Abstract
Objective
Data from two double-blind, randomized, Phase III studies were analysed to investigate the ability of Routine Assessment of Patient Index Data 3, DAS28 (CRP), modified (M)-DAS28 (CRP) and Simplified or Clinical Disease Activity Indices to predict structural damage progression in RA.
Methods
This post hoc analysis included data from the 2-year Abatacept vs adaliMumab comParison in bioLogic-naïvE RA subjects with background MTX (AMPLE) trial in biologic-naïve patients with active RA (<5 years) and an inadequate response to MTX, and the 12-month treatment period of the Assessing Very Early Rheumatoid arthritis Treatment (AVERT) trial in MTX-naïve patients with early RA (⩽2 years) and poor prognostic indicators. Adjusted logistic regression analysis assessed the relationship between baseline disease activity and structural damage progression (defined as change from baseline greater than the smallest detectable change) at 12 and 24 months in AMPLE and 6 and 12 months in AVERT. Areas under the receiver operating characteristic curves for the impact of baseline disease activity on structural damage progression were calculated.
Results
Adjusted logistic regression analyses included all randomized and treated patients in AMPLE (N = 646) and those who received abatacept plus MTX or MTX monotherapy in AVERT (N = 235). Baseline Routine Assessment of Patient Index Data 3, DAS28 (CRP) and M-DAS28 (CRP) scores significantly predicted structural progression at months 12 and 24 in AMPLE (P < 0.05) and months 6 and 12 in AVERT (P < 0.01), and were stronger predictors than Simplified or Clinical Disease Activity Indices.
Conclusion
In this post hoc analysis of two patient populations with RA, Routine Assessment of Patient Index Data 3, DAS28 (CRP) and M-DAS28 (CRP) were good at predicting structural damage.
Trial registration
ClinicalTrials.gov, http://clinicaltrials.gov: NCT00929864 (AMPLE); NCT01142726 (AVERT).
Keywords: CDAI, clinical trial, composite indices, DAS28 (CRP), disease activity, DMARDs, M-DAS28 (CRP), RAPID3, RA, SDAI
Rheumatology key messages
The first study to compare five measures of disease activity in two different RA populations.
Routine Assessment of Patient Index Data 3 is similar to DAS28 (CRP) and modified DAS28 (CRP) in predicting structural damage progression.
Routine Assessment of Patient Index Data 3, DAS28 (CRP) and modified DAS28 (CRP) are stronger predictors of structural damage progression than the Simplified or Clinical Disease Activity Indices.
Introduction
Prognostic factor research, which aims to identify disease measures associated with certain endpoints [1], is important for understanding disease progression and improving patient outcomes through individualized treatment strategies. In RA, high disease activity is associated with progression of joint damage, and is therefore a useful prognostic factor in guiding treatment decisions [2].
DAS28 (CRP), a validated measure of disease activity routinely used in RA clinical trials [3], is calculated from the number of tender and swollen joints (tender joint count, TJC; swollen joint count, SJC), Patient Global Assessment (PGA) of disease activity and CRP. The modified version, M-DAS28 (CRP), includes CRP, SJC in 28 joints (SJC28) and the Evaluators’ Global Assessment (EvGA) of disease activity, but excludes TJC and PGA [4]. Logistic regression analyses have shown that DAS28 (CRP) [5] and M-DAS28 (CRP) [4] positively predict joint damage and disease progression. However, in addition to the time required for the testing itself, the need for laboratory analysis means that results are not immediately available to the patient and physician at the time of the visit. Simplified measures of disease activity, namely the Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI), are highly associated with DAS28 (CRP) [6–8]. The CDAI does not require laboratory testing, thereby providing more immediate results to the clinician, but both SDAI and CDAI still require formal joint counts (SJC and TJC). The Routine Assessment of Patient Index Data 3 (RAPID3) is a pooled index of the three patient-reported ACR core data set measures—physical function, pain and PGA of disease activity [9–11]. However, the utility of RAPID3 in predicting structural damage progression is largely unknown, particularly in comparison with DAS28 (CRP) and M-DAS28 (CRP).
Abatacept, an inhibitor of T-cell co-stimulation, is effective in the treatment of RA across a range of different patient populations [12–17]. The AMPLE (Abatacept vs adaliMumab comParison in bioLogic-naïvE RA subjects with background MTX; NCT00929864) [18, 19] and AVERT (Assessing Very Early Rheumatoid arthritis Treatment; NCT01142726) [13] trials studied two distinct populations comprising patients with established active RA who were biologic-naïve and those with early RA, respectively.
This post hoc analysis of data from the AMPLE and AVERT trials examined the relationship between RAPID3 and other measures of baseline disease activity, and their ability to predict structural damage progression up to 24 months in patients with established or early RA.
Methods
Patient population and study design
Details of the study design and patient inclusion/exclusion criteria for the AMPLE and AVERT trials have been published in full previously [13, 18, 19].
Briefly, the AMPLE trial was a 2-year randomized, investigator-blinded study that enrolled adults (⩾18 years old) with active RA for ⩽5 years who were naïve to biologic therapy and had an inadequate response to MTX. Patients were randomized to receive s.c. abatacept 125 mg weekly or adalimumab 40 mg every 2 weeks, both administered in combination with stable-dose MTX [18, 19]. The primary endpoint in AMPLE was treatment non-inferiority, assessed according to the proportion of patients achieving ACR20 at 12 months [18].
The AVERT trial enrolled adults (⩾18 years old) with RA who had persistent symptoms for ⩽2 years, active clinical synovitis in at least two joints for ⩾8 weeks and DAS28 (CRP) ⩾3.2; were positive for anti-CCP2 antibodies; and were naïve to treatment with MTX or biologic therapy [13]. The trial included a 12-month treatment period in which patients were randomized (1:1:1) to s.c. abatacept 125 mg plus MTX, s.c. abatacept monotherapy or MTX monotherapy. At 12 months, patients with DAS28 (CRP) <3.2 could enter the 12-month withdrawal period, during which all treatment was stopped. After month 15, patients in the withdrawal period who experienced a flare of RA were eligible to enter a re-exposure period with open-label s.c. abatacept 125 mg plus MTX. The co-primary endpoint in the AVERT trial was DAS28 (CRP) <2.6 at months 12 and 18 [13].
The AMPLE and AVERT study protocols were approved by the institutional review boards and/or independent ethics committees at the participating sites. Due to the post hoc nature of this analysis, obtaining patient consent was not necessary.
Assessments
Disease activity at baseline was assessed in both the AVERT and AMPLE trials using RAPID3, DAS28 (CRP), M-DAS28 (CRP), CDAI and SDAI scoring systems. M-DAS28 (CRP) was calculated using the following equation: M-DAS28 (CRP) = 0.49 × ln (CRP) + 0.15 × SJC28 + 0.22 × EvGA + 1 [4].
In the AMPLE trial, radiographs of the hands and feet were taken on day 1 and at 12 and 24 months, and scored using the modified Sharp/van der Heijde scoring system [18–20]. In the AVERT trial, contrast MRI of the wrist and hand of the major affected upper limb were conducted at baseline and at 6, 12, 18 and 24 months. MRI osteitis, synovitis and erosion scores were assessed at each time point.
For the AMPLE trial, radiographic disease progression was defined as the change from baseline in modified total Sharp score (mTSS) greater than the smallest detectable change [18, 19]. For the AVERT trial, MRI erosion progression was defined as the change from baseline greater than the smallest detectable change [13]. For both trials, the smallest detectable change was calculated as s.d./[square root (2) × 1.96], where s.d. is the standard deviation of paired differences of change from baseline in total score between two readers [21].
Statistical analysis
For the purposes of the present post hoc analysis, data from the AMPLE and AVERT trials were analysed separately. Analysis of the AMPLE trial included pooled data for all randomized, treated patients from both the abatacept plus MTX and adalimumab plus MTX treatment arms. To be consistent with how patients are routinely treated in clinical practice, analysis of the AVERT trial included pooled data for all randomized, treated patients from the abatacept plus MTX and MTX monotherapy treatment groups, but not from the abatacept monotherapy group.
The cumulative probability of change in mTSS from baseline to 12 months was plotted for the AMPLE trial data, and the cumulative probability of change in MRI erosion score from baseline to 12 months was plotted for the AVERT trial data.
Unadjusted and adjusted logistic regression analyses were carried out on all randomized, treated patients and used to assess the relationship between baseline measures of disease activity, and radiographic progression at 12 and 24 months in the AMPLE trial and MRI erosion progression at 6 and 12 months in the AVERT trial. Odds ratios were adjusted for treatment (abatacept vs adalimumab), baseline radiographic total score, sex (male vs female), age (in 5-year increments), weight (in 10-kg increments) and RA duration (per year) in the AMPLE trial and for treatment (abatacept + MTX and abatacept alone vs MTX), baseline MRI erosion score, prior CS use (yes vs no), sex (male vs female), age (in 5-year increments), weight (in 10-kg increments) and RA duration (per year) in the AVERT trial.
The area under the receiver operating characteristic curve for the impact of baseline disease activity [RAPID3, DAS28 (CRP), M-DAS28 (CRP), SDAI and CDAI] on radiographic progression at 12 and 24 months in the AMPLE trial or on MRI erosion progression at 6 and 12 months in the AVERT trial was calculated.
Results
Patient population
In the AMPLE trial, a total of 646 patients were randomized and treated: 318 patients received abatacept plus MTX and 328 patients received adalimumab plus MTX [18]. Of the randomized, treated patients in AMPLE, 274 (86.2%) abatacept patients and 269 (82.0%) adalimumab patients completed month 12 of the study [18], and 252 (79.2%) abatacept patients and 245 (74.7%) adalimumab patients completed month 24 [19].
This retrospective analysis of the AVERT trial included only the abatacept plus MTX and MTX monotherapy treatment arms. A total of 351 patients were randomized and treated: 119 patients received abatacept plus MTX and 116 received MTX monotherapy [13]. Of the randomized, treated patients in AVERT, 103 (86.6%) patients in the abatacept plus MTX arm and 96 (82.8%) in the MTX arm completed the 12-month treatment period.
Baseline demographics and clinical characteristics for both trials have been reported previously [13, 18, 19]; key demographics and clinical characteristics of the two RA populations of AMPLE and AVERT are shown in Table 1. The mean age in the AMPLE and AVERT studies was 51 and 46–49 years, and the mean symptom duration was 1.7–1.9 and 0.50–0.58 years, respectively. In both studies, patients had highly inflammatory disease, severe disease activity [mean DAS28 (CRP) 5.3–5.5 and HAQ-Disability Index 1.4–1.5] and poor prognostic factors at baseline.
Table 1.
Baseline characteristics for patients with RA included in AMPLE and AVERT (overall ITT populations)
| AMPLE | AVERT | |||
|---|---|---|---|---|
| Characteristics | Abatacept + MTX (n = 318) | Adalimumab + MTX (n = 328) | Abatacept + MTX (n = 119) | MTX (n = 116) |
| Age, years | 51.4 (12.6) | 51.0 (12.8) | 46.4 (13.2) | 49.1 (12.4) |
| Weight, kg | 80.8 (20.3) | 80.1 (20.7) | 73.0 (17.7) | 74.1 (17.1) |
| Female, n (%) | 259 (81.4) | 270 (82.3) | 95 (79.8) | 89 (76.7) |
| White race, n (%) | 257 (80.8) | 256 (78.0) | 100 (84.0) | 102 (87.9) |
| RA symptom duration, years | 1.9 (1.4) | 1.7 (1.4) | 0.58 (0.50) | 0.50 (0.49) |
| RF positive, n (%) | 240 (75.5) | 254 (77.4) | 113 (95.0) | 110 (94.8) |
| Tender joint counta | 25.4 (15.3) | 26.3 (15.8) | 14.0 (7.7) | 12.8 (7.8) |
| Swollen joint counta | 15.8 (9.8) | 15.9 (10.0) | 11.2 (6.9) | 10.7 (7.0) |
| CRP, mg/dl | 1.6 (2.1) | 1.5 (2.8) | 1.8 (2.8) | 1.7 (2.2) |
| Physician Global Assessment | 58.8 (18.6) | 58.8 (18.9) | 58.4 (19.1) | 58.6 (20.3) |
| DAS28 (CRP) | 5.5 (1.1) | 5.5 (1.1) | 5.5 (1.3) | 5.3 (1.3) |
| HAQ-DI | 1.5 (0.7) | 1.5 (0.7) | 1.5 (0.68) | 1.4 (0.65) |
| Pain (0–100 mm VAS) | 63.1 (22.3) | 65.5 (21.8) | 62.4 (20.8) | 59.5 (18.3) |
Data are mean (s.d.) unless stated otherwise. AMPLE: Abatacept vs adaliMumab comParison in bioLogic-naïvE RA subjects with background MTX; AVERT: Assessing Very Early Rheumatoid arthritis Treatment; HAQ-DI: HAQ-Disability Index; ITT: intent-to-treat; VAS: visual analogue scale. aIn AMPLE, a total of 68 joints were assessed for tenderness and 66 were assessed for swelling; in AVERT, 28 joints were assessed for tenderness and swelling.
Impact of disease activity at baseline on radiographic and MRI disease progression
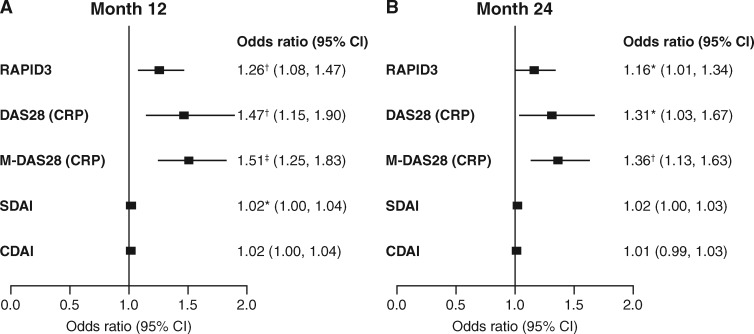
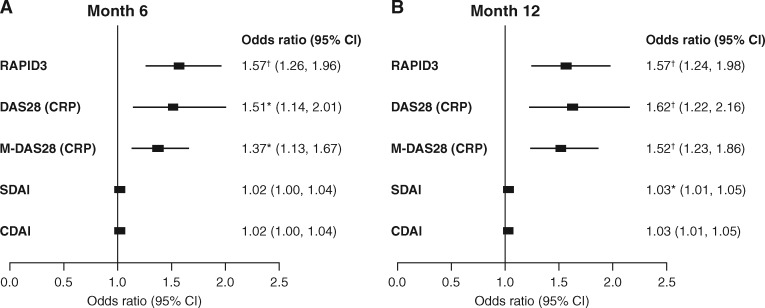
The cumulative probability of a change in mTSS in the AMPLE study and the cumulative probability of a change in MRI erosion score in the AVERT study are shown in Fig. 1. Unadjusted and adjusted logistic regression analyses, showing the relationship between baseline measures of disease activity and joint disease progression, are shown in supplementary Tables S1 and S2, available at Rheumatology online. Adjusted logistic regression analysis for all randomized and treated patients demonstrated that RAPID3, DAS28 (CRP) and M-DAS28 (CRP) scores at baseline were significant predictors of structural progression at months 12 and 24 in the AMPLE trial (P < 0.05; Fig. 2) and at months 6 and 12 in the AVERT trial (P < 0.01; Fig. 3). Baseline SDAI was a significant predictor of radiographic progression at month 12 (odds ratio 1.02, 95% CI 1.00, 1.04; P < 0.05) but not at month 24 in the AMPLE trial, and of MRI erosion progression at month 12 (odds ratio 1.03, 95% CI 1.01, 1.05; P < 0.01) but not at month 6 in the AVERT trial (Figs 2 and 3). By contrast, baseline CDAI was not a significant predictor at any time point in either study (Figs 2 and 3).
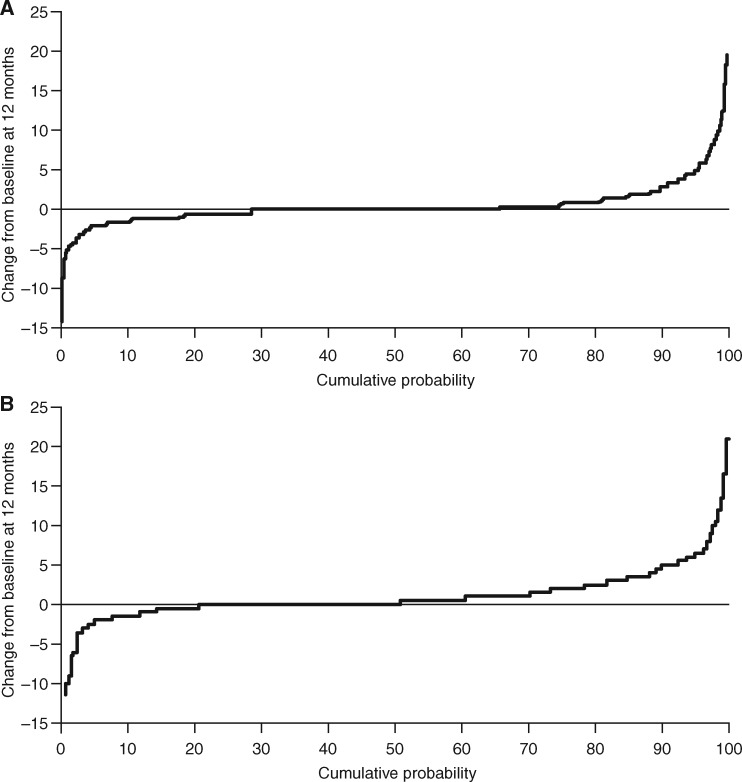
Fig. 1.
Cumulative probability of change in (A) mTSS (AMPLE) and (B) MRI erosion score (AVERT)
The analysis includes pooled data for all randomized and treated patients in each study (ITT population); for the AVERT trial, only the abatacept plus MTX and MTX monotherapy arms were included in the analysis. AMPLE: Abatacept vs adaliMumab comParison in bioLogic-naïvE RA subjects with background MTX; AVERT: Assessing Very Early Rheumatoid arthritis Treatment; ITT: intent-to-treat; mTSS: modified total Sharp score.
Fig. 2.
Relationship between baseline disease activity and radiographic progression at months 12 and 24 (AMPLE)
The adjusted logistic regression model included pooled data for all randomized and treated patients (ITT population). Odds ratios were adjusted for the following factors: treatment (abatacept vs adalimumab), baseline radiographic total score, sex (male vs female), age (in 5-year increments), weight (in 10-kg increments) and RA duration (per year). Radiographic progression was defined as change from baseline in mTSS greater than the smallest detectable change, which is calculated as s.d./[square root (2) × 1.96], where s.d. is that of the paired differences of change from baseline in total score between two readers. *P < 0.05; †P < 0.01; ‡P < 0.001. AMPLE: Abatacept vs adaliMumab comParison in bioLogic-naïvE RA subjects with background MTX; CDAI: Clinical Disease Activity Index; ITT: intent-to-treat; M-DAS28 (CRP): modified DAS28 (CRP); mTSS: modified total Sharp score; RAPID3: Routine Assessment of Patient Index Data 3; SDAI: Simplified Disease Activity Index.
Fig. 3.
Relationship between baseline disease activity and MRI erosion progression at months 6 and 12 (AVERT)
The adjusted logistic regression model included pooled data for all randomized and treated patients (ITT population); only data for the abatacept plus MTX and MTX monotherapy arms were included in the analysis. Odds ratios were adjusted for the following factors: treatment (abatacept + MTX and abatacept alone vs MTX), baseline MRI erosion score, prior corticosteroid use (yes vs no), sex (male vs female), age (in 5-year increments), weight (in 10-kg increments) and RA duration (per year). MRI erosion progression was defined as change from baseline greater than the smallest detectable change, calculated as s.d./[square root (2) × 1.96], where s.d. is that of the paired differences of change from baseline in total score between two readers. *P < 0.01; †P < 0.001. AVERT: Assessing Very Early Rheumatoid arthritis Treatment; CDAI: Clinical Disease Activity Index; ITT: intent-to-treat; M-DAS28 (CRP): modified DAS28 (CRP); RAPID3: Routine Assessment of Patient Index Data 3; SDAI: Simplified Disease Activity Index.
Adjusted logistic regression analysis also showed that there was no impact of treatment arm in either the AMPLE (abatacept plus MTX vs adalimumab plus MTX) or AVERT (abatacept plus MTX vs MTX monotherapy) trials on measures of baseline disease activity as predictors of radiographic outcomes (data not shown).
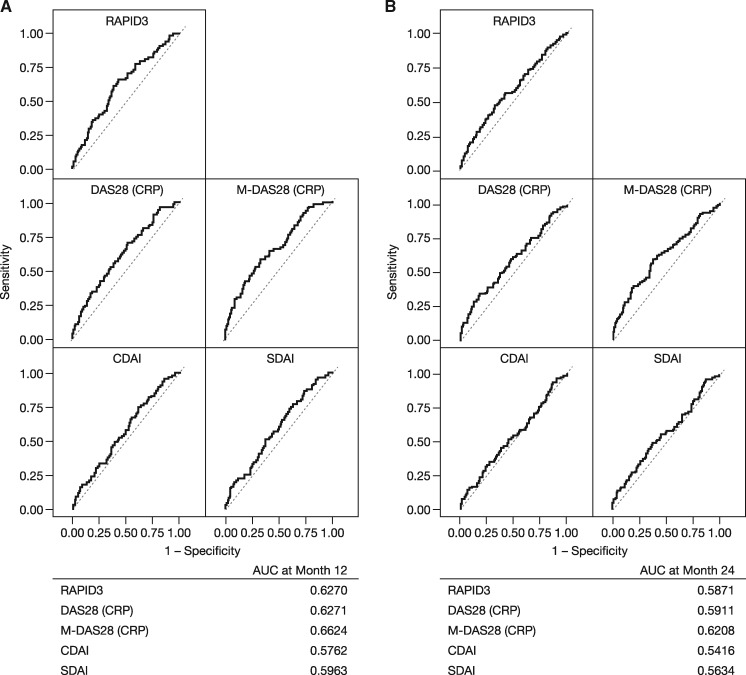
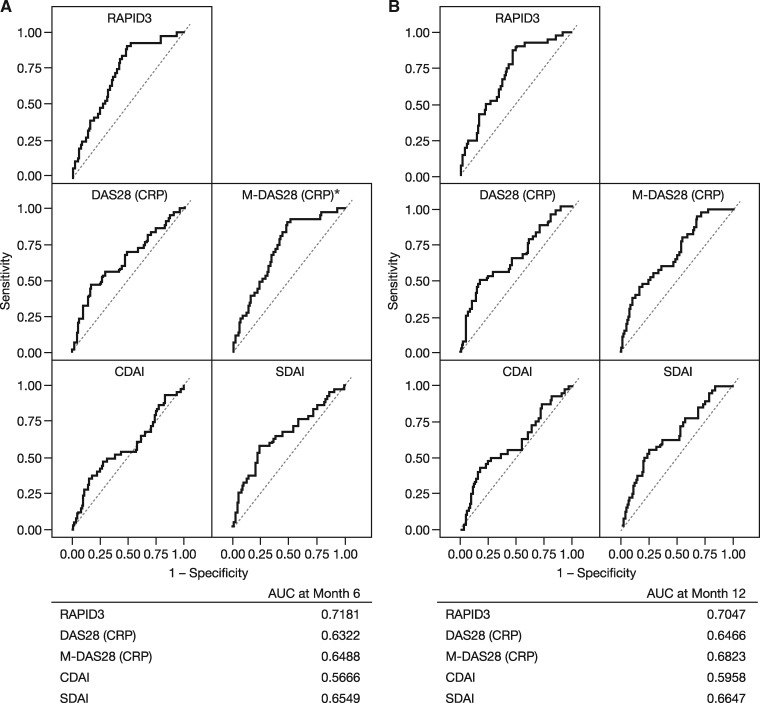
Analysis of receiver operating characteristic curves showed that RAPID3, DAS28 (CRP) and M-DAS28 (CRP) had higher predictive values (area under the curve) than CDAI or SDAI for radiographic progression at months 12 and 24 in the AMPLE trial (Fig. 4), and for MRI progression at months 6 and 12 in the AVERT trial (Fig. 5). Of the five disease activity measures evaluated, in the AMPLE trial, M-DAS28 (CRP) was the strongest predictor of radiographic progression; however, RAPID3 was as good at predicting structural damage as DAS28 (CRP; Fig. 4). In the AVERT trial, RAPID3 was the strongest predictor of MRI progression among the disease activity measures evaluated (Fig. 5).
Fig. 4.
ROC curves: baseline disease activity vs radiographic progression at (A) 12 and (B) 24 months (AMPLE)
The analysis includes pooled data for all randomized, treated patients from the AMPLE ITT population without missing data for 1- and 2-year radiographic progression for all baseline disease activity measures (n = 578 at 1 year and n = 506 at 2 years). AMPLE: Abatacept vs adaliMumab comParison in bioLogic-naïvE RA subjects with background MTX; AUC: area under the curve; CDAI: Clinical Disease Activity Index; ITT: intent-to-treat; M-DAS28 (CRP): modified DAS28 (CRP); RAPID3: Routine Assessment of Patient Index Data 3; ROC: received operating characteristic; SDAI: Simplified Disease Activity Index.
Fig. 5.
ROC curves: baseline disease activity vs MRI progression at (A) 6 and (B) 12 months (AVERT)
The analysis includes pooled data for all randomized, treated patients (abatacept plus MTX and MTX monotherapy arms only) from the AVERT ITT population without missing scores for 6-month or 1-year MRI erosion progression for all baseline disease activity measures [n = 215 for M-DAS28 (CRP) and n = 217 for all other measures at 6 months, and n = 183 for all measures at 2 years]. aM-DAS28 (CRP) was calculated based on a different statistical model [which also included DAS28 (CRP) and RAPID3] due to a different number of patients with non-missing values. In this model, the AUC for DAS28 (CRP) and RAPID3 at month 6 were 0.6281 and 0.7157, respectively. AUC: area under the curve; AVERT: Assessing Very Early Rheumatoid arthritis Treatment; CDAI: Clinical Disease Activity Index; ITT: intent-to-treat; M-DAS28 (CRP): modified DAS28 (CRP); RAPID3: Routine Assessment of Patient Index Data 3; ROC: received operating characteristic; SDAI: Simplified Disease Activity Index.
Discussion
In the AMPLE [18, 19] and AVERT [13] trials, sustained clinical, functional and radiographic benefits were observed with abatacept plus MTX treatment for 12 months. The two distinct patient populations in these trials were further analysed in this post hoc analysis to examine the ability of different measures of baseline disease activity, namely RAPID3, DAS28 (CRP), M-DAS28 (CRP), CDAI and SDAI, to predict structural damage progression. This adjusted logistic regression analysis, the first to directly compare these five different measures, demonstrated that RAPID3 is as good at predicting structural damage progression as DAS28 (CRP) and M-DAS28 (CRP).
A previous logistic regression analysis showed that baseline time-integrated DAS28 (CRP) positively predicted joint damage and disease progression in patients with early (<1 year) active RA [5]. In a separate logistic regression analysis of patients with established RA who had active disease despite treatment with MTX, baseline DAS28 (CRP), SDAI and CDAI all showed a significant, albeit relatively small in some cases, correlation with radiographic progression [4]. However, this analysis also showed that modified versions of these scores, M-DAS28 (CRP), M-SDAI and M-CDAI—all characterized by the exclusion of TJC28 and the PGA—had a greater ability to predict radiographic progression than their respective original disease activity measures. Thus, these results suggest that the association of clinical activity and subsequent radiographic progression can be strengthened by modifications to these scores [4]. Furthermore, both M-DAS28 (CRP) and DAS28 (CRP) were stronger predictors of radiographic progression than SDAI, CDAI or their modified counterparts [3, 4].
RAPID3 has been shown to provide similar quantitative information to DAS28 (CRP) and CDAI [10, 11]. In addition, in patients with moderate-to-severe RA who had an inadequate response to MTX, the ability of RAPID3 (with or without SJC) to predict a HAQ-Disability Index score ⩽0.5 (normal) or no worsening of HAQ-Disability Index or Genant-mTSS after 2 years of tocilizumab treatment was similar to that of SDAI and Boolean remission criteria [22]. In the present adjusted logistic regression analysis, data from the AMPLE trial showed that M-DAS28 (CRP) was the strongest predictor of radiographic progression. However, RAPID3 was as good at predicting structural damage as DAS28 (CRP) and M-DAS28 (CRP). Data from the AVERT trial demonstrated that RAPID3 was the strongest predictor of MRI progression. Additionally, data from both studies showed that RAPID3, M-DAS28 (CRP) and DAS28 (CRP) were all stronger predictors of structural damage progression than SDAI and CDAI.
There are certain limitations inherent to post hoc analyses and therefore these data should be considered in context. Neither of the original studies was designed or powered to test the ability of baseline disease activity measures to predict structural outcomes in patients with RA. In addition, the sample size was relatively small, particularly for analysis of the AVERT trial data, and these findings would benefit from validation in a larger patient population. The AMPLE and AVERT trials represent two different patient populations with divergent baseline demographics and disease characteristics and therefore data could not be pooled to create a larger sample size. However, adjusted logistic regression analysis by treatment group in both studies showed no impact of treatment arm on the ability of different measures of baseline disease activity to predict structural joint damage, allowing pooled data sets that included all randomized, treated patients in AMPLE and those who received abatacept plus MTX or MTX monotherapy in AVERT to be used. Finally, the impact of additional factors such as ACPA status or smoking history/status remains to be investigated, although such data may be difficult to collect from multicentre studies.
In summary, the present post hoc analysis, the first to directly compare five measures of disease activity, demonstrated that the assessment of disease activity at baseline according to RAPID3 had a similar ability to M-DAS28 (CRP) and DAS28 (CRP) to predict the risk of progression of structural joint damage in these clinical trial populations, regardless of therapy.
Supplementary Material
Acknowledgements
We would like to thank June Ye and Xiaoni Liu for their contribution to the statistical analyses. Professional medical writing and editorial assistance was provided by Catriona McKay, PhD, Stacey Reeber, PhD, and Bu Reinen, PhD, at Caudex, and was funded by Bristol-Myers Squibb. All authors (E.C.K., H.A.A., Y.Y. and M.J.B.) made substantial contributions to the study conception and design and/or acquisition of data, analysis and interpretation of data, drafting of the manuscript and revising it critically for important intellectual content. All authors had final approval of the version of the article to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was supported by Bristol-Myers Squibb.
Disclosure statement: E.C.K. has received grant/research support from AbbVie, Amgen, Gilead, Lilly, Merck, Pfizer, PuraPharm and Sanofi; has received fees as a consultant/advisory board member from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celltrion, Crescendo, F. Hoffmann-La Roche, Genentech, Gilead, Janssen, Lilly, Merck, Pfizer, Sandoz, Sanofi-Genzyme and Samsung Bioepsis; and has received honoraria for speaking from Amgen, AbbVie, Bristol-Myers Squibb, F. Hoffmann-La Roche, Janssen, Merck, Pfizer, Sanofi Genzyme and UCB. H.A.A. is a shareholder and employee of Bristol-Myers Squibb. Y.Y. has received grant/research support from Bristol-Myers Squibb, Celgene, Genentech and Sanofi; and has received fees as a consultant from Celgene, Genentech and Bristol-Myers Squibb. M.J.B. is a shareholder of Johnson & Johnson and Pfizer; has received fees as a consultant from AstraZeneca, Boehringer Ingelheim, Genentech/Roche, Horizon, Janssen, Novartis, Pfizer and Sanofi; and has served on a speaker bureau for AbbVie, Celgene, Novartis and Sanofi.
References
- 1. Riley RD, Hayden JA, Steyerberg EW. et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med 2013;10:e1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albrecht K, Zink A.. Poor prognostic factors guiding treatment decisions in rheumatoid arthritis patients: a review of data from randomized clinical trials and cohort studies. Arthritis Res Ther 2017;19:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prevoo ML, van’t Hof MA, Kuper HH. et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 4. Baker JF, Conaghan PG, Smolen JS. et al. Development and validation of modified disease activity scores in rheumatoid arthritis: superior correlation with magnetic resonance imaging-detected synovitis and radiographic progression. Arthritis Rheumatol 2014;66:794–802. [DOI] [PubMed] [Google Scholar]
- 5. Salaffi F, Carotti M, Ciapetti A. et al. Relationship between time-integrated disease activity estimated by DAS28-CRP and radiographic progression of anatomical damage in patients with early rheumatoid arthritis. BMC Musculoskelet Disord 2011;12:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smolen J, Breedveld F, Schiff M. et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244–57. [DOI] [PubMed] [Google Scholar]
- 7. Aletaha D, Smolen J.. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23:S100–8. [PubMed] [Google Scholar]
- 8. Aletaha D, Nell VP, Stamm T. et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pincus T, Swearingen CJ, Bergman MJ. et al. RAPID3 (Routine Assessment of Patient Index Data) on an MDHAQ (Multidimensional Health Assessment Questionnaire): agreement with DAS28 (Disease Activity Score) and CDAI (Clinical Disease Activity Index) activity categories, scored in five versus more than ninety seconds. Arthritis Care Res (Hoboken) 2010;62:181–9. [DOI] [PubMed] [Google Scholar]
- 10. Pincus T, Bergman MJ, Yazici Y. et al. An index of only patient-reported outcome measures, routine assessment of patient index data 3 (RAPID3), in two abatacept clinical trials: similar results to disease activity score (DAS28) and other RAPID indices that include physician-reported measures. Rheumatology (Oxford) 2008;47:345–9. [DOI] [PubMed] [Google Scholar]
- 11. Pincus T, Yazici Y, Bergman MJ.. RAPID3, an index to assess and monitor patients with rheumatoid arthritis, without formal joint counts: similar results to DAS28 and CDAI in clinical trials and clinical care. Rheum Dis Clin North Am 2009;35:773–8. [DOI] [PubMed] [Google Scholar]
- 12. Bathon J, Robles M, Ximenes AC. et al. Sustained disease remission and inhibition of radiographic progression in methotrexate-naive patients with rheumatoid arthritis and poor prognostic factors treated with abatacept: 2-year outcomes. Ann Rheum Dis 2011;70:1949–56. [DOI] [PubMed] [Google Scholar]
- 13. Emery P, Burmester GR, Bykerk VP. et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis 2015;74:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emery P, Durez P, Dougados M. et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial). Ann Rheum Dis 2010;69:510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Genovese MC, Becker JC, Schiff M. et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005;353:1114–23. [DOI] [PubMed] [Google Scholar]
- 16. Schiff M, Bessette L.. Evaluation of abatacept in biologic-naïve patients with active rheumatoid arthritis. Clin Rheumatol 2010;29:583–91. [DOI] [PubMed] [Google Scholar]
- 17. Westhovens R, Robles M, Ximenes AC. et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis 2009;68:1870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinblatt ME, Schiff M, Valente R. et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum 2013;65:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiff M, Weinblatt ME, Valente R. et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis 2014;73:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Heijde DM, van Riel PL, Nuver-Zwart IH. et al. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet 1989;1:1036–8. [DOI] [PubMed] [Google Scholar]
- 21. Navarro-Compán V, van der Heijde D, Ahmad HA. et al. Measurement error in the assessment of radiographic progression in rheumatoid arthritis (RA) clinical trials: the smallest detectable change (SDC) revisited. Ann Rheum Dis 2013;1067–70. [DOI] [PubMed] [Google Scholar]
- 22. Khawaja MN, Bergman MJ, Yourish J. et al. Routine Assessment of Patient Index Data 3 and the American College of Rheumatology/European League Against Rheumatism provisional remission definitions as predictors of radiographic outcome in a rheumatoid arthritis clinical trial with tocilizumab. Arthritis Care Res (Hoboken) 2017;69:609–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.