Abstract
Objectives
Smoking is a major risk factor for the development of both cardiovascular disease (CVD) and RA and may cause attenuated responses to anti-rheumatic treatments. Our aim was to compare disease activity, CVD risk factors and CVD event rates across smoking status in RA patients.
Methods
Disease characteristics, CVD risk factors and relevant medications were recorded in RA patients without prior CVD from 10 countries (Norway, UK, Netherlands, USA, Sweden, Greece, South Africa, Spain, Canada and Mexico). Information on CVD events was collected. Adjusted analysis of variance, logistic regression and Cox models were applied to compare RA disease activity (DAS28), CVD risk factors and event rates across categories of smoking status.
Results
Of the 3311 RA patients (1012 former, 887 current and 1412 never smokers), 235 experienced CVD events during a median follow-up of 3.5 years (interquartile range 2.5–6.1). At enrolment, current smokers were more likely to have moderate or high disease activity compared with former and never smokers (P < 0.001 for both). There was a gradient of worsening CVD risk factor profiles (lipoproteins and blood pressure) from never to former to current smokers. Furthermore, former and never smokers had significantly lower CVD event rates compared with current smokers [hazard ratio 0.70 (95% CI 0.51, 0.95), P = 0.02 and 0.48 (0.34, 0.69), P < 0.001, respectively]. The CVD event rates for former and never smokers were comparable.
Conclusion
Smoking cessation in patients with RA was associated with lower disease activity and improved lipid profiles and was a predictor of reduced rates of CVD events.
Keywords: rheumatoid arthritis, epidemiology, behaviour, quality of life, outcome measures
Rheumatology key messages
Smoking cessation in patients with RA was associated with improved disease activity and improved lipid profile.
Smoking cessation in patients with RA was a predictor of fewer CVD events.
Health professionals should strongly recommend and encourage smoking cessation to patients with RA.
Introduction
Patients with RA experience an increased risk of cardiovascular disease (CVD) as a result of an intricate interplay between chronic inflammation and traditional CVD risk factors [1–5]. This risk is comparable to that of patients with diabetes mellitus [6].
A strong link between tobacco smoking and CVD events has been identified in the general population as well as in patients with RA [3, 7]. Indeed, a meta-analysis from 2015 revealed that RA patients who were smokers had a 50% higher CVD morbidity compared with non-smokers [3]. Moreover, smoking is a well-established modifiable risk factor in the development of RA and causes accelerated disease progression as well as an attenuated response to DMARDs [8–11]. Thus smoking cessation in RA patients may represent an opportunity to ‘kill two birds with one stone’. Unsurprisingly, smoking cessation is recommended in the European Society of Cardiology guidelines for CVD prevention, the EULAR recommendations for CVD risk management in RA and the EULAR recommendations for the management of early arthritis [12–14].
In the general population, smoking is associated with increased lipid levels, while smoking cessation leads to improved lipid profiles [15, 16]. However, this association has not been investigated in patients with RA. For the general population, a 50% CVD risk reduction has been observed within the first 2 years following smoking cessation, while risk of ischaemic stroke is comparable to that of never smokers 5 years after smoking cessation [17–19]. Data on the effect of smoking cessation and prevention of future CVD events in RA are limited to a retrospective medical records–based study that found a significant reduction in the risk of hospitalization due to CVD events in former compared with never smokers [20].
The aim of this study was to compare disease activity, CVD risk factors and the risk of future CVD events in RA patients across current, former and never smokers in a large multinational register.
Methods
Study population
The study population has previously been described in detail [21, 22]. In short, the cohort consists of patients with physician diagnosis of RA (1987 or 2010 American College of Rheumatology criteria for RA [23, 24]) from 10 countries (UK, Norway, Netherlands, USA, Sweden, Greece, South Africa, Spain, Canada and Mexico) for whom both traditional and RA-specific CVD risk factors, including RA disease activity measures, as well as drugs used for treatment of RA were collected [21].
The study was carried out according to the Declaration of Helsinki and was approved by the ethical boards/committees at each centre. Informed consent was obtained from the study participants where required. The ATACC-RA (A TransAtlantic Cardiovascular risk Calculator for Rheumatoid Arthritis) register was built at the Mayo Clinic after anonymous (de-identified) patient data were transferred from each centre to the Mayo Clinic. The ATACC-RA register was transferred to Diakonhjemmet, Oslo, Norway. In addition, a General Data Protection Regulation evaluation has been approved by the Data Protection Officer at Diakonhjemmet (25/4-2019). This study was approved by the South-East Health Authorities Ethical Committee (2019/807).
Variable definitions
The following CVD risk factors were collected at baseline: age, sex, smoking status (current, former, never), systolic and diastolic blood pressure (BP), lipid levels [total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC), triglycerides], BMI and a family history of CVD, diabetes mellitus and hypertension. Use of lipid-lowering and antihypertensive medications was also recorded [21, 22]. In addition, non-HDL levels were calculated by subtracting HDLC from TC.
The following RA-specific factors were recorded: RF positivity, ACPA positivity, ESR, CRP and 28-joint DAS using ESR (DAS28-ESR) [25]. RF and ACPA (only available in a subset of patients) were considered positive based on the tests performed at each centre. CRP was unavailable in one cohort, and DAS28 was unavailable in another cohort. Data on the use of NSAIDs, cyclooxygenase-2 inhibitors, glucocorticoids and synthetic and biologic DMARDs were collected [21, 22].
CVD events during follow-up were defined according to guidelines as acute coronary syndrome (unstable angina pectoris, ST elevation and non-ST elevation myocardial infarction), chronic ischaemic heart disease (stable angina pectoris), coronary revascularization (e.g. percutaneous coronary intervention and coronary artery bypass grafting), coronary death, other CVD death, cerebrovascular events (ischaemic cerebrovascular accident and transient ischemic attack) and peripheral vascular events (with and without revascularization procedures, peripheral artery disease) [21, 22]. Cases of confirmed cerebral haemorrhage, non-coronary cardiac death, non-ischemic cardiovascular disease, heart failure and aortic aneurysm were not included as CVD events [21, 22].
Statistical methods
Descriptive statistics are expressed as numbers (%) for dichotomized variables, while mean (s.d.) and median with interquartile range (IQR) were applied for normally and non-normally distributed continuous variables, respectively. Independent samples t-tests, analysis of variance and χ2 tests were applied as appropriate for crude group comparisons. Non-normally distributed variables were logarithmically transformed before conducting the analyses.
Analyses of covariance were applied to perform adjusted comparisons of RA-specific and cardiovascular variables across smoking status. All analyses were adjusted for age and sex, while lipoprotein and BP variables were also adjusted for statin and antihypertensive use, respectively.
The first occurrence of a CVD event was compared across smoking status in adjusted Cox proportional hazards regression models. Three different models adjusted for age, sex, antihypertensive and lipid-lowering therapy use were constructed in order to compare former vs current smokers, former vs never smokers and current vs never smokers. In addition, an adjusted Cox model that included all three smoking statuses (former, current and never) was plotted on a graph to visualize cumulative CVD event rates. Proportional hazard assumptions were tested and confirmed graphically (log-log curves). The analyses included only the first CVD event in each patient; subsequent CVD events in the same patient were not included.
To examine differences in RA disease activity across smoking status, patients were divided into either remission/low disease activity or moderate/high disease activity according to the DAS28 [25]. Results are presented as crude percentages in a bar graph with P-values from logistic regression adjusted for age, sex and disease duration.
Two-sided P-values <0.05 were considered significant. All analyses were performed using the Statistical Package for the Social Sciences for Windows, version 21.0 (IBM, Armonk, NY, USA).
Results
A total of 3311 patients were included in the present study, including 1012 former smokers, 887 current smokers and 1412 never smokers. The median follow-up was 3.54 years (interquartile range 2.5–6.1), which was comparable in the three groups (data not shown). During follow-up, 235 patients experienced one or more CVD events. As shown in Table 1, there were significant differences in age (ranging from 53.6 years among current smokers to 58.8 years in the former smokers) and female sex (ranging from 86.5% in those who had never smoked to 62.8% among those who were former smokers) across smoking status groups. Furthermore, current smokers had lower BMI, longer disease durations and used less lipid-lowering and antihypertensive therapies compared with former and never smokers. There were also significant differences in the use of various anti-rheumatic drugs among patients with different smoking statuses.
Table 1.
Patient characteristics
| Characteristics | Former smokers (n = 1012) | Current smokers (n = 887) | Never smoked (n = 1412) |
|---|---|---|---|
| Age, mean (s.d.), years | 58.77 (12.30)** | 53.59 (11.72)** | 55.33 (15.41)* |
| Sex (female), n (%) | 636 (62.8)* | 596 (67.2)** | 1221 (86.5)** |
| BMI, mean (s.d.) | 27.47 (4.90)** | 25.95 (4.74)* | 27.03 (5.13)** |
| Disease duration, years, median (IQR) | 1.74 (0.25–10.59)** | 4.22 (0.07–6.00)* | 3.08 (0.45–11.00)** |
| RF positive, n (%) | 656 (66.3)* | 632 (72.3) | 885 (63.9)** |
| Anti-CCP positive, n (%) | 597 (61.0) | 529 (63.3)* | 766 (56.4)* |
| Extra-articular RA, n (%) | 48 (6.3) | 42 (5.9) | 82 (8.1) |
| Statin, n (%) | 111 (11.0)* | 69 (7.8) | 175 (12.4)** |
| Anti-hypertensive, n (%) | 220 (21.7)** | 137 (15.5) | 331 (23.5)** |
| MTX, n (%) | 398 (39.7)** | 263 (29.8)* | 635 (45.1)** |
| HCQ, n (%) | 128 (12.7) | 95 (10.8)** | 287 (20.4)** |
| DMARD, n (%) | 515 (51.1)** | 368 (41.6)** | 824 (58.5)** |
| Biologic, n (%) | 155 (15.4)* | 111 (12.6) | 250 (17.8)** |
| Steroids, n (%) | 240 (23.8) | 215 (24.3)* | 412 (29.3)* |
P < 0.05,
P < 0.001.
There were no significant differences in ESR, CRP or DAS28 across smoking status (Table 2).
Table 2 Crude data and adjusted comparisons across smoking status
| Variables | Crude data, mean (s.d.) | Adjusted comparisons, median (IQR) | ||||
|---|---|---|---|---|---|---|
| Former smokers | Current smokers | Never smoked | Former vs current | Former vs never | Current vs never | |
| Rheumatologic variables | ||||||
| ESR, mm/h | 24.52 (22.32) | 23.94 (20.20) | 24.11 (21.00) | −0.01 (−4.44–4.41) | −5.16 (−10.58–0.27) | −4.47 (−11.00–2.06) |
| CRP, mg/L | 7.10 (2.00–20.84) | 8.00 (1.90–20.00) | 5.00 (1.60–13.00) | 2.58 (−2.51–7.68) | 2.04 (−2.67–6.76) | −1.07 (−5.93–3.80) |
| DAS28 | 3.88 (1.72) | 4.08 (1.55) | 3.90 (1.79) | −0.21 (−0.67–0.25) | −0.36 (−0.77–0.06) | −0.23 (−0.70–0.24) |
| Cardiovascular variables | ||||||
| TC, mmol/L | 5.31 (1.11) | 5.35 (1.15) | 5.13 (1.14) | −0.07 (−0.18–0.04) | 0.19 (0.09–0.29)** | 0.29 (0.18–0.39)** |
| TG, mmol/L | 1.43 (0.72) | 1.51 (0.82) | 1.31 (0.74) | −0.09 (−0.16 to −0.01)* | 0.09 (0.02–0.15)* | 0.19 (0.12–0.26)** |
| HDLC, mmol/L | 1.51 (0.45) | 1.41 (0.43) | 1.53 (0.46) | 0.10 (0.05–0.14)** | 0.02 (−0.02–0.06) | −0.08 (−0.12 to −0.03)** |
| LDLC, mmol/L | 3.17 (1.01) | 3.30 (0.98) | 3.03 (0.97) | −0.15 (−0.24 to −0.05)* | 0.12 (0.03–0.20)* | 0.29 (0.20–0.38)** |
| Non-HDLC, mmol/L | 3.79 (1.03) | 3.94 (1.07) | 3.60 (1.03) | −0.16 (−0.27 to −0.06)* | 0.17 (0.08–0.26)** | 0.36 (0.27–0.46)** |
| TC/HDLC, mmol/L | 3.76 (1.16) | 4.03 (1.17) | 3.56 (1.02) | 0.26 (0.15–0.37)** | −0.08 (−0.18–0.01) | −0.36 (−0.46–0.27)** |
| Systolic BP, mmHg | 142.74 (22.32) | 138.85 (22.51) | 136.18 (21.91) | 0.21 (−1.71–2.13) | 3.41 (1.77–5.05)** | 3.23 (1.53–4.92)** |
| Diastolic BP, mmHg | 82.87 (11.09) | 81.52 (10.46) | 79.14 (10.76) | 0.69 (−0.29–1.68) | 2.63 (1.73–3.53)** | 1.98 (1.08–2.87)** |
All analyses adjusted for age and sex. Lipoprotein variable analyses adjusted for statin use. BP variable analyses adjusted for antihypertensive use.
P < 0.05,
P < 0.001.
TG, triglycerides.
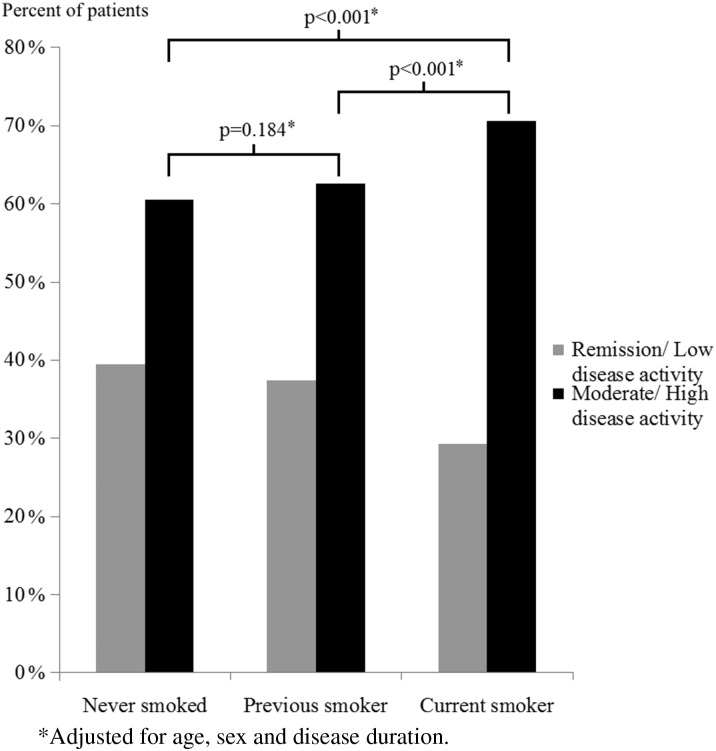
However, when patients were clustered into groups according to their DAS28 scores (remission/low disease activity vs moderate/high disease activity; Fig. 1), a greater proportion of current smokers had moderate or high disease activity (70.6%) compared with former and never smokers (62.5% and 60.4%, respectively), which remained highly significant after adjustment for age, sex and disease duration (P < 0.001 for both).
Fig. 1.
Disease activity across smoking status
Patients who had never smoked had more favourable CVD risk profiles than former and current smokers after adjusting for age, sex and use of relevant CVD preventive medication (Table 2). RA patients who had never smoked had significantly lower TC, triglycerides, LDLC, non-HDLC, systolic BP and diastolic BP, while their HDLC levels were significantly higher compared with the two other groups. Moreover, former smokers had significantly lower triglycerides, LDLC and non-HDLC compared with current smokers. Former smokers also had significantly higher HDLC than current smokers. However, there were no significant differences in systolic or diastolic BP levels when comparing former and current smokers.
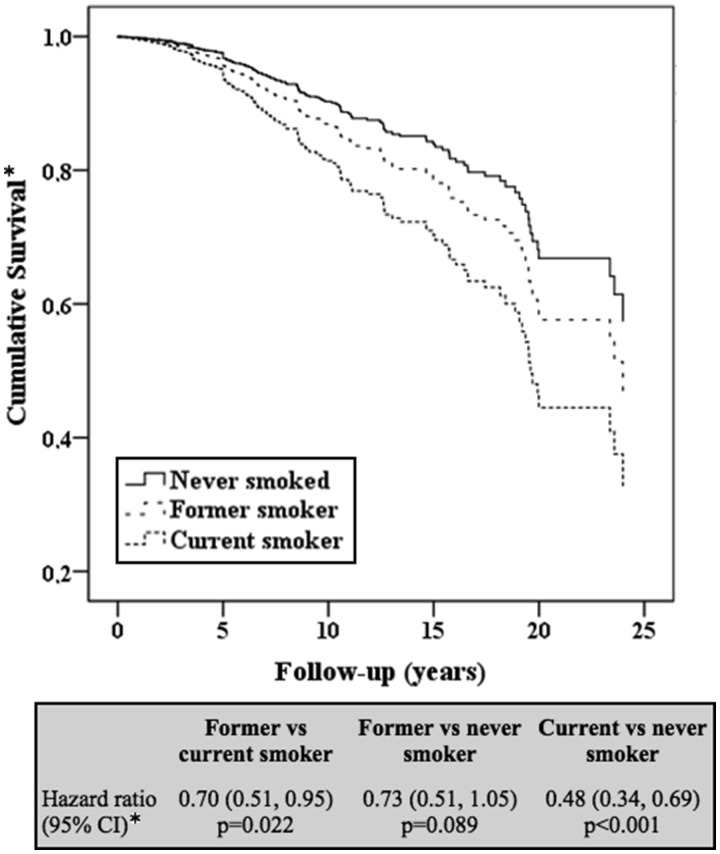
Cox models adjusted for age, sex, lipid-lowering therapies and antihypertensive use are presented in Fig. 2. Compared with current smokers, former and never smokers had a 30% (P = 0.022) and 50% (P < 0.001) lower risk of future CVD, respectively. However, the risk for future CVD was not significantly different in former and never smokers. These results were robust in additional Cox models that included additional covariates (data not shown).
Fig. 2.
Adjusted Cox proportional hazards regression models comparing CVD risk across smoking status in RA patients
Discussion
In a large cohort of RA patients, we showed that the risk of future CVD events is significantly lower in RA patients who have stopped smoking compared with those who continue smoking. Interestingly, while there were no significant differences in CVD event rates across never smokers and former smokers, the event rate was significantly higher among RA patients who were current smokers. This difference may be explained, at least partly, by a continuum in terms of worsening CVD risk factor profiles from never smokers to former smokers to current smokers in RA patients and is supported by existing evidence on the impact of smoking on CVD events and risk factor profiles [17, 26, 27]. Another cause may be that smokers were more likely to have higher disease activity, which is an important RA-associated CVD risk factor.
We did not observe significant differences in DAS28 when it was considered as a continuous variable across smoking status groups. However, former smokers were more likely to be in remission or have moderate disease activity compared with current smokers. This was also found in a register study comparing disease activity across smoking status among anti-CCP-positive RA patients in the USA where never and former smokers had lower disease activity compared with current smokers [28]. In contrast, Fisher et al. [29] reported that in a North American population of 16 521 RA patients, smoking status did not influence disease activity. However, this study used the composite measure clinical disease activity index, which does not include acute phase reactants. In a Swedish RA cohort, Andersson et al. [30] reported that RA disease activity measures, including HAQ, visual analogue scale pain or DAS28, did not improve following smoking cessation. This study used DAS28 as a composite score for disease activity (n = 1460), while interpretation of the results were also hampered by the retrospective design and the fact that only 127 patients stopped smoking after inclusion [30]. In a cohort study by Castañeda et al. [31] it was suggested that prolonged smoking exposure may favour a pro-inflammatory state leading to increased disability and higher risk of CVD in these patients.
Previous studies from the general population have presented evidence that despite weight gain, HDLC levels tend to increase in persons who quit smoking [16, 32]. To date, the effect of smoking cessation on other lipoprotein fractions, such as LDLC, is inconclusive [32]. To our knowledge, the present study provides the first data on the possible effect of smoking cessation on lipid levels in RA patients. It is well documented that inflammation suppresses lipoprotein levels and, accordingly, there is a tendency that treatment with inflammation-lowering drugs leads to rising lipid levels [13, 33]. In our study, current smokers were more likely to have moderate or high disease activity, which would presumably lead to decreased lipid levels. Despite this, we found that the lipoproteins were higher in current smokers, apart from HDLC, which was lower, suggesting a beneficial effect of smoking cessation on lipid profiles in RA patients.
Although the CVD preventive effect of smoking cessation is well documented in the general population [34–36], the information about the impact of smoking cessation in RA patients has until now been limited [20]. In a study by Joseph et al. [20], UK primary care medical records were explored to identify smoking habits, prescription of smoking cessation therapy and hospitalization among some 5000 RA patients. In adjusted analyses, the authors found that the hazard ratio (HR) of hospitalization for CVD events in current vs never smokers was 2.23, while the corresponding HR for current vs former smokers was 1.51. Our findings are along the same lines, as we demonstrated that the HR for a CVD event in former vs current smokers was 0.70 in adjusted analyses, while it was 0.48 in never vs current smokers. However, in contrast to the study by Joseph et al. [20], we did not find a significant difference in the CVD event rates across former and never smokers, although there was a numerical trend in the same direction as the findings in the study by Joseph et al. This inconsistent finding may be explained by the fact that our data did not include time since smoking cessation nor pack-years of smoking. Nonetheless, our results back up the hypothesis that smoking cessation in RA patients leads to reduced CVD risk, approaching the low risk observed in never smokers. The underlying mechanisms for this risk reduction may lie, at least partly, in the other results from our study; in particular, improved CVD risk factor profiles and reduced disease activity [37, 38]. Furthermore, smoking has direct effects on the vessel walls, which are known to aggravate atherogenesis, including increased arterial stiffness and endothelial dysfunction, which was not measured in our study [39, 40].
Our finding that smoking cessation is associated with lower RA disease activity further supports the importance of smoking cessation in RA patients. The benefits from smoking cessation on RA disease-specific outcomes and long-term effects on RA disease have been investigated to a limited extent. Still, it is currently advised that rheumatology health professionals encourage smoking cessation in RA patients [12, 13, 41]. To our knowledge there is only one published pilot study and one ongoing randomized controlled trial on smoking cessation in patients with RA [42, 43].
The strengths of this study are that we have a large sample size including several cohorts of patients with RA from 10 different countries from different continents. This makes the results internationally transferable and not just limited to a specific geographical area. The cohorts included were either population based or applied enrolment of consecutive patients, a factor that could reduce the risk of selection bias. This international study format could be considered a strength since the results may be regarded as more likely to be generalizable and not limited to a specific country or area. In addition, within the cohort there were a large number of CVD events, which strengthens our results.
Several limitations should also be considered for interpretation of our results. First, smoking cessation was self-reported. Despite the fact that self-reported outcomes may vary in quality and reliability, existing evidence supports the validity of self-reported smoking status [44–46]. Second, smoking status data was collected at baseline and time of smoking cessation, smoking duration and pack-years were not collected. Hence we could not assess the dose response and time from smoking cessation to a CVD event. Furthermore, the fact that we did not have access to follow-up data on smoking cessation during the study period is a limitation since we do not know which patients may have stopped or reinitiated smoking after baseline. Thus, hypothetically, if more people had stopped smoking during follow-up this would have strengthened our results further. In case of the opposite, that more patients had started smoking after the baseline visit, our results regarding smoking cessation would still be significant. Third, this is a study evaluating the effect of lifestyle-related changes on future CVD events, and although we have made an effort to adjust our analyses for relevant confounders, we cannot exclude the possibility that other concurrent non-measured lifestyle changes (e.g. increased frequency of physical activity and nutrition factors) may have confounded our results. Moreover, we did not have available data to investigate possible differences in socio-economic status or psychological traits, such as conscientiousness, which may have influenced our results. Differences in the latter variables may also be possible explanations for the discrepancies observed across smoking status in the use of cardioprotective and anti-rheumatic medications.
In conclusion, smoking cessation appears to have several beneficial effects in patients with RA. Our data suggest that patients who stop smoking experience improved RA disease activity, a more favourable lipid profile and lower frequency of CVD events. We argue that these findings underline the importance of a larger focus among health professionals on recommending and encouraging smoking cessation in patients with RA.
Acknowledgements
ATACC-RA members: Eric L. Matteson, Aamer Sandoo, Elke Arts, Jaap Fransen, Lena Innala, Evi Zampeli, Alfonso Corrales, Daniel Solomon, Katherine Liao, Mart van de Laar, Harald Vonkeman, Inger Meek, Elaine Huseni, Robert Overman, Iris Colunga-Pedraz, Dionicio Galarza-Delgardo and José Ramón Azpiri-López. IKR, EI and AGS outlined the hypothesis, analysed the data and drafted the first version of the manuscript. SR and GW contributed substantially to the subsequent versions. All authors contributed to the analyses and interpretation of the data and read and commented on drafts and approved the final version of the manuscript. IKR was supported by funding from Trygfonden (ID: 119298).
Funding: This work was supported by a collaborative agreement for independent research from Eli Lilly. Eli Lilly had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure statement: TKK has received fees for speaking and/or consulting and/or received research funding to Diakonhjemmet Hospital from AbbVie, Biogen, Celltrion, Eli Lilly, MSD, Mylan, Novartis, Oktal, Orion Pharma, Hospira/Pfizer, Sandoz, Sanofi and UCB. CAH has received research funding from Pfizer Canada and UCB. MAGG received grants/research support from AbbVie, MSD, Jansen and Roche and consultation fees/participation in company-sponsored speakers bureaus from Pfizer, Lilly, Sobi, Celgene, Novartis, Roche and Sanofi. AGS received speaker honoraria and/or consulting fees from KPMG, Novartis, Bayer and AbbVie.
References
- 1. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D.. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- 2. Schieir O, Tosevski C, Glazier RH, Hogg-Johnson S, Badley EM.. Incident myocardial infarction associated with major types of arthritis in the general population: a systematic review and meta-analysis. Ann Rheum Dis 2017;76:1396–404. [DOI] [PubMed] [Google Scholar]
- 3. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA.. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One 2015;10:e0117952.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wibetoe G, Ikdahl E, Rollefstad S. et al. Cardiovascular disease risk profiles in inflammatory joint disease entities. Arthritis Res Ther 2017;19:153.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panoulas VF, Stavropoulos-Kalinoglou A, Metsios GS. et al. Association of interleukin-6 (IL-6)-174G/C gene polymorphism with cardiovascular disease in patients with rheumatoid arthritis: the role of obesity and smoking. Atherosclerosis 2009;204:178–83. [DOI] [PubMed] [Google Scholar]
- 6. Lindhardsen J, Ahlehoff O, Gislason GH. et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 2011;70:929–34. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies, Geneva: World Health Organization, 2017. https://apps.who.int/iris/handle/10665/255874 (18 November 2019, date last accessed).
- 8. Sugiyama D, Nishimura K, Tamaki K. et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2010;69:70–81. [DOI] [PubMed] [Google Scholar]
- 9. Di Giuseppe D, Discacciati A, Orsini N, Wolk A.. Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther 2014;16:R61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang K, Yang SM, Kim SH. et al. Smoking and rheumatoid arthritis. Int J Mol Sci 2014;15:22279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malm K, Bremander A, Arvidsson B. et al. The influence of lifestyle habits on quality of life in patients with established rheumatoid arthritis—a constant balancing between ideality and reality. Int J Qual Stud Health Well-Being 2016;11:30534.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Combe B, Landewe R, Daien CI. et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017;76:948–59. [DOI] [PubMed] [Google Scholar]
- 13. Agca R, Heslinga SC, Rollefstad S. et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. [DOI] [PubMed] [Google Scholar]
- 14. Piepoli MF, Corra U, Adamopoulos S. et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol 2014;21:664–81. [DOI] [PubMed] [Google Scholar]
- 15. Takata K, Imaizumi S, Kawachi E. et al. Impact of cigarette smoking cessation on high-density lipoprotein functionality. Circ J 2014;78:2955–62. [DOI] [PubMed] [Google Scholar]
- 16. Forey BA, Fry JS, Lee PN, Thornton AJ, Coombs KJ.. The effect of quitting smoking on HDL-cholesterol – a review based on within-subject changes. Biomarker Res 2013;1:26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mons U, Muezzinler A, Gellert C. et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ 2015;350:h1551.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rigotti NA, Clair C.. Managing tobacco use: the neglected cardiovascular disease risk factor. Eur Heart J 2013;34:3259–67. [DOI] [PubMed] [Google Scholar]
- 19. Kawachi I, Colditz GA, Stampfer MJ. et al. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Arch Intern Med 1994;154:169–75. [PubMed] [Google Scholar]
- 20. Joseph RM, Movahedi M, Dixon WG, Symmons DP.. Risks of smoking and benefits of smoking cessation on hospitalisations for cardiovascular events and respiratory infection in patients with rheumatoid arthritis: a retrospective cohort study using the Clinical Practice Research Datalink. RMD Open 2017; 3:e000506.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crowson CS, Rollefstad S, Ikdahl E. et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018;77:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crowson CS, Rollefstad S, Kitas GD. et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One 2017;12:e0174656.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 24. Aletaha D, Neogi T, Silman AJ. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 25. Prevoo ML, van ’t Hof MA, Kuper HH. et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 26. Critchley JA, Capewell S.. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290:86–97. [DOI] [PubMed] [Google Scholar]
- 27. Ambrose JA, Barua RS.. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004;43:1731–7. [DOI] [PubMed] [Google Scholar]
- 28. Sokolove J, Wagner CA, Lahey LJ. et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: a cross-sectional analysis of US veterans. Rheumatology 2016;55:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fisher MC, Hochberg MC, El-Taha M. et al. Smoking, smoking cessation, and disease activity in a large cohort of patients with rheumatoid arthritis. J Rheumatol 2012;39:904–9. [DOI] [PubMed] [Google Scholar]
- 30. Andersson ML, Bergman S, Soderlin MK.. The effect of stopping smoking on disease activity in rheumatoid arthritis (RA). Data from BARFOT, a multicenter study of early RA. Open Rheumatol J 2012;6:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Castaneda S, Martin-Martinez MA, Gonzalez-Juanatey C. et al. Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: baseline data of the CARMA Project. Semin Arthritis Rheum 2015;44:618–26. [DOI] [PubMed] [Google Scholar]
- 32. Gepner AD, Piper ME, Johnson HM. et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J 2011;161:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Svenson KL, Lithell H, Hallgren R, Vessby B.. Serum lipoprotein in active rheumatoid arthritis and other chronic inflammatory arthritides. II. Effects of anti-inflammatory and disease-modifying drug treatment. Arch Intern Med 1987;147:1917–20. [PubMed] [Google Scholar]
- 34. Mallaina P, Lionis C, Rol H. et al. Smoking cessation and the risk of cardiovascular disease outcomes predicted from established risk scores: results of the Cardiovascular Risk Assessment among Smokers in Primary Care in Europe (CV-ASPIRE) study. BMC Public Health 2013;13:362.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iso H, Date C, Yamamoto A. et al. Smoking cessation and mortality from cardiovascular disease among Japanese men and women: the JACC Study. Am J Epidemiol 2005;161:170–9. [DOI] [PubMed] [Google Scholar]
- 36. Hjermann I, Velve Byre K, Holme I, Leren P.. Effect of diet and smoking intervention on the incidence of coronary heart disease. Report from the Oslo Study Group of a randomised trial in healthy men. Lancet 1981;2:1303–10. [DOI] [PubMed] [Google Scholar]
- 37. Asthana A, Johnson HM, Piper ME. et al. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. Am Heart J 2010;160:458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shiels MS, Katki HA, Freedman ND. et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kool MJ, Hoeks AP, Struijker Boudier HA, Reneman RS, Van Bortel LM.. Short- and long-term effects of smoking on arterial wall properties in habitual smokers. J Am Coll Cardiol 1993;22:1881–6. [DOI] [PubMed] [Google Scholar]
- 40. Grassi D, Desideri G, Ferri L. et al. Oxidative stress and endothelial dysfunction: say NO to cigarette smoking! Curr Pharm Des 2010;16:2539–50. [DOI] [PubMed] [Google Scholar]
- 41. Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Douglas KM. et al. Cigarette smoking associates with body weight and muscle mass of patients with rheumatoid arthritis: a cross-sectional, observational study. Arthritis Res Ther 2008;10:R59.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aimer P, Treharne GJ, Stebbings S. et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: a randomized controlled pilot trial. Arthritis Care Res 2017;69:28–37. [DOI] [PubMed] [Google Scholar]
- 43. Roelsgaard IK, Thomsen T, Ostergaard M. et al. The effect of an intensive smoking cessation intervention on disease activity in patients with rheumatoid arthritis: study protocol for a randomised controlled trial. Trials 2017;18:570.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D.. Assessment of validity of self-reported smoking status. Health Rep 2012;23:47–53. [PubMed] [Google Scholar]
- 45. Kvalvik LG, Nilsen RM, Skjærven R. et al. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatr Res 2012;72:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Patrick DL, Cheadle A, Thompson DC. et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health 1994;84:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]