Abstract
The high-resistive grain boundaries are the bottleneck for Li+ transport in Li7La3Zr2O12 (LLZO) solid electrolytes. Herein, high-conductive LLZO thin films with cubic phase and amorphous domains between crystalline grains are prepared, via annealing the repetitive LLZO/Li2CO3/Ga2O3 multi-nanolayers at 600 °C for 2 h. The amorphous domains may provide additional vacant sites for Li+, and thus relax the accumulation of Li+ at grain boundaries. The significantly improved ionic conductivity across grain boundaries demonstrates that the high energy barrier for Li+ migration caused by space charge layer is effectively reduced. Benefiting from the Li+ transport paths with low energy barriers, the presented LLZO thin film exhibits a cutting-edge value of ionic conductivity as high as 6.36 × 10−4 S/cm, which is promising for applications in thin film lithium batteries.
Keywords: Solid electrolytes, LLZO, Thin film, Energy barrier, Ionic conductivity
Introduction
As the rise of 5G mobile telecommunication network, the power consumption of mobile terminals is expected to significantly increase [1–3]. Thin film lithium batteries (TFLBs) with high energy density, long cycle life, and excellent safety hold great promise for the integrated power sources in the intelligent terminals, such as smart cards [4]. To date, most of the workable TFLBs are based on LiPON solid electrolyte [5]. But the low ionic conductivity of LiPON limits the performance of TFLBs. Garnet Li7La3Zr2O12 (LLZO) is another promising alternative, due to its high ionic conductivity, wide electrochemical window, and stability against to Li metal anodes [6–10]. However, it remains a challenge to fabricate LLZO thin films with high ionic conductivity [11, 12].
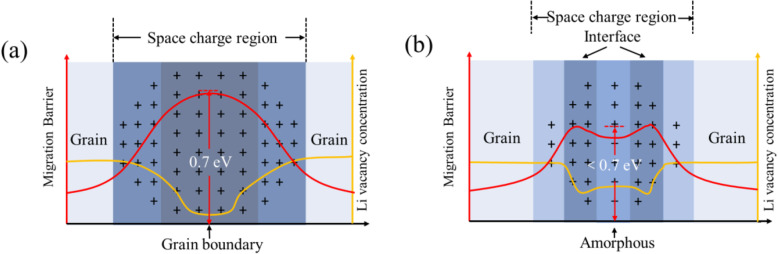
It is well-known that the energetically favorable paths for Li+ transport are one of the keys to achieving high ionic conductivity in solids [13, 14]. For the case of polycrystalline LLZO thin films, there are two energy barriers that determine the Li+ conducting performance. One is related to Li+ transport within a grain. The lattice sites possibly occupied by Li+ are energetically nonequivalent, and thus Li+ must get over an energy barrier (EBg) when it hops between these sites [15–18]. The other one is related to Li+ transport across the grain boundaries (GBs) [19, 20]. The lattice defects at GBs would cause the accumulation of Li+. A space charge layer would form because the unoccupied possible sites for Li+ around GBs are depleted (orange line in Fig. 1a). The space charge effect results in a high migration energy barrier (EBgb, red line in Fig. 1a) [21]. Typically, EBgb (~ 0.7 eV) is much higher than EBg (~ 0.3 eV) for the case of LLZO [20].
Fig. 1.
Illustration of the migration barrier and Li vacancy concentration at the conventional grain boundary (a), and the grain boundary with amorphous domains (b)
It has been reported that the possible sites for Li+ occupation in the LLZO with cubic phase, which are tetrahedral 24d site (Li1) and distorted octahedral 96 h site (Li2), are close to energetically equivalent [16, 22, 23]. Therefore, it is generally believed that the EBg in the cubic LLZO is moderate (~ 0.3 eV). Although the cubic phase of LLZO is metastable at room temperature (RT), the strategies to stabilize it through the doping of high valence cations, such as Al3+, Ga3+, and Ta5+, have been well developed [24–33]. Lobe et al. reported Al-doped LLZO thin films with ionic conductivity of 1.2 × 10−4 S/cm and activation energy of 0.47 eV [34]. It is generally believed that the high concentration of Li+ in the crystal lattice may further help to lower EBg [11, 13]. LLZO thin films with activation energy of 0.38 ± 0.02 eV have been prepared by introducing extra Li2O during thin film deposition [12, 35]. Li2O effectively compensated the lithium loss during sputtering-deposition. On the other hand, the strategy to address the conduction issues derived from high EBgb is few, although it is well-known the high-resistive GBs is the bottleneck for Li+ transport in LLZO [14, 21].
In this work, we demonstrate a LLZO thin film with amorphous domains between crystalline grains. The amorphous domains could provide extra Li+ vacancies [21, 36–38] and a lower migration barrier (~ 0.6 eV) [36] at GBs (Fig. 1b), which would weaken the space charge effect and lower EBgb (< 0.7 eV) [21, 38]. The presented LLZO thin film is prepared via repeatedly depositing the sequentially stacked nanolayers of LLZO, Li2CO3, and Ga2O3, and the following annealing (Fig. 2). The ultrathin thicknesses of each layer facilitate the interdiffusion in the multilayered structure, in turn enable Ga2O3 to help to stabilize the cubic phase of LLZO, and Li2CO3 to compensate the Li loss during deposition and annealing. Through carefully tuning the temperature of annealing, the LLZO thin film with the desired cubic phase and amorphous domains between grains was obtained. The electrochemical impedance measurement suggests the presented LLZO thin film solid electrolyte achieves a high ionic conductivity of 6.36 × 10−4 S/cm.
Fig. 2.
Schematics of fabrication procedures of the presented LLZO thin films
Methods
Fabrication of Ga-LLZO Thin Film Solid-State Electrolyte
The ultrathin layers of LLZO, Li2CO3, and Ga2O3 were sequentially deposited by radio frequency magnetron sputtering on polished MgO (100) substrates in pure Ar atmosphere. A multilayered thin film with the thickness of ~ 1500 nm (± 10%) was obtained by repeatedly deposited the triple-layered unit for 80 cycles (Figure S1). The targets of Li7La3Zr2O12 (99%), Li2CO3(99%), and Ga2O3(99.9%) mounted on 190 mm × 55 mm Cu backing plates are provided by Zhongnuo New Materials Manufacturing Co., China. The LLZO target used here is with desired cubic phase (Figure S2) and its density is 5.35 g/cm3. The pressure for the deposition is 1 Pa. The power density for LLZO deposition was 2.38 W cm−2, and 1.90 W cm−2 for Li2CO3 and Ga2O3. The as-deposited multilayered thin films were further annealed in pure oxygen (99.99%) for 2 h at 600 °C, 700 °C, and 800 °C, respectively.
Characterization
The thickness of each single layer of LLZO, Li2CO3, and Ga2O3 was determined by a step profiler (see details in Note S1 and Table S1). The crystallographic structure of the thin film was determined using X-ray diffraction (XRD), with Cu-Kα source and 2θ in the range from 10 to 60°. The chemical composition was characterized using time-of-flight secondary ion mass spectrometry (TOF-SIMS) and high-resolution transmission electron microscopy (HRTEM) equipped with an energy dispersive X-ray spectroscopy (EDX) detector. The ionic conductivity was determined in an in-plane test configuration at room temperature (25 °C), via measuring electrochemical impendence spectroscopy (EIS) with the applied frequency ranged from 3 × 106 to 1 Hz with a constant 30 mV AC amplitude. The aluminum contacts on the top of LLZO thin films were fabricated using direct current magnetron sputtering. The data of EIS was processed using the Zview software.
Results and Discussion
The LLZO thin film samples and their process parameters were summarized in Table 1. Sample #800-1 without Li-supplementary and Ga-doping exhibits a Li-deficient phase of La2Zr2O7 (LZO) after annealing at 800 °C for 2 h (Fig. 3a). After introducing Ga2O3 and Li2CO3, the diffraction peaks belonging to the cubic phase of LLZO are observed in the XRD pattern of #800-2 (Fig. 3b). This suggests that Ga dopant and extra Li would be favorable for the formation and/or stabilization of the desired cubic phase of LLZO. However, a strong diffraction peak at 28.2° indexed to LZO remains in the XRD pattern of #800-2. As the annealing temperature decreases to 700 °C, the intensity of the diffraction peak at 28.2° declined appreciably (Fig. 3c). These observations indicate that the high temperature annealing may lead to a severe Li loss even though extra Li is introduced. Through further reducing the annealing temperature to 600 °C, the thin film with a major phase of cubic LLZO and a negligible diffraction peak of LZO were obtained (Fig. 3d). Our observations are consistent with previous literature [11, 12], which report that the formation of the cubic phase in Ga-doped LLZO thin films is triggered at 600 °C, and LZO may form within 700 to 800 °C.
Table 1.
Samples of LLZO thin film solid electrolyte and their preparation parameters
| Samples | Annealing temperature (°C) | Gallium doping | Extra lithium |
|---|---|---|---|
| #800-1 | 800 | × | × |
| #800-2 | 800 | √ | √ |
| #700-1 | 700 | √ | √ |
| #600-1 | 600 | √ | √ |
Fig. 3.

XRD patterns of #800-1 (a), #800-2 (b), 700-1 (c), and #600-1 (d), and the standard diffraction patterns for cubic LLZO (e) and LZO (f)
Meanwhile, there are no diffraction peaks of Li2CO3 or Ga2O3 observed in the XRD patterns (Fig. 3). In addition, the compositional depth profile of #600-1 obtained using TOF-SIMS shows that the signal of CO32− is very low through the whole thin film (orange line in Fig. 4). And the competent content of Li in #600-1 is demonstrated by the high intensity of the recorded counts of 6Li+ (red line in Fig. 4). Thus, Li2CO3 in the multilayered thin film should have completely decomposed after annealing at 600 °C for 2 h, and effectively compensated the Li loss during thin film deposition and heat treatment. In addition, the undesired reaction between LLZO and CO2, which may form a low-conductive layer of Li2CO3, should be effectively prevented by the annealing atmosphere of pure oxygen. This inference is consistent with the measured high ionic conductivity of #600-1 (see below).
Fig. 4.

TOF-SIMS depth profiles of #600-1: 6Li+ (red), La3+ (green), Zr4+ (black), Ga3+ (indigo), CO32− (orange)
TOF-SIMS characterization also reveals the even distribution of 6Li+, La3+, Zr4+, and Ga3+ throughout the thin film #600-1 (Fig. 4). Typically, the interdiffusion of the precursors should be the speed control step in solid-state reactions. Huang et al. reported that the interdiffusion distance of the Ga2O3 and LLZO precursor layers was about 10–20 nm during an annealing process at 700 to 900 °C for 2 h. Thus, the thickness of each precursor layer in this study was set to be less than 10 nm. The multilayered structure based on the nanolayers of LLZO, Li2CO3, and Ga2O3 fabricated here, facilitates the homogenous mixing of the precursors via reducing their necessary diffusion length significantly. The uneven distribution of doped element observed in the LLZO thin films derived from the thicker precursor layers [11] are not observed here. An enrichment of Li in the interphase layer between the deposited thin film and MgO substrate can be observed. This should ascribe to the diffusion of Li+ into MgO lattice [34].
Briefly, the multilayers of LLZO/Li2CO3/Ga2O3 are well-mixed and reacted, benefiting from the sufficient interdiffusion among these ultrathin layers. Moreover, the reaction kinetics in the multilayered thin films with doped Ga and extra Li are optimized at 600 °C, for the sake of trying to prepare the cubic phase of LLZO with a low EBg.
As mentioned above, the Li+ conducting performance of LLZO is notably influenced by the structures at GBs (Fig. 1). The microstructure of #600-1 is carefully characterized using HRTEM. The crossed structure, which is a typical indicator of the reactions between LLZO and H2O or CO2 [35], can be observed in the HRTEM images. However, the XRD pattern and TOF-SIMS depth profile of #600-1 suggest that the as-prepared LLZO thin films prevent from reacting with H2O or CO2. Thus, it is reasonable to ascribe the formation of crossed structure to the exposure of LLZO thin films to air during the preparation of testing samples. Remarkably, amorphous domains between crystalline grains are observed (Fig. 5a, b). It indicates that #600-1 LLZO thin film should be not fully crystallized after annealing, which is consistent with the relative large full width at half maximum (FWHM) observed in the XRD pattern of #600-1 (Fig. 3d). EDX mapping reveals the uniform distribution of Ga, La, O, and Zr over the crystalline grains and amorphous domains (Fig. 5c–f). Therefore, we propose that the amorphous domains are composed of glassy Li-Ga-La-Zr-O oxides. It has been known that amorphous LLZO is a Li+ conductor. Its typical ionic conductivity and activation energy are 1 × 10−6 S/cm and ~ 0.6 eV, respectively [36]. The Li+-conductive amorphous domains would improve the physical contact between crystalline grains, and thus, the paths for Li+ transport in the thin films are with a better continuity [20]. More importantly, the amorphous domains between the grains are potential to provide additional vacant sites for Li+ [21, 36–38]. The electrostatic repulsion between Li+ would be reduced, compared with the conventional LLZO GBs in which the possible sites for Li+ occupation are depleted [19, 20]. In other words, the amorphous domains may diminish the cacoethic space charge effects and lower the EBgb for Li+ transport across GBs (Fig. 1b). Consequently, it is reasonable to expect a reduced grain boundary resistance (Rgb) in the present LLZO thin film solid electrolyte #600-1.
Fig. 5.
HRTEM images (a, b) and elemental mapping (c for Ga, d for La, e for O, f for Zr) of LLZO thin film #600-1
The EIS measurements of the presented LLZO thin films are conducted with the in-plane test configuration shown in Fig. 6a. Their total ionic conductivities (σtotal) can be calculated according to the equation:
| 1 |
Fig. 6.
a The in-plane test configuration for EIS measurements. b The Nyquist plot of impedance spectrum of LLZO thin film #600-1 measured at room temperature, insert shows the equivalent circuit for EIS analysis
where L is the distance between the two contacting electrodes, S is the electrode area, and Rtotal is the total resistance of LLZO thin film determined through EIS measurements. The Nyquist plots of the measured impedance spectra (Fig. 6b and Figure S2a and S2b) are fitted with the equivalent circuit depicted in the inserts, which consists of a series combination of a constant phase element (CPE) with two circles of a resistor in parallel with a CPE. Rbulk and Rgb in the equivalent circuit represent the bulk resistance and the grain boundary resistance of the LLZO thin film. The grain boundary ionic conductivities (σgb) of LLZO thin films are also normalized to the distance of two parallel contacting electrodes, and can be calculated according to the following equation [39]:
| 2 |
where Cbulk and Cgb are the bulk capacitance and the grain boundary capacitance, which can be calculated using the equation (3) based on the fitted values of their corresponding R (Rbulk and Rgb) and CPE (CPEbulk and CPEgb) [34, 40].
| 3 |
The geometrical parameters (L and S) and the fitted values of the elements in the equivalent circuit (Rtotal, Rbulk,Rgb, Cbulk, and Cgb) are summarized in Table S2. Table 2 summarizes the calculated σbulk, σgb, and σtotal at room temperature of the presented LLZO thin films. σtotal of #800-1 is lower than 10−8 S/cm since it is dominated by the Li-poor phase of LZO. The samples with Ga dopant and extra Li, #800-2, #700-1, and #600-1, possess the σtotal of 5.63 × 10−7, 3.89 × 10−5, and 6.36 × 10−4 S/cm, respectively. This trend may be caused by two reasons. First, the proportion of high-resistive LZO in the prepared thin films is trimmed down as the annealing temperature is reduced, which is demonstrated by their XRD patterns (Fig. 3b–d). Second, the intensities of the diffraction peaks of #600-1 are much lower than that of the other two. Its low crystallinity may be related to the formation of amorphous domains between crystalline grains. As mentioned above, the amorphous domains between crystalline grains may lower the energy barrier for Li+ transport across GBs (Fig. 1). In addition, the grain size of #600-1 is about 50 nm (Figure S3), which is smaller than the common values (hundreds of nanometers) reported in previous studies and may lead to a greater number of high-resistive GBs. However, the ionic conductivity of #600-1 reaches a cutting-edge value. These facts give a good indication that the strategy presented here to lower the energy barrier for Li+ transport across GBs is effective. The analysis of EIS data indeed shows that σgb of #600-1 is closed to 2 orders of magnitude higher than that of #700-1, although it is difficult to quantify σbulk and σgb of #800-1 and #800-2 because of their high grain boundary resistance.
Table 2.
Bulk ionic conductivities (σbulk), grain boundary ionic conductivities (σgb), and total ionic conductivities (σtotal) at room temperature of the presented LLZO thin films
| Sample name | σbulk (S/cm) | σgb (S/cm) | σtotal (S/cm) |
|---|---|---|---|
| #800-1 | / | / | 7.86 × 10−9 |
| #800-2 | / | / | 5.63 × 10−7 |
| #700-1 | 1.03 × 10−4 | 6.23 × 10−6 | 3.89 × 10−5 |
| #600-1 | 1.33 × 10−3 | 1.21 × 10−4 | 6.36 × 10−4 |
Conclusions
In summary, LLZO thin films with cubic phase and amorphous domains between crystalline grains were obtained through introducing Ga dopant and extra Li, and carefully optimizing annealing temperature. Firstly, the small energy disparity between Li+ sites in the LLZO lattice of the cubic phase leads to a low energy barrier for Li+ transport within crystalline grains. More importantly, the amorphous domains provide additional Li+ vacant sites around GBs and thus lower the energy barriers for Li+ transport across GBs via relaxing the space charge effects. As a result, benefiting from the Li+ transport paths with low migration energy barriers, the presented LLZO thin film exhibits an ionic conductivity of 6.36 × 10−4 S/cm at room temperature, which is attractive for applications in TFLBs.
Supplementary information
Additional file 1: Reduced energy barrier for Li+ transport across grain boundaries with amorphous domains in LLZO thin-films. Note S1: The determination of thicknesses of each single layer of LLZO, Li2CO3, and Ga2O3.Table S1. The thickness of each single-layer thin film. Table S2. The geometrical parameters (L and S) of electrodes and the fitted values of the elements in the equivalent circuit (Rtotal, Rbulk, Rgb, Cbulk, and Cgb) of the different thin films for calculating σtotal, σbulk, and σgb at room temperature. Figure S1. The thickness of #600-1 (1.516 μm) determined in its cross-sectional SEM image. Figure S2. XRD patterns of the LLZO target used in this study. Figure S3. The grain size of #600-1(~50 nm) determined by SEM image. Figure S4. The Nyquist plots of impedance spectra of LLZO thin-films #700-1 (a), #800-1 (green in b), and #800-2 (brown in b) measured at room temperature, inserts show the equivalent circuits for EIS analysis.
Acknowledgements
Not applicable.
Abbreviations
- Li
Lithium
- Li7La3Zr2O12 (LLZO)
Lithium lanthanum zirconate
- La2Zr2O7 (LZO)
Lanthanum zirconate
- Li2CO3
Lithium carbonate
- Ga2O3
Gallium(III) oxide
- MgO
Magnesium Oxide
- Ga
Gallium
- La
Lanthanum
- O
Oxygen
- Zr
Zirconium
- Al
Aluminum
- Ta
Tantalum
- Ar
Argon
- Cu
Copper
- TFLBs
Thin film lithium batteries
- LiPON
Lithium phosphorus oxynitride
- Li2O
Lithium oxide
- EBg
Migration energy barrier for Li+ transport within a grain
- EBgb
Migration energy barrier for Li+ transport across the grain boundaries
- GBs
Grain boundaries
- σtotal
Total ionic conductivity
- σgb
Grain boundary ionic conductivity
- σbulk
Bulk ionic conductivity
- Cbulk
Bulk capacitance
- Cgb
Grain boundary capacitance
- R
Resistor
- CPE
Constant phase element
- L
Distance between the two contacting electrodes
- S
Electrode area
- XRD
X-ray diffraction
- TOF-SIMS
Time-of-flight secondary ion mass spectrometry
- HRTEM
High-resolution transmission electron microscopy
- EDX
Energy dispersive X-ray spectroscopy
- EIS
Electrochemical impendence spectroscopy
- AC
Alternating current
Authors’ Contributions
Yanlin Zhu, Xiaokun Zhang, Zongkai Yan, and Yong Xiang conceived and designed the experiments. Yanlin Zhu, Shuai Liu, and Zongkai Yan performed the experiments. Yanlin Zhu, Xiaokun Zhang, and Zongkai Yan analyzed the data. Yanlin Zhu, Yilan Pan, and Xiaokun Zhang wrote the paper. All authors read and approved the final manuscript.
Funding
This research work was financially supported by the National Science Funds of China (Grant No. 21905040), the Fundamental Research Funds for Central Universities (Contract No. ZYGX2019Z009), and the startup funds from the University of Electronic Science and Technology of China.
Availability of Data and Materials
The authors declare that the materials and data are promptly available to readers without undue qualifications for material transfer agreements. All data generated or analyzed during this study are included in this article.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yanlin Zhu, Email: yanlinzhu@outlook.com.
Shuai Wu, Email: 201722170226@std.uestc.edu.cn.
Yilan Pan, Email: ylpan@uestc.edu.cn.
Xiaokun Zhang, Email: zxk@uestc.edu.cn.
Zongkai Yan, Email: yanzongkai@uestc.edu.cn.
Yong Xiang, Email: xyg@uestc.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s11671-020-03378-x.
References
- 1.Ostfeld AE, Gaikwad AM, Khan Y, Arias AC. High-performance flexible energy storage and harvesting system for wearable electronics. Sci Rep. 2016;6:26122. doi: 10.1038/srep26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janek J, Zeier WG. A solid future for battery development. Nature Energy. 2016;1(9):1–4. doi: 10.1038/nenergy.2016.141. [DOI] [Google Scholar]
- 3.García Núñez C, Manjakkal L, Dahiya R. Energy autonomous electronic skin. npj Flexible Electronics. 2019;3(1):1–24. doi: 10.1038/s41528-018-0045-x. [DOI] [Google Scholar]
- 4.Chang J, Shang J, Sun Y, Ono LK, Wang D, Ma Z, Huang Q, Chen D, Liu G, Cui Y, Qi Y, Zheng Z. Flexible and stable high-energy lithium-sulfur full batteries with only 100% oversized lithium. Nat Commun. 2018;9(1):4480. doi: 10.1038/s41467-018-06879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Ma C, Chi M, Liang C, Dudney NJ. Solid electrolyte: the key for high-voltage lithium batteries. Adv Energy Materials. 2015;5(4):1401408. doi: 10.1002/aenm.201401408. [DOI] [Google Scholar]
- 6.Teng S, Tan J, Tiwari A. Recent developments in garnet based solid state electrolytes for thin film batteries. Curr Opinion Solid State Materials Sci. 2014;18(1):29–38. doi: 10.1016/j.cossms.2013.10.002. [DOI] [Google Scholar]
- 7.Albertus P, Babinec S, Litzelman S, Newman A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nature Energy. 2017;3(1):16–21. doi: 10.1038/s41560-017-0047-2. [DOI] [Google Scholar]
- 8.Gao Z, Sun H, Fu L, Ye F, Zhang Y, Luo W, Huang Y. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv Mater. 2018;30(17):e1705702. doi: 10.1002/adma.201705702. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Geng Z, Han C, Fu Y, Li S, He Y-b, Kang F, Li B. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J Power Sources. 2018;389:120–134. doi: 10.1016/j.jpowsour.2018.04.019. [DOI] [Google Scholar]
- 10.Shoji M, Cheng EJ, Kimura T, Kanamura K. Recent progress for all solid state battery using sulfide and oxide solid electrolytes. J Physics D. 2019;52(10):103001. doi: 10.1088/1361-6463/aaf7e2. [DOI] [Google Scholar]
- 11.Pfenninger R, Struzik M, Garbayo I, Stilp E, Rupp JLM. A low ride on processing temperature for fast lithium conduction in garnet solid-state battery films. Nature Energy. 2019;4(6):475–483. doi: 10.1038/s41560-019-0384-4. [DOI] [Google Scholar]
- 12.Rawlence M, Filippin AN, Wäckerlin A, Lin TY, Cuervo-Reyes E, Remhof A, Battaglia C, Rupp JLM, Buecheler S. Effect of gallium substitution on lithium-ion conductivity and phase evolution in sputtered Li7–3xGaxLa3Zr2O12 thin films. ACS Applied Materials & Interfaces. 2018;10(16):13720–13728. doi: 10.1021/acsami.8b03163. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Richards WD, Ong SP, Miara LJ, Kim JC, Mo Y, Ceder G. Design principles for solid-state lithium superionic conductors. Nat Mater. 2015;14(10):1026–1031. doi: 10.1038/nmat4369. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Xie J, Shi F, Lin D, Liu Y, Liu W, Pei A, Gong Y, Wang H, Liu K, Xiang Y, Cui Y. Vertically aligned and continuous nanoscale ceramic-polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity. Nano Lett. 2018;18(6):3829–3838. doi: 10.1021/acs.nanolett.8b01111. [DOI] [PubMed] [Google Scholar]
- 15.Buschmann H, Dolle J, Berendts S, Kuhn A, Bottke P, Wilkening M, Heitjans P, Senyshyn A, Ehrenberg H, Lotnyk A, Duppel V, Kienle L, Janek J. Structure and dynamics of the fast lithium ion conductor “Li7La3Zr2O12”. Phys Chem Chem Phys. 2011;13(43):19378–19392. doi: 10.1039/c1cp22108f. [DOI] [PubMed] [Google Scholar]
- 16.Wagner R, Redhammer GJ, Rettenwander D, Senyshyn A, Schmidt W, Wilkening M, Amthauer G. Crystal structure of garnet-related Li-ion conductor Li7–3xGaxLa3Zr2O12: fast Li-ion conduction caused by a different cubic modification? Chemistry Materials. 2016;28(6):1861–1871. doi: 10.1021/acs.chemmater.6b00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernstein N, Johannes MD, Hoang K. Origin of the structural phase transition in Li7La3Zr2O12. Phys Rev Lett. 2012;109(20):205702. doi: 10.1103/PhysRevLett.109.205702. [DOI] [PubMed] [Google Scholar]
- 18.Dhivya L, Murugan R. Effect of simultaneous substitution of Y and Ta on the stabilization of cubic phase, microstructure, and Li(+) conductivity of Li7La3Zr2O12 lithium garnet. ACS Appl Mater Interfaces. 2014;6(20):17606–17615. doi: 10.1021/am503731h. [DOI] [PubMed] [Google Scholar]
- 19.Yu S, Siegel DJ. Grain boundary contributions to Li-ion transport in the solid electrolyte Li7La3Zr2O12 (LLZO) Chemistry of Materials. 2017;29(22):9639–9647. doi: 10.1021/acs.chemmater.7b02805. [DOI] [Google Scholar]
- 20.Shiiba H, Zettsu N, Yamashita M, Onodera H, Jalem R, Nakayama M, Teshima K. Molecular dynamics studies on the lithium ion conduction behaviors depending on tilted grain boundaries with various symmetries in garnet-type Li7La3Zr2O12. The Journal of Physical Chemistry C. 2018;122(38):21755–21762. doi: 10.1021/acs.jpcc.8b06275. [DOI] [Google Scholar]
- 21.Xu X, Liu Y, Wang J, Isheim D, Dravid VP, Phatak C, Haile SM (2020) Variability and origins of grain boundary electric potential detected by electron holography and atom-probe tomography. Nat Mater 19:887–893 [DOI] [PubMed]
- 22.Xie H, Alonso JA, Li Y, Fernández-Díaz MT, Goodenough JB. Lithium distribution in aluminum-free cubic Li7La3Zr2O12. Chemistry of Materials. 2011;23(16):3587–3589. doi: 10.1021/cm201671k. [DOI] [Google Scholar]
- 23.Awaka J, Takashima A, Kataoka K, Kijima N, Idemoto Y, Akimoto J. Crystal structure of fast lithium-ion-conducting cubic Li7La3Zr2O12. Chem. Lett. 2011;40(1):60–62. doi: 10.1246/cl.2011.60. [DOI] [Google Scholar]
- 24.Allen JL, Wolfenstine J, Rangasamy E, Sakamoto J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J Power Sources. 2012;206:315–319. doi: 10.1016/j.jpowsour.2012.01.131. [DOI] [Google Scholar]
- 25.Chen Y-T, Jena A, Pang WK, Peterson VK, Sheu HS, Chang H, Liu R-S. Voltammetric enhancement of Li-ion conduction in Al-doped Li7–xLa3Zr2O12 solid electrolyte. J Phys Chem C. 2017;121(29):15565–15573. doi: 10.1021/acs.jpcc.7b04004. [DOI] [Google Scholar]
- 26.Ramakumar S, Deviannapoorani C, Dhivya L, Shankar LS, Murugan R. Lithium garnets: synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Progress Mater Sci. 2017;88:325–411. doi: 10.1016/j.pmatsci.2017.04.007. [DOI] [Google Scholar]
- 27.Jin Y, McGinn PJ. Al-doped Li7La3Zr2O12 synthesized by a polymerized complex method. J Power Sources. 2011;196(20):8683–8687. doi: 10.1016/j.jpowsour.2011.05.065. [DOI] [Google Scholar]
- 28.Mukhopadhyay S, Thompson T, Sakamoto J, Huq A, Wolfenstine J, Allen JL, Bernstein N, Stewart DA, Johannes MD. Structure and stoichiometry in supervalent doped Li7La3Zr2O12. Chem Mater. 2015;27(10):3658–3665. doi: 10.1021/acs.chemmater.5b00362. [DOI] [Google Scholar]
- 29.Qian X, Wang D, Zhang Y, Wu H, Pennycook SJ, Zheng L, Poudeu PFP, Zhao L-D. Contrasting roles of small metallic elements M (M = Cu, Zn, Ni) in enhancing the thermoelectric performance of n-type PbM0.01Se. J Mater Chem A. 2020;8(11):5699–5708. doi: 10.1039/D0TA00174K. [DOI] [Google Scholar]
- 30.Rangasamy E, Wolfenstine J, Sakamoto J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ionics. 2012;206:28–32. doi: 10.1016/j.ssi.2011.10.022. [DOI] [Google Scholar]
- 31.Shao C, Yu Z, Liu H, Zheng Z, Sun N, Diao C. Enhanced ionic conductivity of titanium doped Li7La3Zr2O12 solid electrolyte. Electrochimica Acta. 2017;225:345–349. doi: 10.1016/j.electacta.2016.12.140. [DOI] [Google Scholar]
- 32.Thompson T, Wolfenstine J, Allen JL, Johannes M, Huq A, David IN, Sakamoto J. Tetragonal vs. cubic phase stability in Al – free Ta doped Li7La3Zr2O12 (LLZO) J Mater Chem A. 2014;2(33):13431–13436. doi: 10.1039/C4TA02099E. [DOI] [Google Scholar]
- 33.Wang D, Zhong G, Dolotko O, Li Y, McDonald MJ, Mi J, Fu R, Yang Y. The synergistic effects of Al and Te on the structure and Li+-mobility of garnet-type solid electrolytes. J Mater Chem A. 2014;2(47):20271–20279. doi: 10.1039/C4TA03591G. [DOI] [Google Scholar]
- 34.Lobe S, Dellen C, Finsterbusch M, Gehrke HG, Sebold D, Tsai CL, Uhlenbruck S, Guillon O. Radio frequency magnetron sputtering of Li7La3Zr2O12 thin films for solid-state batteries. J Power Sources. 2016;307:684–689. doi: 10.1016/j.jpowsour.2015.12.054. [DOI] [Google Scholar]
- 35.Sastre J, Tzu-Ying Lin TY, Filippin AN, Priebe A, Avancini E, Michler J, Tiwari AN, Romanyuk YE, Buecheler S. Aluminum-assisted densification of cosputtered lithium garnet electrolyte films for solid-state batteries. ACS Appl. Energy Mater. 2019;2:8511–8524. doi: 10.1021/acsaem.9b01387. [DOI] [Google Scholar]
- 36.Garbayo I, Struzik M, Bowman WJ, Pfenninger R, Stilp E, Rupp JLM. Glass-type polyamorphism in Li-garnet thin film solid state battery conductors. Adv Energy Mater. 2018;8(12):1702265. doi: 10.1002/aenm.201702265. [DOI] [Google Scholar]
- 37.Zheng Z, Song S, Wang Y. Sol-gel-processed amorphous lithium ion electrolyte thin films: structural evolution, theoretical considerations, and ion transport processes. Solid State Ionics. 2016;287:60–70. doi: 10.1016/j.ssi.2016.02.006. [DOI] [Google Scholar]
- 38.Huang Y, He Y, Sheng H, Lu X, Dong H, Samanta S, Dong H, Li X, Kim DY, Mao HK, Liu Y, Li H, Li H, Wang L. Li-ion battery material under high pressure: amorphization and enhanced conductivity of Li4Ti5O12. National Sci Rev. 2019;6(2):239–246. doi: 10.1093/nsr/nwy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu JF, Guo X. Origin of the low grain boundary conductivity in lithium ion conducting perovskites: Li3xLa0.67-xTiO3. Phys Chem Chem Phys. 2017;19(8):5880–5887. doi: 10.1039/C6CP07757A. [DOI] [PubMed] [Google Scholar]
- 40.Loho C, Djenadic R, Mundt P, Clemens O, Hahn H. On processing-structure-property relations and high ionic conductivity in garnet-type Li5La3Ta2O12 solid electrolyte thin films grown by CO2 -laser assisted CVD. Solid State Ionics. 2017;313:32–44. doi: 10.1016/j.ssi.2017.11.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Reduced energy barrier for Li+ transport across grain boundaries with amorphous domains in LLZO thin-films. Note S1: The determination of thicknesses of each single layer of LLZO, Li2CO3, and Ga2O3.Table S1. The thickness of each single-layer thin film. Table S2. The geometrical parameters (L and S) of electrodes and the fitted values of the elements in the equivalent circuit (Rtotal, Rbulk, Rgb, Cbulk, and Cgb) of the different thin films for calculating σtotal, σbulk, and σgb at room temperature. Figure S1. The thickness of #600-1 (1.516 μm) determined in its cross-sectional SEM image. Figure S2. XRD patterns of the LLZO target used in this study. Figure S3. The grain size of #600-1(~50 nm) determined by SEM image. Figure S4. The Nyquist plots of impedance spectra of LLZO thin-films #700-1 (a), #800-1 (green in b), and #800-2 (brown in b) measured at room temperature, inserts show the equivalent circuits for EIS analysis.
Data Availability Statement
The authors declare that the materials and data are promptly available to readers without undue qualifications for material transfer agreements. All data generated or analyzed during this study are included in this article.