Abstract
Objectives
The effectiveness of TNF inhibitors in RA has been shown to be affected by obesity. No such effect has been found for abatacept and rituximab, while for tocilizumab results are ambiguous. Additionally, it remains unresolved whether sex is an effect modifier for obesity. We investigated the impact of obesity on the drug effectiveness of conventional synthetic or biologic DMARDs, taking into account potential sex-specific differences.
Methods
Data from 10 593 RA patients included in the German observational cohort study Rheumatoid Arthritis: oBservation of BIologic Therapy (RABBIT) since 2009 were analysed. Patients had to have a BMI ≥18.5 kg/m2, at least one follow-up and 6 months of observation time. The influence of obesity on drug effectiveness was investigated by regression analysis, adjusting for potential confounders.
Results
Obesity had a negative impact on improvement in the DAS with 28 joints using ESR as an inflammation marker of –0.15 (95% CI: –0.26; –0.04) units for women receiving conventional synthetic DMARDs, –0.22 (95% CI: –0.31; –0.12) units for women receiving TNF inhibitors, –0.22 (95% CI: –0.42; –0.03) units for women receiving tocilizumab and –0.41 (95% CI: –0.74; –0.07) units for men receiving tocilizumab. Overall, no negative obesity effects on the effectiveness of rituximab and abatacept were found.
Conclusion
Obesity has a negative impact on the effectiveness of cytokine-targeted but not cell-targeted therapies in daily practice, affecting more outcomes and therapies in women than in men. Overall, no effects of obesity on treatment effectiveness were found for rituximab and abatacept.
Keywords: rheumatoid arthritis, obesity, sex, drug dosage
Rheumatology key messages
Obesity reduces the real-world effectiveness of the cytokine-targeted therapies, TNF inhibitors and tocilizumab.
Overall, no obesity effects were found for cell-targeted therapies rituximab and abatacept.
In women, obesity affects numerous measures of effectiveness, while in men only a few.
Introduction
The prevalence of obesity has been increasing globally for decades [1]. Obesity is currently viewed as a mild chronic inflammatory disease and has been shown to be a risk factor for developing RA [2]. White adipose tissue produces cytokines such as TNF and IL-6 [3], which have pro-inflammatory activity and are involved in RA, e.g. via T- and B-cell recruitment and activation [4]. These cytokines and cell populations are targets of conventional synthetic (cs) or biologic (b) DMARDs, thus it has increasingly become of interest to elucidate how obesity influences the response to specific therapies.
Obesity makes it more difficult for RA patients to reduce overall disease activity, achieve remission or regain good physical abilities, in both the short and the long term [5–10], and the effect differs between DMARD modes of action [11]. At the same time, a protective effect of higher levels of BMI regarding radiographic joint damage has been found [10, 12, 13]. CRP levels have been reported to be positively associated with BMI in female RA patients, but not in males [14]. Male obese patients, contrary to the general population, have even been reported to present lower CRP levels than non-obese patients [15].
While for csDMARDs, a negative influence of obesity on disease activity and physical function has been only occasionally reported [16, 17], more evidence exists for patients receiving TNF inhibitors (TNFi). In particular, obesity significantly decreases the likelihood of remission with TNFi therapy, and the likelihood of improvement in the DAS with 28 joints using ESR as an inflammation marker (DAS28-ESR) or one of its components within 6 or 12 months after the initiation of treatment [11,18–21]. Despite these results, there is still not sufficient evidence to conclude that obesity affects the response to a specific class of medication [22].
For abatacept (ABA) [23–26] as well as rituximab (RTX) [27], no influence of obesity on any relevant measure of therapy effectiveness has been determined thus far. For tocilizumab (TOC), the picture is less clear. Several studies did not find an influence of baseline BMI on the effectiveness of TOC [28–30]. On the other hand, a potential influence of patients’ weight is suggested by other studies [31, 32]. However, one of the studies only considered a dosage of 162 mg s.c. every other week, suggesting an underdosing for obese patients [32]. This limitation may also have been present in other studies that neglected the issue of underdosing substances in obese patients.
Past studies have confirmed that the influence BMI exerts over RA disease activity may differ between the sexes. The association between obesity and a higher disease activity has been reported for women rather than for men, both in general terms [33] and particularly regarding inflammation markers CRP and ESR [5].
To the best of our knowledge, however, no study has yet investigated the combined influence of obesity, therapy and sex on the effectiveness of commonly used DMARDs in a systematic way, taking into account the potential influence of dosage. The aim of this study was to close this gap. As measures of effectiveness, we primarily considered improvement regarding the DAS28-ESR, as well as improvement in its components during the first 6 months of treatment.
Methods
Patients
Data from the German biologics register Rheumatoid Arthritis: oBservation of BIologic Therapy (RABBIT), an ongoing prospective, observational cohort study initiated in 2001 to observe the long-term safety and effectiveness of treatment with bDMARDs and other new therapies in RA patients, was used. Patients are enrolled in RABBIT with the start of a biologic, biosimilar or targeted synthetic DMARD, or with a csDMARD treatment after at least one prior csDMARD therapy. They are subsequently observed for a minimum of 5 and up to 10 years disregarding treatment changes or withdrawals.
At the time of enrolment, after 3 and 6 months and then every 6 months during the time of observation, information is collected from rheumatologists and patients including demographics, clinical status including joint counts, treatment, laboratory tests and patient-reported outcomes. Further details have been described elsewhere [34]. All patients provided written informed consent before enrolment. The RABBIT study received approval by the Ethics Committee of Charité – Universitätsmedizin Berlin.
Inclusion criteria for patients in this analysis were enrolment between January 2009 and April 2019 with the start of a csDMARD or bDMARD treatment, a BMI ≥18.5 kg/m2, at least one follow-up and the possibility of at least half a year of observation time (at least 3 months on the therapy prescribed at enrolment), leading to the inclusion of 10 593 patients (of 13 062 enrolled between 2009 and 2019). Sixty-four patients switched between s.c. and i.v. route of administration within 6 months of enrolment or their route of administration was unknown, and they were discarded for s.c. vs i.v. subgroup analyses. Since the physician’s global assessment of disease activity was only ascertained in RABBIT as of 2013, the Clinical Disease Activity Index (CDAI) and the Simplified Disease Activity Index (SDAI) were only available for a subset of 4716 patients.
BMI categories
BMI categories were defined according to the World Health Organization definition, i.e. normal weight if 18.5 ≤ BMI < 25 kg/m2, overweight if 25 ≤ BMI < 30 kg/m2 and obese if BMI ≥30 kg/m2. To assess the impact of obesity, comparisons were made between obese patients on one hand and normal or overweight patients on the other hand.
Therapies and dosages
Therapies were grouped according to their mechanism of action. Five drugs or groups of drugs were considered: csDMARDs (e.g. MTX, SSZ, LEF and chloroquine), TNFi [adalimumab, certolizumab, etanercept, golimumab and infliximab (INF)], ABA, RTX and TOC. For etanercept, INF and RTX, both the originator drug and the available biosimilars (SB4, GP2015, CT-P13, SB2 and GP2013) were considered. For the other bDMARDs, only originators were prescribed to patients.
To investigate whether patients received the recommended standard dosage of bDMARDs, the mean dosage in mg per week was calculated. For weight-dependent dosed drugs INF and TOC i.v., intervals were defined for the standard dosage to account for variations in the dose due to potential package-size related constraints. In particular, a deviation of up to ±15% from the standard dose was regarded as the standard for TOC i.v. The standard INF dose of 3 mg/kg of body weight per 8 weeks has been discussed as possibly being too low [35], and the large filling size of 100 mg promotes imprecise dosing of INF. Thus a deviation of up to –15% and +50% was regarded as the standard here. The other bDMARDs were prescribed with fixed doses, but may also suffer under- or overdosing due to variations in the application interval. Regarding the route of administration, adalimumab, certolizumab, etanercept and golimumab were prescribed as a s.c. application, while INF and RTX were prescribed as an i.v. application. ABA and TOC were prescribed both as i.v. and (since 2012 and 2014, respectively) as s.c. applications.
Outcomes
As measures for treatment effectiveness, the improvement in the DAS28-ESR and its components, i.e. swollen joint count, tender joint count, ESR level and patient global health assessment, during the first 6 months of treatment were considered. In addition, we compared the improvement in the physician’s global assessment of disease activity, CDAI, SDAI, CRP level and physical function (via the Hannover Functional Status Questionnaire [36]) and binary outcomes based on DAS28-ESR, i.e. remission (DAS28-ESR <2.6) and good EULAR response (DAS28-ESR ≤3.2 and improvement >1.2). For details on the CDAI and SDAI scores, see Smolen and Aletaha [37].
Statistical analysis
For some variables, data were missing for a considerable proportion of patients, e.g. 10% of CRP values at the start of treatment (T0) and 27% after 6 months of treatment (T2) (see supplementary Table S1, available at Rheumatology online). To predict missing values and to perform 10 imputations of each missing value, regression models were fitted [38]. Imputations were combined to calculate combined estimates. To prevent undesired effects of strongly skewed distributions, the CRP and ESR levels, swollen and tender joint counts, and CDAI and SDAI scores were logarithmized. For the calculation of regression coefficients in imputation models and of mean values, the values were reverse-transformed, applying a correction for non-linearity (second-degree Taylor polynomial).
The influence of obesity on drug effectiveness was investigated either by multiple linear regression to calculate mean obesity effects for continuous outcomes, or by multiple Poisson regression with a robust error variance to calculate obesity risk ratios for binary outcomes. Estimates were adjusted for age at RA onset, baseline value of the outcome of interest, number of prior bDMARDs (categories: ≥1 vs none), glucocorticoid (GC) therapy (dose during the first 3 months of treatment in categories: ≤5 mg/day, >5 mg/day and <15 mg/day, ≥15 mg/day), number of comorbidities (categories: 0, 1, 2, ≥3), joint erosions, seropositivity (RF and/or ACPA) and smoking habits (categories: ever, never and unknown). When analysing improvement in physical function, DAS28-ESR was additionally considered for adjustment. The selection of adjustment factors was guided by findings in the literature [39–43]. Age at RA onset was used instead of age to avoid collinearities with disease duration. GC doses were considered to adjust for confounding by indication since patients with high disease activity and poor prognostic factors have a higher likelihood of receiving GCs [44]. We assumed that the possible influence of obesity is sex-specific and may differ clearly between therapies. For this reason, we separately estimated the effects for all, resulting in 2 (obesity yes/no) × 2 (male/female) × 5 (therapy) = 20 subgroups. This corresponds to an additive modelling of interactions [45]. For details, see the Supplementary Methods, available at Rheumatology online. To draw overarching conclusions regarding therapies, P-values for obesity effects were (conservatively) adjusted using Bonferroni correction. Calculations were carried out with the software packages SAS, version 9.4 (SAS Institute, Cary, USA), and R, version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
A total of 10 593 patients from the RABBIT cohort were analysed, including 7845 women (2192 obese) and 2748 men (718 obese). At baseline, obese patients were comparable to non-obese in age (mean 58 years in both groups) and sex (75% women in obese vs 74% women in non-obese). Obese patients were less likely to be seropositive (71 vs 79%) and to present erosive joint changes (39 vs 51%) than non-obese, but more often had three or more comorbidities (46 vs 30%) relative to non-obese patients. Women had worse physical function than men, with 65 vs 72% of full physical function on average. This difference was particularly notable when comparing obese women and men (58 vs 68% of full physical function on average). However, women also had lower overall CRP values (mean 10 vs 15 mg/l), were considerably less likely to ever smoke (47 vs 75%) and received lower GC doses on average (6.8 vs 7.8 mg/day in the last 6 months) than men (see Table 1 for patient characteristics stratified for sex and three BMI groups; see supplementary Table S2, available at Rheumatology online, for the subgroup of enrolments after 2012).
Table 1.
Baseline patient characteristics in sex/BMI categories for 10 593 patients
| Normal weight | Overweight | Obesity | ||||
|---|---|---|---|---|---|---|
| Parameter | Women | Men | Women | Men | Women | Men |
| N = 3092 | N = 773 | N = 2561 | N = 1257 | N = 2191 | N = 718 | |
| 80.0% | 20.0% | 67.1% | 32.9% | 75.3% | 24.7% | |
| Age, years | 55.6 (13.9) | 57.7 (13.5) | 59.4 (12.3) | 58.8 (11.3) | 58.2 (11.6) | 57.4 (10.7) |
| BMI, kg/m2 | 22.3 (1.7) | 23.0 (1.6) | 27.3 (1.4) | 27.2 (1.4) | 34.8 (4.4) | 33.3 (3.2) |
| Smoking habits | ||||||
| Never smoked [n, (%)] | 1409 (45.6) | 199 (25.7) | 1265 (49.4) | 258 (20.5) | 1053 (48.0) | 151 (21.0) |
| Ever smoked [n (%)] | 1530 (49.5) | 550 (71.2) | 1142 (44.6) | 959 (76.3) | 1017 (46.4) | 540 (75.2) |
| Smoking habits unknown [n (%)] | 153 (4.9) | 24 (3.1) | 154 (6.0) | 40 (3.2) | 122 (5.6) | 27 (3.8) |
| Disease activity | ||||||
| ESR | 25.7 (19.2) | 30.3 (24.3) | 28.1 (20.4) | 29.7 (23.2) | 30.7 (21.2) | 28.5 (22.0) |
| CRP | 9.5 (8.9) | 16.0 (15.4) | 10.0 (9.1) | 14.7 (13.9) | 11.8 (10.1) | 13.6 (12.4) |
| Swollen joint count | 4.9 (4.2) | 5.1 (4.4) | 5.0 (4.3) | 5.1 (4.5) | 5.1 (4.4) | 5.0 (4.5) |
| Tender joint count | 7.0 (5.9) | 6.8 (6.0) | 7.8 (6.6) | 7.2 (6.2) | 8.6 (7.1) | 7.7 (6.6) |
| Patient global health assessment | 5.4 (2.2) | 5.4 (2.1) | 5.7 (2.1) | 5.7 (2.1) | 6.1 (2.0) | 5.8 (2.0) |
| DAS28-ESR | 4.7 (1.3) | 4.7 (1.4) | 4.9 (1.3) | 4.8 (1.4) | 5.1 (1.2) | 4.8 (1.3) |
| DAS28-ESR <3.2 [n (%)] | 415 (13.4) | 111 (14.4) | 253 (9.9) | 160 (12.7) | 159 (7.2) | 84 (11.7) |
| 3.2 ≤ DAS28-ESR < 5.1 [n (%)] | 1549 (50.1) | 358 (46.4) | 1200 (46.9) | 555 (44.1) | 938 (42.8) | 340 (47.3) |
| 5.1 ≤ DAS28-ESR [n (%)] | 1128 (36.5) | 303 (39.2) | 1108 (43.3) | 542 (43.1) | 1095 (50.0) | 295 (41.0) |
| Disease history, function | ||||||
| Disease duration, years | 10.0 (9.1) | 7.7 (8.2) | 9.8 (9.0) | 7.2 (7.3) | 8.7 (8.4) | 6.3 (6.6) |
| Joint erosions [n (%)] | 1628 (54.5) | 374 (50.1) | 1228 (50.3) | 549 (46.1) | 801 (38.8) | 284 (41.2) |
| Number of previous csDMARDs | 2.2 (1.1) | 1.8 (1.0) | 2.1 (1.1) | 1.8 (1.0) | 2.0 (1.1) | 1.8 (0.9) |
| Number of previous bDMARDs | 0.4 (0.9) | 0.3 (0.7) | 0.4 (0.8) | 0.3 (0.8) | 0.4 (0.8) | 0.4 (0.8) |
| % of full physical function | 70.3 (22.0) | 74.7 (20.9) | 64.6 (22.5) | 72.7 (21.6) | 58.3 (22.4) | 67.6 (22.7) |
| Comorbidities | ||||||
| No comorbidities [n (%)] | 981 (31.7) | 249 (32.2) | 504 (19.7) | 270 (21.5) | 261 (11.9) | 100 (13.9) |
| One comorbidity [n (%)] | 795 (25.7) | 192 (24.8) | 595 (23.2) | 327 (26.0) | 457 (20.8) | 177 (24.7) |
| Two comorbidities [n (%)] | 549 (17.8) | 128 (16.6) | 524 (20.5) | 237 (18.9) | 455 (20.8) | 134 (18.7) |
| ≥3 comorbidities [n (%)] | 767 (24.8) | 204 (26.4) | 938 (36.6) | 423 (33.7) | 1019 (46.5) | 307 (42.8) |
| Sum of comorbidities | 1.7 (2.0) | 1.8 (2.1) | 2.3 (2.2) | 2.2 (2.2) | 2.8 (2.4) | 2.6 (2.4) |
| Autoantibody status | ||||||
| RF or ACPA positive [n (%)] | 2512 (81.2) | 606 (78.4) | 1949 (76.1) | 965 (76.8) | 1544 (70.4) | 522 (72.7) |
| Glucocorticoid therapy | ||||||
| Any glucocorticoids in last 6 months [n (%)] | 1706 (55.2) | 452 (58.5) | 1449 (56.6) | 725 (57.7) | 1245 (56.8) | 398 (55.5) |
| Glucocorticoid dose in last 6 monthsa | 6.8 (3.4) | 7.5 (3.9) | 6.7 (3.2) | 7.7 (3.9) | 6.9 (3.3) | 8.2 (4.5) |
| DMARD therapy | ||||||
| csDMARDs [n (%)] | 883 (28.6) | 240 (31.0) | 822 (32.1) | 416 (33.1) | 792 (36.1) | 240 (33.4) |
| TNFi [n (%)] | 1451 (46.9) | 379 (49.0) | 1162 (45.4) | 583 (46.4) | 957 (43.7) | 316 (44.0) |
| Abatacept [n (%)] | 165 (5.3) | 32 (4.1) | 117 (4.6) | 64 (5.1) | 109 (5.0) | 44 (6.1) |
| Rituximab [n (%)] | 210 (6.8) | 47 (6.1) | 175 (6.8) | 72 (5.7) | 100 (4.6) | 44 (6.1) |
| Tocilizumab [n (%)] | 383 (12.4) | 75 (9.7) | 285 (11.1) | 122 (9.7) | 234 (10.7) | 74 (10.3) |
Means (s.d.) are reported if not otherwise specified. Non-integer numbers for categorical variables resulting from multiple imputation are rounded. aConsidering only patients who received glucocorticoids. DAS28-ESR: DAS with 28 joints using ESR as an inflammation marker; csDMARD: conventional synthetic DMARD; bDMARD: biologic DMARD.
Joint effect of obesity, sex and therapy
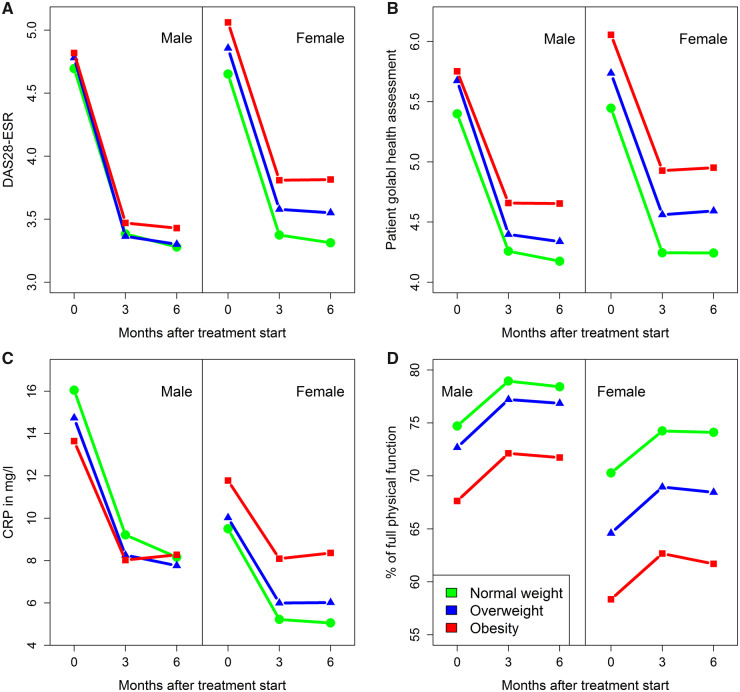
As visualized in trajectory curves representing the time points at baseline, 3 and 6 months after the start of treatment, for women there were generally more differences between the BMI groups than for men. Pronounced differences between the sexes can be observed for levels of CRP and for percentage of full physical function (see Fig. 1 and supplementary Fig. S1, available at Rheumatology online).
Fig. 1.
Evolution of measures of therapy effectiveness over time
Mean values are shown at baseline, 3 and 6 months after the start of treatment, separately for women and men as well as for BMI groups (normal weight: 18.5 ≤ BMI < 25 kg/m2, overweight: 25 ≤ BMI < 30 kg/m2, obesity: ≥30 kg/m2). The following outcomes are shown: (A) improvement in DAS28-ESR, (B) improvement in patient global health assessment, (C) improvement in CRP in mg/l and (D) improvement in physical function (Hannover Functional Status Questionnaire). DAS28-ESR: DAS with 28 joints using ESR as an inflammation marker.
For women treated with TNFi or csDMARDs as well as for all patients treated with TOC, obesity had a negative influence on improvement in DAS28-ESR after 6 months of treatment. The mean impact of obesity on the improvement in DAS28-ESR was –0.15 units (95% CI: −0.26; −0.04) for women receiving csDMARDs, −0.22 units (95% CI: –0.31; –0.12) for women receiving TNFi, −0.22 units (95% CI: −0.42; −0.03) for women receiving TOC and −0.41 units (95% CI: −0.74; –0.07) for men receiving TOC (see Fig. 2). This means that, for example, an obese man receiving TOC is expected to improve his DAS28-ESR by 0.41 points less over half a year after start of treatment than a non-obese man receiving the same therapy. The weaker DAS28-ESR response in obese women seemed to be mainly associated with a weaker ESR/inflammation response in the csDMARD group, while in the TNFi group it appeared to be additionally associated with a weaker response regarding joint pain and the patient global health assessment. For patients treated with TOC, significant associations with obesity were observed for joint pain or (only in women) swelling, and an association with a weaker ESR response was observed only among men (see Fig. 2 and supplementary Fig. S2, available at Rheumatology online).
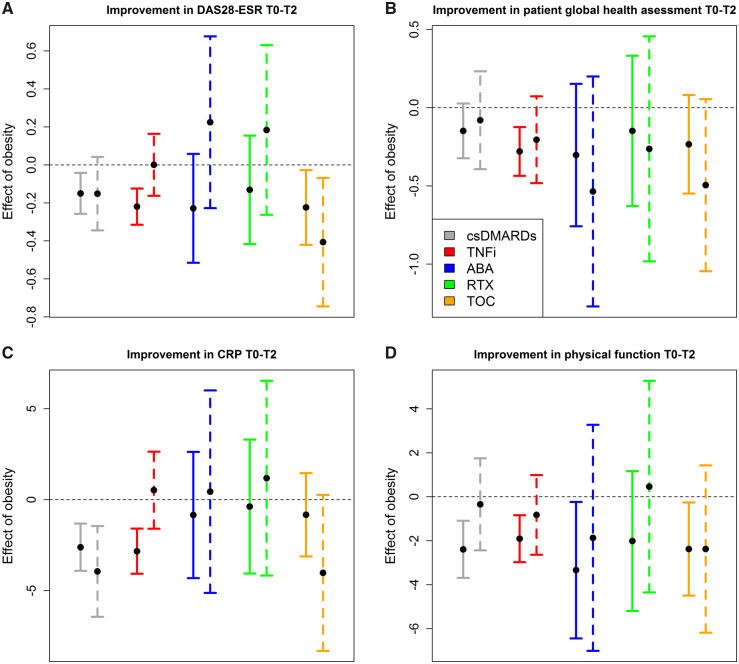
Fig. 2.
Adjusted effects of obesity on measures of therapy effectiveness
Shown are mean effects of obesity and their 95% CIs for 10 subgroups of patients. In each of the five treatment groups (csDMARDs, TNFi, ABA, RTX and TOC) obesity effects of women (left, solid line) and men (right, dashed line) are presented for the following outcomes: (A) improvement in DAS28-ESR, (B) improvement in patient global health assessment, (C) improvement in CRP and (D) improvement in percentage of full physical function (Hannover Functional Status Questionnaire), 6 months (T2) after start of treatment (T0). Estimates were obtained by linear regression, adjusting for age at RA onset, baseline value of the outcome of interest, number of prior bDMARDs (categories: ≥1 vs none), GC therapy (dose during first 3 months of treatment in categories: ≤5 mg/day, >5 mg/day and <15 mg/day, ≥15 mg/day), number of comorbidities (categories: 0, 1, 2, ≥3), joint erosions, seropositivity (RF and/or ACPA) and smoking habits (categories: ever, never and unknown). When analysing improvement of physical function, DAS28-ESR was additionally considered for adjustment. csDMARD: conventional synthetic; bDMARD: biologic DMARD; TNFi: TNF inhibitors; ABA: abatacept; RTX: rituximab; TOC: tocilizumab; GC: glucocorticoid; DAS28-ESR: DAS with 28 joints using ESR as an inflammation marker
A significant difference between sex-specific obesity effects was found for ESR reduction. The mean impact of obesity on the improvement in ESR values after 6 months was –3.81 units (95% CI: –5.16; –2.46) for women receiving TNFi, while it was 0.83 units (95% CI: –1.50; 3.17) for men (see supplementary Fig. S2, available at Rheumatology online). For all other measures, the difference in effect size was not statistically significant.
The mean effect of obesity on physical function in women was –2.40 percentage points (95% CI: –3.69; −1.10) of full physical function in the csDMARD group, −1.91 percentage points (95% CI: –2.98; –0.84) in the TNFi group, –3.34 percentage points (95% CI: –6.44; −0.24) in the ABA group and –2.38 percentage points (95% CI: –4.50; –0.26) in the TOC group (see Fig. 2).
Remission and good EULAR response were assessed for patients presenting a DAS28-ESR ≥2.6 and a DAS28-ESR ≥3.2 at baseline, respectively. The effects of obesity on these outcomes showed a pattern similar to the results for the improvement in DAS28-ESR (supplementary Fig. S3, available at Rheumatology online). The risk ratio of obesity for achieving remission after 6 months was 0.80 (95% CI: 0.66; 0.97) among women receiving csDMARDs, 0.73 (95% CI: 0.61; 0.88) among women receiving TNFi and 0.59 (95% CI: 0.35; 0.99) for men receiving TOC. The risk ratio for achieving a good EULAR response was 0.83 (95% CI: 0.72; 0.95) among women receiving TNFi.
All estimates are documented in Table 2. In supplementary Fig. S4, available at Rheumatology online, separate effects for s.c. and i.v. routes of administration are presented for ABA and TOC.
Table 2.
Estimates for the effect of obesity on different outcomes
| Outcome | csDMARDs | TNFi | ABA | RTX | TOC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |
| DAS28-ESR | −0.15 | −0.15 | −0.22 | 0.001 | −0.23 | 0.22 | −0.13 | 0.18 | −0.22 | −0.41 |
| improvement | (−0.26; −0.04) | (−0.34; 0.04) | (−0.31; −0.12) | (−0.16; 0.16) | (−0.52; 0.06) | (−0.23; 0.68) | (−0.42; 0.15) | (−0.26; 0.63) | (−0.42;−0.03) | (−0.74;−0.07) |
| 0.006 | 0.125 | <0.0001* | 0.994 | 0.117 | 0.330 | 0.367 | 0.419 | 0.026 | 0.018 | |
| ESR | −3.51 | −5.70 | −3.81 | 0.83 | −4.08 | 2.20 | −0.37 | 5.87 | 1.13 | −6.32 |
| improvement | (−4.97; −2.06) | (−8.65; −2.76) | (−5.16; −2.46) | (−1.50; 3.17) | (−8.07;−0.08) | (−4.17; 8.57) | (−4.34; 3.59) | (−0.39; 12.12) | (−1.52; 3.78) | (−11.68; −0.96) |
| <0.0001* | 0.0002* | <0.0001* | 0.483 | 0.046 | 0.497 | 0.853 | 0.066 | 0.402 | 0.021 | |
| CRP | −2.62 | −3.95 | −2.84 | 0.52 | −0.84 | 0.44 | −0.38 | 1.18 | −0.83 | −4.03 |
| improvement | (−3.92; −1.32) | (−6.44;−1.45) | (−4.08;−1.59) | (−1.60; 2.64) | (−4.31; 2.63) | (−5.31; 6.01) | (−4.07; 3.31) | (−4.17; 6.54) | (−3.12; 1.46) | (−8.31; 0.26) |
| <0.0001* | 0.002* | <0.0001* | 0.627 | 0.633 | 0.877 | 0.839 | 0.664 | 0.474 | 0.065 | |
| Swollen joint count | 0.08 | 0.05 | 0.07 | 0.44 | 0.36 | 0.05 | 0.09 | 0.06 | −0.57 | −0.35 |
| improvement | (−0.20; 0.36) | (−0.42; 0.53) | (−0.17; 0.31) | (0.04; 0.85) | (−0.35; 1.07) | (−1.06; 1.16) | (−0.61; 0.78) | (−1.02; 1.15) | (−1.03;−0.10) | (−1.20; 0.50) |
| 0.569 | 0.823 | 0.583 | 0.031 | 0.317 | 0.932 | 0.807 | 0.912 | 0.016 | 0.420 | |
| Tender joint count | −0.19 | −0.41 | −0.39 | −0.26 | −0.64 | 0.14 | −0.63 | 0.33 | −1.29 | −1.65 |
| improvement | (−0.60; 0.23) | (−1.14; 0.33) | (−0.74;−0.03) | (−0.89; 0.38) | (−1.72; 0.43) | (−1.53; 1.82) | (−1.68; 0.41) | (−1.29; 1.94) | (−2.01;−0.57) | (−2.91;−0.39) |
| 0.374 | 0.279 | 0.032 | 0.423 | 0.241 | 0.866 | 0.235 | 0.692 | 0.0004* | 0.010 | |
| Improvement | −0.15 | −0.08 | −0.28 | −0.20 | −0.30 | −0.54 | −0.15 | −0.26 | −0.23 | −0.50 |
| in patient global | (−0.32; 0.03) | (−0.39; 0.23) | (−0.44; −0.12) | (−0.48; 0.07) | (−0.76; 0.15) | (−1.27; 0.20) | (−0.63; 0.33) | (−0.98; 0.46) | (−0.55; 0.08) | (−1.05; 0.06) |
| health assessment | 0.098 | 0.616 | 0.0004* | 0.148 | 0.193 | 0.154 | 0.546 | 0.474 | 0.145 | 0.078 |
| Improvement | −2.40 | −0.35 | −1.91 | −0.83 | −3.34 | −1.87 | −2.02 | 0.46 | −2.38 | −2.38 |
| in % of full physical | (−3.69; −1.10) | (−2.44; 1.74) | (−2.98;−0.84) | (−2.64; 0.98) | (−6.44;−0.24) | (−7.01; 3.27) | (−5.20; 1.16) | (−4.36; 5.27) | (−4.50;−0.26) | (−6.18; 1.43) |
| function | 0.0003* | 0.745 | 0.001* | 0.370 | 0.035 | 0.476 | 0.213 | 0.852 | 0.028 | 0.220 |
| Improvement in | −0.22 | 0.16 | −0.31 | −0.05 | −0.42 | −0.20 | −0.76 | 0.05 | −0.80 | −0.54 |
| physician’s global assess- | (−0.50; 0.06) | (−0.34; 0.66) | (−0.56;−0.07) | (−0.47; 0.37) | (−1.04; 0.20) | (−1.29; 0.90) | (−1.77; 0.26) | (−1.29; 1.39) | (−1.31;−0.29) | (−1.38; 0.30) |
| ment of disease activity | 0.118 | 0.526 | 0.010 | 0.814 | 0.187 | 0.725 | 0.144 | 0.939 | 0.002* | 0.208 |
| CDAI | −0.62 | −0.36 | −1.37 | 0.59 | −2.18 | 0.57 | −5.46 | 0.52 | −3.14 | −2.00 |
| improvement | (−1.80; 0.56) | (−2.48; 1.77) | (−2.39;−0.36) | (−1.17; 2.34) | (−4.81; 0.45) | (−4.04; 5.17) | (−9.76;−1.15) | (−5.19; 6.23) | (−5.29;−1.00) | (−5.56; 1.56) |
| 0.306 | 0.742 | 0.008 | 0.512 | 0.105 | 0.810 | 0.013 | 0.859 | 0.004* | 0.271 | |
| SDAI | −2.87 | −4.75 | −4.79 | −0.30 | −3.98 | 3.78 | −9.02 | 4.29 | −5.98 | −1.47 |
| improvement | (−5.16; −0.58) | (−8.82; −0.67) | (−6.76;−2.82) | (−3.80; 3.19) | (−9.06; 1.11) | (−5.48; 13.04) | (−17.19;−0.85) | (−6.62; 15.20) | (−10.13;−1.84) | (−8.24; 5.30) |
| 0.014 | 0.023 | <0.0001* | 0.865 | 0.126 | 0.423 | 0.031 | 0.441 | 0.005 | 0.670 | |
| Achievement of | 0.80 | 0.85 | 0.73 | 1.01 | 0.57 | 1.12 | 0.996 | 1.26 | 0.89 | 0.59 |
| remission | (0.66; 0.97) | (0.63; 1.16) | (0.61; 0.88) | (0.79; 1.28) | (0.27; 1.23) | (0.53; 2.37) | (0.50; 1.99) | (0.57; 2.78) | (0.68; 1.15) | (0.35; 0.99) |
| 0.026 | 0.314 | 0.001* | 0.941 | 0.151 | 0.766 | 0.990 | 0.565 | 0.359 | 0.044 | |
| Achievement of | 0.94 | 0.79 | 0.83 | 1.04 | 0.59 | 1.08 | 0.86 | 1.22 | 0.92 | 0.76 |
| good EULAR | (0.80; 1.11) | (0.59; 1.05) | (0.72; 0.95) | (0.85; 1.28) | (0.35; 1.02) | (0.59; 1.98) | (0.51; 1.43) | (0.62; 2.41) | (0.74; 1.15) | (0.50; 1.17) |
| response | 0.468 | 0.105 | 0.009 | 0.676 | 0.059 | 0.798 | 0.559 | 0.559 | 0.451 | 0.211 |
For each outcome and therapy/sex stratum, estimate (above), 95% CI (middle) and P-value (below) are given. For the outcomes ‘achievement of remission’ and ‘achievement of good EULAR response’, risk ratios are given as estimates. For all other outcomes, mean obesity effects are given as estimates. The P-values corresponding to estimates significant at 5% significance level are marked in bold, the P-values corresponding to estimates remaining significant after Bonferroni adjustment for multiple testing to draw overarching conclusions on the levels of therapy/sex groups are additionally marked with an asterisk (*). DAS28-ESR: DAS with 28 joints using ESR as an inflammation marker; CDAI: Clinical Disease Activity Index; SDAI: Simplified Disease Activity Index.
To draw overarching conclusions across the 12 considered outcomes for sex/therapy groups, we additionally looked at the significance of obesity effects when adjusted for the multiple outcomes by Bonferroni correction. This approach is rather conservative, given there is some degree of correlation between outcomes. The groups associated with an impact of obesity on one or several aspects of effectiveness are women and men receiving csDMARDs, women receiving TNFi and women receiving TOC (see Table 2). While for women, there are several significant outcomes in each of these therapy groups, 13 in total, for men receiving csDMARDs there are just two significant outcomes (improvement of ESR and CRP). There is thus a broader evidence for an impact of obesity on treatment effectiveness in women than in men.
Dosage
The proportions of patients treated with bDMARDs who received the standard dose, or an under- or overdose in the first 3 months of treatment are reported in Table 3. Overall, ∼95% of patients received the standard dosage. This still holds true when considering obese or non-obese patients separately (supplementary Tables S3 and S4, available at Rheumatology online). The highest percentages of under- or overdoses could be observed for INF (17.5% overdoses), ABA i.v. (16.3% underdoses) and RTX (9.8% underdoses). No relevant differences in patient characteristics could be observed at baseline between the three dosage groups (see supplementary Table S5, available at Rheumatology online).
Table 3.
Dosage for individual drugs for patients receiving a bDMARD
| Drug | Dosage | |||
|---|---|---|---|---|
| Standard | Under | Over | Total | |
| Etanercept (originator) | 1310 (94.4) | 71 (5.1) | 7 (0.5) | 1388 |
| Etanercept (SB4, biosimilar) | 457 (98.5) | 7 (1.5) | 0 (0) | 464 |
| Etanercept (GP2015, biosimilar) | 102 (98.1) | 2 (1.9) | 0 (0) | 104 |
| Infliximab (originator) | 107 (78.1) | 6 (4.4) | 24 (17.5) | 137 |
| Adalimumab (originator) | 1259 (98.9) | 5 (0.4) | 9 (0.7) | 1273 |
| Certolizumab (originator) | 710 (94.8) | 9 (1.2) | 30 (4.0) | 749 |
| Golimumab (originator) | 362 (97.3) | 5 (1.3) | 5 (1.3) | 372 |
| Abatacept s.c. (originator) | 292 (99.3) | 2 (0.7) | 0 (0) | 294 |
| Abatacept i.v. (originator) | 150 (81.5) | 30 (16.3) | 4 (2.2) | 185 |
| Rituximab (originator) | 581 (90.2) | 63 (9.8) | 0 (0) | 644 |
| Tocilizumab s.c. (originator) | 326 (94.8) | 8 (2.3) | 10 (2.9) | 344 |
| Tocilizumab i.v. (originator) | 682 (90.8) | 36 (4.8) | 33 (4.4) | 751 |
| Total | 6338 (94.5) | 245 (3.7) | 122 (1.8) | 6705 |
Data are presented as n (%). The available dosage information during the first 3 months of treatment for the drugs shown represents 6705 of 7200 patients receiving a biologic DMARD, i.e. 93.1% of those patients. In case of missing data, the dosage intended at baseline is considered. For tocilizumab and abatacept, s.c. and i.v. routes of administration are shown separately. One abatacept patient was not considered in this table due to an unknown route of administration.
Discussion
Summary
When performing an overarching assessment of obesity effects on all 12 considered outcomes, adjusting for multiple comparisons, the groups associated with an impact of obesity on one or several aspects of treatment effectiveness are women and men receiving csDMARDs, women receiving TNFi and women receiving TOC, while no effect of obesity on effectiveness could be proven for RTX and ABA. In particular, the findings confirm a negative effect of obesity in women on the effectiveness of csDMARDs and TNFi regarding the reduction of disease activity and gain in physical function over half a year after start of treatment. This is consistent with previous literature. In the case of disease activity, for patients receiving csDMARDs obesity primarily affects inflammation, but not the joint counts. For women receiving TNFi, effects on tender joints and patient global health assessment play a role in addition to inflammation. An influence of obesity on the reduction of disease activity and on the improvement in physical function was also detected for women receiving TOC, which is notable given that thus far no significant effect of obesity (as opposed to large body weight) has been reported in the literature for this therapy. This may be due to previous studies not assessing the effect of obesity in a sex-specific manner and, in some cases, to a potential lack of power resulting from small sample sizes. Interestingly, in female TOC patients obesity appears to affect the reduction of disease activity primarily regarding joint counts and not inflammation.
After Bonferroni adjustment, the only significant obesity effects in men are on the improvement of ESR and CRP levels among patients receiving csDMARDs, while the effects observed in women are considerably more numerous. A statistically significant difference between sex-specific obesity effects was only observed on the improvement of the ESR level for patients receiving TNFi.
Among bDMARDs, the greater impact of obesity on TNFi and TOC effectiveness might be partly due to their targeting of single cytokines, while ABA and RTX target immune cell populations. Since white adipose tissue produces pro-inflammatory cytokines such as TNF and IL-6, the higher fat mass in adipose RA patients might lead to higher concentrations of these [46], affecting the therapeutic response. However, it has been suggested recently that adipocytes may play a role in activating T cells and recruiting B cells as well [47, 48].
Strengths and limitations
To the best of our knowledge, this study is the first comprehensive assessment of the impact of obesity on the effectiveness of DMARDs in RA treatment, considering the effects of obesity in a treatment and sex-specific manner, for a large set of outcomes and with a large number of patients.
Nevertheless, some obesity/sex/therapy strata were small. Thus, while cell-targeted treatments were least affected by obesity, the smaller total numbers of patients receiving ABA (531) and RTX (648) compared with the other therapies (csDMARDs, 3393; TNFi, 4848; TOC, 1173) may have prevented the detection of a potential obesity effect for some outcomes. Similarly, the number of women in the study was considerably larger than men, thus the power to detect obesity effects was smaller in men than in women. In addition, we only compared obesity effects, not directly treatment effects, which is difficult in an observational study. Some of the observed obesity effects are small and might not be clinically relevant.
Finally, obesity was ascertained only via its proxy BMI, while body fat mass was not measured directly. This is a drawback since men have more muscle mass than women. Higher levels of CRP and ESR have been significantly associated with adiposity in women, but not in men [14]. While fat mass is associated with the CRP level, adjustment for fat mass attenuates the corresponding association of the BMI [15]. Thus, the observed association of obesity with the CRP level might be attenuated in women if fat mass was observed directly. Measurement of body fat mass would be even more desirable, as activity of pro-inflammatory cytokines may lead to weight loss and cachexia after RA onset, affecting primarily muscle mass [49]. Consequently, when enrolled in our study several years after RA onset, patients may show a reduced BMI but continue to be obese.
Other factors with the potential to explain in part a stronger impact of obesity on effectiveness in women that were not ascertained in this study include physical activity and adherence. The potential impact of adherence on effectiveness is probably restricted to therapies using the s.c. route of administration; while ∼75% of patients receiving a bDMARD correspond to the s.c. route of administration, both RTX and (partly) ABA are applied i.v.
Conclusions
Obesity has a negative impact on the effectiveness of cytokine-targeted but not cell-targeted therapies in daily practice. This has a statistically significant effect in considerably more outcomes and therapies for women than for men. When assessing a large number of outcomes jointly, no effects of obesity were found on the effectiveness of cell-targeted therapies RTX and ABA. These DMARDs might thus be particularly worthwhile treatment options for obese women. Further investigations into the reasons for the different patterns observed for cell-targeted and cytokine-targeted therapies are desirable.
Supplementary Material
Acknowledgements
The authors acknowledge the invaluable contributions of all participating consultant rheumatologists and their patients. In particular, we would like to thank those rheumatologists who enrolled the highest numbers of patients: J. Kaufmann, T. Klopsch, C. Eisterhues, J. Braun, I. Schwarze, A. Liebhaber, A. Krause, K. Rockwitz, S. Zinke, C. Kneitz, C. Möbius, E. Ständer, H. Tony, S. Berger, A. Gräßler, C. Kühne, S. Remstedt, W. Ochs, E. Wilden, M. Bohl-Bühler, S. Wassenberg, H. Kellner, G. Burmester, F. Haas, M. Röser, A. Bruckner, S. Balzer, H. Fricke-Wagner, H. Bergerhausen, W. Harmuth, G. Wiesmüller, S. Lebender, A. Bussmann, F. Hamann, C. Stille, M. Feuchtenberger, E. Edelmann, H. Tremel, B. Krummel-Lorenz, H. Körber, K. Krüger, L. Meier, A. Kapelle, L. Müller, A. Thiele, M. Schmitt-Haendle, U. Prothmann, D. Pick, K. Karberg, H. Brandt, K. Weiß, J. Kekow, A. Seifert, U. Müller-Ladner, K. Manger, C. Baumann, D. Krause, M. Aringer, M. Worsch, A. Roßbach, M. Zänker, H. Streibl, M. Backhaus, H. Schulze-Koops, C. Herzberg, M. Grünke, N. Heel, P. Herzer, A. Reck, F. Wiesent, G. Dahmen, N. Blank, R. Max, T. Eidner, R. Dockhorn, G. Zeh, K. Winkler, H. Menne, U. von Hinüber, W. Demary, H. Sörensen, M. Schneider, A. Gause, C. Bielecke, T. Marycz, B. Häckel, K. Alliger, H. Euler, F. Moosig, C. Iking-Konert, J. Häntsch, H. Wernitzsch, A. Claußnitzer, E. Riechers, R. Schmidt, F. Arndt, H. Schibinger, P. Fuchs, M. Aurich and R. Boldemann. We also acknowledge the significant contributions of Peter Herzer, Munich, and Matthias Schneider, Düsseldorf, as members of the advisory board.
Funding: This work was supported by the German Federal Ministry of Education and Research within the METARTHROS consortium (grant number 01EC1407D). RABBIT is supported by a joint, unconditional grant from AbbVie, Amgen, Bristol-Myers Squibb, Celltrion, Fresenius Kabi, Hexal AG, Lilly, MSD Sharp & Dome, Mylan, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis and UCB. The principal investigators and their team had full academic freedom in study design and conduct, data analysis and publication of results. These stipulations were delineated in their contract with the sponsors. For the purpose of information, all funding companies received the manuscript 30 days prior to submission. Publication of this article was not contingent on their approval. The data interpretation, drafting, critical revision and approval of the final manuscript were performed solely by the authors.
Disclosure statement: Y.M. has received speaking fees from Pfizer. B.M. has received speaking fees from AbbVie, Bristol-Myers Squibb, MSD Sharp & Dome, Pfizer, Roche and UCB. A.S. has received speaking fees from Bristol-Myers Squibb, MSD Sharp & Dome, Pfizer and Roche. A.Z. has received speaking fees from AbbVie, Janssen, Pfizer, Roche and Sanofi-Aventis. S.R. has participated in studies for BMG Pharma, AbbVie, Pfizer, Bristol-Myers Squibb, MSD Sharp & Dome, UCB, Roche, Chugai, Sanofi and Biogen. The other authors have declared no conflicts of interest.
References
- 1.GBD Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crowson CS, Matteson EL, Davis JM. et al. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russolillo A, Iervolino S, Peluso R. et al. Obesity and psoriatic arthritis: from pathogenesis to clinical outcome and management. Rheumatology (Oxford) 2013;52:62–7. [DOI] [PubMed] [Google Scholar]
- 4. Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y. et al. Obesity in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:450–62. [DOI] [PubMed] [Google Scholar]
- 5. George MD, Østergaard M, Conaghan PG. et al. Obesity and rates of clinical remission and low MRI inflammation in rheumatoid arthritis. Ann Rheum Dis 2017;76:1743–6. [DOI] [PubMed] [Google Scholar]
- 6. Liu Y, Hazlewood GS, Kaplan GG. et al. Impact of obesity on remission and disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2017;69:157–65. [DOI] [PubMed] [Google Scholar]
- 7. Lupoli R, Pizzicato P, Scalera A. et al. Impact of body weight on the achievement of minimal disease activity in patients with rheumatic diseases: a systematic review and meta-analysis. Arthritis Res Ther 2016;18:297.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nikiphorou E, Norton S, Young A. et al. The association of obesity with disease activity, functional ability and quality of life in early rheumatoid arthritis: data from the Early Rheumatoid Arthritis Study/Early Rheumatoid Arthritis Network UK prospective cohorts. Rheumatology (Oxford) 2018;57:1194. [DOI] [PubMed] [Google Scholar]
- 9. Schulman E, Bartlett SJ, Schieir O. et al. Overweight, obesity, and the likelihood of achieving sustained remission in early rheumatoid arthritis: results from a Multicenter Prospective Cohort Study. Arthritis Care Res (Hoboken) 2018;70:1185–91. [DOI] [PubMed] [Google Scholar]
- 10. Vidal C, Barnetche T, Morel J. et al. Association of body mass index categories with disease activity and radiographic joint damage in rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol 2015;42:2261–9. [DOI] [PubMed] [Google Scholar]
- 11. Shan J, Zhang J.. Impact of obesity on the efficacy of different biologic agents in inflammatory diseases: a systematic review and meta-analysis. Joint Bone Spine 2019;86:173–83. [DOI] [PubMed] [Google Scholar]
- 12. Baker JF, Østergaard M, George M. et al. Greater body mass independently predicts less radiographic progression on X-ray and MRI over 1-2 years. Ann Rheum Dis 2014;73:1923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westhoff G, Rau R, Zink A.. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum 2007;56:3575–82. [DOI] [PubMed] [Google Scholar]
- 14. Giles JT, Bartlett SJ, Andersen R. et al. Association of body fat with C-reactive protein in rheumatoid arthritis. Arthritis Rheum 2008;58:2632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George MD, Giles JT, Katz PP. et al. Impact of obesity and adiposity on inflammatory markers in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2017;69:1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandberg MEC, Bengtsson C, Källberg H. et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann Rheum Dis 2014;73:2029–33. [DOI] [PubMed] [Google Scholar]
- 17. Levitsky A, Brismar K, Hafström I. et al. Obesity is a strong predictor of worse clinical outcomes and treatment responses in early rheumatoid arthritis: results from the SWEFOT trial. RMD Open 2017;3:e000458.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gremese E, Carletto A, Padovan M. et al. Obesity and reduction of the response rate to anti-tumor necrosis factor α in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res (Hoboken) 2013;65:94–100. [DOI] [PubMed] [Google Scholar]
- 19. Heimans L, van den Broek M, le Cessie S. et al. Association of high body mass index with decreased treatment response to combination therapy in recent-onset rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2013;65:1235–42. [DOI] [PubMed] [Google Scholar]
- 20. Iannone F, Fanizzi R, Notarnicola A. et al. Obesity reduces the drug survival of second line biological drugs following a first TNF-α inhibitor in rheumatoid arthritis patients. Joint Bone Spine 2015;82:187–91. [DOI] [PubMed] [Google Scholar]
- 21. Klaasen R, Wijbrandts CA, Gerlag DM. et al. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum 2011;63:359–64. [DOI] [PubMed] [Google Scholar]
- 22. George MD, Baker JF. The obesity epidemic and consequences for rheumatoid arthritis care. Curr Rheumatol Rep 2016;18:6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. D’Agostino MA, Alten R, Mysler E. et al. Body mass index and clinical response to intravenous or subcutaneous abatacept in patients with rheumatoid arthritis. Clin Rheumatol 2017;36:2655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gardette A, Ottaviani S, Sellam J. et al. Body mass index and response to abatacept in rheumatoid arthritis. Eur J Clin Invest 2016;46:1048–52. [DOI] [PubMed] [Google Scholar]
- 25. Iannone F, Courvoisier DS, Gottenberg JE. et al. Body mass does not impact the clinical response to intravenous abatacept in patients with rheumatoid arthritis. Analysis from the “pan-European registry collaboration for abatacept (PANABA). Clin Rheumatol 2017;36:773–9. [DOI] [PubMed] [Google Scholar]
- 26. Mariette X, Alten R, Nüßlein HG. et al. The effect of body mass index on clinical response to abatacept as a first-line biologic for rheumatoid arthritis: 6-month results from the 2-year, observational, prospective ACTION study. Joint Bone Spine 2017;84:571–6; [DOI] [PubMed] [Google Scholar]
- 27. Ottaviani S, Gardette A, Roy C. et al. Body mass index and response to rituximab in rheumatoid arthritis. Joint Bone Spine 2015;82:432–6. [DOI] [PubMed] [Google Scholar]
- 28. Gardette A, Ottaviani S, Sellam J. et al. Body mass index and response to tocilizumab in rheumatoid arthritis: a real life study. Clin Rheumatol 2016;35:857–61. [DOI] [PubMed] [Google Scholar]
- 29. Pappas D, Etzel C, Crabtree M. et al. THU0150 Impact of comorbidity burden and obesity on the effectiveness of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis 2018;77:295. [DOI] [PubMed] [Google Scholar]
- 30. Pers Y-M, Godfrin-Valnet M, Lambert J. et al. Response to tocilizumab in rheumatoid arthritis is not influenced by the body mass index of the patient. J Rheumatol 2015;42:580–4. [DOI] [PubMed] [Google Scholar]
- 31. Burmester GR, Rubbert-Roth A, Cantagrel A. et al. Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann Rheum Dis 2016;75:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kivitz A, Olech E, Borofsky M. et al. Subcutaneous tocilizumab versus placebo in combination with disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:1653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jawaheer D, Olsen J, Lahiff M. et al. Gender, body mass index and rheumatoid arthritis disease activity: results from the QUEST-RA Study. Clin Exp Rheumatol 2010;28:454–61. [PMC free article] [PubMed] [Google Scholar]
- 34. Richter A, Meißner Y, Strangfeld A. et al. Primary and secondary patient data in contrast: the use of observational studies like RABBIT. Clin Exp Rheumatol 2016;34:S79–86. [PubMed] [Google Scholar]
- 35. De Vries HS, Van Oijen MGH, Driessen RJB. et al. Appropriate infliximab infusion dosage and monitoring: results of a panel meeting of rheumatologists, dermatologists and gastroenterologists. Br J Clin Pharmacol 2011;71:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raspe HH, Hagedorn U, Kohlmann T, Matussek S. Der Funktionsfragebogen Hannover (FFbH): ein Instrument zur Funktionsdiagnostik bei polyartikulären Gelenkerkrankungen, in Wohnortnahe Betreuung Rheumakranker - Ergebnisse sozialwissenschaftlicher Evaluation eines Modellversuchs. Stuttgart: Schattauer, 1990. [Google Scholar]
- 37. Smolen JS, Aletaha D.. Scores for all seasons: SDAI and CDAI. Clin Exp Rheumatol 2014;32(5 Suppl 85):S-75–9. [PubMed] [Google Scholar]
- 38. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. [DOI] [PubMed] [Google Scholar]
- 39. Albrecht K, Zink A. Poor prognostic factors guiding treatment decisions in rheumatoid arthritis patients: a review of data from randomized clinical trials and cohort studies. Arthritis Res Ther 2017;19:68.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baganz L, Richter A, Albrecht K. et al. Are prognostic factors adequately selected to guide treatment decisions in patients with rheumatoid arthritis? A collaborative analysis from three observational cohorts. Semin Arthritis Rheum 2019;48:976–82. [DOI] [PubMed] [Google Scholar]
- 41. Dusa D, Hanzu-Pazara L, Pana C, Tudorache M, Suta M. The impact of smoking on the activity of rheumatoid arthritis. ARS Medica Tomitana 2015;4:201–5. [Google Scholar]
- 42. Markatseli TE, Papagoras C, Drosos AA.. Prognostic factors for erosive rheumatoid arthritis. Clin Exp Rheumatol 2010;28:114–23. [PubMed] [Google Scholar]
- 43. Smolen JS, Landewé R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 44. Makol A, Davis JM, Crowson CS. et al. Time trends in glucocorticoid use in rheumatoid arthritis: results from a population-based inception cohort, 1980-1994 versus 1995-2007. Arthritis Care Res (Hoboken) 2014;66:1482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rothman KJ. Modern epidemiology. Little, Brown and Company, 1986. [Google Scholar]
- 46. Francisco V, Pino J, Gonzalez-Gay MA. et al. Adipokines and inflammation: is it a question of weight? Br J Pharmacol 2018;175:1569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huh JY, Park YJ, Ham M. et al. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells 2014;37:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Procaccini C, De Rosa V, Galgani M. et al. Role of adipokines signaling in the modulation of T cells function. Front Immunol 2013;4:332.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giles JT, Ling SM, Ferrucci L. et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum 2008;59:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.