Abstract
Objectives
Peficitinib, a novel Janus kinase (JAK) inhibitor, demonstrated promising results in treating RA in phase 3 clinical trials. This in vitro study was undertaken to characterize the pharmacological properties of peficitinib and investigate the involvement of JAK and signal transducer and activator of transcription (STAT) pathways in the pathological processes of SSc, which is also an autoimmune disease.
Methods
Phosphorylation levels of STAT molecules were assessed in peripheral blood mononuclear cells collected from patients with RA or SSc and healthy subjects, and in skin specimens obtained from 19 patients with SSc. In vitro inhibition of STAT phosphorylation and cytokine/chemokine production by peficitinib, tofacitinib and baricitinib were also characterized.
Results
Higher spontaneous STAT1 or STAT3 phosphorylation was observed in peripheral T-cells and monocytes from patients with RA and SSc compared with healthy subjects. In skin sections from patients with SSc, phosphorylated STAT3–positive cells were found in almost all cases, irrespective of disease subtype or patient characteristics. Conversely, phosphorylated STAT1-positive cells were observed only in samples from untreated patients with diffuse disease of short duration. Peficitinib inhibited STAT phosphorylation induced by various cytokines, with comparable efficacy to tofacitinib and baricitinib. Peficitinib also suppressed cytokine and chemokine production by peripheral blood mononuclear cells and skin fibroblasts.
Conclusion
Our results suggest that JAK/STAT pathways are constitutively activated in SSc and RA, and that the JAK inhibitor may represent a novel therapeutic option for SSc.
Keywords: Janus kinase, signal transducer and activator of transcription, peficitinib, RA, SSc
Rheumatology key messages
JAK inhibitors show promising clinical outcomes in treating several autoimmune diseases.
Systemic sclerosis is an autoimmune disease in which the JAK/STAT pathway is constitutively activated.
As a JAK inhibitor, peficitinib might be a novel option for treating systemic sclerosis.
Introduction
The refractory chronic autoimmune disease SSc is characterized by angiopathy resulting from extensive organ fibrosis and vascular endothelial hyperplasia. Disease classification is based on the aggressiveness of the disease or severity of sclerosis, namely diffuse or limited [1]. Effective treatment is currently lacking, therefore patients with SSc, particularly those with diffuse disease, experience high rates of morbidity and mortality [2]. Although the full disease pathogenesis remains unknown, fibroblast activation, vascular dysfunction and immune dysregulation are believed to contribute to the pathogenesis of SSc [3].
The proinflammatory cytokine, IL-6, is elevated in SSc and correlates with disease activity [4]. However, randomized controlled trials of tocilizumab, an anti-IL-6 receptor alpha antibody, failed to show statistically significant improvements in skin thickening and lung function in adults with progressive SSc [5, 6]. These results support the complexity and pleiotropic aspects of SSc, with further cumulative evidence suggesting the involvement of other T-cell-derived cytokine signal pathways, especially T-helper 2 cell-derived cytokines, including IL-4, IL-13 and TGF-β [7]. Strategies involving multiple cytokine targets may therefore improve the clinical outcomes of SSc in comparison with single-cytokine targeting strategies.
Therapeutic cytokine signal targeting is already well established in the treatment of RA, with the emergence of several effective biologic (b)DMARDs [8]. Additionally, orally available Janus kinase (JAK) inhibitors, which improve quality of life in patients with RA, have recently been introduced and are recommended in combination with bDMARDs for the management of early disease when unfavourable prognostic markers are present [9]. Several cytokines transduce their signalling via JAK/signal transducer and activator of transcription (STAT) pathways in various combinations (JAK1, JAK2, JAK3, tyrosine kinase-2/STAT1, 2, 3, 4, 5a, 5b, 6) [10, 11].
A growing body of evidence is accumulating indicating that JAK/STAT pathways are involved in many pathological processes in autoimmune diseases [11], and three effective JAK inhibitors are currently available for clinical indications: tofacitinib and baricitinib, for RA, and ruxolitinib, for myelofibrosis and polycythaemia [12–15]. JAK inhibitors are therefore increasingly recognized as useful options for treating immune-related diseases, and a large number of clinical trials are now underway to expand the potential indications of JAK inhibitors, for example, in SLE, alopecia areata and inflammatory bowel disease [16, 17]. It has also been proposed that JAK inhibitors may be efficacious in SSc, because of multitargeted cytokine signal inhibition [18]. Nevertheless, to date, little attention has been given to the application of JAK inhibitors in treating SSc.
Peficitinib, a novel pan-JAK inhibitor, is a more potent inhibitor of tyrosine kinase-2 activity than the currently marketed JAK inhibitors, and has demonstrated efficacy in phase 3 clinical trials in RA [14, 15, 19, 20]. The aim of this study was to assess the therapeutic potential of peficitinib in SSc, by characterizing the in vitro pharmacological profile of peficitinib in inhibiting the JAK/STAT pathway.
Methods
Study design, patients and ethics
This was an in vitro study of blood samples obtained from 29 bDMARD-naïve patients with RA, 21 patients with SSc and 10 healthy subjects at the University of Occupational and Environmental Health, Japan. Patients with RA were clinically diagnosed based on ACR/EULAR 2010 classification criteria for RA [21, 22], and clinical diagnosis of SSc was made based on the 2013 ACR/EULAR classification criteria for SSc [23, 24]. Skin biopsy specimens were also obtained from 19 patients with SSc. The study was approved by the Human Ethics Review Committees of the University of Occupational and Environmental Health, Japan, and Astellas Pharma, Inc. Each subject provided written informed consent.
Test compounds
All test compounds (tofacitinib, baricitinib and peficitinib) were synthesized at Astellas Pharma, Inc. (Tokyo, Japan).
Baseline phosphorylation levels of STAT
Aliquots of human whole blood (100 μl) were stained with V450-conjugated anti-CD3 antibody (BD Biosciences, San Jose, CA, USA) to surface stain CD3+ T cells before fixation with 1 × BD Phosflow Lyse/Fix buffer (BD Biosciences) for 10 min at 37°C. After washing and permeabilization with Perm buffer III (BD Biosciences) for 30 min at 4°C, the cells were washed and stained with anti-phospho STAT antibodies (BD Biosciences) at 4°C in the dark for 60 min. After final washing and resuspension in 200 μl wash buffer, the cells were kept on ice until flow cytometer analysis. STAT phosphorylation levels in CD3+ T cells or monocytes were expressed as the mean fluorescence intensity values of cells staining positive for phosphorylated STATs from which the mean fluorescence intensity values of unstained cells were subtracted. Monocytes were defined by forward and side scatter flow cytometer values. The required sample size for this study was determined based on the findings of a previous report [25].
Cytokine stimulation–induced STAT phosphorylation assays
Peripheral blood mononuclear cells (PBMCs), isolated using density gradient centrifugation with Lympholyte-H Cell Separation Media (Cedarlane, ON, Canada), were suspended in RPMI1640 medium (Wako, Tokyo, Japan) containing 10% foetal bovine serum and 1% (v/v) penicillin–streptomycin, and stained with V450-conjugated anti-CD3 antibody for 10 min at 37°C. CD3-stained PBMCs or serum-starved normal human dermal fibroblast cells (1.0 × 105 cells/sample) cultured in serum-free DMEM medium (Merck, Darmstadt, Germany) were pre-incubated with the test compounds at designated concentrations for 10 min at 37°C and treated with recombinant human cytokines for an additional 15 min. The recombinant human cytokines were: IL-2 (30 ng/ml; R&D Systems, Minneapolis, MN, USA); IL-4 (3 ng/ml; R&D Systems); IL-6 (30 ng/ml; R&D Systems); IL-13 (30 ng/ml; R&D Systems) or IFN-α (1000 U/ml; Abcam, Cambridge, UK) for the PBMC assay. IL-6 (10 or 30 ng/ml; R&D Systems) or IFN-α2b (100 ng/ml; Miltenyi Biotec, Bergisch Gladbach, Germany) were used for the fibroblast assay.
After cytokine stimulation, the cells were fixed with Fix buffer I (BD Biosciences) for 10 min at 37°C and incubated with Perm buffer III for 30 min on ice. The cells were then washed and stained with anti-phospho STAT antibodies at 4°C in the dark for 30–90 min. Finally, the cells were washed and resuspended in wash buffer, and kept on ice until flow cytometry analysis.
Cytokine/chemokine production assays
For anti-CD3 antibody/anti-CD28 antibody-stimulated cytokine release assay, PBMC suspensions (100 μl/well, 1 × 105 cells/well) were seeded into anti-CD3 antibody (Thermo Fisher Scientific, Waltham, MA, USA) pre-coated 96-well tissue culture plates and incubated with test compounds for 30 min at 37°C. The cells were then stimulated with anti-CD28 antibody (0.5 μg/ml; Thermo Fisher Scientific) solution and incubated for 24 h at 37°C. For the IL-2–stimulated cytokine release assay, PBMC suspensions (100 μl/well, 1 × 105 cells/well) were pre-incubated with the test compounds for 30 min at 37°C before stimulation with IL-2 (100 ng/ml) and incubation for 72 h at 37°C. Normal human dermal fibroblast cells (100 μl/well, 1 × 104 cells/well) were seeded into flat-bottomed 96-well tissue culture plates 2 days before stimulation with IL-6 (100 ng/ml) or IFN-α (1000 U/ml) for 24 h and drug treatment. Culture supernatants were harvested after incubation, and cytokine concentrations were determined using a BD CBA Flex Set System (BD Biosciences). Cell viability was also measured using a CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, Madison, WI, USA).
Immunohistochemical analysis
Immunohistochemical analysis was performed on formalin-fixed, paraffin-embedded skin sections obtained from 19 patients with SSc and anti-phospho STAT1 (Tyr701) and 3 (Tyr705) antibodies (Cell Signalling Technology, Danvers, MA, USA). Paraffin-embedded HeLa cell pellets (Cell Signalling Technology) were used as negative controls, and IFN-α-treated pellets (Cell Signalling Technology) were used as positive controls. Antibodies labelled with horseradish peroxidase (Agilent Technologies Inc., Santa Clara, CA, USA) were used as the secondary antibody.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 7 software (version 7.03; GraphPad Software, La Jolla, CA, USA). P <0.05 was considered to be significant.
Results
Constitutive STAT phosphorylation in RA and SSc
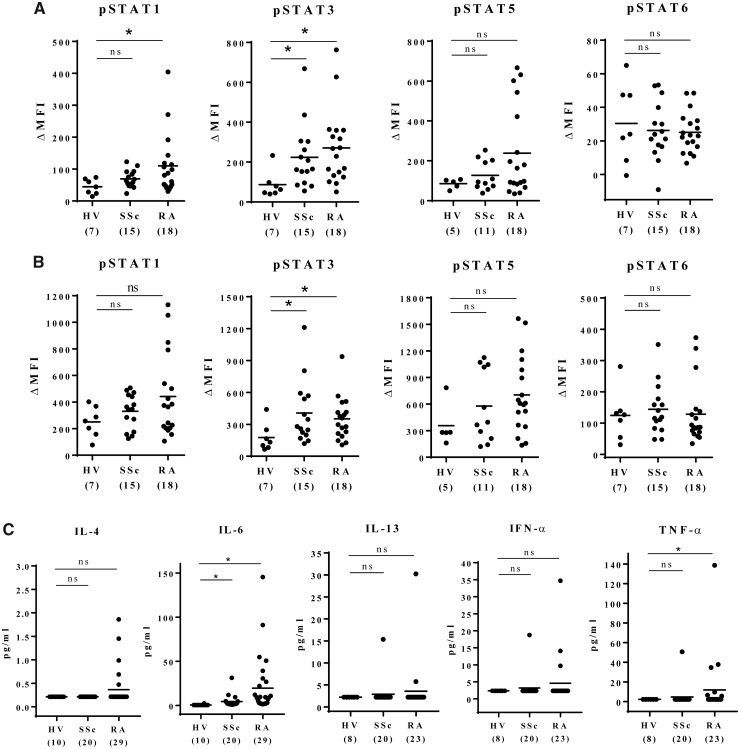
Constitutive phosphorylation of STAT in PBMCs in healthy subjects and patients with RA or SSc is shown in Fig. 1A and B. STAT1 phosphorylation in CD3+ T cells was significantly higher in patients with RA than in healthy subjects, and STAT3 phosphorylation levels in CD3+ T cells and monocytes were significantly higher in both patients with RA and patients with SSc than in healthy subjects. Although relatively high phosphorylated (p)STAT5 levels were seen in both patients with RA and patients with SSc compared with healthy subjects, the differences were not statistically significant. No significant difference was observed in pSTAT6.
Fig. 1.
Constitutive activation of STATs in peripheral blood mononuclear cells from patients with RA and SSc
Basal phosphorylated STATs level in CD3+ T-cells (A) and in monocytes (B). (C) Plasma cytokine levels were assessed by bead-based immunoassays. Samples with cytokine levels that were below the assay’s lower limit of detection were assigned the values of the midpoint between the lower limit of detection and zero. The horizontal bars in the figure indicate the means. Numbers in brackets on the abscissa represent the number of subjects in each group. Statistical analyses were performed using Dunn’s multiple comparisons test (HV vs SSc, HV vs RA). *P < 0.05. HV: healthy control; ns: not significant; MFI: mean fluorescence intensity; STAT: signal transducer and activator of transcription.
In addition, we measured plasma IFN-α, TNF-α and IL-13 levels using a BD Cytometric Bead Array Flex Set System, and IL-4 and IL-6 levels using a BD Cytometric Bead Array Enhanced Sensitivity Flex Set System (Fig. 1C). However, plasma cytokines were below the kit detection limit in most patients and healthy subjects.
Inhibitory effect of JAK inhibitors on cytokine-induced STAT phosphorylation
To compare the inhibitory activity of each JAK inhibitor against JAK/STAT pathway signalling, we assessed cytokine-stimulated STAT phosphorylation. Peficitinib effectively suppressed STAT phosphorylation in all cytokine/JAK/STAT pathways tested in PBMCs from healthy subjects (Table 1A). In PBMCs derived from patients with RA (Table 1B) and patients with SSc (Table 1C), tofacitinib and baricitinib suppressed STAT phosphorylation with IC50 values comparable with or lower than peficitinib in all tested JAK/STAT pathways. None of the three JAK inhibitors showed significant changes in IC50 in blood samples from SSc patients compared with those from RA patients or healthy subjects.
Table 1.
Inhibitory effect of peficitinib on cytokine-stimulated STAT phosphorylation in PBMCs
| A; Healthy subjects | ||||
|---|---|---|---|---|
| Cytokine | Cell | Associated JAKs | Readout | IC50 (nM) [95% CI] (n) |
| Peficitinib | ||||
| IL-2 | T-cell | JAK1, JAK3 | pSTAT5 | 41.4 [20.4, 84.2] (n = 4) |
| IL-4 | T-cell | JAK1, JAK3 | pSTAT6 | 94.1 [56.7, 156.0] (n = 4) |
| Monocyte | 181.3 [89.3, 367.9] (n = 4) | |||
| IL-6 | T-cell | JAK1, JAK2, TYK2 | pSTAT1 | 69.8 [37.4, 130.4] (n = 4) |
| IL-13 | Monocyte | JAK1, JAK2, TYK2 | pSTAT6 | 239.8 [125.9, 456.7] (n = 3) |
| IFN-α | T-cell | JAK1, TYK2 | pSTAT1 | 35.8 [19.1, 67.3] (n = 4) |
| B; Patients with RA | ||||||
|---|---|---|---|---|---|---|
| Cytokine | Cell | Associated JAKs | Readout | IC50 (nM) [95%CI] (n) | ||
| Peficitinib | Tofacitinib | Baricitinib | ||||
| IL-2 | T-cell | JAK1, JAK3 | pSTAT5 | 27.6 [20.9, 36.4] (n = 5) | 8.4, 31.1 (n = 2) | 12.6 (n = 1) |
| IL-4 | T-cell | JAK1, JAK3 | pSTAT6 | 64.2 [37.2, 110.8] (n = 4) | 35.0 [17.5, 69.8] (n = 4) | 38.5 [18.0, 82.6] (n = 4) |
| Monocyte | 127.6 [83.1, 195.9] (n = 4) | 66.7 [27.1, 164.1] (n = 4) | 45.0 [21.5, 94.2] (n = 4) | |||
| IL-6 | T-cell | JAK1, JAK2, TYK2 | pSTAT1 | 62.4 [37.4, 104.0] (n = 5) | 38.9 [20.3, 74.7] (n = 5) | 12.8 [3.8, 43.0] (n = 5) |
| IL-13 | Monocyte | JAK1, JAK2, TYK2 | pSTAT6 | 87.0 [58.0, 130.4] (n = 8) | 44.9 [21.0, 95.8] (n = 3) | 32.1 [14.6, 70.4] (n = 4) |
| IFN-α | T-cell | JAK1, TYK2 | pSTAT1 | 25.5 [19.0, 34.4] (n = 6) | 21.1, 34.3 (n = 2) | 11.6, 25.6 (n = 2) |
| C; Patients with SSc | ||||||
|---|---|---|---|---|---|---|
| Cytokine | Cell | Associated JAKs | Readout | IC50 (nM) [95% CI] (n) | ||
| Peficitinib | Tofacitinib | Baricitinib | ||||
| IL-2 | T-cell | JAK1, JAK3 | pSTAT5 | 58.5 [31.1, 110.0] (n = 3) | 13.8 [8.1, 23.4] (n = 3) | 6.8 [1.6, 28.0] (n = 3) |
| IL-4 | T-cell | JAK1, JAK3 | pSTAT6 | 64.1 [45.4, 90.3] (n = 5) | 26.4 [11.4, 61.3] (n = 3) | 14.2 [11.6, 17.3] (n = 3) |
| Monocyte | 99.3 [60.8, 162.2] (n = 5) | 58.3 [19.4, 174.9] (n = 3) | 18.3 [10.8, 30.8] (n = 3) | |||
| IL-6 | T-cell | JAK1, JAK2, TYK2 | pSTAT1 | 79.9 [52.9, 120.4] (n = 5) | 42.5 [20.5, 88.1] (n = 3) | 12.8 [5.5, 29.9] (n = 3) |
| IL-13 | Monocyte | JAK1, JAK2, TYK2 | pSTAT6 | 127.3, 190.2 (n = 2) | 64.2, 107.2 (n = 2) | 20.9, 30.5 (n = 2) |
| IFN-α | T-cell | JAK1, TYK2 | pSTAT1 | 26.7 [17.4, 41.0] (n = 7) | 16.1 [8.0, 32.5] (n = 4) | 4.6 [3.3, 6.5] (n = 4) |
PBMCs isolated from healthy subjects (A), patients with RA (B) and patients with SSc (C) were stimulated with IL-2, IL-4, IL-6, IL-13 or IFN-α in the presence of JAK inhibitors. Cytokine-mediated STAT phosphorylation was measured by flow cytometry. The IC50 values calculated using Sigmoid–Emax model non-linear regression analysis are shown with 95% CIs. IC50, geometric mean of concentrations from different donors that provoke a response halfway between the unstimulated baseline and maximum induction by cytokine stimulation. Each value was shown for the case of n ≤2.
JAK: Janus kinase; PBMCs: peripheral blood mononuclear cells; TYK2: tyrosine kinase-2.
Inhibitory effect of peficitinib on cytokine production
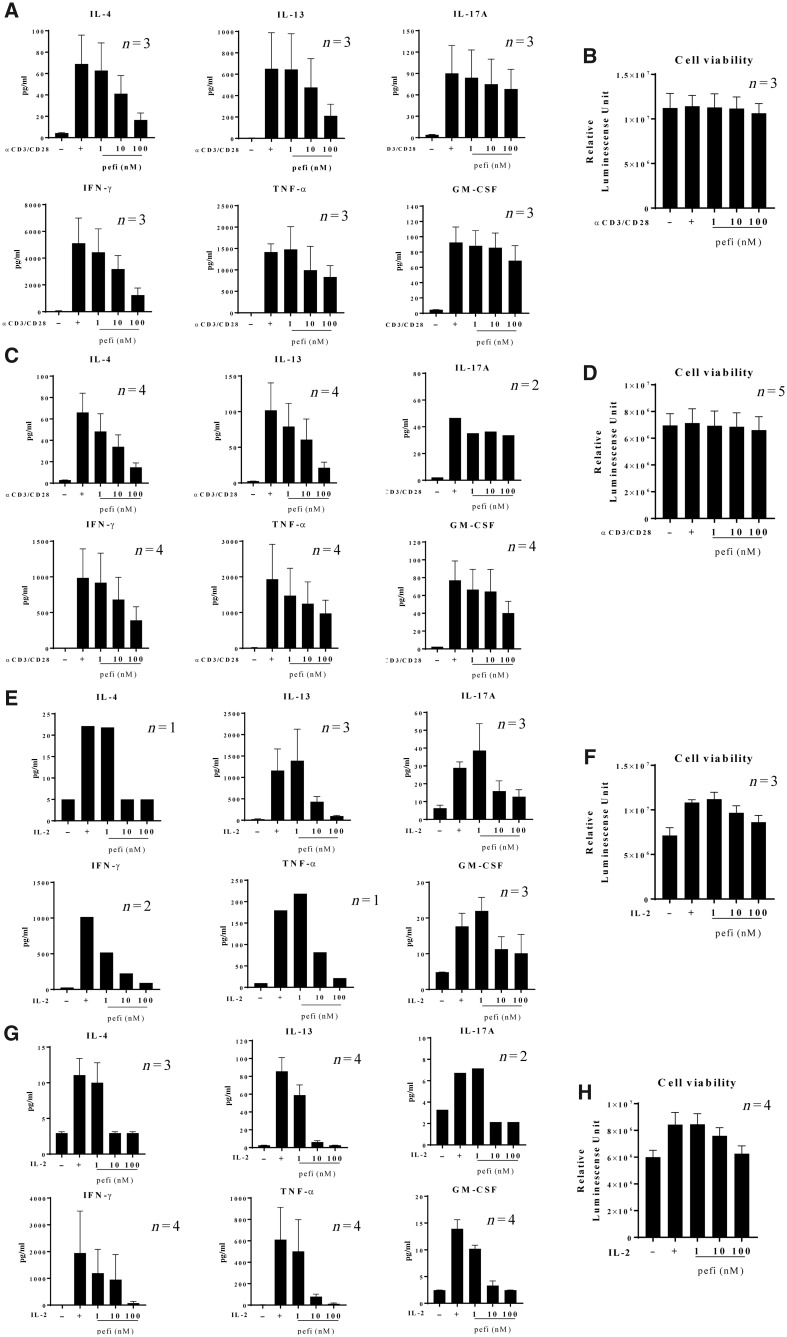
Fig. 2 shows the suppressive effect of peficitinib on cytokine production induced by TCR stimulation with anti-CD3/anti-CD28 antibody (Fig. 2A and C) or IL-2 stimulation (Fig. 2E and G). TCR or IL-2 stimulation significantly induced IL-4, IL-13, IL-17A, IFN-γ, TNF-α and GM-CSF. Peficitinib suppressed IL-4, IL-13, IFN-γ and TNF-α production in a dose-related manner without affecting cell viability (Fig. 2B, D, F and H). Peficitinib had no clear suppressive effect on IL-17A or GM-CSF production as a result of TCR-stimulation, but almost completely suppressed production of these cytokines as a result of IL-2 stimulation. Peficitinib treatment had no effect on cell viability.
Fig. 2.
Suppressive activity of peficitinib on proinflammatory cytokine production by peripheral blood mononuclear cells
Peripheral blood mononuclear cells isolated from patients with RA (A, B, E, F) or SSc (C, D, G, H) were stimulated with anti-CD3/anti-CD28 antibodies for 24 h (A–D) or recombinant human IL-2 for 72 h (E–H) in the presence of the indicated concentration of peficitinib. Proinflammatory cytokine production in the culture supernatants were assessed by bead-based immunoassays. Cell viability was assessed with CellTiter-Glo (B, D, F, H). Data represents mean or mean with S.E.M. of three to five independent experiments using different donors. pefi: peficitinib.
STAT phosphorylation in skin sections from patients with SSc
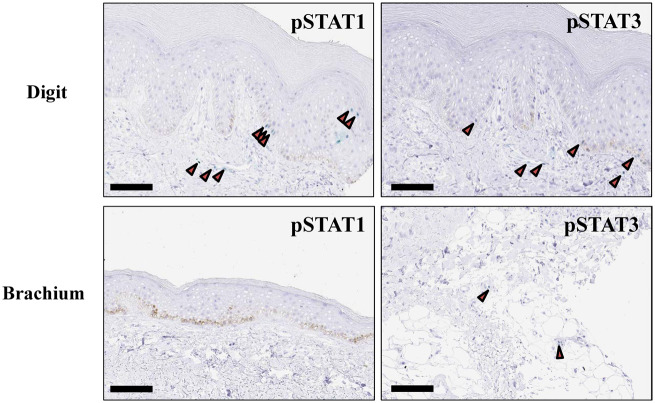
To investigate the activation status of the JAK/STAT pathway in topical skin lesion sites of SSc, we performed immunohistochemical analysis on skin sections from 19 patients with SSc. The clinical characteristics of these patients are detailed in Table 2. Ten patients with lcSSc and nine with dcSSc were evaluated; in each patient, two skin sections from digit and brachium were stained with anti pSTAT1 or pSTAT3 antibody (Table 2). No pSTAT1-positive cell was observed in skin sections from the group with limited disease, while pSTAT1-positive keratinocytes and endothelial cells were detected in three of nine skin sections from those with diffuse disease. Interestingly, these three pSTAT1-positive sections were derived from untreated patients with short disease duration. In contrast, pSTAT3-positive cells were detected regardless of disease type and patient background. Representative immunohistochemistry images are shown in Fig. 3.
Table 2.
Baseline characteristics and pSTAT1 and pSTAT3 activation in skin sections from patients with SSc
| pSTAT1 score | pSTAT3 score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KCs/ECs and fibroblasts | KCs/ECs and fibroblasts | ||||||||||||
| Sex | Age | Duration (month) | Treatment | Disease type | m-Rodnan TSS score | Anti- Scl-70 | Anti- centromere | Anti-RNA polymerase III | Digit | Brachium | Digit | Brachium | |
| SSc-1 | F | 76 | 36 | None | Limited | 9 | 1 | 191 | 0 | 0/0 | 0/0 | 1/1 | 0/1 |
| SSc-2 | F | 70 | 48 | None | Limited | 4 | 1 | 135 | 0 | 0/0 | 0/0 | 0/1 | 0/1 |
| SSc-3 | M | 71 | 4 | None | Limited | 2 | 1 | 5 | 0 | 0/0 | 0/0 | 1/1 | 1/1 |
| SSc-4 | F | 80 | 240 | None | Limited | 0 | 1 | 81.9 | 5 | 0/0 | 0/0 | 0/1 | 0/1 |
| SSc-5 | F | 76 | 36 | None | Limited | 2 | 13 | 165 | <5.0 | 0/0 | 0/0 | 0/0 | 0/0 |
| SSc-6 | F | 87 | 2 | None | Limited | 10 | 1.9 | 142(+) | <5.0 | 0/0 | 0/0 | 0/1 | 0/1 |
| SSc-7 | F | 75 | 156 | None | Limited | 4 | <1.0 | 108 | 63.8 | 0/0 | 0/0 | 0/1 | 0/0 |
| SSc-8 | F | 57 | 348 | None | Limited | 20 | <1.0 | 164 | 0 | 0/0 | 0/0 | 0/1 | 0/1 |
| SSc-9 | M | 68 | 2 | None | Limited | 2 | 1 | 145 | <5.0 | 0/0 | 0/0 | 0/0 | 0/0 |
| SSc-10 | F | 72 | 11 | None | Limited | 4 | 10.9 | 141 | <5.0 | 0/0 | 0/0 | 0/0 | 0/1 |
| SSc-11 | F | 81 | 48 | i.v. CYC, TAC | Diffuse | 15 | ≧850 | <5.0(-) | <5.0 | 0/0 | 0/0 | 0/1 | 0/1 |
| SSc-12 | F | 66 | 3 | None | Diffuse | 8 | >850 | 168 | 10.9 | 1/1 | 0/0 | 1/1 | 0/1 |
| SSc-13 | F | 66 | 2 | None | Diffuse | 3 | 631 | <5.0 | <5.0 | 0/1 | 0/0 | 0/1 | 0/1 |
| SSc-14 | F | 37 | 36 | None | Diffuse | 2 | 419 | 5 | 5 | 0/1 | 0/0 | 0/0 | 0/0 |
| SSc-15 | F | 34 | 131 | TAC | Diffuse | 30 | 461 | 40.4 | <5.0 | 0/0 | 0/0 | 0/0 | 0/1 |
| SSc-16 | F | 72 | 240 | i.v. CYC | Diffuse | 12 | 699 | <5.0 | <5.0 | 0/0 | 0/0 | 0/1 | 0/0 |
| SSc-17 | F | 83 | 25 | TAC, Amb | Diffuse | 26 | 0 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/1 |
| SSc-18 | F | 79 | 288 | TAC | Diffuse | 24 | <1.0 | 153 | <5.0 | 0/0 | 0/0 | 0/1 | 0/1 |
| SSc-19 | F | 59 | 6 | TAC | Diffuse | 18 | <1.0 | <5.0 | 9.3 | 0/0 | 0/0 | 0/0 | 0/1 |
Skin sections were stained with anti pSTAT1 or pSTAT3 antibody. Semiquantitative scoring of the staining was performed as follows: 0: no visible staining, 1: mild staining or staining in very few cells, 2: moderate staining, 3: very intense staining in the majority of cells.
Amb: Ambrisentan; anti Scl-70: anti-scleroderma-70 antibody; ECs: endothelial cells; KCs: skin keratinocytes; m-Rodnan TSS score: modified Rodnan total skin thickness score; TAC: tacrolimus.
Fig. 3.
Phosphorylated STAT1 and phosphorylated STAT3 expression in SSc skin biopsies
Representative immunohistochemical images of digit and brachium biopsies from SSc patient (SSc-12). Scale bar = 100 μm. STAT: signal transducer and activator of transcription.
Inhibitory effect of peficitinib on dermal fibroblast function
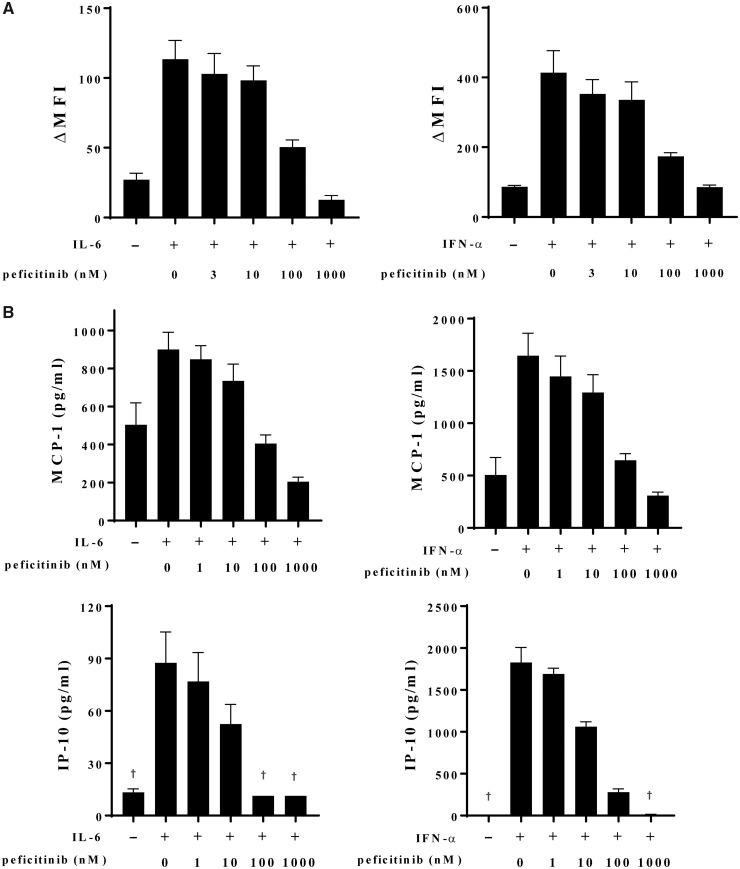
Finally, the effects of peficitinib on the expression of pSTAT1 or pSTAT3 and the production of chemokines from normal human dermal fibroblasts stimulated with IL-6 or IFN-α were evaluated. Peficitinib suppressed STAT1 and STAT3 phosphorylation induced by IL-6 or IFN-α in a dose-related manner (Fig. 4A). Monocyte chemoattractant protein-1 and IFN-γ-induced protein-10 production induced by IL-6 or IFN-α were also suppressed by peficitinib in a dose-related manner (Fig. 4B).
Fig. 4.
Peficitinib inhibition of cytokine-induced STATs phosphorylation and chemokine production in skin fibroblasts
(A) Normal human dermal fibroblasts (NHDFs) were stimulated with IL-6 or IFN-α in the presence of indicated concentrations of peficitinib, and STAT phosphorylation was assessed by flow cytometry. Data are expressed as the mean values with S.E.M. of three independent experiments using fibroblasts from different lots. (B) Chemokine productions from IL-6- or IFN-α-stimulated NHDFs were measured by bead-based immunoassays. Data are expressed as the mean values with S.E.M. of three independent experiments using fibroblasts from a single lot. †The data include values below the detection limit. MFI, mean fluorescence intensity; MCP-1: monocyte chemoattractant protein-1; IP-10: IFN-γ–induced protein-10; STAT: signal transducer and activator of transcription.
Discussion
The efficacy of JAK inhibitors in RA has encouraged their development for other systemic autoimmune disorders, including psoriasis, ulcerative colitis and SLE [19]. These autoimmune diseases share multiple symptoms, and genetic and environmental risk factors, and can often develop concurrently in an individual patient. Patients with multiple autoimmune diseases have been frequently reported as having polyautoimmunity, which supports the presence of a common pathological pathway for these diseases [26]. Therefore, exploring the potential of a drug approved in one autoimmune disease for the treatment of other autoimmune disorders can be considered a feasible study approach. Indeed, several studies [27, 28] have revealed the prevalence of polyautoimmunity in patients with SSc, and meta-analysis of genomic studies has shown the JAK/STAT pathway to be one of the potential common target pathways for the treatment of RA and SSc [29]. Recent clinical evidence also suggests that JAK inhibition might be useful in dermatological autoimmune disease [30–32]. SSc is an autoimmune disease characterized by dermatological manifestation resulting from immune dysregulation, vasculopathy and fibrosis, but to date little attention has been paid to the potential efficacy of JAK inhibitors in this indication. Therefore, in this study, we investigated the in vitro pharmacological profile of peficitinib, a novel JAK inhibitor, using peripheral blood from patients with autoimmune disease, and explored the therapeutic potential for peficitinib in treating SSc. First, we compared basal STAT phosphorylation levels in circulating PBMCs from patients with RA, patients with SSc, and healthy subjects. Although the low number of samples for flow cytometry should be considered as a limitation of our study, constitutive STAT1 activation was found in CD3+ T cells in patients with RA, with significantly higher STAT3 phosphorylation levels being observed in both patients with RA and patients with SSc compared with healthy individuals. As the phosphorylation of STAT in PBMCs could be triggered by circulating cytokines, which could be crucial for the pathogenesis of RA and SSc, we measured plasma concentrations to confirm that the higher baseline STAT1 and STAT3 levels resulted from higher circulating levels of plasma cytokines. Although we found no correlation between individual plasma cytokine concentrations and STAT phosphorylation levels (data not shown), our results suggest that combinations of circulating inflammatory cytokines could have caused constitutive STAT phosphorylation and activated the PBMCs, thus inducing systemic inflammatory disease.
We also examined the effect of JAK inhibitors on cytokine-induced STAT phosphorylation in PBMCs from healthy subjects, and patients with RA and SSc. As each cytokine utilizes a specific combination of JAKs and STATs for the transduction of downstream signals [33], it is assumed that JAK inhibitors with different selectivity for different JAKs may exhibit varying inhibitory profiles for cytokine downstream signalling. However, unexpectedly, all three JAK inhibitors effectively suppressed the STAT phosphorylation induced by IL-2, IL-4, IL-6, IL-13 and IFN-α with almost comparable potency in our cell-based pSTAT inhibition assays. As ∼60 cytokines utilize JAK/STAT pathway signalling [19], further data are needed to elucidate whether JAK selectivity leads to selective regulation of cellular functions. Another important finding was that IC50 values for peficitinib in RA were comparable with those in SSc, suggesting that the clinical dosage of peficitinib for SSc will be similar to RA. This is also observed for tocilizumab, for which the clinically effective dose for SSc is the same as that for RA (162 mg administered by weekly s.c. injection) [5, 34].
In SSc, immunological activation is crucial for the induction of subsequent angiopathy and tissue fibrosis. Infiltrated T cells in skin lesions showed an active phenotype even in pre-fibrotic state [35]. IL-4 and IL-13 are considered to be the fibrogenic cytokines produced by these infiltrated T cells, and many in vitro and in vivo studies support the idea that these cytokines promote fibrosis by inducing the differentiation of fibroblasts into myofibroblasts and stimulating extracellular matrix production [36]. Th1 cytokines including IFN-γ and TNF-α are also considered to participate in the perpetuation of inflammation by inducing IL-6 release from primary cultured SSc fibroblasts [37]. These observations are consistent with the higher serum level of these cytokines in patients with SSc and the positive correlation with disease progression [38, 39]. Tofacitinib and baricitinib inhibit human T-cell cytokine production and lymphocyte function [40, 41]; we therefore also investigated the pharmacological activity of peficitinib on cytokine production by PBMCs. In addition, as TCR engagement also induces JAK/STAT activation and contributes to initial T-cell priming and cytokine and activation marker expression, which enables T cells to respond to various cytokines [42, 43], we examined cytokine production both in the priming phase of T-cell activation (by 24-h TCR engagement) and the growing phase (by IL-2 stimulation for 72 h). Peficitinib effectively suppressed IL-4, IL-13, IFN-γ and TNF-α production induced by TCR stimulation without affecting cell viability, but no clear suppressive effects on IL-17A and GM-CSF production were observed; all cytokines tested (including IL-17A and GM-CSF) induced by IL-2 stimulation were suppressed by peficitinib. Taken together, these results suggest that peficitinib is a potent JAK inhibitor that can broadly inhibit cytokine signalling.
We also explored the involvement of the JAK/STAT pathway in skin topical lesions of SSc. Notably, pSTAT1-positive cells were detected in sections derived from three SSc treatment-naïve patients with diffuse disease of short duration. Although responsible cytokines remain unclear, this may indicate that aggressive inflammation is dominant during the early disease phase. Indeed, IL-6 is a well-known predictor of early disease stage, mortality risk and worse skin involvement [44], but the contribution of IL-6 in SSc declines with disease progression when other cytokines, such as IL-4, IL-13 or TGF-β, have a greater role [45–47]. Another potential STAT1 activator is type I IFN, and although there is little evidence of type I IFN commitment in SSc pathology, immune complexes can induce IFN-α production; indeed, a high IFN signature has been reported in peripheral blood cells from patients with early SSc, as well as SLE and RA [48]. In inflammatory conditions, this cytokine network may induce fibroblast activation, leading to the production of various chemokines, such as monocyte chemoattractant protein-1 and IFN-γ-induced protein-10 [49]. Aberrant chemokine production by fibroblasts attracts lymphocytes, and as infiltrated lymphocytes in turn release pro-inflammatory cytokines, an inflammatory loop may ensue [50]. Thus, JAK inhibitors are expected to show at least comparable efficacy to IL-6 signalling blockade, and may potentially exert greater efficacy than tocilizumab by inhibiting a broad array of cytokine signalling.
In summary, our results suggest that JAK/STAT pathways are constitutively activated in both RA and SSc, and that JAK inhibitors broadly suppress JAK/STAT pathway signals, resulting in downstream direct and indirect inhibition of cytokine production and fibroblast function. Although a single cytokine targeting strategy is highly efficient in the treatment of RA, SSc is a connective tissue disease in which a complex of autoimmune, inflammation and fibrosis processes leads to irreversible organ damage, and many cytokines participate in disease progression. Therefore, JAK inhibition may have potential in the treatment of autoimmune diseases including SSc.
Acknowledgements
The authors would like to thank Takashi Emori for helpful suggestions during manuscript drafting. We would also like to thank Masashi Maeda, Kaori Hanaoka and Yuka Kawato for carefully checking the quality of the data. Editorial support was provided by Cello Health MedErgy.
Funding: This work was supported by Astellas Pharma, Inc. Editorial support was provided by Julia Donnelly, PhD, for Cello Health MedErgy (Europe) and funded by Astellas Pharma, Inc.
Disclosure statement: Y.K., E.I., H.F. and Y.F. are employees of Astellas Pharma, Inc. Y.N. is currently an employee of LSI Medience Corporation. S.K. received personal fees from Bristol-Myers, Pfizer, Eli Lilly and Takeda for work other than the submitted work. S.N. received personal fees from Bristol-Myers, Sanofi, AbbVie, Eisai, Eli Lilly, Chugai, Asahi Kasei and Pfizer, and grants from Mitsubishi Tanabe, Takeda, Novartis and MSD for work other than the submitted work. Y.T. received personal fees from Astellas Pharma, Inc., Eli Lilly, Sanofi, Pfizer, YL Biologics, GlaxoSmithKline, UCB, Novartis, Janssen and Asahi Kasei, grants from Ono, MSD and Taisho–Toyama, and grants and personal fees from Daiichi-Sankyo, Chugai, AbbVie, Bristol-Myers, Mitsubishi Tanabe, Eisai and Takeda for work other than the submitted work.
References
- 1. Wollheim FA. Classification of systemic sclerosis. Visions and reality. Rheumatology 2005;44:1212–16. [DOI] [PubMed] [Google Scholar]
- 2. Poudel DR, Derk CT.. Mortality and survival in systemic sclerosis: a review of recent literature. Curr Opin Rheumatol 2018;30:588–93. [DOI] [PubMed] [Google Scholar]
- 3. Cutolo M, Soldano S, Smith V.. Pathophysiology of systemic sclerosis: current understanding and new insights. Expert Rev Clin Immunol 2019;15:753–64. [DOI] [PubMed] [Google Scholar]
- 4. Muangchant C, Pope JE.. The significance of interleukin-6 and C-reactive protein in systemic sclerosis: a systematic literature review. Clin Exp Rheumatol 2013;31:122–34. [PubMed] [Google Scholar]
- 5. Khanna D, Denton CP, Jahreis A. et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 2016;387:2630–40. [DOI] [PubMed] [Google Scholar]
- 6. Khanna D, Denton CP, Lin CJF. et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann Rheum Dis 2018;77:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furue M, Mitoma C, Mitoma H. et al. Pathogenesis of systemic sclerosis–current concept and emerging treatments. Immunol Res 2017;65:790–7. [DOI] [PubMed] [Google Scholar]
- 8. Rein P, Mueller RB.. Treatment with biologicals in rheumatoid arthritis: an overview. Rheumatol Ther 2017;4:247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smolen JS, Landewe R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka Y. Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. J Biochem 2015;158:173–9. [DOI] [PubMed] [Google Scholar]
- 11. O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A.. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 2013;72(Suppl 2):ii111–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka Y. The JAK inhibitors: do they bring a paradigm shift for the management of rheumatic diseases? Rheumatology 2019;58:i1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakayamada S, Kubo S, Iwata S, Tanaka Y.. Chemical JAK inhibitors for the treatment of rheumatoid arthritis. Expert Opin Pharmacother 2016;17:2215–25. [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi T, Tanaka Y, Tanaka S. et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis 2019;78:1305–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanaka Y, Takeuchi T, Tanaka S. et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3). Ann Rheum Dis 2019;78:1320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choy EH. Clinical significance of Janus kinase inhibitor selectivity. Rheumatology 2019;58:953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vainchenker W, Leroy E, Gilles L. et al. JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Res 2018;7:82.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhattacharyya S, Wang W, Wei J, Varga J.. Pharmacological inhibition of JAK/STAT signaling by tofacitinib prevents experimental organ fibrosis: novel therapy for systemic sclerosis [abstract]. Arthritis Rheumatol 2018;70 (suppl 10) https://acrabstracts.org/abstract/pharmacological-inhibition-of-jak-stat-signaling-by-tofacitinib-prevents-experimental-organ-fibrosis-novel-therapy-for-systemic-sclerosis/ (18 October 2019, date last accessed). [Google Scholar]
- 19. T Virtanen A, Haikarainen T, Raivola J, Silvennoinen O.. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs 2019;33:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamaguchi H, Amano Y, Moritomo A. et al. Discovery and structural characterization of peficitinib (ASP015K) as a novel and potent JAK inhibitor. Bioorg Med Chem 2018;26:4971–83. [DOI] [PubMed] [Google Scholar]
- 21. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 22. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 23. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 25. Isomäki P, Junttila I, Vidqvist KL, Korpela M, Silvennoinen O.. The activity of JAK-STAT pathways in rheumatoid arthritis: constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology 2015;54:1103–13. [DOI] [PubMed] [Google Scholar]
- 26. Matusiewicz A, Stróżyńska‐Byrska J, Olesińska M.. Polyautoimmunity in rheumatological conditions. Int J Rheum Dis 2019;22:386–91. [DOI] [PubMed] [Google Scholar]
- 27. Elhai M, Avouac J, Kahan A, Allanore Y.. Systemic sclerosis at the crossroad of polyautoimmunity. Autoimmun Rev 2013;12:1052–7. [DOI] [PubMed] [Google Scholar]
- 28. Hudson M, Rojas-Villarraga A, Coral-Alvarado P. et al. Polyautoimmunity and familial autoimmunity in systemic sclerosis. J Autoimmun 2008;31:156–9. [DOI] [PubMed] [Google Scholar]
- 29. Márquez A, Kerick M, Zhernakova A. et al. Meta-analysis of Immunochip data of four autoimmune diseases reveals novel single-disease and cross-phenotype associations. Genome Med 2018;10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Welsch K, Holstein J, Laurence A, Ghoreschi K.. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol 2017;47:1096–107. [DOI] [PubMed] [Google Scholar]
- 31. Hosking AM, Juhasz M, Mesinkovska NA.. Topical Janus kinase inhibitors: a review of applications in dermatology. J Am Acad Dermatol 2018;79:535–44. [DOI] [PubMed] [Google Scholar]
- 32. Shreberk-Hassidim R, Ramot Y, Zlotogorski A.. Janus kinase inhibitors in dermatology: a systematic review. J Am Acad Dermatol 2017;76:745–53.e19. [DOI] [PubMed] [Google Scholar]
- 33. Seif F, Khoshmirsafa M, Aazami H. et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal 2017;15:23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitchell E, Jones G.. Subcutaneous tocilizumab for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol 2016;12:103–14. [DOI] [PubMed] [Google Scholar]
- 35. Kalogerou A, Gelou E, Mountantonakis S. et al. Early T cell activation in the skin from patients with systemic sclerosis. Ann Rheum Dis 2005;64:1233–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCormick SM, Heller NM.. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015;75:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antonelli A, Fallahi P, Ferrari SM. et al. Systemic sclerosis fibroblasts show specific alterations of interferon-γ and tumor necrosis factor-α–induced modulation of interleukin 6 and chemokine ligand 2. J Rheumatol 2012;39:979–85. [DOI] [PubMed] [Google Scholar]
- 38. Hasegawa M, Fujimoto M, Kikuchi K, Takehara K.. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol 1997;24:328–32. [PubMed] [Google Scholar]
- 39. Bălănescu P, Lădaru A, Bălănescu E. et al. IL-17, IL-6 and IFN-gamma in systemic sclerosis patients. Rom J Intern Med 2015;53:44–9. [DOI] [PubMed] [Google Scholar]
- 40. Migita K, Miyashita T, Izumi Y. et al. Inhibitory effects of the JAK inhibitor CP690, 550 on human CD4+ T lymphocyte cytokine production. BMC Immunol 2011;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kubo S, Nakayamada S, Sakata K. et al. Janus kinase inhibitor baricitinib modulates human innate and adaptive immune system. Front Immunol 2018;9:1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tomita K, Saijo K, Yamasaki S. et al. Cytokine-independent Jak3 activation upon T cell receptor (TCR) stimulation through direct association of Jak3 and the TCR complex. J Biol Chem 2001;276:25378–85. [DOI] [PubMed] [Google Scholar]
- 43. Saemann MD, Zeyda M, Diakos C, Szekeres A. et al. Suppression of early T-cell-receptor-triggered cellular activation by the Janus kinase 3 inhibitor WHI-P-154. Transplantation 2003;75:1864–72. [DOI] [PubMed] [Google Scholar]
- 44. Desallais L, Avouac J, Frechet M. et al. Targeting IL-6 by both passive or active immunization strategies prevents bleomycin-induced skin fibrosis. Arthritis Res Ther 2014;16:R157.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shima Y. The benefits and prospects of interleukin-6 inhibitor on systemic sclerosis. Mod Rheumatol 2019;29:294–301. [DOI] [PubMed] [Google Scholar]
- 46. Denton CP, Ong VH, Xu S. et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann Rheum Dis 2018;77:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Assassi S, Mayes MD.. What does global gene expression profiling tell us about the pathogenesis of systemic sclerosis? Curr Opin Rheumatol 2013;25:686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chizzolini C, Brembilla NC, Montanari E, Truchetet ME.. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev 2011;10:276–81. [DOI] [PubMed] [Google Scholar]
- 49. Hasegawa M, Fujimoto M, Matsushita T. et al. Serum chemokine and cytokine levels as indicators of disease activity in patients with systemic sclerosis. Clin Rheumatol 2011;30:231–7. [DOI] [PubMed] [Google Scholar]
- 50. King J, Abraham D, Stratton R.. Chemokines in systemic sclerosis. Immunol Lett 2018;195:68–75. [DOI] [PubMed] [Google Scholar]