Abstract
Objective
To describe a configurable mobile health (mHealth) framework for integration of physiologic and environmental sensors to be used in studies focusing on the domain of pediatric asthma.
Materials and Methods
The Biomedical REAl-Time Health Evaluation (BREATHE) platform connects different sensors and data streams, contextualizing an individual’s symptoms and daily activities over time to understand pediatric asthma’s presentation and its management. A smartwatch/smartphone combination serves as a hub for personal/wearable sensing devices collecting data on health (eg, heart rate, spirometry, medications), motion, and personal exposures (eg, particulate matter, ozone); securely transmitting information to BREATHE’s servers; and interacting with the user (eg, ecological momentary assessments). Server-side integration of electronic health record data and spatiotemporally correlated information (eg, weather, traffic) elaborates on these observations. An initial panel study involving pediatric asthma patients was conducted to assess BREATHE.
Results
Twenty subjects were enrolled, during which BREATHE accrued seven consecutive days of continuous data per individual. The data were used to confirm knowledge about asthma (use of controller inhalers, time-activity behaviors, personal air pollution exposure), and additional analyses provided insights into within-day associations of environmental triggers and asthma exacerbations. Exit surveys focusing on mHealth usability, while positive, noted several translational challenges.
Discussion
Based on these promising results, a longitudinal panel study to evaluate individual microenvironments and exposures is ongoing. Lessons learned thus far reflect the need to address various usability aspects, including convenience and ongoing engagement.
Conclusion
BREATHE enables multi-sensor mHealth studies, capturing new types of information alongside an evolving understanding of personal exposomes.
Keywords: mHealth/telemedicine, environmental health, asthma
INTRODUCTION
Pediatric asthma is one of the most prevalent childhood diseases in the United States, affecting upwards of 6 million individuals.1 A primary reason for hospitalization for those under age 10, asthma is a leading cause of student absenteeism: almost 14 million school days are lost per year due to asthmatic symptoms.2–7 Uncontrolled asthma can limit the ability of a child to participate in outdoor or athletic activities, and pediatric patients with poorly controlled asthma report a decreased health-related quality of life.8 A deeper understanding of the factors influencing pediatric asthma and its effective management are needed.
Given the growing potential of mobile health (mHealth), in 2014 the National Institutes of Health (NIH) launched the Pediatric Research using Integrated Sensor Monitoring Systems (PRISMS) initiative. PRISMS’ mandate was to link cutting-edge wearable and environmental sensors with new informatics platforms to enable epidemiological studies of pediatric asthma.9 As part of this effort, the Los Angeles PRISMS Center designed BREATHE (Biomedical REAl-Time Health Evaluation), an mHealth integration framework for both off-the-shelf and newly developed sensors, with software for real-time data collection and interaction with subjects. BREATHE combines sensors with a smartwatch/phone platform, information from external resources (eg, electronic health record [EHR], local traffic, weather, air quality services), and server-side analysis to provide context around health-related events (eg, asthma symptoms/attacks) as they occur. As data accrues, a longitudinal picture of the individual is established, elucidating specific triggers and clinical response.
This article overviews BREATHE. We start with a summary of related mHealth research, providing perspective around our platform’s design and objectives. We describe BREATHE’s hardware and software architecture including sensors, devices, data sources, and collection framework. We also present initial results from a study demonstrating BREATHE’s abilities and lessons learned around the translational challenges of mHealth studies.
BACKGROUND AND SIGNIFICANCE
Early examples of technology to instruct and monitor asthmatic patients show positive impact on patient education and adherence.10–15 Reviews of digital asthma self-management systems find benefit in regards to outcomes,16–18 highlighting a variety of interventional approaches including education, electronic action plans, and reminders.19–25 However, these prior demonstrations mostly relied on self-reporting, with no incorporation of sensor-based technologies to automate data collection. Recently, several mHealth efforts have looked at asthma and related data collection and analysis issues. One of the first Apple ResearchKit apps captured regional air quality and health tracking.26,27 One study assessed the use of Bluetooth-enabled asthma inhalers in a large population to inform public policy around environmental exposures.28 More generally, under NIH’s Big Data to Knowledge program, the Mobile Data to Knowledge Center and Mobilize Center examined computational infrastructure related to machine learning at scale for mHealth data.29,30 These projects were contemporaneous to BREATHE, allowing us to learn from our colleagues to design an open, end-to-end platform for multiple sensor and data integration able to support epidemiological studies.
MATERIALS AND METHODS
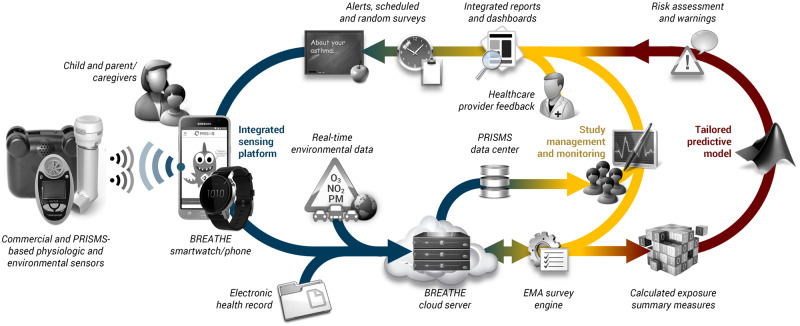
Figure 1 illustrates BREATHE’s high-level design, an interactive ecosystem involving different types of end-users: children with pediatric asthma and their caregivers; clinicians overseeing patient care; and researchers, including epidemiologists, biostaticians, and other health service researchers. BREATHE serves as an informatics layer that connects sensor developers by enabling data collection in a standardized framework; and facilitates data transfer and curation, with information relayed to a final data repository. Table 1 overviews the variety of data BREATHE presently collects.
Figure 1.
High-level architecture overview for the BREATHE platform. The system comprises three interacting components addressing sensor and data integration, user interaction, and analysis. A smartwatch/smartphone is the cornerstone for sensor connectivity and communication with the child/caregiver. BREATHE sends data to a cloud-based server in real-time, enabling continuous monitoring of events. Real-time dashboards permit biomedical researchers (eg, epidemiologists, environmental health scientists) to track subject progress and identify study issues. Healthcare providers can also view data through customized reports. Data are accrued over time in the backend to develop an individually tailored understanding of each child’s asthma. BREATHE: Biomedical REAl-Time Health Evaluation; EMA: ecological momentary assessment; PM: particulate matter.
Table 1.
BREATHE enables collection of a variety of data (physiologic, environmental, behavioral, clinical) to shed light on the diverse factors that contribute to pediatric asthma and its management
| Sensors | Self-reported measures | Geospatial data | Electronic health record |
|---|---|---|---|
|
|
|
|
Note: The platform provides multiple ways to collect real-time, contextualized information to support epidemiological studies.
Abbreviations: BREATHE: Biomedical REAl-Time Health Evaluation; EMA: ecological momentary assessment; GPS: global positioning system; PFTs: pulmonary function tests; VOCs: volatile organic compounds.
Integrating different sensors
We use an Android-based smartwatch and smartphone combination to facilitate ongoing interaction and data acquisition in our target demographic:
Smartwatch: BREATHE’s smartwatch serves as the primary hub for connecting registered devices via Bluetooth low energy (BLE) protocol. An on-watch app combines real-time data streams from local environmental and physiological sensors in real-time alongside on-device measurements (eg, heart rate, accelerometry). Using the smartwatch for sensor connectivity provides more reliable data collection, buffering, and transmission in a multi-sensor monitoring study—particularly in children—as it is wearable throughout the day and across different activities (eg, school, exercise). The smartwatch also provides a simple interface for limited sensor/device interaction (eg, indicating if proper spirometry is captured). All smartwatch data are directed to the smartphone for timestamping and geotagging.
Smartphone: As the power and processing resources of a smartwatch are limited, we use a smartphone to perform more computationally intensive tasks including data encryption and transmission to and from BREATHE’s cloud-based servers. The smartphone, with its larger screen, permits more involved user interactions including completion of ecological momentary assessment (EMA) surveys (see below).
One advantage of this setup for the pediatric population is that users do not have to always carry the smartphone given a wireless network or BLE connection to the smartwatch that remains active when in range (20–100 m, depending on the device and power31,32). Table 2 presents current commercial and PRISMS-specific sensors linked to BREATHE. We record metadata regarding the sensors, smartwatch, and smartphone (eg, sensor state, battery levels) to document the state in which data are captured. Given the variety of (proprietary) data formats from different sensors and manufacturers, we defined a standardized BREATHE JSON model for data transfer and storage to normalize representation (existing standards, like Open mHealth,37 were used when possible). In creating our platform, we also recognized the need to accommodate other modes of sensor connectivity. For example, some systems do not implement Bluetooth but instead use WiFi or their own centralized servers. We therefore established an application program interface to BREATHE’s server. The server is implemented in a Health Insurance Portability and Accountability Act-compliant Amazon Web Services framework and handles real-time requests including data pushes using hybrid encryption.38 Once data are received and decrypted, they are parsed, reformatted into BREATHE’s JSON format, and relayed to PRISMS’ Data Software and Coordinating Integration Center (DSCIC) for long-term archiving.
Table 2.
Current list of commercial and PRISMS-based sensors connected to the BREATHE platform
| Device/sensor | Variable | Provider | Communication |
|---|---|---|---|
| Moto 360 Smartwatch | Heart rate | Motorola | BLE |
| 3-axis accelerometry | BLE | ||
| 3-axis gyroscopy | BLE | ||
| AirBeam v1.033 | PM2.5 (air quality) | HabitatMap | BLE |
| Temperature | |||
| Relativity humidity | |||
| Asma-1 BT spirometer34 | FEV1 | Vitalograph | BLE |
| PEFR | |||
| Bluetooth inhaler sensor35 | Controller and rescue medication usage | Propeller Health | Central server |
| MA200 (personal device)36 | Black carbon | PRISMS Columbia/AethLabs | BLE |
| Brown carbon | |||
| MA350 + Dylos (residential device)36 | PM2.5 | PRISMS Columbia/AethLabs | Central server |
| Black carbon | |||
| Brown carbon | |||
| ASU sensor | O3 | PRISMS Arizona State University | BLE |
| Volatile organic compounds | |||
| Humidity | |||
| Temperature |
Note: BREATHE uses a smartwatch to enable data stream integration from local sensors and a smartphone for data transmission. Additional data collection to create a complete picture of the child’s health and real-time environment is made possible by accessing various online resources.
Abbreviations: ASU: Arizona State University; BLE: Bluetooth low energy; BREATHE: Biomedical REAl-Time Health Evaluation; FEV1: forced expiratory volume in 1 s; PEFR: peak expiratory flow rate; PRISMS: Pediatric Research using Integrated Sensor Monitoring Systems.
Deploying EMA surveys
EMA entails repeated sampling of subjects’ current behaviors and experiences in real-time and natural settings. Its aim is to minimize recall bias, maximize validity, and allow study of microprocesses that influence behavior in real-world contexts.39 Here, EMA enables an unfolding view of an individual’s reports about his/her asthma and environment over time. BREATHE allows EMA delivery in different ways:
Scheduled EMAs: Akin to a standardized daily or periodic questionnaire (eg, for assessing asthma symptoms), EMA delivery is tailored to an individual’s daily schedule (interval-contingent prompting). For instance, EMAs are not sent during school hours, but instead on waking up, immediately after school, and in the early evening.
Random EMAs: In line with traditional EMA methods, random surveys occur within a predesignated window of time to inform behaviors throughout the day (signal-contingent prompting).
Triggered EMAs: BREATHE’s background sensor monitoring mobile app processes incoming data streams to trigger system “intents” that will in real-time request users to answer EMA surveys “in the moment” on the smartphone when a given event (or context) of interest occurs (eg, usage of an emergency inhaler, worsening personal air quality; sensor-informed event-contingent prompting). Triggers are defined for a given study/sensor and comprise a logical rule or algorithm that outputs a Boolean value; and an action to execute, such as a specific EMA survey, given positive activation.
A subset of questions was always included in each EMA survey regardless of type, covering: asthma control (based on the Asthma Control Test and adapted to the past hour) on symptoms of chest tightness, wheezing, trouble breathing, and cough; context (eg, what were you doing and where were you before the phone alert appeared); nearby air pollution sources; and compliance with proper use and placement of the air monitor.
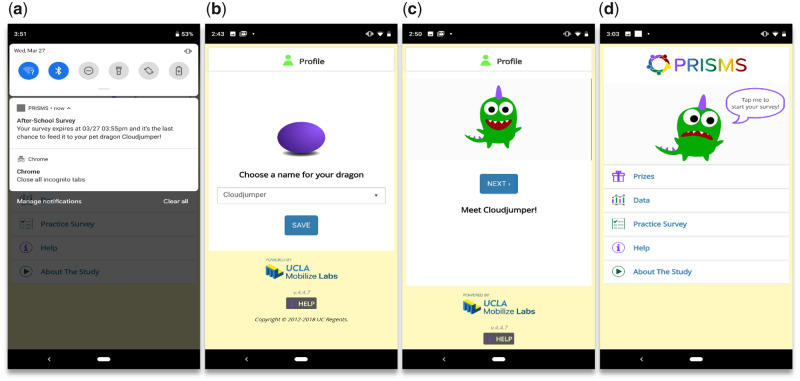
To support EMA survey design, delivery, and collation, we use ohmage40 for mobile data storage and a custom-built accompanying PRISMS smartphone app (Figure 2). Notifications are sent to the smartphone with a set number of repeating alerts before the EMA survey expires after a few minutes (given sufficient lapse in time, a response is no longer deemed “in the moment”). To entice younger children, we use gamification and personalization methods, allowing users to name a pet dragon character that “hatches” when the app is first started. The character “consumes” surveys as food and broadcasts a “feed me” message when an EMA survey is available to “eat.” Prizes are then awarded based on how well the dragon is kept full (ie, the level of survey completion). Markedly, a significant amount of user interaction and telemetry-related metadata is generated from the EMA process, providing insight about the individual and their level of mHealth engagement: the status of what happens to each notification (eg, suppressed, ignored, expired), the smartphone connectivity, and the degree of EMA survey interaction and adherence are compiled (eg, ignored, completed, partially completed).
Figure 2.
Screenshots of BREATHE’s ecological momentary assessment phone app. (A) A notification pops up on the smartphone at certain times throughout the day to collect information on symptoms, or if a sensed event triggers a specific survey. (B–D) To engage younger children in answering the surveys, gamification is employed, including the use of an animated dragon the user personally names when first starting the app. The pet dragon can be “fed” and kept happy whenever a survey is successfully answered, resulting in rewards. BREATHE: Biomedical REAl-Time Health Evaluation.
Elucidating a more complete patient context
Broadly, context provides the basis upon which to properly understand observed data and to subsequently make informed decisions. By way of illustration, an asthma attack that occurs when a child is outside playing in a neighborhood close to a busy freeway is very different from an occurrence when he/she is inside, sleeping. In some situations, localized sensor data may not be enough (or even be available) in which case external information must be sought. Context therefore motivates BREATHE’s goal of incorporating as much relevant and timely information as possible to fully characterize the patient, environment, and circumstances around observed daily activities:
Environmental asthma triggers: BREATHE gathers information around the major types of environmental asthma triggers: regional air pollution; local-scale air pollution, primarily from traffic; and greenness. Several online resources provide related real-time environmental information based on geographic location. Working with the Environmental Protection Agency’s AirNow system, BREATHE accesses real-time regional air quality (particulate matter, PM2.5; NO2, O3) from a national sensor network, and meteorological data. Different types of traffic data allow for further modeling of local air quality: information about traffic speed regarding freeways and major roadways/highways allows for pollutant concentration and dispersion modeling;41 and in Los Angeles, city streets are embedded with sensors to provide continuous traffic volume, speed, and congestion data.42 Proximity to parks and areas of greenness are given by a normalized difference vegetation index.
EHR integration: An important source of insight resides in the patient’s medical record: data on prescribed medications, allergies, current health status, and past exacerbations are critical to understanding the individual’s current situation and future risk. Working with pediatric asthma specialists, we identified key information to inform retrospective analysis of a subject’s data and prospective patient monitoring within clinical and epidemiological studies. Through our institutional clinical data warehouse, this patient information is automatically extracted from our EHR, which BREATHE then utilizes to provide relevant subject-level information. Notably, we endeavor to remap information to existing ontologies and data models, leveraging efforts like NIH’s Children’s Health Exposure Analysis Resource for clarity and compatibility.
Supporting different types of studies
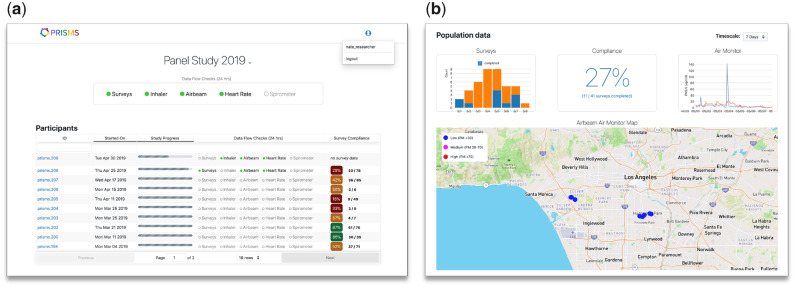
We developed BREATHE to support several use cases, including sensor development and testing studies that involve lab and field evaluation experiments; epidemiological studies deploying sensors prospectively to collect primary exposure and health outcome data through passive, observational data collection and/or real-time, context-sensitive interaction (eg, triggering an EMA survey after detecting a peak in combustion-related PM2.5); and health studies using secondary sources of existing sensor measurement data, such as from citizen sensor networks (eg, Purple Air), regulatory monitoring data (eg, AirNow), and from earlier sensor-based studies conducted within the PRISMS ecosystem. To facilitate these studies BREATHE has several supportive dashboards for subject and study management (Figure 3). For example, study coordinators use BREATHE’s online portal to register participants under a study protocol and to assign/release devices. The researcher dashboard provides real-time aggregate and drill-down individual views of sensor data streams, EMA survey completion rates, and other BREATHE data to monitor participants’ progress. Collectively, these dashboards support enrollment and quick interventions/troubleshooting in the case of prolonged loss of data transmission or other issues.
Figure 3.
BREATHE real-time dashboards supporting the implementation of epidemiological studies. To facilitate study recruitment and monitoring processes we created specific interfaces to expedite subject enrollment and troubleshooting. (A) An overall screen shows subject enrollment and a snapshot of each participant’s status toward a snapshot of each participant’s status toward study completion, the status of data transmission from their sensors, as well as the rate of EMA survey completion. Color coding and progress bars are used to provide visual cues to quick gage study progress. (B) An aggregate view of all participants’ data currently being monitored. The “air monitor” panel shows the time-series of personal PM2.5 concentrations from two ongoing participants (blue and orange lines) being monitored simultaneously in this example. Similarly, the map illustrates GPS data transmission with concurrent levels of PM2.5 being measured, at a coarse spatial resolution to protect privacy. This overview interface allows study researchers to drill-down into specific subjects. BREATHE: Biomedical REAl-Time Health Evaluation; EMA: ecological momentary assessment; GPS: global positioning system; PM: particulate matter.
Demonstrating BREATHE
We used BREATHE in an initial longitudinal panel study of 20 children recruited from the UCLA Pediatric Asthma Center of Excellence for monitoring over a 1-week period during May–December 2018. Individuals were consented in the clinic and trained in the use of the smartwatch/smartphone, EMA app, and three sensors (personal PM2.5; spirometry; inhaler). Baseline heart rate and spirometry values were collected for calibration purposes. The day following recruitment, a detailed baseline questionnaire was conducted over the phone by a trained research coordinator with the child and caregiver to collect asthma-related health and environmental data (perceptions about symptom control, typical activity patterns of the child, household operation conditions, indoor sources of air pollution, etc.). EHR-related data for the subjects were extracted, deidentified, and made available as part of the study dataset. We conducted telephone exit surveys after the 1-week study period to gather feedback on BREATHE, asking about users’ experiences, adherence, and challenges they encountered with the devices or the study. Participants were compensated for their time. The UCLA Institutional Review Board approved all study procedures and forms.
RESULTS
Data were collected on all 20 subjects (184 subject-days), with varying degrees of data completion (Table 3). On average, 1.1 GBs of data were gathered per individual, representing >530 000 observations/individual. Figures 4 and 5 depict some of this data. Participants were on average 13 years old (range, 9.8–16.4). Mean (SD) daily, personal PM2.5, relative humidity (RH), and temperature (T) exposures were 9.7 µg/m³ (12.3), 45.9% (5.1), and 80.6F (4.7), respectively, with 1-min integrated values similar on average but more variable (PM2.5 9.5 µg/m³ [17.4], RH 46.8% [6.9], and T 80.5F [5.4]).
Table 3.
Percentage of total monitoring time during a 1-week longitudinal panel study with complete data for continuous sensors (spirometry and medications are summarized differently), including periods of sleep
| Device/sensor | 25% | 50% Median | 75% | 100% Max |
|---|---|---|---|---|
| AirBeam | 13.7% | 22.2% | 36.2% | 83.3% |
| Accelerometer (smartwatch) | 19.5% | 40.2% | 50.4% | 75.2% |
| Gyroscope (smartwatch) | 24.5% | 45.4% | 55.4% | 75.3% |
| Heart rate (smartwatch) | 6.5% | 16.4% | 32.4% | 63.0% |
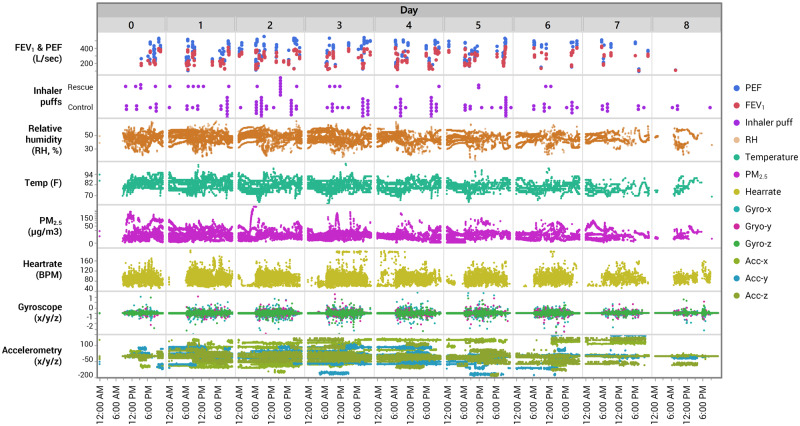
Figure 4.
Plots showing the highly time-resolved nature of continuous and intermittent data streams across all subjects. Information on personal environment (PM2.5, relative humidity, temperature), motion and physical activity (accelerometry, gyroscopy, heart rate), medication usage (rescue/control inhalers), and spirometry (FEV1, PEF) are illustrated. FEV1: forced expiratory volume in 1 s; PEF: peak expiratory flow.
Figure 5.
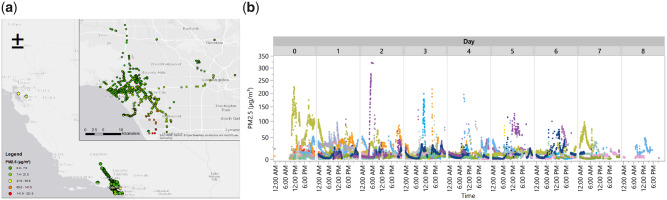
Examples of data collection from BREATHE. (A) Map of GPS trajectories across all subjects, correlated with 1-min PM2.5 concentrations. (B) Temporal variation in personal PM2.5 concentrations colored by subject. BREATHE: Biomedical REAl-Time Health Evaluation; GPS: global positioning system; PM: particulate matter.
Asthma inhaler medication sensors were provided to all 20 participants (6 rescue only, 6 controller only, 8 both); however, 3 participants encountered data connectivity issues. Of the 17 participants with active sensor(s), 15 used at least one of their sensor-enabled inhalers during the 1-week study period (10 used control, 6 used rescue). The average number of puffs dispensed was higher for controller inhalers (mean 10.8 puffs, SD 7.6, min 1, max 24 puffs), which are typically prescribed to be used on a regular daily basis, compared to rescue medications (mean 3.8 puffs, SD 3.2, min 1, max 8), which are taken as needed for symptoms. Only one of four participants with active rescue and control sensors used both (18 controller puffs, 1 rescue puff) while the remaining three used their controller inhaler only during the study. Cough, wheeze, and chest tightness were reported on 11%, 10%, and 11% of (n = 82) person-days per EMA responses.
Participants were asked to conduct three “good” spirometry (peak flow) maneuvers both in the morning and in the evening, with a maximum of six attempts per session. The quality of spirometry reading is reported by the sensor. Overall, 487 maneuvers were attempted by 18 participants (2 had spirometry data transmission issues). Of 77 total person-days with spirometry data, participants completed a minimum of six attempts on 40 (52%) days, and 76 days had at least one good maneuver (regardless of number of attempts). However, the minimum six “good” maneuvers were only obtained on 21 (28%) subject-days, with 55 (72%) days having at least one (median three) “good” maneuver. We calculated predicted lung function based on age, gender, and height for FEV1 (forced expiratory volume in 1 s [L]) and PEF (peak expiratory flow rate [L/s]) based on equations from Knudson et al.43 Mean Knudson-predicted FEV1 was 2.69 L (SD 0.61) and mean predicted PEF 5.93 L/s (SD 1.06) for the 20 participants. Daily mean percent predicted FEV1 was 71.5% (SD 26.3%) and PEF was 64.7% (SD 25.6%, n = 29 subject-days with AM and PM measurements) (Table 4).
Table 4.
Predicted (based on age, gender, and height) and calculated FEV1 and PEF (based on measurements, reported as % predicted) over study subjects
| Parameter | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Knudson predicted values | |||||
| FEV1 (L) | 20 participants | 2.69 | 0.61 | 1.66 | 3.91 |
| PEF (L/s) | 20 participants | 5.93 | 1.06 | 4.11 | 8.10 |
| Study measures, reported as percent of predicted | |||||
| FEV1 (% predicted), AM | 62 subject-days | 95 | 18 | 49 | 124 |
| FEV1 (% predicted), PM | 61 subject-days | 96 | 19 | 48 | 127 |
| PEF (% predicted), AM | 62 subject-days | 89 | 23 | 38 | 133 |
| PEF (% predicted), PM | 61 subject-days | 95 | 23 | 37 | 133 |
| PEF lability (%), (PEFam − PEFpm)/PEFam | 30 subject-days (AM and PM; excl. 1 outlier) | −2.8 | 17.1 | −40.5 | 35.9 |
Abbreviations: FEV1: forced expiratory volume in 1 s; PEF: peak expiratory flow; PM: particulate matter.
Asthma symptoms collected in EMAs generally represented good agreement with the inhaler medication use data. The percentage of cough, wheeze, and chest tightness symptoms reported was higher (∼25%) on days when rescue inhalers were used (at least one puff) compared to ∼8–9% on days when rescue inhalers were not used. Similarly, the percentage of cough, wheeze, and chest tightness symptoms was smaller (∼7%) on days when controller inhaler medication were used (at least one puff) compared to no use (∼11–13%). However, these comparisons were not statistically significant given the small sample size.
Example data analyses
Ongoing analyses on this dataset include modeling time-activity patterns and determinants of personal exposures, in addition to investigating exposure–response associations with the goal of identifying acute triggers of asthma exacerbations at a within-day to daily level. Initial examination of global positioning system (GPS) trajectories from our data revealed that participants spent on average approximately 87% of the time indoors. GPS trajectories are being used to construct activity space polygons and to assign momentary geospatial exposures (eg, traffic, greenness, proximity to parks, etc.). Context-sensitive (triggered) EMA’s are informing analyses investigating the effects of peak exposures (number of peaks, height of peak, dose) and specific air pollution sources related to primary combustion on acute asthma exacerbation, assessed several times within-day based on asthma symptom scores and rescue inhaler use. These analyses will advance our understanding of how multiple, co-occurring exposures coming from different sources with potentially lagged effects can interact with behaviors to increase the risk of an asthma attack.
Exit survey results
Usability
Of the 19 completed exit surveys, most children reported being satisfied (12, 63%) or very satisfied (6, 32%) with their overall experience using BREATHE and found it somewhat easy (10, 53%) or very easy (9, 47%) to use. Parents/caretakers were similarly very satisfied (9, 47%) or satisfied (8, 42%) with their child’s overall experience, with only 2 (11%) reporting being dissatisfied. Most parents agreed (13, 68%) or strongly agreed (5, 26%) that their child’s experience using BREATHE was enjoyable and easy to remember using as instructed (12 [63%] agree, 4 [21%] strongly agree, and 3 [15%] disagree). When asked if using BREATHE every day was burdensome or took too much time or effort for their child, most parents disagreed (7, 37%) or strongly disagreed (4, 21%), although 7 (37%) agreed and 1 (5%) strongly agreed. Most parents (14, 74%) also did not think that carrying the kit every day was uncomfortable for their child. With respect to the smartphone app specifically, of 17 respondents, 14 (82%) and 3 (18%) thought the smartphone app was very easy or somewhat easy to use, respectively.
The most commonly mentioned additional features suggested for ease of use or more pleasant experience were smaller or less bulky devices (especially the air monitor); more options to bundle the devices together; and integrating more sensors into the smartwatch (if not using only a smartwatch) with more durable protection (ie, more “sports” friendly). When asked what they would like to see to make BREATHE more useful to learn about their child’s asthma and triggers, the majority of parents mentioned seeing more information, especially about air pollutant exposures and how they relate to exacerbations specifically at school or during certain activities; more tracking of symptom responses; and reminders to use medication as scheduled.
mHealth study perceptions
Most respondents (17 out of 19, 89%) would be willing to participate in a similar study using mHealth technologies for understanding asthma if asked again (2 responded maybe). When asked about the longest duration of time the parent and child would consider acceptable if given the option of using BREATHE for an extended period to learn more about the child’s environmental exposures, experiences, and asthma, 2 weeks (7, or 41% of n = 17), 4 weeks (5, 29%), and 1 week (5, 29%) were the most frequent responses. One participant responded “Spring” because of the seasonal nature of the child’s asthma, another with an open-ended “any” duration of time, and one with 8 weeks as the maximum duration. Two participants mentioned in-school months as more challenging given limited device access while at school.
DISCUSSION
We designed BREATHE as a comprehensive mHealth platform to combine contemporary data sources to characterize symptoms and context more fully in daily environments. As a sensor integration framework, BREATHE simplifies connectivity to smart devices and server-side communication, allowing multiple sensors to be combined in a single study. As an information aggregator, BREATHE enables personal-level data collection through EMAs and retrieves additional external data (eg, environmental, clinical) to paint a complete picture of the individual. As a research enablement tool, BREATHE facilitates real-time study execution and sensor deployment through a variety of dashboards informing subject compliance and system status monitoring; combined views of subject data to develop new insights; as well as final deposit of all collected data into a repository. Collectively, BREATHE’s components aim to help build deeper, data-driven views of a disease by constructing detailed, individualized chronologies illuminating etiology and management over time. Our initial results, reported here, demonstrate an end-to-end system capable of executing mHealth studies. Building from this effort, a larger, formal panel study is now in progress using BREATHE and various PRISMS-based sensors, assessing personal microenvironments and exposures over a 2-week period in 40 participants. One of two funded NIH mHealth informatics PRISMS Centers, our sister site at the University of Utah44 addresses longitudinal residential monitoring, providing a complementary perspective. As PRISMS draws to a close, BREATHE will be released as an open source project with documentation and associated datasets through the U24.
Some lessons learned
Collaborating across the spectrum of mHealth stakeholders surfaced recurring themes around system complexity and usability, as well as subject privacy and confidentiality. We briefly touch upon these issues below.
Unintended consequences of multiple sensor studies
The appeal of deploying multiple sensors concurrently in a single study led to a quick realization of the potential interactions between devices and sensor triggers. For example, alpha testing of some sensors uncovered conflicting BLE implementations that would disconnect other devices when deployed together. Piloted study designs showed during testing that too many EMAs could be sent throughout the day; and in some situations, multiple different triggers were occurring simultaneously, creating an overly long series of questions to be answered. Thus, while enabling complex study designs, BREATHE did not allow researchers to anticipate the complexity arising from implementing multiple sensors and EMA surveys.
Usability
Despite the fast-paced change in mHealth technologies, usability remains a challenge. Usability is paramount to ensure that the end-user employs the sensors correctly (thereby providing correct contextual data and measurements), easily and routinely engages with the system when asked, and ultimately feels that using the system is acceptable, worth ongoing effort, and with benefit. mHealth usability encompasses multiple considerations and we address three here:45
Battery life and convenience: While sensors and smart devices are decreasing in physical size, battery life has not significantly improved, thus introducing added user burden to ensure devices are appropriately charged. At a minimum, sensors and devices must last throughout a full day of usage without recharging. BREATHE attempts to balance ongoing wireless communication (typically the largest cause of power usage) between devices and sampling frequency using different algorithms. Newer approaches to powering sensors may help.46 Pragmatically, having multiple devices (eg, watch, phone, sensors) that use different charging methods (eg, USB 2, USB-C, proprietary) is problematic and confusing for many users. Consensus and integration around the design of sensors and devices will further usability and consumer adoption.
Physical sensor/device appearance: In working with pediatric-aged individuals, we were sensitive to how they may feel if seen wearing a sensor. We posed questions regarding the physical appearance of devices to participants. Interestingly, while one PRISMS sensor collaboration indicated that children in their study liked sensor packaging to be in bright colors, our local testing instead found that individuals wanted neutral colors (eg, black), so carried devices would be inconspicuous to peers. This difference highlights the fact that no single design is likely to universally work, and flexibility is imperative to achieve acceptance across various users.
User interaction: Many mHealth studies suffer from user fatigue and disinterest once the novelty fades. More passive measurements are desirable ensuring minimal complexity with interactions designed to be continuously informative and rewarding (eg, gamification) and/or actionable. Many parents/caregivers and even the children themselves in our study were interested in seeing the data that BREATHE collected, hoping that it would directly help with asthma control. We considered the extent of user interaction given its deviation from a purely observational epidemiological study, as the return of information or any intervention may affect and subsequently change behavior (Figure 6). We were also cautious of how we returned data to these individuals given the potential to misinterpret information in the absence of established health evidence (eg, we aggregated continuous PM2.5 concentrations hourly to prevent users from associating minute-level, transient peaks as “harmful” or “concerning” for their health; we also avoided overlaying exposure and health data in the same plot so as not to imply a relationship). We thus created BREATHE’s end-user displays to curtail potential false inferences.
Figure 6.
Return of information to end-users (patients/caregivers). Our design decisions reflected on how a layperson may interpret the information collected by BREATHE and subsequently draw (incorrect) conclusions. (A) Information on FEV1 spirometry with an explanation is provided. (B) Regional air quality information is provided in terms of PM and spatial location, averaged over a period of time to mitigate specific conclusions about location and experienced symptoms. (C) Basic physiological information. BREATHE: Biomedical REAl-Time Health Evaluation; FEV1: forced expiratory volume in 1 s, PM: particulate matter.
Privacy and confidentiality
The PRISMS consortium discussed issues related to GPS information collected using BREATHE. Others describe similar concerns,47 wherein information about frequented locations (ie, home, school) are derivable through analyses. We disclosed the use of GPS to parents/caregivers during subject recruitment; most did not have concerns given our goal to keep information secure. While there is clear value in having this information to understand environmental exposures (eg, duration of time spent in relation to high pollution areas), generating deidentified GPS tracks for other researchers that preserves both spatial proximity and subject privacy is an additional step that must be taken. BREATHE captures GPS and sends this data to the PRISMS DSCIC, who is responsible for obfuscating individuals’ locations accordingly.
CONCLUSION
Based on our initial experience, we continue to enhance BREATHE in several ways including the development of more sophisticated EMA trigger logic; adaptive data collection methods, particularly as machine learning-based models accrue information about a given individual over time (and can thus tailor the types and frequency of data gathered to optimize decision-making); and assessment of what data may be usefully incorporated into the EHR.
Our experience thus far exemplifies that comprehensive and long-term testing with different user groups will be necessary to reach mHealth’s full promise. The infrastructure and support to execute these types of studies at scale are not widely in place but platforms like BREATHE can facilitate such development. While our objective in PRISMS is to support epidemiological studies, elements of this information could ultimately be useful in clinical monitoring of symptoms and adherence.
FUNDING
This work was supported by NIH/NIBIB U54 EB022002.
AUTHOR CONTRIBUTIONS
AATB is the Director of the Los Angeles PRISMS Center and Principal Investigator of the NIH funding, and wrote this manuscript. AH, RR, NJ, MKR, SO, FL, SE, ED, GD, FG, MS, and RH are all part of the Los Angeles PRISMS U54 and contributed to the overall design of the described system, evaluation, and the manuscript. All authors provided content and/or review of the submitted article.
ACKNOWLEDGEMENTS
The authors would like to thank members of the PRISMS consortium, including Ed Sakabu, Wren Reynolds, Cathy Trance, and Lauren Cullen of UCLA Mobilize Labs; Lisa Valencia of USC; Ken Craig and Changsy Chang of Sonoma Technology; Dimitrios Stripelis and Dr Jose-Luis Ambite of the U24 DSCIC (U24 EB021996); Drs Steven Chillrud and Matthew Perzanowski of Columbia University (U01 EB021983); Jeff Blair of AethLabs; and Dr NJ Tao of Arizona State University (U01 EB021980). The authors would also like to thank Dr Meredith Barrett of Propeller Health for useful comments on the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Center for Disease Control (CDC). Most Recent Asthma Data. 2018. https://www.cdc.gov/-asthma/most_recent_data.htm Accessed March 1, 2019.
- 2.American Lung Association. Asthma & Children Fact Sheet. 2018. http://www.lung.org/lung-disease/asthma/resources/facts-and-figures/asthma-children-fact-sheet.html#4 Accessed March 1, 2019.
- 3. Bloom B, Cohen RA, Freeman G.. National Center for Health Statistics (2010) Summary health statistics for US children: National Health Interview Survey. Vital Health Stat 2009; 10 (247): 1–82. [PubMed] [Google Scholar]
- 4. McDermott KW, Stocks C, Freeman WJ. Overview of Pediatric Emergency Department Visits, 2015 Agency for Healthcare Research and Quality (AHRQ). 2018. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb242-Pediatric-ED-Visits-2015.pdf Accessed March 1, 2019. [PubMed]
- 5. Meng Y-Y, Babey SH, Wolstein J.. Asthma-related school absenteeism and school concentration of low-income students in California. Prev Chronic Dis 2012; 9 (1): E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowler MG, Davenport MG, Garg R.. School functioning of US children with asthma. Pediatrics 1992; 90 (6): 939–44. [PubMed] [Google Scholar]
- 7. Lodha R, Puranik M, Kattal N, Kabra S.. Social and economic impact of childhood asthma. Indian Pediatr 2003; 40 (9): 874–9. [PubMed] [Google Scholar]
- 8. Juniper EF, Guyatt GH, Feeny DH, Ferrie P, Griffith LE, Townsend M.. Measuring quality of life in children with asthma. Qual Life Res 1996; 5 (1): 35–46. [DOI] [PubMed] [Google Scholar]
- 9. Sward KA, Bui AA, Ambite JL, Dellarco M. Pediatric Research Using Integrated Sensor Monitoring Systems (PRISMS): applying sensor technology and informatics to better understand asthma. In: Proc AMIA Fall Symp, 2016; Chicago, IL.
- 10. Finkelstein J, Cabrera MR, Hripcsak G.. Internet-based home asthma telemonitoring: can patients handle the technology? Chest 2000; 117 (1): 148–55. [DOI] [PubMed] [Google Scholar]
- 11. Krishna S, Francisco BD, Balas EA, Konig P, Graff GR, Madsen RW.. Internet-enabled interactive multimedia asthma education program: a randomized trial. Pediatrics 2003; 111 (3): 503–10. [DOI] [PubMed] [Google Scholar]
- 12. Rasmussen LM, Phanareth K, Nolte H, Backer V.. Internet-based monitoring of asthma: a long-term, randomized clinical study of 300 asthmatic subjects. J Allergy Clin Immunol 2005; 115 (6): 1137–42. [DOI] [PubMed] [Google Scholar]
- 13. Chan DS, Callahan CW, Hatch-Pigott VB, et al. Internet-based home monitoring and education of children with asthma is comparable to ideal office-based care: results of a 1-year asthma in-home monitoring trial. Pediatrics 2007; 119 (3): 569–78. [DOI] [PubMed] [Google Scholar]
- 14. Jan R-L, Wang J-Y, Huang M-C, Tseng S-M, Su H-J, Liu L-F.. An Internet-based interactive telemonitoring system for improving childhood asthma outcomes in Taiwan. Telemed J E Health 2007; 13 (3): 257–68. [DOI] [PubMed] [Google Scholar]
- 15. McLean S, Chandler D, Nurmatov U, et al. Telehealthcare for asthma: a Cochrane review. CMAJ 2011; 183 (11): E733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mosnaim GS, Powell LH, Rathkopf M.. A review of published studies using interactive Internet tools or mobile devices to improve asthma knowledge or health outcomes. Pediatr Allergy Immunol Pulmonol 2012; 25 (2): 55–63. [Google Scholar]
- 17. Morrison D, Wyke S, Agur K, et al. Digital asthma self-management interventions: a systematic review. J Med Internet Res 2014; 16 (2): e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hui CY, Walton R, McKinstry B, Jackson T, Parker R, Pinnock H.. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc 2017; 24 (3): 619–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rikkers-Mutsaerts E, Winters AE, Bakker MJ, et al. ; for the SMASHING Study Group. Internet-based self-management compared with usual care in adolescents with asthma: a randomized controlled trial. Pediatr Pulmonol 2012; 47 (12): 1170–9., [DOI] [PubMed] [Google Scholar]
- 20. Holtz B, Whitten P.. Managing asthma with mobile phones: a feasibility study. Telemed J E Health 2009; 15 (9): 907–9. [DOI] [PubMed] [Google Scholar]
- 21. Nkoy FL, Stone BL, Fassl BA, et al. Development of a novel tool for engaging children and parents in asthma self-management. AMIA Annu Symp Proc 2012; 2012: 663–72. [PMC free article] [PubMed] [Google Scholar]
- 22. Voorend-van Bergen S, Vaessen-Verberne AA, Landstra AM, et al. Monitoring childhood asthma: web-based diaries and the asthma control test. J Allergy Clin Immunol 2014; 133 (6): 1599–605. e1592. [DOI] [PubMed] [Google Scholar]
- 23. Lv Y, Zhao H, Liang Z, et al. A mobile phone short message service improves perceived control of asthma: A randomized controlled trial. Telemed J E Health 2012; 18 (6): 420–6. [DOI] [PubMed] [Google Scholar]
- 24. Gahleitner F, Legg J, Holland E, Pearson S, Roberts G.. The validity and acceptability of a text‐based monitoring system for pediatric asthma studies. Pediatr Pulmonol 2016; 51 (1): 5–12. [DOI] [PubMed] [Google Scholar]
- 25. Huckvale K, Morrison C, Ouyang J, Ghaghda A, Car J.. The evolution of mobile apps for asthma: an updated systematic assessment of content and tools. BMC Med 2015; 13 (1): 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan Y-FY, Wang P, Rogers L, et al. The Asthma Mobile Health Study, a large-scale clinical observational study using ResearchKit. Nat Biotechnol 2017; 35 (4): 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan Y-FY, Bot BM, Zweig M, et al. The Asthma Mobile Health Study, smartphone data collected using. Sci Data 2018; 5 (1): 180096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barrett M, Combs V, Su JG, Henderson K, Tuffli M; The AIR Louisville Collaborative. AIR Louisville: addressing asthma with technology, crowdsourcing, cross-sector collaboration, and policy. Health Affairs 2018; 37 (4): 525–34. [DOI] [PubMed] [Google Scholar]
- 29. Kumar S, Abowd GD, Abraham WT, et al. Center of excellence for mobile sensor data-to-knowledge (MD2K). JAMIA 2015; 22 (6): 1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ku JP, Hicks JL, Hastie T, Leskovec J, Ré C, Delp SL.. The mobilize center: an NIH big data to knowledge center to advance human movement research and improve mobility. J Am Med Inform Assoc 2015; 22 (6): 1120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Collotta M, Pau G, Talty T, Tonguz OK.. Bluetooth 5: a concrete step forward toward the IoT. IEEE Commun Mag 2018; 56 (7): 125–31. [Google Scholar]
- 32. Lonzetta MA, Cope P, Campbell J, Mohd JB, Hayajneh T.. Security vulnerabilities in Bluetooth technology as used in IoT. J Sens Actuator Netw 2018; 7 (3): 28. [Google Scholar]
- 33.Talkingspace.org. 2019. http://www.takingspace.org/aircasting/airbeam/ Accessed May 23, 2019.
- 34.Vitalograph. Asma-1 BT. 2019. https://vitalograph.com/product/162431/asma-1-bt Accessed May 23, 2019.
- 35.Propeller Health. 2019. https://www.propellerhealth.com/ Accessed May 23, 2019.
- 36.AethLabs. microAeth Family. 2019. https://aethlabs.com/microaeth Accessed May 23, 2019.
- 37.Open mHealth. Open Source Data Integration Tools. 2019. http://www.openmhealth.org/ Accessed May 26, 2019.
- 38. Silva BM, Rodrigues J, Canelo F, Lopes IC, Zhou L.. A data encryption solution for mobile health apps in cooperation environments. J Med Internet Res 2013; 15 (4): e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shiffman S, Stone AA, Hufford MR.. Ecological momentary assessment. Annu Rev Clin Psychol 2008; 4 (1): 1–32. [DOI] [PubMed] [Google Scholar]
- 40. Tangmunarunkit H, Hsieh C-K, Longstaff B, et al. Ohmage: a general and extensible end-to-end participatory sensing platform. ACM Trans Intell Syst Technol 2015; 6 (3): 1–21. [Google Scholar]
- 41. Snyder MG, Venkatram A, Heist DK, Perry SG, Petersen WB, Isakov V.. RLINE: a line source dispersion model for near-surface releases. Atmos Environ 2013; 77: 748–56. [Google Scholar]
- 42.City of Los Angeles, Department of Transportation. LADOT Live Traffic Information. 2012. http://trafficinfo.lacity.org/about-atsac.php. Accessed March 24, 2019.
- 43. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B.. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 1983; 127 (6): 725–34. [DOI] [PubMed] [Google Scholar]
- 44. Sward K, Patwari N, Gouripeddi R, Facelli J. An infrastructure for generating exposomes: Initial lessons from the Utah PRISMS platform. In: International Society of Exposure Science Annual Meeting, 2017; Research Triangle Park, NC.
- 45. Merchant R, Inamdar R, Henderson K, et al. Digital health intervention for asthma: Patient-reported value and usability. JMIR mHealth Uhealth 2018; 6 (6): e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dieffenderfer J, Goodell H, Bent B, et al. Wearable wireless sensors for chronic respiratory disease monitoring. In: IEEE Body Sensor Networks (BSN), Vol. 12, 2015; Cambridge, MA. [Google Scholar]
- 47. Kotz D, Gunter CA, Kumar S, Weiner JP.. Privacy and security in mobile health: A research agenda. Computer (Long Beach Calif) 2016; 49 (6): 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]