Abstract
Objective
To evaluate the efficacy of electroacupuncture (EA) on treating insomnia in patients with depression.
Patients and Methods
In a patient-assessor-blind, randomized and sham controlled trial, 90 depression patients with insomnia were assigned into three different groups, receiving EA in the treatment group, superficial acupuncture at sham points in the control group A, or Streitberger non-insertion sham acupuncture in the control group B. Treatment was applied 3 times weekly for 8 consecutive weeks. The primary outcome was measured using the Pittsburgh Sleep Quality Index (PSQI). Secondary outcomes were sleep parameters including sleep efficiency (SE), total sleep time (TST) and numbers of sleep awakenings (SA) recorded in the actigraphy, as well as applying the Hamilton Rating Scale for Depression (HAMD-17), Self-Rating Depression Scale (SDS) and Hamilton Rating Scale for Anxiety (HAMA). Assessments were performed at the baseline (week 0), week 4, week 8, and week 12. Linear mixed-effects models were used for analyses and all statistical tests were two-sided.
Results
Patients in the EA group had more significant improvement in PSQI scores than those in the control groups over time (respectively p<0.001 and p=0.04 for treatment and time interaction). At 8-week posttreatment, the EA group reported a reduction of −6.64 points in PSQI scores compared with −2.23 points in the control group A (95% CI= −5.74 to −2.39) and −2.94 points in the control group B (95% CI= −5.73 to −2.47). Compared with the two control groups, significant between-group differences were seen in SE (both p<0.01) and TST (both p<0.01) at week 8; similar results can be found in HAMD-17, SDS, and HAMA scores as well. However, there were no between-group differences in SA (respectively p=0.24 and p=0.08) after 8-weeks of treatment.
Conclusion
Electroacupuncture may improve the sleep quality of patients with depression.
Trial Registration
Chinese Clinical Trial Registry (ChiCTR); URL: http://www.chictr.org.cn/showproj.aspx?proj=12327; Trial ID: ChiCTR-IIR-16008058.
Keywords: insomnia, depression, electroacupuncture, randomized controlled trial
Introduction
Insomnia can be found in more than 80% of patients with major depressive disorder (MDD);1,2 and is characterized by reduced sleep quantity or quality, with difficulty falling asleep, frequent nighttime awakenings, and/or early morning awakening.3 Depression and insomnia are closely related, affecting a person’s social life, behavioral passivity, and general health.4 Reduced sleep quantity or quality increases the risk of the onset and development of depression, which in turn increases the risk of developing and worsening sleep disturbance.5,6
MDD patients with significant insomnia complaints comprise a particularly challenging group to treat. Acupuncture is a widely recognized alternative therapy with long-term clinical and economic benefits.7 Previous studies of acupuncture on primary insomnia suggested that acupuncture may alleviate symptoms of insomnia such as sleep efficiency, total sleep time, and depressive or anxious moods.8–10 Insomnia in MDD patients has been regarded as a relevant symptom rather than a comorbid disorder and remains unexamined in these terms. Therefore, well-designed studies that focus on insomnia-targeted therapies for patients with comorbid depression and insomnia are urgently needed.11
To verify our hypothesis that acupuncture with specific acupoints has real rather than placebo effects on treating insomnia and alleviating depression, we designed this strictly randomized controlled trial. In this trial, we aimed to observe the effects of electroacupuncture (EA) treatment on sleep disorders and depressive moods in comparison to two different sham acupuncture controls.
Patients and Methods
Design
We conducted a single-center, patient-assessor-blinded, randomized controlled trial at the acupuncture department in Shanghai Municipal Hospital of Traditional Chinese Medicine (TCM) in Shanghai, China. Eligible patients (n=90) were randomly assigned into one of three groups, receiving electroacupuncture in the treatment group, superficial acupuncture in the control group A or non-insertion sham acupuncture in the control group B. After 1 week of washout period, treatment was given 3 times a week (every other day) for 8 consecutive weeks. Patients were assessed at 4 time points, at the baseline (1 week before treatment), week 4, week 8, and week 12. The trial was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Ethics Committee of Shanghai Municipal Hospital of TCM (2015SHL-KY-21) and was published on November, 2016.12 The Consolidated Standards of Reporting Trials (CONSORT)13 and acupuncture reporting guidelines, Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA)14 were followed.
Participants
All participants were recruited through hospital-based posters, from the outpatient clinic, and from the website of Shanghai Municipal Hospital of TCM. The trial was conducted between June 2016 and May 2018. Participants were screened by questionnaires administered over the phone or in face-to-face interviews at the hospital. Additionally, they were asked to wear a wrist actigraphy to monitor sleep quality for 1 night and return it the next day. Eligible participants were asked to sign the written informed consent form before the intervention began.
The inclusion criteria of this study were: 1) aged 18 to 70 years; 2) diagnosis of depression according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV);15 3) HAMD-17 score of 20–35, indicating mild to moderate severity of depression; 4) had complaint of insomnia at the first screening; 5) PSQI score was more than 7, suggesting presence of sleep disorders; 6) sleep efficiency recorded in the actigraphy was lower than 85%, indicating poor sleep quality; 7) signed a written informed consent form for the clinical trial.
Participants were excluded if they: 1) had secondary depressive disorders caused by organic diseases, medicine, or psychotic disorders including schizophrenia, etc.; 2) were in the depressive episode of bipolar disorder, or suffering from dysthymia, reactive depression and depressive syndrome caused by other diseases; 3) had alcohol abuse or drug dependence; 4) had serious cardiovascular, liver, kidney or hematopoietic system disease; 5) had infections, ulcers and scars on the skin around the selected acupoints; 6) had received acupuncture treatment in the past year; and 7) were pregnant or breast-feeding.
Treatments
All treatments were performed after skin cleansing, with patients wearing an eye mask and lying supine. All acupuncturists are licensed doctors with more than 5 years of experience in clinical acupuncture treatment. Considering the patients’ mental state, they were allowed to take their routine antidepressant medication (ADM) during the trial and they were instructed not to change the dosage optionally. However, if the change of dosage occurred under the psychiatrist’s advice due to the change of patients’ mental conditions, the details should be recorded in the case report form (CRF).
Treatment Group
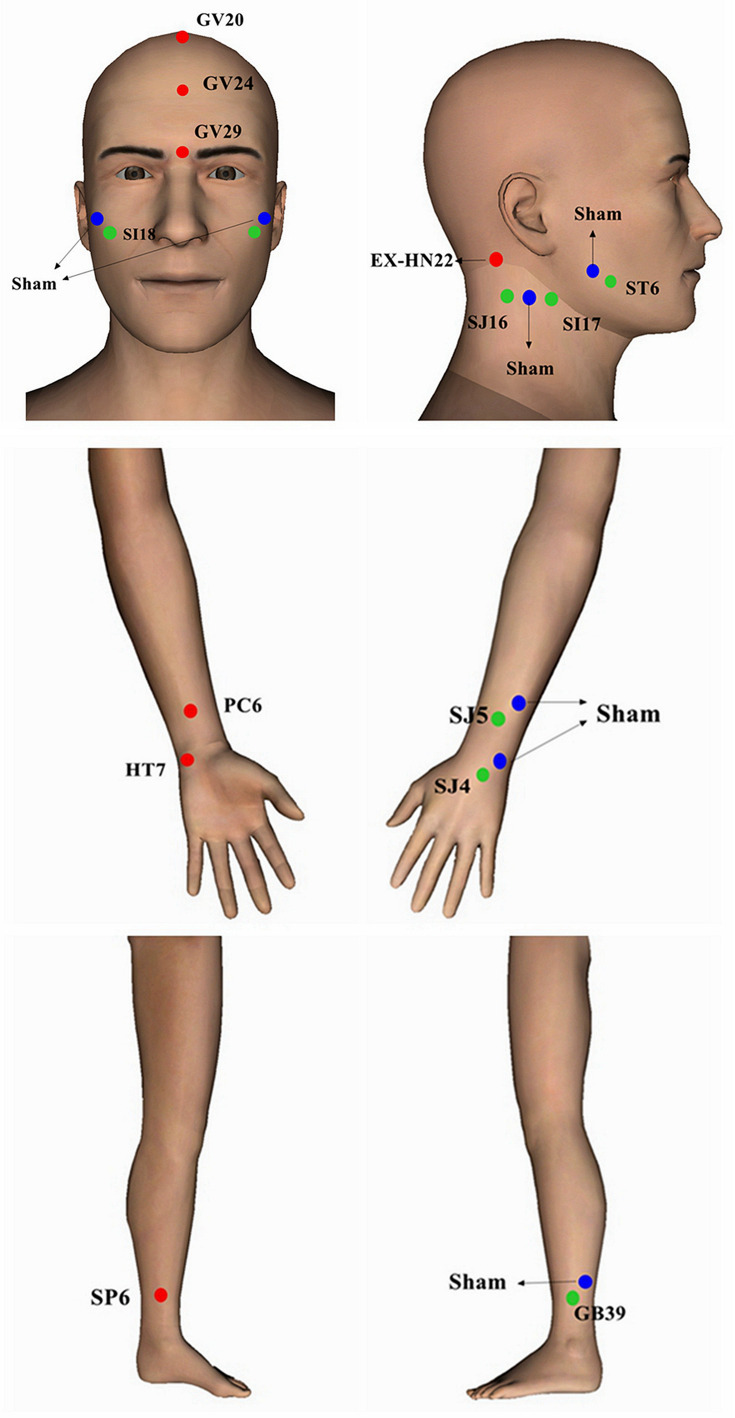
Patients in the treatment group received electroacupuncture treatment. Regular acupuncture method was applied at the following acupuncture points: Baihui (GV20), Shenting (GV24), Yintang (GV29), bilateral Anmian (EX-HN22), Shenmen (HT7), Sanyinjiao (SP6) and Neiguan (PC6). After needle insertion, “Deqi” sensation was obtained. A pair of electrodes of the electro-stimulator (CMNS6-1, Wuxi Jianjian Medical Device Co., Ltd, China) were then connected to needles at GV20 and GV29. The electro-stimulator delivered a continuous wave, square pulse with 0.175 ms width, 30Hz frequency to the patients, and the current intensity from 0.1 to 1mA based on the endurance of each patient.16 30Hz is the low frequency of dense continuous wave and causes the effects of calming, relaxing and analgesia. Each session of the treatment lasted for 30 minutes.
Superficial Acupuncture Group (Control Group A)
In order to control for non-specific effect of EA, the patients in the control group A received superficial acupuncture treatment at the following non-specific sham acupoints (seen in Figure 1): bilateral acupoints 1 cm upper outer of Jiache (ST6), Waiguan (SJ5), Yangchi (SJ4) and Xuanzhong (GB39); acupoint upper outer of Quanliao (SI18); and bilateral acupoints in the middle of Tianyou (SJ16) and Tianrong (SI17). To exclude any possible effects of acupoints on insomnia or depression, the sham acupoints were set located at 1 cm away from the acupoints on the yang meridians, opposite to the acupoints on the yin meridians we chose for real EA in the limbs; and other sham acupoints were set located far away from the Governor Vessel meridian and its branches. No “deqi” sensation was obtained after inserting the needles. A pair of electrodes was connected to the needles at bilateral acupoints 1 cm upper outer of ST6. The switch light of the electro-stimulator was turned on during the treatment, but no current flowed. The treatment time and other needling procedures were the same as the treatment group.
Figure 1.
Real and sham acupoints.
Sham Acupuncture Group (Control Group B)
In order to control for the placebo effect of EA, the participants in the control group B received non-insertion sham acupuncture treatment with the Streitberger needles17 at the same acupoints as the control group A. The patient received a pricking sensation when the blunt needle tip touched the skin, but there was no real needle inserted into the skin. A pair of electrodes was connected to the needles at bilateral acupoints 1 cm upper outer of ST6, with no flowing current during the treatment. The treatment time and other procedures were the same as the treatment group.
Outcome Measurements
Primary Outcome
Pittsburgh Sleep Quality Index (PSQI) was used among all three groups at 8 weeks after treatments as the primary outcome. PSQI is a widely-used questionnaire to assess one’s sleep disorders.18 It is comprised of 19 self-rated items and 5 other-rated items, and the scores include the following indicators: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of medication, and daytime dysfunction. Each indicator is rated from 0 to 3. The accumulated scores of the seven indicators constitute the total score of PSQI (0–21). A patient with a PSQI score higher than 7 indicates sleep disorder,19 and the higher score indicates the worse sleep quality and more severe sleep disorders.
The Secondary Outcomes
The quality of sleep including sleep efficiency (SE), total sleep time (TST) and sleep awakenings (SA) recorded in the actigraphy, as well as the Hamilton Rating Scale for Depression (HAMD-17)20 scores, the Hamilton Rating Scale for Anxiety (HAMA)21 scores and Self-rating Depression Scale (SDS)22 scores from baseline to 8 weeks after inclusion were measured as secondary outcomes. The results of HAMD-17, HAMA and SDS provided the subjective assessment of patients’ mental health state and the actigraphy records provided a relatively objective assessment of patients’ sleep status.
Actigraphy (wActiSleep-BT. LLC, Pensacola, USA), which was worn on the patient’s wrist, can monitor the quality of sleep, such as sleep onset, sleep latency, total sleep time, number of sleep awakenings during the night, duration of sleep, and sleep efficiency. The software ActiLife6 (Version 6.8.1, ActiGraph, LLC) was used to analyze every participant’s sleep condition recorded by the actigraphy. The sleep efficiency was calculated as number of sleep minutes divided by the total number of minutes the patient was in bed. Patients whose sleep efficiency was lower than 85% indicated poor sleep quality and the presence of sleep disorders. The researcher gave the actigraphy to every participant at the first screening, and at week 4, week 8, and week 12, asking them to wear it for one night (mainly from bedtime to rising time) and return it the next day.
Adverse Events
Any adverse event (AE, described as unfavorable or unintended signs, symptoms or diseases occurring during the trial) related to the acupuncture treatment or the administration of antidepressant medication (ADM) was asked to be reported by patients and practitioners. The AE data were assessed in terms of frequency, severity, and causality. The 3-point grading categories were applied: grade 1, mild, grade 2, moderate, grade 3, severe, or medically insignificant. The causality categories were “certain,” “probable,” “possible,” “unlikely,” “conditional,” and “not assessable.” The incidence of AEs was presented as the number of AEs per number of acupuncture sessions (%).
Sample Size Calculation
The calculation of sample size was based on an open study on acupuncture for aging patients with depression and sleep disorders.23 In that reference study, the designer used PSQI as the main assessment indicator and recruited 24 patients in each group to receive true or sham acupuncture treatment. The basal PSQI score of the acupuncture group was 8.04±4.0, which significantly reduced (−53.23%; p<0.01) after 10 sessions of treatment. Researchers then suggested further study in controlled trials on the effects of acupuncture on younger patients with depression or insomnia. We considered 20% dropout rate and determined the sample size of each group as 30 participants. With 1:1:1 allocation to each group, therefore, 90 participants were ultimately recruited.
Randomization and Blinding
A simple randomization method was used in this trial. An independent researcher (Bo Dong) generated a random number table to divide 90 participants into three groups by SPSS 20.0. This researcher then made random allocation cards and sealed each card in an opaque envelope. The number on these cards indicated patient registration time and group allocation for this trial. Another researcher (Shanshan Li) gave the sealed envelopes to acupuncturists (Wei Li and Huangan Wu) who were the only ones who knew the treatment assignments. Other researchers, including the outcome assessor (Xuan Yin) and the statistician (Jie Ma), were all blinded to the group assignments. Patients were asked to wear an eye mask during each session of treatment. Each treatment was conducted in a closed unit with drape. All researchers received training about the specification of this research method before and were required to strictly adhere to the task separation principle.
Statistical Analysis
All analyses were performed on the intention-to-treat (ITT) population of participants who had received at least one treatment. Missing data were replaced according to the principle of the last observation carried forward. Data analyses were performed with the use of the statistical software SPSS 20.0. All data were entered twice by two researchers to ensure accuracy. The linear mixed-effects model, with degrees of freedom calculated by Kenward-Roger approximation, was used to do the longitudinal analysis of different timepoints among groups in the repeated measurements, the PSQI scores as the primary outcome. The one-way analysis of variance (ANOVA) was used to compare other measurement data among groups from the baseline to follow-up. Student’s t-test was used to compare the between-group differences; rank sum test was used for ranked data while χ2 test was adopted to analyze categorical data. The significance level used for statistical analysis with 2-tailed testing was 5%. Data values were mainly presented as Mean±SD.
Results
Participants' Characteristics
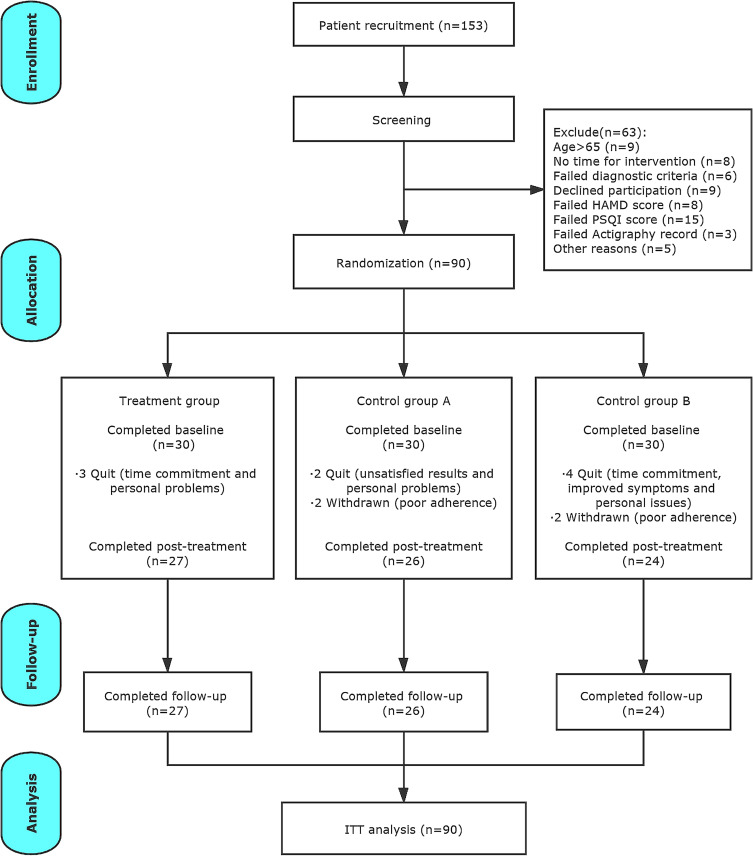
We screened a total of 153 participants, and 63 of them were excluded due to various reasons (details in Figure 2). The remaining 90 participants were randomly assigned into three groups. A total of 13 participants dropped out during the 12-week study period; 9 participants among them quit because of time commitment, personal issues, and unsatisfactory effects; 4 participants were withdrawn from the trial due to their poor adherence. There were no missing data in other 77 participants who completed the study.
Figure 2.
CONSORT flowchart.
Baseline features of all participants are presented at Table 1. There were no significant differences identified in the demographic and clinical characteristics among the three groups (all p>0.05).
Table 1.
Demographic and Clinical Characteristics of the Participants
| Treatment Group (n=30) | Control Group A (n=30) | Control Group B (n=30) | χ2/F-value | ANOVA P-value | ||
|---|---|---|---|---|---|---|
| Age, years | 47.30±14.89 | 49.80±15.13 | 46.77±15.57 | 0.34 | 0.71 | |
| Duration, years | 5.67±5.70 | 7.48±6.23 | 5.89±5.64 | 0.85 | 0.43 | |
| Gender, n (%) | Male | 11 (36.7) | 10 (33.3) | 11 (36.7) | 0.10 | 0.95 |
| Female | 19 (63.3) | 20 (66.7) | 19 (63.3) | |||
| Marriage, n (%) | Married | 4 (13.3) | 7 (23.3) | 7 (23.3) | 3.09 | 0.60 |
| Not married | 24 (80.0) | 22 (73.3) | 23 (76.7) | |||
| Divorced/widowed | 2 (6.7) | 1 (3.3) | 0 (0.0) | |||
| Alcohol, n (%) | Y | 8 (26.7) | 6 (20.0) | 8 (26.7) | 0.48 | 0.79 |
| N | 22 (73.3) | 24 (80.0) | 22 (73.7) | |||
| Education, n (%) | Graduate | 3 (10.0) | 4 (13.3) | 5 (16.7) | 2.28 | 0.69 |
| Undergraduate | 12 (40.0) | 11 (36.7) | 7 (23.3) | |||
| High school and below | 15 (50.0) | 15 (50.0) | 18 (60.0) | |||
| Previous treatment, n (%) | Y | 15 (50.0) | 21 (70.0) | 15 (50.0) | 3.26 | 0.20 |
| N | 15 (50.0) | 9 (30.0) | 15 (50.0) | |||
| Coffee, n (%) | Y | 14 (46.7) | 10 (33.3) | 13 (43.3) | 1.19 | 0.55 |
| N | 16 (53.3) | 20 (66.7) | 17 (56.7) | |||
| Cigarettes, n (%) | Y | 7 (23.3) | 6 (20.0) | 6 (20.0) | 0.13 | 0.94 |
| N | 23 (76.7) | 24 (80.0) | 24 (80.0) | |||
| Course of disease, years | 5.68±5.70 | 7.48±6.22 | 6.35±5.85 | 0.85 | 0.43 | |
| PSQI | 16.47±1.89 | 16.13±2.70 | 16.87±4.83 | 0.36 | 0.70 | |
| HAMD-17 | 28.17±5.53 | 27.13±4.62 | 27.40±6.46 | 0.28 | 0.76 | |
| SDS | 43.20±10.50 | 41.40±10.40 | 43.73±8.98 | 0.45 | 0.64 | |
| HAMA | 22.33±8.76 | 22.97±9.25 | 24.87±8.42 | 0.67 | 0.51 | |
| Actigraphy | SE, % | 76.73±5.04 | 77.32±4.20 | 77.49±5.17 | 0.21 | 0.81 |
| TST, minutes | 326.67±61.59 | 336.17±46.83 | 324.10±59.97 | 0.38 | 0.69 | |
| SA, times | 22.57±6.55 | 20.07±5.91 | 22.07±6.86 | 1.26 | 0.29 | |
Abbreviations: PSQI, Pittsburgh Sleep Quality Index; SDS, Self-Rating Depression Scale; HAMD-17, Hamilton Rating Scale for Depression; HAMA, Hamilton Rating Scale for Anxiety; SE, sleep efficiency; TST, total sleep time; SA, sleep awakening.
Primary Outcome
Table 2 presents the PSQI scores of the patients in the three groups during the trial. The PSQI scores of the EA group was compared to those of the control group A and to those of the control group B, and the results were analyzed to reveal changes from baseline to 4-week follow-up. Patients in the EA group reported greater improvement in PSQI scores than those in the control groups over time (respectively p<0.001 and p=0.04 for treatment and time interaction). It indicated that the EA treatment had more significant impact on the downward trend of PSQI scores than the superficial acupuncture treatment and sham EA treatment over time. At 8-week posttreatment (primary endpoint), the EA group reported a reduction of −6.64 points compared with −2.23 points in the control group A (95% CI= −5.74 to −2.39) and −2.94 points in the control group B (95% CI= −5.73 to −2.47).
Table 2.
Changes of PSQI Scores from Baseline to Follow-Up
| Treatment Group (n=30) | Control Group A (n=30) | Control Group B (n=30) | T vs CAb | T vs CBb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | Within-Group Effect Sizea | Mean±SD | Within-Group Effect Sizea | Mean±SD | Within-Group Effect Sizea | Estimate (SE) | t | P-valuec | Estimate (SE) | t | P-valuec | ||
| PSQI | Week 0 | 16.47±1.89 | 16.13±2.70 | 16.87±4.83 | 0.28 (0.07) | 3.96 | <0.001 | 0.20 (0.09) | 2.40 | 0.04 | |||
| Week 4 | 12.13±3.44 | −0.62 | 15.10±3.62 | −0.16 | 14.97±3.18 | −0.23 | |||||||
| Week 8 | 9.83±3.11 | −0.14 | 13.90±3.38 | −0.17 | 13.93±3.22 | −0.16 | |||||||
| Week 12 | 11.40±3.52 | 0.10 | 14.47±3.33 | 0.08 | 14.10±3.00 | 0.27 | |||||||
Notes: aEffect size by Cohen’s d formula. bTreatment and time interaction from the linear mixed-effects model. cP-value by Bonferroni correction
Abbreviations: PSQI, Pittsburgh Sleep Quality Index; T, treatment group; CA, control group A; CB, control group B; SE, standard error.
Secondary Outcomes
The changes of HAMD-17, SDS and HAMA scores of patients in the three groups during the trial are shown in Table 3. There were statistically significant differences in self-rated depression and the anxiety scales among the three groups from baseline to follow-up (all p<0.01). Compared with patients receiving superficial or sham acupuncture, those taking EA treatment group saw a significant reduction in HAMD-17, SDS, and HAMA scores at week 4, 8 and 12 (all p<0.01). These results suggested that EA could not only improve MDD patients’ sleep quality, but could also effectively enhance their mental status.
Table 3.
Patients’ Mood State from Baseline to Follow-Up
| Treatment Group (n=30) | Control Group A (n=30) | Control Group B (n=30) | ANOVA P-value |
T vs CA | T vs CB | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | Within-Group Effect Sizea | Mean±SD | Within-Group Effect Sizea | Mean±SD | Within-Group Effect Sizea | P-valueb | P-valueb | |||
| HAMD-17 | Week 0 | 28.17±5.53 | 27.13±4.62 | 27.40±6.46 | 0.76 | |||||
| Week 4 | 17.77±6.03 | −0.67 | 25.33±6.51 | −0.16 | 25.77±6.75 | −0.12 | <0.001 | <0.001 | <0.001 | |
| Week 8 | 11.20±6.91 | −0.45 | 21.97±6.95 | −0.24 | 25.30±7.29 | −0.03 | <0.001 | <0.001 | <0.001 | |
| Week 12 | 12.53±7.87 | 0.09 | 21.10±7.54 | −0.06 | 27.07±7.00 | 0.12 | <0.001 | <0.001 | <0.001 | |
| SDS | Week 0 | 43.20±10.50 | 41.40±10.40 | 43.73±8.98 | 0.64 | |||||
| Week 4 | 30.57±6.62 | −0.58 | 36.07±12.92 | −0.22 | 38.80±10.44 | −0.25 | 0.009 | 0.35 | 0.003 | |
| Week 8 | 26.13±7.58 | −0.30 | 35.13±8.57 | −0.04 | 38.46±8.16 | −0.02 | <0.001 | <0.001 | <0.001 | |
| Week 12 | 28.53±7.22 | 0.16 | 35.17±8.00 | 0.002 | 38.30±8.61 | −0.01 | <0.001 | 0.011 | <0.001 | |
| HAMA | Week 0 | 22.33±8.76 | 22.97±9.25 | 24.87±8.42 | 0.51 | |||||
| Week 4 | 14.27±6.73 | −0.46 | 21.07±8.90 | −0.10 | 22.43±9.14 | −0.14 | <0.001 | 0.012 | <0.001 | |
| Week 8 | 9.27±8.06 | −0.32 | 18.17±8.67 | −0.16 | 22.03±10.10 | −0.02 | <0.001 | <0.001 | <0.001 | |
| Week 12 | 10.53±7.53 | −0.08 | 16.80±8.29 | −0.08 | 20.90±8.17 | −0.06 | <0.001 | 0.026 | <0.001 | |
Notes: aEffect size by Cohen’s d formula. bP-value by Bonferroni correction.
Abbreviations: HAMD-17, Hamilton Rating Scale for Depression; SDS, Self-Rating Depression Scale; HAMA, Hamilton Rating Scale for Anxiety; T, treatment group; CA, control group A; CB, control group B.
Table 4 presents the actigraphy records, including the SE, TST and SA data from baseline to follow-up among the three groups. There were significant differences in SE and TST from week 4 to week 12 among the three groups (all p<0.01). No difference was found in SA at week 8 among the three groups (p=0.093), but there were statistical differences in SA at week 4 and week 12 (p=0.018 and p=0.009). Compared with patients in the superficial acupuncture group, patients in the EA group made no improvement in SE, TST and SA at week 4 (p=0.64, p=0.44, and p=0.95 respectively). Significant between-group differences were found in SA and TST at week 8 and week 12 (all p<0.05), indicating that patients receiving EA treatment had longer sleep duration and higher sleep efficiency than those receiving superficial acupuncture after 8 weeks of intervention. Compared with patients in the sham acupuncture group, there were significant between-group differences in SE and TST at week 4, week 8 and week 12 (all p<0.01); there was also a statistical difference in SA at all the three timepoints (all p<0.05). These objective findings suggested that EA played a positive role in prolonging patient’s sleep time, decreasing sleep awakenings, and thus increasing sleep efficiency.
Table 4.
Actigraphy Records from Baseline to Follow-Up
| Treatment Group (n=30) | Control Group A (n=30) | Control Group B (n=30) | ANOVA P-value |
T vs CA | T vs CB | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | Within-Group Effect Sizea | Mean±SD | Within-Group Effect Sizea | Mean±SD | Within-Group Effect Sizea | P-valueb | P-valueb | |||
| SE, % | Week 0 | 76.73±5.04 | 77.32±4.20 | 77.49±5.17 | 0.81 | |||||
| Week 4 | 82.10±5.05 | 0.47 | 81.42±5.91 | 0.37 | 77.24±5.51 | −0.02 | 0.002 | 1.00 | 0.007 | |
| Week 8 | 84.60±6.03 | 0.22 | 79.57±6.92 | −0.14 | 77.94±6.78 | 0.06 | <0.001 | 0.03 | 0.001 | |
| Week 12 | 83.93±6.38 | −0.05 | 79.76±6.30 | 0.01 | 77.32±6.69 | −0.05 | <0.001 | 0.11 | 0.002 | |
| TST, minutes | Week 0 | 326.67±61.59 | 336.17±46.83 | 324.10±59.97 | 0.69 | |||||
| Week 4 | 370.57±65.15 | 0.33 | 358.73±52.35 | 0.22 | 320.87±59.27 | −0.03 | 0.004 | 0.88 | 0.024 | |
| Week 8 | 382.70±64.29 | 0.09 | 334.37±57.23 | −0.22 | 325.90±64.85 | 0.01 | 0.001 | 0.024 | 0.009 | |
| Week 12 | 378.27±62.21 | −0.03 | 339.90±51.02 | 0.05 | 329.30±65.99 | 0.03 | 0.006 | 0.09 | 0.04 | |
| SA, times | Week 0 | 22.57±6.55 | 20.07±5.91 | 22.07±6.86 | 0.29 | |||||
| Week 4 | 17.73±5.38 | −0.37 | 17.63±6.05 | −0.20 | 21.93±7.93 | −0.01 | 0.018 | 1.00 | 0.16 | |
| Week 8 | 16.63±7.10 | −0.09 | 19.07±6.54 | 0.11 | 20.77±8.12 | −0.07 | 0.093 | 0.34 | 0.08 | |
| Week 12 | 16.77±7.63 | 0.01 | 21.03± | 0.24 | 21.60±7.83 | 0.05 | 0.009 | 0.27 | 0.15 | |
Notes: aEffect size by Cohen’s d formula bP-value by Bonferroni correction
Abbreviations: SE, sleep efficiency; TST, total sleep time; SA, sleep awakening; T, treatment group; CA, control group A; CB, control group B.
The Credibility of Blinding
We randomly selected 14 patients of the treatment group and 15 patients of the sham acupuncture group and asked them about the group assignment at the end of the intervention. Twelve participants in the EA group thought they took the real treatment, and the other two had no idea; six participants in the sham acupuncture group believed they had the real acupuncture treatment, six thought they had the sham treatment, and three were unsure. No statistical difference was found between groups in the proportion of participants (χ2=2.00, p=0.157).
Adverse Events
Of the 14 patients in the EA group, three reported hand numbness and pain at acupoints as adverse events. In control group A, one patient complained of local hematoma at Yintang (GV29) and one reported dizziness after one session of treatment. In control group B, one subject reported dizziness after treatment as an adverse event. All adverse events were evaluated as mild in severity. The incidences of AEs in the three groups were 10%, 6.7% and 3.3%, respectively.
Discussion
Our findings showed that 8 weeks of EA treatment improved MDD patients’ PSQI scores, increased sleep efficacy, prolonged total sleep time, and reduced the sleep awakening times, suggesting that EA treatment can effectively improve MDD patients’ quality of sleep. Patients with EA treatment also made progress in their mental health status with significant improvements in HAMD-17, SDS and HAMA scores after treatment. There were no severe adverse effects reported during the trial showing that EA treatment can be safely applied in comorbid depression and insomnia treatment. Compared with two control groups, EA was more effective than superficial acupuncture at sham acupoints as well as the non-insertion placebo acupuncture, indicating acupuncture is superior to non-specific acupuncture needling insertion and to a placebo in improving MDD patients’ sleep quality and mental health.
Many MDD patients have not received adequate treatment yet, and still more are nonresponsive to current interventions.24 Cognitive behavior therapy for insomnia (CBT-I) represents an attractive option for comorbid MDD and insomnia patients,25 but it is often unavailable for patients in developing countries due to the cultural difference, high cost, and lack of qualified psychotherapists.26 Antidepressant medication can improve both the depression symptoms and the sleep architecture, but many ADM have unwanted adverse effects on sleep, notably by worsening insomnia, daytime sleepiness, or sedation.27,28 Sleep disturbance does not always resolve with ADM, for many patients, insomnia persists even after mood symptoms have been adequately treated. With the high prevalence of depression but low penetration of CBT-I in the People's Republic of China, the situation becomes more dire.29 The high prevalence of untreated patients suggests a need to promote accessible and easily implemented interventions. Acupuncture is safe and acceptable to patients and has been used to treat depressive disorders and related sleep disturbances for a long time.30 However, previous RCTs found that EA had a relatively slight effect on treating depression-related insomnia,31,32 and the findings are contraindicated with ours. These different results could be explained by differences in the trial design including the treatment duration and the acupoint specificity, as well as acupuncturists’ skills and patient characteristics. And thus, we conducted this trial with a longer intervention period and used experienced acupuncturists to further explore EA’s effects on comorbid depression and insomnia. We also added acupoints on limbs in conjunction with the main acupoints of the Governor Vessel in our trial, to help regulate the meridian qi, calm nerves, and relax the patient.33,34 The results showed that few patients dropped out in our trial. To control the dropping out rate, the researchers kept communication with patients in all three groups by WeChat or by phone, and scheduled each session of treatment to reduce their waiting time. Besides, patients were given some guidance on lifestyles and advices on sleeping habits and they were free to consult for health problems. We also provided transportation allowance of 200 RMB (about 30 US dollars) for each patient when they completed the 8-week treatment.
The mechanisms of action of acupuncture for sleep disorder have not been clearly elucidated. Some experimental researches suggest that acupuncture may aid mood imbalances35 and minimize sleep disturbances.36 Animal studies found that acupuncture can alleviate the depressive-like behaviors in rats by inhibiting the inflammatory mediators in the hippocampus and prefrontal cortex via modulation of NF-κB pathway.37 Our previous study found that EA could ameliorate behaviors of depressive model rats by restoring hippocampus CA1 synaptic plasticity, which might be mainly mediated by regulating 5-HT receptor levels.38 Since acupuncture works on multi-level and multi-target, the possible mechanism may be explained by normalizing the function of the HPA axis negative feed reflex39 and regulating the expression of particular genes as well.40
There are two limitations in our trial. A notable limitation is the accuracy of the actigraphy records. We required all patients to wear the actigraphy only for one night before, during and after intervention; the results may be affected by patients who were sensitive to changes in sleep habits. Compared to polysomnography, actigraphy may underestimate total sleep time and sleep efficiency and cause bias.41 However, we hope the adequate randomization would minimize the weakness as these patients could be randomly distributed in each group. Besides, a clear minimal important difference (MID) or minimal clinically important difference (MCID) of the change in PSQI has not been established. The MCID should be discussed from the perspective of outcome measurement, otherwise the change difference from baseline should be converted into a response rate for inter-group comparison.
In future studies, we should try to overcome these limitations and also prolong the follow-up period to observe the lasting effect of acupuncture treatment. Future work should be carried out in multiple health centers with large sample sizes to further verify the generalization of the results in this study.
Conclusion
Our trial provided subjective and objective evidence for the efficacy and safety of electroacupuncture on treating patients with comorbid depression and insomnia. The results suggest that EA is an effective and safe alternative therapy for insomnia in MDD patients.
Acknowledgments
The study was partly supported by grants from the National Natural Science Foundation of China (No 81973943), Shanghai Hospital Development Center (No SHDC12016124), and Shanghai Municipal Health Commission (2019LJ06), and sponsored by Shanghai Sailing Program (No 20YF1446200). We would like to thank Dr Philippa Hazlewood from the International Education College, Shanghai University of Traditional Chinese Medicine, for her editorial support.
Abbreviations
MDD, major depressive disorder; EA, electroacupuncture; TCM, Traditional Chinese Medicine; PSQI, Pittsburgh Sleep Quality Index; SDS, Self-Rating Depression Scale; HAMD, Hamilton Rating Scale for Depression; HAMA, Hamilton Rating Scale for Anxiety; SE, sleep efficiency; TST, total sleep time; SA, sleep awakening; AE, adverse event; ADM, antidepressant medication; CRF, case report form; RCT, randomized controlled trial; ITT, intention-to-treat; ANOVA, analysis of variance; CBT-I; cognitive behavior therapy for insomnia; MID, minimal important difference; MCID, minimal clinically important difference.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author (Shifen Xu) upon reasonable request. Also, the individual deidentified participant data is available after contacting the corresponding author via email (xu_teacher2006@126.com). The data will be available immediately following publication without an end date.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. doi: 10.4088/JCP.v66n1008 [DOI] [PubMed] [Google Scholar]
- 2.Srisurapanont M, Likhitsathian S, Chua HC, et al. Clinical and sociodemographic correlates of severe insomnia in psychotropic drug-free, Asian outpatients with major depressive disorder. J Affect Disord. 2015;186:26–31. doi: 10.1016/j.jad.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 3.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 4.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- 5.Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37(2):239–244. doi: 10.5665/sleep.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao YP, Han Y, Ma J, et al. Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: meta-analysis and systematic review. Neurosci Biobehav Rev. 2017;75:257–273. doi: 10.1016/j.neubiorev.2017.01.032 [DOI] [PubMed] [Google Scholar]
- 7.Wu XK, Stener-Victorin E, Kuang HY, et al. Effect of acupuncture and clomiphene in Chinese women with polycystic ovary syndrome. JAMA. 2017;317(24):2502–2514. doi: 10.1001/jama.2017.7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193–200. doi: 10.1016/j.sleep.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Shi Y. Efficacy comparison of different acupuncture treatments for primary insomnia: a Bayesian analysis. Evid Based Complement Alternat Med. 2019;2019:8961748. doi: 10.1155/2019/8961748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shergis JL, Ni X, Jackson ML, et al. A systematic review of acupuncture for sleep quality in people with insomnia. Complement Ther Med. 2016;26:11–20. doi: 10.1016/j.ctim.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 11.Carney CE, Edinger JD, Kuchibhatla M, et al. Cognitive behavioral insomnia therapy for those with insomnia and depression: a randomized controlled clinical trial. Sleep. 2017;40(4):zsx019. doi: 10.1093/sleep/zsx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin X, Xu J, Dong B, et al. Efficacy and safety of electroacupuncture on treating depression related sleep disorders: study protocol of a randomized controlled trial. Evid Based Complement Alternat Med. 2016;2016:1069597. doi: 10.1155/2016/1069597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2012;340(7748):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugh MP, Altman DG, Richard H, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. J Evid Based Med. 2015;3(3):35–46. [DOI] [PubMed] [Google Scholar]
- 15.Segal DL. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR). American Psychiatric Association; 2000. [Google Scholar]
- 16.Fang J. Acupuncture and Moxibustion Technique. Beijing: People’s Medical Publishing House; 2012. [Google Scholar]
- 17.Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. 1998;352(9125):364–365. doi: 10.1016/S0140-6736(97)10471-8 [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, Rd RC, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 19.Guo S, Sun W, Liu C, Wu S. Structural validity of the Pittsburgh sleep quality index in Chinese undergraduate students. Front Psychol. 2016;7:1126. doi: 10.3389/fpsyg.2016.01126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- 22.Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12(12):63. doi: 10.1001/archpsyc.1965.01720310065008 [DOI] [PubMed] [Google Scholar]
- 23.Zuppa C, Prado CH, Wieck A, Zaparte A, Barbosa A, Bauer ME. Acupuncture for sleep quality, BDNF levels and immunosenescence: a randomized controlled study. Neurosci Lett. 2015;587:35–40. doi: 10.1016/j.neulet.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 24.Kok RM, Reynolds CF 3rd. Management of depression in older adults: a review. JAMA. 2017;317(20):2114–2122. doi: 10.1001/jama.2017.5706 [DOI] [PubMed] [Google Scholar]
- 25.Blom K, Jernelöv S, Rück C, Lindefors N, Kaldo V. Three-year follow-up comparing cognitive behavioral therapy for depression to cognitive behavioral therapy for insomnia for patients with both diagnoses. Sleep. 2017;40(8):zsx108. doi: 10.1093/sleep/zsx108 [DOI] [PubMed] [Google Scholar]
- 26.Ye YY, Chen NK, Chen J, et al. Internet-based cognitive-behavioural therapy for insomnia (ICBT-i): a meta-analysis of randomised controlled trials. BMJ Open. 2016;6(11):e010707. doi: 10.1136/bmjopen-2015-010707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manber R, Buysse DJ, Edinger J, et al. Efficacy of cognitive-behavioral therapy for insomnia combined with antidepressant pharmacotherapy in patients with comorbid depression and insomnia: a randomized controlled trial. J Clin Psychiatry. 2016;77(10):e1316–e1323. doi: 10.4088/JCP.15m10244 [DOI] [PubMed] [Google Scholar]
- 28.Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63. doi: 10.1007/s11920-017-0816-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu L, Xie J, Long J, et al. Epidemiology of major depressive disorder in Mainland China: a systematic review. PLoS One. 2013;8(6):e65356. doi: 10.1371/journal.pone.0065356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia MK, McQuade J, Haddad R, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013;31(7):952–960. doi: 10.1200/JCO.2012.43.5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeung WF, Chung KF, Tso KC, Zhang SP, Zhang ZJ, Ho LM. Electroacupuncture for residual insomnia associated with major depressive disorder: a randomized controlled trial. Sleep. 2011;34(6):807. doi: 10.5665/SLEEP.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung KF, Yeung WF, Yu YM, et al. Acupuncture for residual insomnia associated with major depressive disorder: a placebo- and sham-controlled, subject- and assessor-blind, randomized trial. J Clin Psychiatry. 2015;76(6):e752. doi: 10.4088/JCP.14m09124 [DOI] [PubMed] [Google Scholar]
- 33.Ding L, Wang J, Yang H, Yu J. “Governor vessel daoqi method of acupuncture” combined with estazolam for insomnia: a randomized controlled trial. Zhongguo zhen jiu = Chin Acupunct Moxibustion. 2018;38(5):4633–4637. [DOI] [PubMed] [Google Scholar]
- 34.Kim YI, Kim SS, Sin RS, Pu YJ, Ri G, Rim KS. Study on the cerebral blood flow regulatory features of acupuncture at acupoints of the governor vessel. Med Acupunct. 2018;30(4):192–197. doi: 10.1089/acu.2018.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu S, Li S, Shen X, Meng X, Lao L. Effects of electroacupuncture on depression in a rat model. Acupunct Electrother Res. 2011;36(3–4):259. doi: 10.3727/036012911803634166 [DOI] [PubMed] [Google Scholar]
- 36.Garland SN, Xie SX, Li Q, Seluzicki C, Basal C, Mao JJ. Comparative effectiveness of electro-acupuncture versus gabapentin for sleep disturbances in breast cancer survivors with hot flashes: a randomized trial. Menopause. 2017;24:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu J, Shao R-H, Jin S-Y, Hu L, Tu Y, Guo J-Y. Acupuncture ameliorates inflammatory response in a chronic unpredictable stress rat model of depression. Brain Res Bull. 2017;128:106–112. doi: 10.1016/j.brainresbull.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 38.Han X, Wu H, Yin P, et al. Electroacupuncture restores hippocampal synaptic plasticity via modulation of 5-HT receptors in a rat model of depression. Brain Res Bull. 2018;139:256–262. doi: 10.1016/j.brainresbull.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 39.You W, Shi YJ, Han YJ, Jia BH, Tu Y. [Effect of electroacupuncture of “Baihui” (GV 20)-”Yintang” (EX-HN 3) on the expression of glucocorticoid and glucocorticoid receptor mRNA of the chronic stress model rats]. Zhen Ci Yan Jiu. 2010;35(4):261–266. Chinese. [PubMed] [Google Scholar]
- 40.Dongmei D, Xiuyan Y, Liping C. Hippocampal gene expression in a rat model of depression after electroacupuncture at the Baihui and Yintang acupoints. Neural Regen Res. 2014;9(1):76–83. doi: 10.4103/1673-5374.125333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aili K, Åström-Paulsson S, Stoetzer U, Svartengren M, Hillert L. Reliability of actigraphy and subjective sleep measurements in adults: the design of sleep assessments. J Clin Sleep Med. 2017;13(1):39–47. doi: 10.5664/jcsm.6384 [DOI] [PMC free article] [PubMed] [Google Scholar]