Rheumatology key message
Burosumab ought to be considered in the management of iron-induced FGF23-mediated hypophosphataemic osteomalacia.
Dear Editor, Refractory iron-induced fibroblast growth factor 23 (FGF23) mediated hypophosphataemic osteomalacia is an uncommon complication of parenteral iron therapy. Treatment thus far in case reports has consisted of iron cessation and phosphate substitution [1–4]. To our knowledge, we describe the first reported use of burosumab therapy for this condition.
We present a 32-year-old man with severe Crohn’s disease and iron-deficiency anaemia. Computed tomography angiography revealed focal ileocolic venous portal hypertension with recurrent lower intestinal blood loss. A transjugular intrahepatic portosystemic shunt was not considered feasible and regular infusions of 250 mg ferric carboxymaltose fortnightly were instituted over oral therapy, given the extensive gastrointestinal disease.
After one year, the patient developed severe foot and leg pain and was diagnosed with multiple insufficiency metatarsal and tarsal fractures. Laboratory workup revealed profound hypophosphataemia (0.38 mmol/l), elevated FGF23 (320 RU/ml) and no pathogenic variants in PHEX, ENPP1, SLC34A3, DMP1 or KLOTHO. He was started on Sandoz phosphate, alfacalcidol and was switched from ferric carboxymaltose to iron isomaltoside. There was no appreciable clinical benefit and tolerability to oral phosphate supplementation was poor, owing to an increase in diarrhoea.
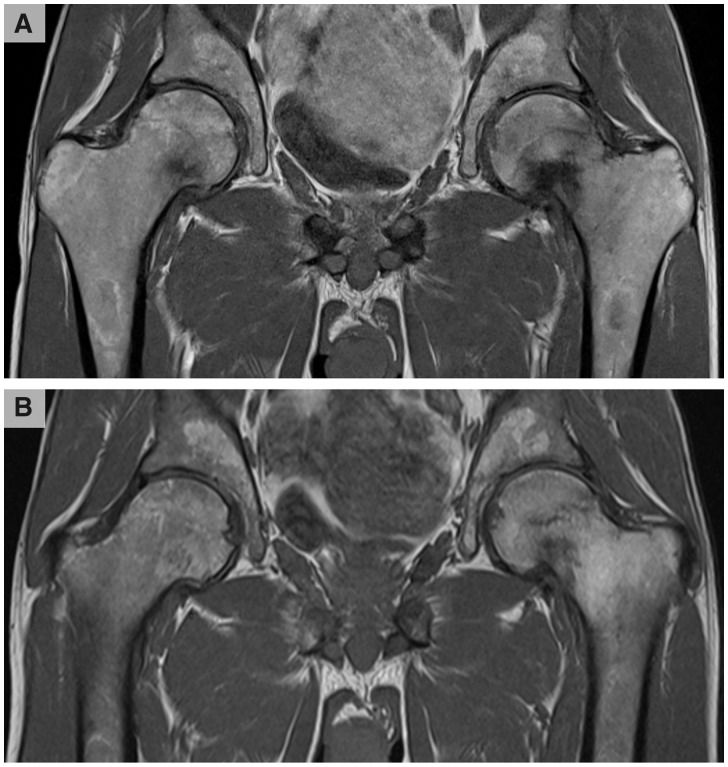
One year later, he developed progressive severe left hip pain after minor trauma. MRI demonstrated a femoral pseudofracture. He was switched to central intravenous phosphate replacement for ten hours a day, five days a week with partial weight bearing. Follow-up MRI three months later demonstrated a complete left femoral fracture, new right femoral pseudofracture and multiple pelvic fractures (Fig. 1A).
Fig. 1.
T1-weighted MRI of the patient’s pelvis prior to and after three doses of burosumab (0.3 mg/kg)
(A) demonstrates a complete left femoral intramedullary subcapital fracture, right femoral pseudofracture and multiple pelvic fractures. (B) shows complete resolution of the right femoral neck pseudofracture and near complete healing of the left femoral fracture and pelvic fractures.
Given the progression of his bone disease and likely need for bilateral hip arthroplasty, the patient was started on burosumab (0.3 mg/kg) subcutaneously every four weeks. After the first dose, he reported a significant resolution of symptoms mirrored with improvements in laboratory measures. Serum phosphate levels improved from 0.38 mmol/l to 1 mmol/l and alkaline phosphatase levels reduced from 218 IU/l to 175 IU/l. Furthermore, subsequent MRI showed complete resolution of the right femoral head fracture and near complete healing of the left femoral and pelvic fractures (Fig. 1B).
The mechanism of iron-induced FGF23 synthesis is incompletely understood, with increases in FGF23 from both iron treatment and iron deficiency [5]. High levels of FGF23 cause diminished bone mineralisation by reducing renal 1α-hydroxylase activity and renal tubular phosphate reabsorption. Additionally, ferric carboxymaltose-induced FGF23 elevation has been suggested to cause secondary hyperparathyroidism and calcitriol deficiency, which subsequently further adds to the hypophosphataemia [6]. Burosumab is a human recombinant monoclonal antibody that binds FGF23 and is licensed for paediatric X-linked hypophosphataemia [7].
We report the first documented use of burosumab in refractory iron-induced FGF23-mediated osteomalacia with successful outcomes including avoidance of costly orthopaedic surgery. This case further highlights the wider clinical advantages of burosumab in other FGF23-mediated diseases.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript. M.K.J. is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Disclosure statement: M.K.J. has received funding for investigator-initiated grants from Kyowa Hakko Kirin and, as a clinical centre, for the Axles trial in addition to compensation for speaker fees and consultancy for advisory board membership.
References
- 1. Schouten BJ, Doogue MP, Soule SG, Hunt PJ.. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem 2009;46:167–9. [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto S, Okada Y, Mori H, Fukumoto S, Tanaka Y.. Fibroblast growth factor 23-related osteomalacia caused by the prolonged administration of saccharated ferric oxide. Intern Med 2012;51:2375–8. [DOI] [PubMed] [Google Scholar]
- 3. Shimizu Y, Tada Y, Yamauchi M. et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone 2009;45:814–6. [DOI] [PubMed] [Google Scholar]
- 4. Klein K, Asaad S, Econs M, Rubin JE.. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep 2018;2018:bcr-2017-222851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanudel MR, Laster M, Salusky IB.. Non-renal-related mechanisms of FGF23 pathophysiology. Curr Osteoporos Rep 2018;16:724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolf M, Chertow GM, Macdougall IC. et al. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight 2018;3:124486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Insogna KL, Briot K, Imel EA. et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with x-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res 2018;33:1383–93. [DOI] [PubMed] [Google Scholar]