever to drive evidence into health policy and practice. Aust Health Rev. 2014;38:401–405. doi: 10.1071/AH14032. [DOI] [PubMed] [Google Scholar]
- 17.Kolasinski S.L., Neogi T., Hochberg M.C., et al. 2019 American College of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72:220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs A.M., Jordan J.E., Jennings M., et al. Supporting the evaluation and implementation of musculoskeletal models of care: a globally informed framework for judging readiness and success. Arthritis Care Res. 2017;69:567–577. doi: 10.1002/acr.22948. [DOI] [PubMed] [Google Scholar]
- 19.Porter M.E. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 20.Sikka R., Morath J.M., Leape L. The Quadruple Aim: care, health, cost and meaning in work. BMJ Qual Saf. 2015;24:608–610. doi: 10.1136/bmjqs-2015-004160. [DOI] [PubMed] [Google Scholar]
- 21.Berwick D.M., Nolan T.W., Whittington J. The triple aim: care, health, and cost. Health Aff. 2008;27:759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 22.Elshaug A.G., Rosenthal M.B., Lavis J.N., et al. Levers for addressing medical underuse and overuse: achieving high-value health care. Lancet. 2017;390:191–202. doi: 10.1016/S0140-6736(16)32586-7. [DOI] [PubMed] [Google Scholar]
- 23.Francke A.L., Smit M.C., de Veer A.J.E., Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inf Decis Making. 2008;8 doi: 10.1186/1472-6947-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briggs A.M., Bragge P., Slater H., et al. Applying a health network approach to translate evidence-informed policy into practice: a review and case study on musculoskeletal health. BMC Health Serv Res. 2012;12:394. doi: 10.1186/1472-6963-12-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briggs A.M., Jordan J.E., Speerin R., et al. Models of care for musculoskeletal health: a cross-sectional qualitative study of Australian stakeholders' perspectives on relevance and standardised evaluation. BMC Health Serv Res. 2015;15:509. doi: 10.1186/s12913-015-1173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowak N.A., Rimmasch H., Kirby A., Kellogg C. Right care, right time, right place, every time. Healthc Financ Manag. 2012;66:82–88. [PubMed] [Google Scholar]
- 27.Western Australian Government . Department of Health; 2015. Implementation of models of care and frameworks – progress report 2015. [Google Scholar]
- 28.WHO . World Health Organisation; Copenhagen: Denmark: 2018. The right care, in the right place, at the right time - every time. World Health Day 2018. [Google Scholar]
- 29.WHO . World Health Organisation; Copenhagen, Denmark: 2016. Integrated care models: an overview. [Google Scholar]
- 30.Donetto S., Tsianakas V., Robert G. King's College; London: United Kingdom: 2014. Using experience-based co-design to improve the quality of healthcare: mapping where we are now and establishing future directions. [Google Scholar]
- 31.Wagner E.H., Austin B.T., VonKorff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–544. [PubMed] [Google Scholar]
- 32.Engel G.L. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 33.Briggs A.M., Slater H., Hsieh E., et al. System strengthening to support value-based care and healthy ageing for people with chronic pain. Pain. 2019;160:1240–1244. doi: 10.1097/j.pain.0000000000001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mardian A.S., Hanson E.R., Villarroel L., et al. Flipping the pain care model: a sociopsychobiological approach to high-value chronic pain care. Pain Med. 2020;21(6):1168–1180. doi: 10.1093/pm/pnz336. [DOI] [PubMed] [Google Scholar]
- 35.Lewis J., O’Sullivan P. Is it time to reframe how we care for people with non-traumatic musculoskeletal pain? Br J Sports Med. 2018;52(4):1543–1544. doi: 10.1136/bjsports-2018-099198. [DOI] [PubMed] [Google Scholar]
- 36.de Wit M., Cooper C., Tugwell P., et al. Practical guidance for engaging patients in health research, treatment guidelines and regulatory processes: results of an expert group meeting organized by the World Health Organization (WHO) and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) Aging Clin Exp Res. 2019;31:905–915. doi: 10.1007/s40520-019-01193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Tovim D.I., Dougherty M.L., O'Connell T.J., McGrath K.M. Patient journeys: the process of clinical redesign. Med J Aust. 2008;188:1326–5377. doi: 10.5694/j.1326-5377.2008.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 38.Légaré F.A.R., Stacey D., Turcotte S., Kryworuchko J., Graham I.D., Lyddiatt A., et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;(7) doi: 10.1002/14651858.CD006732.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou L., Ranger T.A., Peiris W., et al. Patients' perceived needs of health care providers for low back pain management: a systematic scoping review. Spine J. 2018;18:691–711. doi: 10.1016/j.spinee.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Chou L., Ellis L., Papandony M., et al. Patients' perceived needs of osteoarthritis health information: a systematic scoping review. PloS One. 2018;13 doi: 10.1371/journal.pone.0195489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou L., Shamdasani P., Briggs A.M., et al. Systematic scoping review of patients' perceived needs of health services for osteoporosis. Osteoporos Int. 2017;28:3077–3098. doi: 10.1007/s00198-017-4167-0. [DOI] [PubMed] [Google Scholar]
- 42.Connelly K., Segan J., Lu A., et al. Patients' perceived health information needs in inflammatory arthritis: a systematic review. Semin Arthritis Rheum. 2019;48:900–910. doi: 10.1016/j.semarthrit.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Beach M.C., Sugarman J., Johnson R.L., et al. Do patients treated with dignity report higher satisfaction, adherence, and receipt of preventive care? Ann Fam Med. 2005;3:331–338. doi: 10.1370/afm.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Wit M., Cooper C., Tugwell P., et al. 2019. Practical guidance for engaging patients in health research, treatment guidelines and regulatory processes: results of an expert group meeting organized by the World Health Organization (WHO) and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rutherford C., Campbell R., Tinsley M., Speerin M., Soars L., Butcher A., et al. Implementing patient-reported outcome measures into clinical practice across NSW: mixed methods evaluation of the first year. Appl Res Qual Life. 2020 doi: 10.1007/s11482-020-09817-2. [DOI] [Google Scholar]
- 46.Bacalao E.J., Greene G.J., Beaumont J.L., Eisentein A., Muftic A., Mandelin A.M., et al. Standardizing and personalizing the treat to target (T2T) approach for rheumatoid arthritis using the Patient-Reported Outcomes Measurement Information System (PROMIS): baseline findings on patient-centred treatment priorities. Clin Rheumatol. 2017;36:1729–1736. doi: 10.1007/s10067-017-3731-5. [DOI] [PubMed] [Google Scholar]
- 47.Bartlett S.J., De Leon E., Orbai A.M., et al. Patient-reported outcomes in RA care improve patient communication, decision-making, satisfaction and confidence: qualitative results. Rheumatology. 2019;59(7):1662–1670. doi: 10.1093/rheumatology/kez506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jester R., Santy-Tomlinson J., Drozd M. The use of Patient Reported Outcome Measures (PROMs) in clinical assessment. Int J Orthop Trauma Nurs. 2018;29:49–53. doi: 10.1016/j.ijotn.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Ishaque S., Karnon J., Chen G., et al. A systematic review of randomised controlled trials evaluating the use of patient-reported outcome measures (PROMs) Qual Life Res. 2019;28:567–592. doi: 10.1007/s11136-018-2016-z. [DOI] [PubMed] [Google Scholar]
- 50.Benham H., Rutherford M., Kirby S., et al. Treat-to-target in rheumatoid arthritis: evaluating the patient perspective using the Patient Opinion Real-Time Anonymous Liaison system: the RA T2T PORTAL study. Int J Rheum Dis. 2019;22:874–879. doi: 10.1111/1756-185X.13514. [DOI] [PubMed] [Google Scholar]
- 51.Bernstein D.N., Fear K., Mesfin A., et al. Patient-reported outcomes use during orthopaedic surgery clinic visits improves the patient experience. Muscoskel Care. 2019;17:120–125. doi: 10.1002/msc.1379. [DOI] [PubMed] [Google Scholar]
- 52.Hill J.C., Dunn K.M., Lewis M., et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59:632–641. doi: 10.1002/art.23563. [DOI] [PubMed] [Google Scholar]
- 53.Kwakkenbos L., Thombs B.D., Khanna D., et al. Performance of the patient-reported outcomes measurement information system-29 in scleroderma: a scleroderma patient-centered intervention network cohort study. Rheumatology. 2017;56:1302–1311. doi: 10.1093/rheumatology/kex055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ministry of Health . Ministry of Health; 2020. Long-term conditions: work programme.https://www.health.govt.nz/our-work/diseases-and-conditions/long-term-conditions/long-term-conditions-work-programme [updated 09 January 2020; cited 2020 12 February]; Available from: [Google Scholar]
- 55.Ministry of Social Development . Office for Disability Issues; Wellington, NZ: 2016. New Zealand disability strategy 2016-2026. [Google Scholar]
- 56.Associate Minister of Health . Health Mo. Ministry of Health; Wellington: 2016. Healthy ageing strategy. [Google Scholar]
- 57.Ministry of Health. The mobility action programme. Ministry of Health; [updated 30 May 2019; cited 2019 20 November]; Available from: https://www.health.govt.nz/our-work/preventative-health-wellness/mobility-action-programme.
- 58.Jones P., editor. MAPping New Zealand Bridging the evidence-practice gap for people with painful musculoskeletal conditions. 2017 Quality Improvement Scientific Symposium. Health Quality & Safety Commission New Zealand; Wellington, New Zealand: 2017. [Google Scholar]
- 59.Baldwin J., Briggs A.M., Bagg W., et al. An osteoarthritis model of care should be a national priority for New Zealand. N Z Med J. 2017;30:78–86. [PubMed] [Google Scholar]
- 60.Bach-Mortensen A.M., Lange B.C.L., Montgomery P. Barriers and facilitators to implementing evidence-based interventions among third sector organisations: a systematic review. Implement Sci : ISCUS. 2018;13:103. doi: 10.1186/s13012-018-0789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Damschroder L.J., Aron D.C., Keith R.E., et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(50) doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belton V. In: Stewart T.J., editor. Springer US; Boston, MA: 2002. Multiple criteria decision analysis : an integrated approach. [Google Scholar]
- 63.Muhlbacher A.C., Kaczynski A. Making good decisions in healthcare with multi-criteria decision analysis: the use, current research and future development of MCDA. Appl Health Econ Health. 2016;14:29–40. doi: 10.1007/s40258-015-0203-4. [DOI] [PubMed] [Google Scholar]
- 64.Marsh K., IJzerman M., Thokala P., et al. Multiple criteria decision analysis for health care decision making-emerging good practices: report 2 of the ISPOR MCDA emerging good practices task Force. Value Health. 2016;19:125–137. doi: 10.1016/j.jval.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 65.de Bekker-Grob E.W., Ryan M., Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21:145–172. doi: 10.1002/hec.1697. [DOI] [PubMed] [Google Scholar]
- 66.Clark M.D., Determann D., Petrou S., et al. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32:883–902. doi: 10.1007/s40273-014-0170-x. [DOI] [PubMed] [Google Scholar]
- 67.Pinto D., Bockenholt U., Lee J., et al. Preferences for physical activity: a conjoint analysis involving people with chronic knee pain. Osteoarthritis Cartilage. 2018;27:240–247. doi: 10.1016/j.joca.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arden N.K., Hauber A.B., Mohamed A.F., et al. How do physicians weigh benefits and risks associated with treatments in patients with osteoarthritis in the United Kingdom? J Rheumatol. 2012;39:1056–1063. doi: 10.3899/jrheum.111066. [DOI] [PubMed] [Google Scholar]
- 69.Tanios N., Wagner M., Tony M., et al. Which criteria are considered in healthcare decisions? Insights from an international survey of policy and clinical decision makers. Int J Technol Assess Health Care. 2013;29:456–465. doi: 10.1017/S0266462313000573. [DOI] [PubMed] [Google Scholar]
- 70.Chua J., Briggs A.M., Hansen P., et al. Choosing interventions for hip or knee osteoarthritis - what matters to stakeholders? A mixed-methods study. Osteoarthritis Cartilage open. 2020 doi: 10.1016/j.ocarto.2020.100062. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hansen P., Devlin N. Oxford research encyclopedia of economics and finance. Oxford University Press; 2019. Multi-criteria decision analysis (MCDA) in healthcare decision-making. [Google Scholar]
- 72.Royal Australian College of General Practitioners . RACGP; East Melbourne, Vic: 2018. Guideline for the management of knee and hip osteoarthritis. [Google Scholar]
- 73.World Health Organization . WHO; Geneva: 2019. WHO guideline: recommendations on digital interventions for health system strengthening. [PubMed] [Google Scholar]
- 74.Slater H., Jordan J.E., Chua J., et al. Young people's experiences of persistent musculoskeletal pain, needs, gaps and perceptions about the role of digital technologies to support their co-care: a qualitative study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-014007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slater H., Deer B.F., Merolli M.A., et al. Use of eHealth technologies to enable implementation of musculoskeletal models of care: the evidence and practice. Best Pract Res Clin Rheumatol. 2016;30:483–502. doi: 10.1016/j.berh.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 76.Greenhalgh T., Wherton J., Papoutsi C., et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. 2017;19:e367. doi: 10.2196/jmir.8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Braithwaite J., Clay-Williams R., Taylor N., et al. Deepening our Understanding of Quality in Australia (DUQuA): an overview of a nation-wide, multi-level analysis of relationships between quality management systems and patient factors in 32 hospitals. Int J Qual Health Care. 2020;32:8–21. doi: 10.1093/intqhc/mzz103. [DOI] [PubMed] [Google Scholar]
- 78.Armitage G.D., Suter E., Oelke N.D., Adair C.E. Health systems integration: state of the evidence. Int J Integrated Care. 2009;9(2) doi: 10.5334/ijic.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Braithwaite J., Mannion R., Matsuyama Y., et al. The future of health systems to 2030: a roadmap for global progress and sustainability. Int J Qual Health Care. 2018;30:823–831. doi: 10.1093/intqhc/mzy242. [DOI] [PubMed] [Google Scholar]
- 80.Starling A. Implementing new models of care: lessons from the new care models programme in England. Int J Care Coord. 2018;21:50–54. doi: 10.1177/2053434518770613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dziedzic K.S., French S., Davis A.M., et al. Implementation of musculoskeletal models of care in primary care settings: theory, practice, evaluation and outcomes for musculoskeletal health in high-income economies. Best Pract Res Clin Rheumatol. 2016;30:375–397. doi: 10.1016/j.berh.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Briggs A.M., Persaud J.G., Deverell M.L., et al. Integrated prevention and management of non-communicable diseases, including musculoskeletal health: a systematic policy analysis among OECD countries. BMJ Global Health. 2019;4 doi: 10.1136/bmjgh-2019-001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.NHS . National Health Service; 2016. Developing a blueprint for the future of NHS and care services United Kingdom. [Google Scholar]
- 84.NHS . South West Academic Health Science Network; United Kingdom: 2020. Models of care.https://modelsofcare.co.uk/ [cited 2020 27/02/2020]; Available from: [Google Scholar]
- 85.Buchbinder R., van Tulder M., Oberg B., et al. Low back pain: a call for action. Lancet. 2018;391:2384–2388. doi: 10.1016/S0140-6736(18)30488-4. [DOI] [PubMed] [Google Scholar]
- 86.Traeger A.C., Buchbinder R., Elshaug A.G., et al. Care for low back pain: can health systems deliver? Bull World Health Organ. 2019;97:423–433. doi: 10.2471/BLT.18.226050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Modica C. The value transformation framework: an approach to value-based care in Federally Qualified Health Centers. J Healthc Qual. 2020;42:106–112. doi: 10.1097/JHQ.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 88.Flood K.L., Booth K., Vickers J., et al. Acute Care for Elders (ACE) team model of care: a clinical overview. Geriatrics. 2018;3 doi: 10.3390/geriatrics3030050. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koff E., Lyons N. Implementing value-based health care at scale: the NSW experience. Med J Aust. 2020;212:104–106. doi: 10.5694/mja2.50470. [DOI] [PubMed] [Google Scholar]
- 90.Bone and Joint Canada . Bone and Joint Canada; Toronto: 2011. National hip fracture toolkit. [Google Scholar]
- 91.NSW Government . NSW Ministry of Health; Sydney, Australia: 2019. Leading better value care.https://www.health.nsw.gov.au/Value/Pages/leading-better-value-care.aspx [cited 2020 10/02/2020]; Available from: [Google Scholar]
- 92.Koff EL N. Implementing value-based health care at scale: the NSW experience. Med J Aust. 2020;212:104–106. doi: 10.5694/mja2.50470. [DOI] [PubMed] [Google Scholar]
- 93.Marchildon G. Canada: health system review. Health Syst Transit. 2013;15:1–179. [PubMed] [Google Scholar]
- 94.Papanicolas I., Woskie L.R., Jha A.K. Health care spending in the United States and other high-income countries. J Am Med Assoc. 2018;319:1024–1039. doi: 10.1001/jama.2018.1150. [DOI] [PubMed] [Google Scholar]
- 95.Peric N., Hofmarcher-Holzhacker M.M., Simon J. Health system performance assessment landscape at the EU level: a structured synthesis of actors and actions. Arch Publ Health. 2017;75:5. doi: 10.1186/s13690-016-0173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Desmeules F., Roy J.S., MacDermid J.C., et al. Advanced practice physiotherapy in patients with musculoskeletal disorders: a systematic review. BMC Muscoskel Disord. 2012;13:107. doi: 10.1186/1471-2474-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mir M.O., Cooney C., O'Sullivan C., et al. The efficacy of an extended scope physiotherapy clinic in paediatric orthopaedics. J Child Orthop. 2016;10:169–175. doi: 10.1007/s11832-016-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robarts S., Kennedy D., MacLeod A.M., et al. A framework for the development and implementation of an advanced practice role for physiotherapists that improves access and quality of care for patients. Healthc Q. 2008;11:67–75. doi: 10.12927/hcq.2008.19619. [DOI] [PubMed] [Google Scholar]
- 99.Tawiah A.K., Borthwick A., Woodhouse L. Advanced Physiotherapy Practice: a qualitative study on the potential challenges and barriers to implementation in Ghana. Physiother Theory Pract. 2018:1–9. doi: 10.1080/09593985.2018.1484535. [DOI] [PubMed] [Google Scholar]
- 100.Ministry of Health and Long-Term Care . Public Information; 2006. Wait time strategy: better access to care.www.health.gov.on.ca/transformation/wait_times/wt_strategy.html [cited 2006 June 15]; Available from: Accessed at: [Google Scholar]
- 101.Fennelly O., Blake C., FitzGerald O., et al. Advanced musculoskeletal physiotherapy practice in Ireland: a National Survey. Muscoskel Care. 2018;16(4):425–432. doi: 10.1002/msc.1351. [DOI] [PubMed] [Google Scholar]
- 102.Victorian Musculoskeletal Clinical Leadership Group . Musculoskeletal Australia; Melbourne: 2018. Victorian model of care for osteoarthritis of the hip and knee.https://www.msk.org.au/wp-content/uploads/2018/07/MoC_Final-report.pdf [Google Scholar]
- 103.Chehade M.J., Gill T.K., Kopansky-Giles D., et al. Building multidisciplinary health workforce capacity to support the implementation of integrated, people-centred Models of Care for musculoskeletal health. Best Pract Res Clin Rheumatol. 2016;30:559–584. doi: 10.1016/j.berh.2016.09.005. [Review] [DOI] [PubMed] [Google Scholar]
- 104.Robarts A framework for the development and implementation of an advanced practice role for physiotherapists that improves access and quality of care for patients. Healthc Q. 2008;11:67–75. doi: 10.12927/hcq.2008.19619. [DOI] [PubMed] [Google Scholar]
- 105.Ontario Go . 2009. Health Force Ontario, new roles in health care.http://www.healthforceontario.ca/WhatIsHFO/NewRoles.aspx [cited 2010 Accessed 10/04]; Available from: [Google Scholar]
- 106.Bryant-Lukosius D., Dicenso A. A framework for the introduction and evaluation of advanced practice nursing roles. J Adv Nurs. 2004;48:530–540. doi: 10.1111/j.1365-2648.2004.03235.x. [DOI] [PubMed] [Google Scholar]
- 107.DiCenso A., Bourgeault I., Abelson J., et al. Utilization of nurse practitioners to increase patient access to primary healthcare in Canada--thinking outside the box. Nurs Leader (Tor Ont) 2010:239–259. doi: 10.12927/cjnl.2010.22281. 23 Spec No 2010. [DOI] [PubMed] [Google Scholar]
- 108.Ahluwalia V., Larsen T.L.H., Kennedy C.A., et al. An advanced clinician practitioner in arthritis care can improve access to rheumatology care in community-based practice. J Multidiscip Healthc. 2019;12:63–71. doi: 10.2147/JMDH.S183397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brennen R., Sherburn M., Rosamilia A. Development, implementation and evaluation of an advanced practice in continence and women's health physiotherapy model of care. Aust N Z J Obstet Gynaecol. 2019;59:450–456. doi: 10.1111/ajo.12974. [DOI] [PubMed] [Google Scholar]
- 110.Crane J., Delany C. Physiotherapists in emergency departments: responsibilities, accountability and education. Physiotherapy. 2013;99:95–100. doi: 10.1016/j.physio.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 111.Goodman D., Harvey D., Cavanagh T., et al. Implementation of an expanded-scope-of-practice physiotherapist role in a regional hospital emergency department. Rural Rem Health. 2018;18:4212. doi: 10.22605/RRH4212. [DOI] [PubMed] [Google Scholar]
- 112.Kennedy D.M., Robarts S., Woodhouse L. Patients are satisfied with advanced practice physiotherapists in a role traditionally performed by orthopaedic surgeons. Physiother Can. 2010;62:298–305. doi: 10.3138/physio.62.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lebec M.T., Jogodka C.E. The physical therapist as a musculoskeletal specialist in the emergency department. J Orthop Sports Phys Ther. 2009;39:221–229. doi: 10.2519/jospt.2009.2857. [DOI] [PubMed] [Google Scholar]
- 114.Matifat E., Perreault K., Roy J.S., et al. Concordance between physiotherapists and physicians for care of patients with musculoskeletal disorders presenting to the emergency department. BMC Emerg Med. 2019;19:67. doi: 10.1186/s12873-019-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morris J., Vine K., Grimmer K. Evaluation of performance quality of an advanced scope physiotherapy role in a hospital emergency department. Patient Relat Outcome Meas. 2015;6:191–203. doi: 10.2147/PROM.S75173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aiken A.B., Atkinson M., Harrison M.M., et al. Reducing hip and knee replacement wait times: an expanded role for physiotherapists in orthopedic surgical clinics. Healthc Q. 2007;10:88–91. doi: 10.12927/hcq..18807. 6. [DOI] [PubMed] [Google Scholar]
- 117.Aiken A.B., Harrison M.M., Hope J. Role of the advanced practice physiotherapist in decreasing surgical wait times. Healthc Q. 2009;12:80–83. doi: 10.12927/hcq.2013.20881. [DOI] [PubMed] [Google Scholar]
- 118.Briggs A.M., Page C.J., Shaw B.R., et al. A model of care for osteoarthritis of the hip and knee: development of a system-wide plan for the health sector in Victoria, Australia. Healthc Policy. 2018;14:47–58. doi: 10.12927/hcpol.2018.25686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fojas M.C., Southerland L.T., Phieffer L.S., et al. Compliance to the Joint Commission proposed Core Measure set on osteoporosis-associated fracture: review of different secondary fracture prevention programs in an open medical system from 2010 to 2015. Arch Osteoporos. 2017;12(16) doi: 10.1007/s11657-017-0307-6. [DOI] [PubMed] [Google Scholar]
- 120.Rice P., Mehan U., Hamilton C., et al. Screening, assessment, and treatment of osteoporosis for the nurse practitioner: key questions and answers for clinical practice--a Canadian perspective. J Am Assoc Nurse Pract. 2014;26:378–385. doi: 10.1002/2327-6924.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sayer J.M., Kinsella R.M., Cary B.A., et al. Advanced musculoskeletal physiotherapists are effective and safe in managing patients with acute low back pain presenting to emergency departments. Aust Health Rev. 2018;42:321–326. doi: 10.1071/AH16211. [DOI] [PubMed] [Google Scholar]
- 122.Briggs A.M., Araujo de Carvalho I. Actions required to implement integrated care for older people in the community using the World Health Organization's ICOPE approach: a global Delphi consensus study. PloS One. 2018;13 doi: 10.1371/journal.pone.0205533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johnson C.D., Haldeman S., Chou R., et al. The global spine care initiative: model of care and implementation. Eur Spine J. 2018;27:925–945. doi: 10.1007/s00586-018-5720-z. [DOI] [PubMed] [Google Scholar]
- 124.Roos E.M., Barton C.J., Davis A.M., et al. GLA:D to have a high-value option for patients with knee and hip arthritis across four continents: good Life with osteoArthritis from Denmark. Br J Sports Med. 2018;52:1544–1545. doi: 10.1136/bjsports-2017-098904. [DOI] [PubMed] [Google Scholar]
- 125.Akesson K., Marsh D., Mitchell P.J., et al. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24:2135–2152. doi: 10.1007/s00198-013-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Briggs A.M., Jordan J.E., Jennings M., et al. Global Alliance for Musculoskeletal Health of the Bone and Joint Decade; Cornwall: 2016. A framework to evaluate musculoskeletal models of care. [Google Scholar]
- 127.Chandran M., Tan M.Z., Cheen M., et al. Secondary prevention of osteoporotic fractures--an “OPTIMAL” model of care from Singapore. Osteoporos Int. 2013;24:2809–2817. doi: 10.1007/s00198-013-2368-8. [DOI] [PubMed] [Google Scholar]
- 128.Li W., Yang M. Fragility Fracture Network (FFN)-China successfully held forum to support FFN Global Call to Action to improve the care of people with fragility fractures. Aging Med. 2018;1:280–281. doi: 10.1002/agm2.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoy D., Geere J.A., Davatchi F., et al. A time for action: opportunities for preventing the growing burden and disability from musculoskeletal conditions in low- and middle-income countries. Best Pract Res Clin Rheumatol. 2014;28:377–393. doi: 10.1016/j.berh.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 130.Sharma S., Blyth F.M., Mishra S.R., et al. Health system strengthening is needed to respond to the burden of pain in low- and middle-income countries and to support healthy ageing. J Global Health. 2019;2 doi: 10.7189/jogh.09.020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lall D., Engel N., Devadasan N., et al. Models of care for chronic conditions in low/middle-income countries: a 'best fit' framework synthesis. BMJ Global Health. 2018;3 doi: 10.1136/bmjgh-2018-001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.World Health Organization . WHO; Geneva: 2019. Integrated care for older people (ICOPE) implementation framework: guidance for systems and services. [Google Scholar]
- 133.Davy C., Bleasel J., Liu H.M., et al. Effectiveness of chronic care models: opportunities for improving healthcare practice and health outcomes: a systematic review. BMC Health Serv Res. 2015;15 doi: 10.1186/s12913-015-0854-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kadu M.K., Stolee P. Facilitators and barriers of implementing the chronic care model in primary care: a systematic review. BMC Fam Pract. 2015;16 doi: 10.1186/s12875-014-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McKillop A., Shaw J., Sheridan N., et al. Understanding the attributes of implementation frameworks to guide the implementation of a model of community-based integrated health care for older adults with complex chronic conditions: a metanarrative review. Int J Integrated Care. 2017;17 doi: 10.5334/ijic.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jessup R.L., O'Connor D.A., Putrik P., et al. Alternative service models for delivery of healthcare services in high-income countries: a scoping review of systematic reviews. 2019;1 doi: 10.1136/bmjopen-2018-024385. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hunter D.J., Bengoa R. World Health Organization (Europe); Denmark: 2017. Leading health system transformation to the next level. Expert meeting: Durham, United Kingdom, 12-13 July 2017. [Google Scholar]
- 138.Hopkins R.B., Tarride J.E., Leslie W.D., et al. Estimating the excess costs for patients with incident fractures, prevalent fractures, and nonfracture osteoporosis. Osteoporos Int. 2013;24:581–593. doi: 10.1007/s00198-012-1997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Greenhalgh T., Jackson C., Shaw S., et al. Achieving research impact through co-creation in community-based health services: literature review and case study. Milbank Q. 2016;94:392–429. doi: 10.1111/1468-0009.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barnett K., Mercer S.W., Norbury M., et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 141.Allen K.D., Bierma-Zeinstra S.M., Foster N.E., et al. OARSI Clinical Trials Recommendations: design and conduct of implementation trials of interventions for osteoarthritis. Osteoarthritis Cartilage. 2015;23:826–838. doi: 10.1016/j.joca.2015.02.772. [DOI] [PubMed] [Google Scholar]
- 142.Pham Q., Wiljer D., Cafazzo J.A. Beyond the randomized controlled trial: a review of alternatives in mHealth clinical trial methods. JMIR mHealth uHealth. 2016;4:e107. doi: 10.2196/mhealth.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Gemert-Pijnen J.E., Nijland N., van Limburg M., et al. A holistic framework to improve the uptake and impact of eHealth technologies. J Med Internet Res. 2011;13:e111. doi: 10.2196/jmir.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Greenhalgh T., Russell J. Why do evaluations of eHealth programs fail? An alternative set of guiding principles. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Biggs J.S., Willcocks A., Burger M., et al. Digital health benefits evaluation frameworks: building the evidence to support Australia's National Digital Health Strategy. Med J Aust. 2019;210(Suppl 6):S9–S11. doi: 10.5694/mja2.50034. [DOI] [PubMed] [Google Scholar]
- 146.NSW Agency for Clinical Innovation . NSW Agency for Clinical Innovation; Sydney: 2013. Understanding program evaluation. An ACI framework. [Google Scholar]
- 147.Moore G.F., Audrey S., Barker M., et al. Process evaluation of complex interventions: medical Research Council guidance. BMJ (Clinical research ed. 2015;350:h1258. doi: 10.1136/bmj.h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Langlois E.V., Tuncalp O., Norris S.L., et al. Qualitative evidence to improve guidelines and health decision-making. Bull World Health Organ. 2018;96 doi: 10.2471/BLT.17.206540. 79-79A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Briggs A.M., Valentijn P.P., Thiyagarajan J.A., et al. Elements of integrated care approaches for older people: a review of reviews. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Threapleton D.E., Chung R.Y., Wong S.Y.S., et al. Integrated care for older populations and its implementation facilitators and barriers: a rapid scoping review. Int J Qual Health Care. 2017;29:327–334. doi: 10.1093/intqhc/mzx041. journal of the International Society for Quality in Health Care/ISQua. [DOI] [PubMed] [Google Scholar]
- 151.Slater H., Briggs A.M. Models of Care for musculoskeletal pain conditions: driving change to improve outcomes. Pain Manag. 2017;7:351–357. doi: 10.2217/pmt-2017-0025. [DOI] [PubMed] [Google Scholar]
- 152.Levesque J.-F., Sutherland K. Combining patient, clinical and system perspectives in assessing performance in healthcare: an integrated measurement framework. BMC Health Serv Res. 2020;20:23. doi: 10.1186/s12913-019-4807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Foster A., Croot L., Brazier J., et al. The facilitators and barriers to implementing patient reported outcome measures in organisations delivering health related services: a systematic review of reviews. J Patient Rep Outcomes. 2018;2:46. doi: 10.1186/s41687-018-0072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rutherford C., Campbell R., Tinsley M., et al. Implementing patient-reported outcome measures into clinical practice across NSW: mixed methods evaluation of the first year. Appl Res Qual Life. 2020 doi: 10.1007/s11482-020-09817-2. [DOI] [Google Scholar]
- 155.Barber C.E., Marshall D., Mosher D., et al. Development of system-level performance measures for evaluation of models of care for inflammatory arthritis in Canada. J Rheumatol. 2016;43:530–540. doi: 10.3899/jrheum.150839. [DOI] [PubMed] [Google Scholar]
- 156.Barber C.E., Patel J.N., Woodhouse L., et al. Development of key performance indicators to evaluate centralized intake for patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2015;17:322. doi: 10.1186/s13075-015-0843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dreinhofer K.E., Mitchell P.J., Begue T., et al. A global call to action to improve the care of people with fragility fractures. Injury. 2018;49:1393–1397. doi: 10.1016/j.injury.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 158.Caneiro J.P., O'Sullivan P.B., Roos E.M., et al. Three steps to changing the narrative about knee osteoarthritis care: a call to action. Br J Sports Med. 2020;54:256–258. doi: 10.1136/bjsports-2019-101328. [DOI] [PubMed] [Google Scholar]
- 159.Briggs A.M., Cross M.J., Hoy D.G., et al. Musculoskeletal health conditions represent a global threat to healthy aging: a report for the 2015 World health Organization World report on ageing and health. Gerontol. 2016;56(Suppl 2):S243–S255. doi: 10.1093/geront/gnw002. [DOI] [PubMed] [Google Scholar]
- 160.Grimshaw J.M., Patey A.M., Kirkham K.R., et al. De-implementing wisely: developing the evidence base to reduce low-value care. BMJ Qual Saf. 2020;29(5):409–417. doi: 10.1136/bmjqs-2019-010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chalmers K., Pearson S.A., Badgery-Parker T., et al. Measuring 21 low-value hospital procedures: claims analysis of Australian private health insurance data (2010-2014) BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-024142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Naylor J.M., Hart A., Mittal R., et al. The effectiveness of inpatient rehabilitation after uncomplicated total hip arthroplasty: a propensity score matched cohort. BMC Muscoskel Disord. 2018;19:236. doi: 10.1186/s12891-018-2134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Briggs A.M., Woolf A.D., Dreinhofer K., et al. Reducing the global burden of musculoskeletal conditions. Bull World Health Organ. 2018;96:366–368. doi: 10.2471/BLT.17.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mitchell P.J., Cooper C., Fujita M., et al. Quality improvement initiatives in fragility fracture care and prevention. Curr Osteoporos Rep. 2019;17:510–520. doi: 10.1007/s11914-019-00544-8. [Review] [DOI] [PubMed] [Google Scholar]
- 165.National Academies of Sciences Engineering and Medicine . 2019. Consensus study report: integrating social care into the delivery of health care: moving upstream to improve the Nation's Health. Washington DC, USA. [PubMed] [Google Scholar]
- 166.Desai S.P.Y.J. Quality measurement and improvement in rheumatology: rheumatoid arthritis as a case study. Arthritis Rheum. 2011;63:3649–3660. doi: 10.1002/art.30605. [DOI] [PMC free article] [PubMed] [Google Scholar]