Abstract
Purpose:
To evaluate the associations of sarcopenia and previous falls with 2-year major osteoporotic fractures (MOFs) in community-dwelling older adults.
Methods:
Four thousand Chinese men and women ≥ 65 years recruited from Hong Kong communities were prospectively followed up. Measures of muscle mass, grip strength, gait speed and falls in the previous year were recorded at baseline, the 2nd year and the 4th year visit for each subject. The associations of fall history, sarcopenia and its components with 2-year MOFs were evaluated using generalized linear mixed models.
Results:
Poor grip strength and poor gait speed were significantly associated with a higher 2-year MOFs risk, with an adjusted OR(95%CI) per one SD decrease of 1.48 (1.17, 1.87) and 1.17 (1.00, 1.36), respectively. Falls in the previous year was a significant predictor for 2-year MOFs risk, with an adjusted OR(95%CI) per one added fall of 1.85 (1.40, 2.44) in men and 1.26 (1.01, 1.58) in women. The adjusted OR(95%CI) of height adjusted appendicular lean muscle mass (ALM/height2) per one SD decrease and sarcopenia for 2-year MOFs risk were 1.34 (0.87, 2.06) and 1.72 (0.92, 3.21) in men, and were 0.73 (0.57, 0.93) and 0.76 (0.39, 1.47) in women, respectively (P for interaction by gender = 0.012 and 0.017, respectively).
Conclusions:
Poor sarcopenia related physical performance and falls in the previous year were significant predictors for 2-year MOFs in community-dwelling older adults. The predictive value of ALM by DXA for near-term fracture risk is limited and different across genders.
Keywords: epidemiology, falls, physical performance, sarcopenia
Introduction
Osteoporotic fractures result in increased morbidity, mortality, and healthcare cost. With global population aging, the corresponding medical burden is increasing exponentially [1]. Over the past 10 years, several measurements including FRAX [2], Garvan Fracture Risk Calculator [3], and Qfracture [4], have been developed for 5- or 10-year fracture risk assessment to aid treatment decisions. However, risk alarming and medical attention based on a relatively short perspective are largely poor both in fracture patients and high risk groups in the community [5, 6]. Healthcare organizations are now beginning to highlight the importance of measuring near-term (i.e. 2 years) fracture risk to improve public awareness and deliver timely treatment [7, 8]. Among the risk factors identified for fracture, sarcopenia [9, 10] and falls [7, 11] are two important components that could be potentially modified in short-term. They could be potential targets for risk identification and intervention to reduce near-term fracture incidence in community-dwelling older adults.
Sarcopenic individuals, defined as having age-related loss of muscle mass, in addition to the loss of strength and physical performance [12], had a higher risk of falls and long-term fracture rate compared with their non-sarcopenic counterparts [10]. A reduction in muscle mass and/or muscle function may lead to an increase in falling tendency, and then the risk of subsequent fractures, as the individual components of sarcopenia were also good predictors for future falls and long-term fractures [13–15]. However, sarcopenia-related evaluations for near-term risk of fracture is scanty. Furthermore, some discrepancies in the association between lean muscle mass and long-term fracture risk had been observed. Studies among men tended to find low lean muscle mass a predictive factor for fracture [14, 16], while that among women were more likely to report a null or reverse association [17, 18]. The inconsistent predictive value of muscle mass across genders may potentially account for the inconsistent performance of sarcopenia reported in the fracture predictions [19, 20]. We also found that sarcopenia was an independent risk predictor for long-term fractures in old men but not old women in a previous study [21]. Further evaluations on the predictive value of sarcopenia and its components for near-term fracture risk in community dwelling older adults are needed, preferably separately for men and women.
Substantial studies have identified long-term fracture risk in relation to falls [22]. However, fall-related risk factors could potentially be even more important in predicting near-term fracture as their fracture predictive ability may decline with increasing follow-up time [23, 24]. Perhaps due to the relatively low incidence of near-term fracture in community-dwelling adults, only few studies on the association between fall-related risk factors and near-term fractures have been conducted among osteoporotic and fractured patients [25–27], and particularly in community-dwelling older adults. Further studies are required to establish the near-term fracture predictive power of previous falls in community-dwelling older adults.
Thus, this study aimed to examine: a) the independent association of the Asian sarcopenia definition and its components [28] (grip strength, gait speed, and height adjusted appendicular lean muscle (ALM/height2)) with the risk of 2-year major osteoporotic fractures (MOFs), and b) the independent association of falls in the previous year with the risk of 2-year MOFs, in the Chinese community-dwelling older adults. Gender difference in the association of each predictor with 2-year MOFs risk was further examined.
Methods
Study participants
The present study was conducted in the Mr. OS and MS. OS Hong Kong cohort. At baseline, two thousand Chinese men and 2000 Chinese women aged ≥ 65 years were recruited from local communities from August 2001 to March 2003 by recruitment notices and talks in community centres and housing estates [29]. Among these 4000 participants, 3427 (85.7%) and 3153 (78.8%) participants were followed up by a visit to the research centre at the end of the 2nd year (the 2nd visit) and at the end of 4th year (the 3rd visit), respectively. A repeated-observations design with three 2-year intervals was adopted (Flow chart see Fig 1). Data from the participants collected in the three visits would be used to predict subsequent 2-year MOF occurred from each visit.
Fig 1.
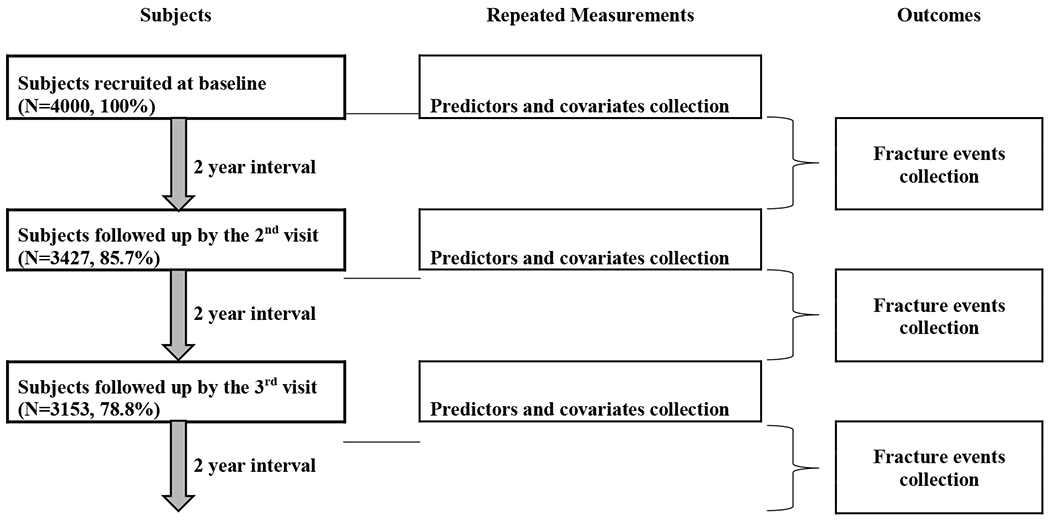
Flow chart for subjects and data collection in the study
Demographical factors and fall history measurements
The interview was conducted using a structured questionnaire at each visit. Information on the clinical risk factors in the FRAX algorithm (including age, gender, previous fracture, parental fracture, current smoking, alcohol drinking, use of glucocorticoids, and rheumatoid arthritis) was collected. Self-reported falls during the 12 months preceding the baseline and each repeated visit were recorded. A fall was defined as any unexpected loss of balance resulting in coming to rest on the ground. Self-reported physical activity level was assessed using the Physical Activity Scale of the Elderly (PASE). Body weight (kilograms) was measured using the Physician Beam Balance Scale (Healthometer, IL. USA) with the participants wearing an examination gown. Body height (centimeters) was measured by a Holtain Harpenden stadiometer (Holtain Ltd., Crosswell, UK). Body mass index (BMI) (kg/m2) was calculated.
Assessment of bone mineral density (BMD) and appendicular lean muscle mass (ALM)
Areal femoral neck BMD and lean mass were measured with dual energy X-ray absorptiometry (DXA) using a Hologic QDR 4,500 W device (Waltham, MA, USA). Centralized quality control procedures, certificated DXA operators, and standardized procedures for scanning were used to ensure measurement reproducibility [30]. The coefficients of variation (CVs) of the scanners estimated using a central phantom was 1.3% and 0.8% for femoral neck BMD and lean mass, respectively. Total ALM was calculated as the sum of appendicular lean mass minus bone mineral content of both arms and legs, with the operator adjusting the cut lines of the limbs according to specific anatomical landmarks [31]. Height adjusted ALM (ALM/height2) was calculated.
Osteoporosis definition and FRAX score calculation
The osteoporosis category was defined using a femoral neck BMD T-score calculated based on the NHANES III reference database for femoral neck measurements in women aged 20 - 29 years [32]. The Hong Kong Chinese-FRAX (version 3.8) 10-year risk estimate of MOFs for each participant was calculated (using the baseline clinical risk factors and femoral neck BMD) with the assistance of the WHO Collaborating Centre for Metabolic Bone Disease, University of Sheffield, UK [19].
Physical function tests and sarcopenia definition
Grip strength was measured using a dynamometer (JAMAR Hand Dynamometer 5030JO; Saimnons Preston, Bolingbrook, IL). Two readings were taken from each side, and the maximum value of the right/left was used for analysis. Participants were asked to walk (at a normal pace for twice) along a straight line 6 meters long in distance, including both the accelerating and decelerating phase. Gait speed in the present study was measured using the best time in seconds to complete the walk. According to the consensus report proposed by the Asian Working Group for Sarcopenia (AWGS) [28], sarcopenia is defined in which a person who has low muscle mass with low muscle strength and/or low physical performance. Gender-specific cut-off values of < 7 kg/m2 for men and < 5.4 kg/m2 for women were used to define low ALM, values of < 26 kg for men and < 18 kg for women to define low grip strength, and values of ≤ 0.8 m/s to define slow gait speed according to the AWGS [28].
Follow-up for fractures
Fracture occurrence was determined by carrying out a search in the Hospital Authority electronic database, which included all visits to Accident and Emergency Departments and outpatient clinics from baseline to October 2013. The database covers all publicly funded hospitals in Hong Kong [33]. Fractures occurring from each visit (baseline, the 2nd visit, and the 3rd visit) through 2 years of follow up were recorded for each subject in the present study. A MOF was defined as a fracture of the hip, clinical spine, wrist or humerus.
Statistical analysis
Differences in baseline characteristics between genders were tested using Student-t test for continuous variables and Chi-Square test for categorical variables. Baseline characteristics, physical and functional measures, and incident fracture events were shown in mean (standard deviation, SD) for continuous variables and frequency (percent) for categorical variables. These results were presented for each gender and visit (1: baseline, 2: the 2nd year, and 3: the 4th year). For each repeated measure, a P value for trend across time points was calculated using linear regression model for continuous variables and using Mantel-Haenszel Chi-Square test for categorical variables.
Repeated measures analysis taken three visits into account was applied (1: baseline, 2: the 2nd year, and 3: the 4th year). To account for within-subject variability and missing values, a generalized linear mixed model [34] was used to estimate the odds ratio (OR) of falls in the previous year, sarcopenia and its components (grip strength, gait speed, and ALM/height2), separately for 2-year MOFs risk. Parameters were estimated by the Restricted Pseudo-Likelihood method and the models followed an independent covariance structure (variance component) to satisfy the convergence criterion. ORs were estimated by adjusting for a) basic adjustments: gender (1 for men and 2 for women), baseline age and time point (1, 2, and 3); b) full adjustments: basic adjustments + continuous values of BMD, BMI, number of falls in the previous year, self-reported physical activity level, and baseline FRAX score. OR per one SD decrease in each continuous variable for MOFs risk was estimated, and 95% confidence interval (CI) was reported for each OR. Estimates were further calculated for each gender. An interaction of each predictor and gender with MOFs risk was examined to assess the difference between genders.
All statistical tests were two-tailed with P < 0.05 being considered as significant. Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
At baseline, the mean age of men was 72.4 (5.0) and 72.6 (5.4) of women. Compared with men, women had a higher prevalence of osteoporosis and higher FRAX score, but a lower prevalence of sarcopenia. (Table 1) Within 2 years after each visit (1: baseline, 2: the 2nd year, and 3: the 4th year), 23 (1.2%), 24 (1.4%) and 24 (1.5%) men had incident MOFs, while 55 (2.8%), 56 (3.3%), and 44 (2.8%) women had incident MOFs. (Table 2)
Table 1.
The baseline characteristics in men and women.
| Baseline characteristics | Mean (SD) / n (%) |
P value | |
|---|---|---|---|
| Men (n=2000) | Women (n= 2000) | ||
| Age (year) | 72.4 (5.0) | 72.6 (5.4) | 0.254 |
| Body mass index (BMI) (kg/m2) | 23.5 (3.1) | 23.9 (3.5) | <0.001 |
| Femoral neck BMD (g/cm2) | 0.7 (0.1) | 0.6 (0.1) | <0.001 |
| FRAX score for MOF | 6.7 (3.3) | 14.6 (8.2) | <0.001 |
| High risk category for MOF | 111 (5.6) | 758 (37.9) | <0.001 |
| Previous fracture (since 50 years old): yes | 274 (13.7) | 416 (20.8) | <0.001 |
| Parental hip fracture: yes | 83 (5.0) | 87 (5.3) | 0.651 |
| Current smoking: yes | 238 (11.9) | 37 (1.9) | <0.001 |
| Alcohol drinking (3 or more units / day): yes | 16 (0.8) | 0 (0.0) | <0.001 |
| Glucocorticoids using (lasting ≥ 3 months): yes | 11 (0.6) | 5 (0.3) | 0.133 |
| Rheumatoid arthritis: yes | 21(1.1) | 38 (1.9) | 0.026 |
| Osteoporosis: yes | 223 (11.2) | 825 (41.3) | <0.001 |
| Sarcopenia: yes | 187 (9.4) | 106 (5.3) | <0.001 |
Notes BMD: bone mineral density; MOF: major osteoporotic fracture.
Difference between genders was tested using Student-t test for continuous variables and Chi-Square test for categorical variables.
Table 2.
Repeart physical and functional measures at three visits in men and women.
| Men, mean (SD) / n (%) |
Women, mean (SD) / n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline visit (n=2000) |
The 2nd visit (2nd year) (n=1745) |
The 3rd visit (4th year) (n=1566) |
P for trenda | Baseline visit (n=2000) |
The 2nd visit (2nd year) (n=1682) |
The 3rd visit (4th year) (n=1586) |
P for trenda | |
| Body mass index (BMI) (kg/m2) | 23.45(3.13) | 23.38(3.07) | 23.35(3.12) | 0.348 | 23.92(3.45) | 23.81(3.41) | 23.81(3.5) | 0.326 |
| Femoral neck BMD (g/cm2) | 0.69(0.11) | 0.69(0.11) | 0.69(0.11) | 0.613 | 0.58(0.10) | 0.58(0.10) | 0.57(0.10) | 0.012* |
| ALM/h eight2 (kg/m2) | 7.20(0.82) | 7.18(0.81) | 7.17(0.82) | 0.239 | 6.06(0.73) | 6.03(0.74) | 6.00(0.73) | 0.013* |
| Grip strength (kg) | 33.90(6.73) | 32.44(6.80) | 31.89(6.66) | <0.001* | 22.31(4.41) | 19.97(4.44) | 20.39(4.41) | <0.001* |
| Gait speed (m/s) | 1.07(0.23) | 1.05(0.23) | 0.99(0.23) | <0.001* | 0.96(0.21) | 0.92(0.22) | 0.87(0.22) | <0.001* |
| Sarcopenia (yes) | 187(9.4) | 203(11.7) | 236(15.1) | 0.001* | 106(5.3) | 164(9.8) | 150(9.5) | <0.001* |
| Previous falls (no. of falls) | ||||||||
| 1 falls | 234(11.7) | 224(12.8) | 177(11.3) | 320(16.0) | 282(16.8) | 252(15.9) | ||
| ≥ 2 falls | 73(3.7) | 81(4.6) | 65(4.2) | 0.617 | 162(8.1) | 128(7.6) | 103(6.5) | 0.116 |
| MOF events from the visit through 2 years of follow up | 23 (1.2) | 24 (1.4) | 24(1.5) | 0.315 | 55 (2.8) | 56 (3.3) | 44 (2.8) | 0.857 |
Note SD: standard deviation; BMD: bone mineral density; ALM: appendicular lean muscle mass; MOF: major osteoporotic fracture.
used linear regression model for continuous variables and Mantel-Haenszel Chi-Square test for categorical variables.
P < 0.05.
In the three repeated measures spanning across 4 years, femoral neck BMD and ALM/height2 declined significantly in women (P for trend was 0.012 and 0.013, respectively), but not in men. Grip strength and gait speed declined significantly in both genders (P for trend all <0.001). Self-reported physical activity level increased significantly in women (P for trend < 0.001), but not in men.
In the generalized linear mixed model, poorer grip strength and poorer gait speed were associated with higher risks of MOFs within 2-year follow-up, with a significant basic-adjusted OR (95%CI) per one decreased SD of 1.60 (1.29, 2.00) and 1.21 (1.05, 1.40), respectively (P for interaction by genders = 0.949 and 0.716, respectively; Table 3). The falls in the previous year was a significant predictor for 2-year MOFs risk in both genders, with a higher basic-adjusted OR(95%CI) per one added fall in men (1.78 (1.35, 2.35)) than women (1.25 (1.02, 1.55), P for interaction = 0.036; Table 3). The associations of ALM/height2 and sarcopenia with 2-year MOFs were significantly different across genders. The basic-adjusted OR (95%CI) of ALM/height2 per one SD decrease for MOFs risk was 1.34 (1.04, 1.72) in men and 0.88 (0.76, 1.03) in women (P for interaction = 0.002; Table 3). The basic-adjusted OR (95%CI) of sarcopenia (yes vs no) for MOFs risk was 2.07 (1.16, 3.68) in men and 0.79 (0.43, 1.45) in women (P for interaction = 0.010; Table 3). Further adjustments for BMI, femoral neck BMD, self-reported physical activity level falls in the previous year, and baseline FRAX score did not essentially change the estimations in the basic models, except that the adjustments attenuated the association of ALM/height2 and sarcopenia with 2-year MOFs risk in men, and ALM/height2 became positively associated with MOF’s risk in women (Table 3). Fig 2 showed the full-adjusted ORs (95%CI) of each predictor for 2-year MOFs risk, separately by genders if gender difference was significant.
Table 3.
Associations of sarcopenia (its components) and fall history with 2-year major osteoporotic fractures risk in men and women.
| Predictors | Model adjustment | OR (95% CI) a |
P for interaction b | ||
|---|---|---|---|---|---|
| All | Men | Women | |||
| Sarcopenia (yes/no) | Age + time point | 1.28 (0.87, 1.90) | 2.07 (1.16, 3.68)* | 0.79 (0.43, 1.45) | 0.010* |
| Full adjustment | 1.18 (0.76, 1.82) | 1.72 (0.92, 3.21) | 0.76 (0.39, 1.47) | 0.017* | |
| Grip strength (kg) | Age + time point | 1.60 (1.29, 2.00)* | 1.38 (1.07, 1.79)* | 1.33 (1.12, 1.58)* | 0.949 |
| Full adjustment | 1.48 (1.17, 1.87)* | 1.23 (0.93, 1.61) | 1.31 (1.09, 1.58)* | 0.977 | |
| Gait speed (m/s) | Age + time point | 1.21 (1.05, 1.40)* | 1.17 (0.91, 1.51) | 1.22 (1.03, 1.44)* | 0.716 |
| Full adjustment | 1.17 (1.00, 1.36)* | 1.05 (0.81, 1.36) | 1.21 (1.01, 1.45)* | 0.703 | |
| ALM/height2 (kg/m2) | Age + time point | 1.02 (0.86, 1.21) | 1.34 (1.04, 1.72) | 0.88 (0.76, 1.03) | 0.002* |
| Full adjustment | 0.88 (0.67, 1.15) | 1.34 (0.87, 2.06) | 0.73 (0.57, 0.93)* | 0.012* | |
| Previous falls (per 1 added fall) | Age + time point | 1.40 (1.19, 1.66)* | 1.78 (1.35, 2.35)* | 1.25 (1.02, 1.55)* | 0.036* |
| Full adjustment | 1.43 (1.20, 1.71)* | 1.85 (1.40, 2.44)* | 1.26 (1.01, 1.58)* | 0.035* | |
Note OR: odds ratio; CI: confidence interval; ALM: appendicular lean muscle mass.
Full adjustment: age, time point, BMI, femoral neck BMD, physical activity level, no. of falls in the previous year, and baseline FRAX score. Gender was also adjusted in the analysis including both genders.
Sarcoepnia is defined according to the Asian Working Group for Sarcopenia (AWGS).
OR (95%CI) per one standard deviation decrease in the predictors of continuous variables.
P for interaction between genders.
P < 0.05.
Fig 2.
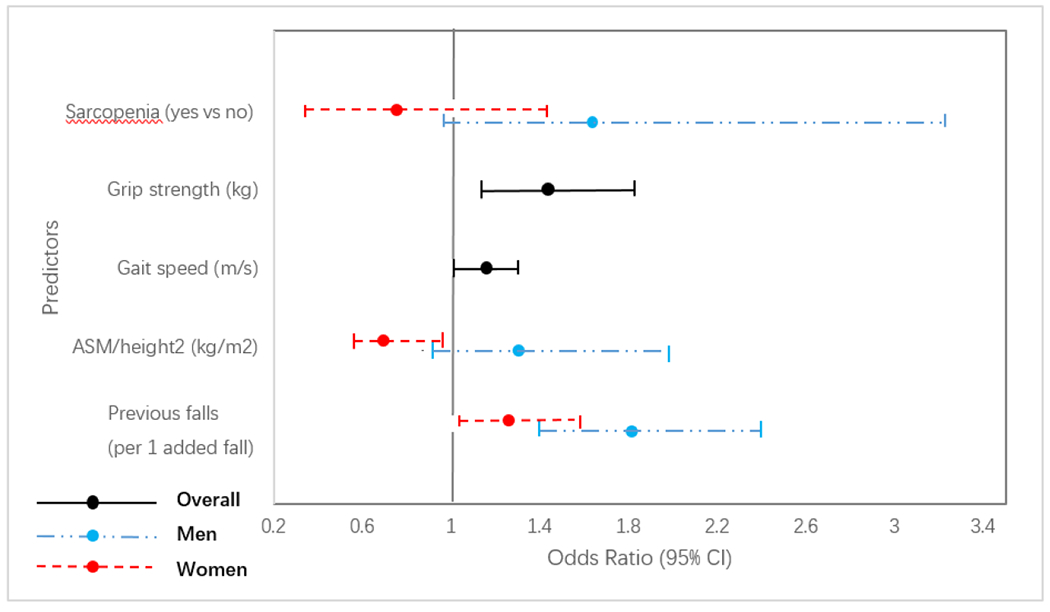
Odds ratios (ORs) of sarcopenia (and its components) and falls history for 2-year major osteoporotic fractures risk in older adults. OR per one standard deviation (SD) decrease was shown for continuous predictors. The models were adjusted for age, gender, time point, BMI, femoral neck BMD, physical activity level, no. of falls in the previous year, and baseline FRAX score. If gender difference was significant, estimates were separately shown for each gender
Exploratory analyses and results
To explore whether the lower ALM/height2 in relation to decreased 2-year MOFs risk in women was also associated with decreased subsequent falling risk within the 2-year follow-up period, a generalized linear mixed model was used to separately estimate the OR of ALM/height2 for the subsequent fall and the 2-year MOFs risk (repeated measures analysis taken two visits into account was applied using data of ALM/height2 at baseline and the 2nd visit, subsequent falls (i.e., self-reported falls in the previous year at the 2nd and the 3rd visit), and subsequent 2-year MOFs from the baseline and the 2nd visit). Only two time-points were used in the exploratory analyses as we have no subsequent fall information after the 3rd visit (the 4th year). Similar analysis was conducted in men for comparison. As shown in Fig 3, after adjusting for BMI, femoral neck BMD, self-reported physical activity level, and baseline FRAX score, low ALM/height2 was significant protector both for 2-year MOFs risk and subsequent falling risk in women (OR (95%CI) = 0.68 (0.51, 0.91) and 0.85 (0.75, 0.97), respectively). In men, low ALM/height2 was significantly associated with higher 2-year MOFs risk (OR (95%CI) = 1.72 (1.01, 2.93)), and marginally significantly with higher subsequent risk for falls (OR (95%CI) = 1.15 (0.98, 1.36)).
Fig 3.
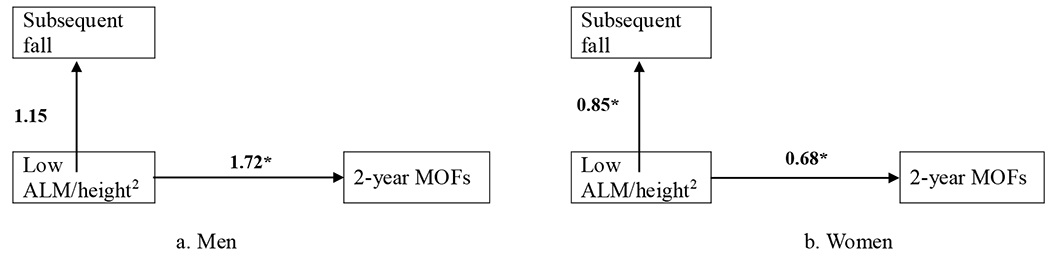
Odds ratio (OR) per one standard deviation (SD) decrease in ALM/height2 for the subsequent fall (yes vs no) and the 2-year MOFs were shown, respectively. The models were adjusted for age, time point, BMI, femoral neck BMD, physical activity level, and baseline FRAX score. * indicate for P value < 0.05
Discussion
In the community-dwelling older adults, poor grip strength and poor gait speed were significantly associated with 2-year MOFs risk without gender difference, irrespective of age, body size, BMD, previous falls, physical activity level and other clinical risk factors of fracture. Each one more fall in the previous year would increase 78% risk of having a MOF in 2 years in men and 25% in women. Appendicular lean muscle mass had an inconsistent association with 2-year MOFs risk across genders. Lower ALM/height2 was a risk factor for 2-year MOFs in older men but not in older women. This difference was more remarkable after further adjustment for BMD, previous falls, physical activity level and other clinical risk factors. Thus accordingly, the Asian sarcopenia definition was a potential predictor for 2-year MOFs risk in older men but not in older women.
Consistently with the associations with long-term fracture risk [13, 14, 22–24], we found that sarcopenia- and fall-related risk factors were also independently associated with 2-year MOFs risk. Poor muscle strength, poor gait speed and previous falls could all independently predict near-term fracture risk in community-dwelling older adults. While BMD changes relatively steadily across the three measurements spanning across 4 years, functional indices change more remarkably. This suggests that previous fall condition and these functional indices have to be closely monitored to assess imminent fracture risk. Moreover, physical function improvement and fall risk reduction could happen rapidly after a short course of intervention [11, 35]. Intervention targeting on improving functional performance and reducing fall risk could potentially markedly reduce near-term fracture incidence. Further randomized trials should also be conducted to examine the potential synergistic benefits of functional performance- and fall-related intervention with pharmacological treatment for their imminent anti-fracture efficacy.
We provided the first direct evidence to show that lean muscle mass could predict near-term fracture differently across genders. Higher ALM/height2 increased 2-year MOFs risk potentially through increasing subsequent falling in older women but tended to be a protective factor in men. This provided a possible explanation to the consistent lack of association and counterintuitive associations observed between DXA-based lean muscle mass or sarcopenia and long-term fracture risk that were previously reported [15, 17, 18, 21, 36–38]. The limited predictive value of lean muscle mass measured by DXA for fracture could be partly attributed to the limitations of DXA in quantifying skeletal muscle mass. DXA based lean mass does include non-skeletal muscle tissue, such as non-contractile connective and fibrotic tissue [17, 39], which may attenuate or even reverse the association of lean mass with muscle function and fracture risk. Age-related decrease in strength outpacing the concomitant decrease in lean mass [40], has also been suggested to play a part in increasing fracture risk [17, 41]. Moreover, increased infiltration of fat into skeletal muscle with aging [42] could also undermine the accuracy of DXA in assessing muscle mass particularly in older adults. However, although a potential higher fat infiltration (as indicated by lower attenuation of skeletal muscle) in older women than men had been reported[42, 43], more direct evidence are needed to demonstrate that older women had a higher fiber or fatty infiltration than older men. In addition, the correction methods accounting for muscle size (namely using height squared: ALM/height2 in the present study) for DXA-based muscle mass might also contribute to the difference. Especially when women are more likely to have a low height than men in general, DXA-based ALM/height2 might be over adjusted by height. However, we found (see Supplementary Table 1) that while similar gender difference in predicting near-term fracture risk by using ALM/weight as ALM/height2 had been done, the gender difference by using ALM/BMI was truly attenuated some (P value for interaction = 0.161) but with declining predictive values in both genders. More evidence is needed before changing current recommendations for the muscle mass correction method. And the reasons why the limitations of DXA-based lean muscle mass might be more obvious in older women deserved further exploration. Nevertheless, our finding suggests that DXA-based lean muscle mass may not be an appropriate indicator for imminent fracture risk in its current form, especially in older women. There is a need to identify better estimates to define skeletal muscle mass based on DXA or other assessments [28, 44], particularly when sarcopenia is now recognized as a muscle disease with an ICD-10 code [45].
Strengths of the study include the large sample of well-characterized older community-dwelling participants who all had 3 longitudinal visits, each with a 2-year follow-up for fracture events. We also have comprehensive data on body measurements, falls history, physical function performance and clinical risk factors for fracture. The limitations include non-random sampling as well as the lack of subjects younger than 65 years and Chinese subjects outside Hong Kong. Therefore, our findings cannot be generalized to the young old or ethnic Chinese elsewhere. This is an epidemiologic observational study with a potential for healthy volunteer bias, and the small nmnber of fracture events from each visit through 2 years of follow up may limit the power to interpret the associations between predictors and near-tenn fracture risk. The gender difference in the associations between lean muscle mass and fracture risk needs further clarification. Self-reported falls might lead to the underreporting of falls not associated with significant injury, and cognitive impairment may influence the recall accuracy in older adults although no appreciably effect had been observed as we reported previously [23]. And using “self-reported falls in the previous year at the 2nd year and the 4th year visit” to represent subsequent falls respectively from baseline and the 2nd year may underestimate the falling risk in the exploratory analyses. No data on muscle quality is available to further distinguish the associations of muscle quantity and muscle quality with near-tenn fracture risk. More studies including various measures of skeletal muscle quality are required to validate and clarity the associations.
Conclusions
Poor grip strength, poor gait speed and falls in the previous year were independent predictors for 2-year MOFs risk in community-dwelling older adults. They should be adopted in the evaluation of near-tenn fracture risk identification in older adults and are potential targets for intervention aiming to reduce imminent fracture risk. Although lower ALM/height2 tended to be a risk factor for 2-year MOFs in older men, it might be associated with lower risk of 2-year MOFs in older women. This limited the predictive value of current Asian definition of sarcopenia for near-term fracture risk in women. Further deliberations on the method of muscle mass estimation are needed.
Supplementary Material
Acknowledgments
The authors wish to thank all participants dedicated contributing to the study and The Chinese University of Hong Kong Jockey Club Centre for Osteoporosis Care and Control supporting the study.
Funding
The study was supported by the National Institutes of Health R01 grant AR049439–01A1 and the Chinese University of Hong Kong Research Grants Council Earmarked Grant CUHK4101/02 M.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest
Yi Su, Freddy Lam, Jason Leung, Wing-Hoi Cheung, Suzanne C Ho and Timothy Kwok declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong (CRE-2003.102). Informed consent was obtained from all individual participants included in the study.
Reference
- 1.He W, Goodkind D, Kowal P (2016) U.S. Census Bureau, International Population Reports, P95/16-1, An Aging world: 2015, U.S. Government Publishing Office, Washington, DC. [Google Scholar]
- 2.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV (2008) Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int 19:1431–1444 [DOI] [PubMed] [Google Scholar]
- 4.Collins GS, Mallett S, Altman DG (2011) Predicting risk of osteoporotic and hip fracture in the United Kingdom: prospective independent and external validation of QFractureScores. BMJ 342:d3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD (2014) Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res 29:1929–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vondracek SF, Minne P, McDermott MT (2008) Clinical challenges in the management of osteoporosis. Clin Interv Aging 3:315–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roux C, Briot K (2017) Imminent fracture risk. Osteoporos Int 28:1765–1769 [DOI] [PubMed] [Google Scholar]
- 8.Pinedo-Villanueva R, Charokopou M, Toth E, Donnelly K, Cooper C, Prieto-Alhambra D, Libanati C, Javaid MK (2019) Imminent fracture risk assessments in the UK FLS setting: implications and challenges. Arch Osteoporos 14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morley JE (2018) Treatment of sarcopenia: the road to the future. J Cachexia Sarcopenia Muscle 9:1196–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, Maier AB (2019) Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Khoury F, Cassou B, Charles MA, Dargent-Molina P (2013) The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ 347:f6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cawthon PM, Fullman RL, Marshall L, Mackey DC, Fink HA, Cauley JA, Cummings SR, Orwoll ES, Ensrud KE (2008) Physical performance and risk of hip fractures in older men. J Bone Miner Res 23:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey NC, Oden A, Orwoll E, et al. (2018) Measures of Physical Performance and Muscle Strength as Predictors of Fracture Risk Independent of FRAX, Falls, and aBMD: A Meta-Analysis of the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res 33:2150–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaap LA, van Schoor NM, Lips P, Visser M (2018) Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci 73:1199–1204 [DOI] [PubMed] [Google Scholar]
- 16.Hars M, Biver E, Chevalley T, Herrmann F, Rizzoli R, Ferrari S, Trombetti A (2016) Low Lean Mass Predicts Incident Fractures Independently From FRAX: a Prospective Cohort Study of Recent Retirees. J Bone Miner Res 31:2048–2056 [DOI] [PubMed] [Google Scholar]
- 17.McLean RR, Kiel DP, Berry SD, Broe KE, Zhang X, Cupples LA, Hannan MT (2018) Lower Lean Mass Measured by Dual-Energy X-ray Absorptiometry (DXA) is Not Associated with Increased Risk of Hip Fracture in Women: The Framingham Osteoporosis Study. Calcif Tissue Int 103:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schott AM, Cormier C, Hans D, et al. (1998) How hip and whole-body bone mineral density predict hip fracture in elderly women: the EPIDOS Prospective Study. Osteoporos Int 8:247–254 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Hao Q, Ge M, Dong B (2018) Association of sarcopenia and fractures in community-dwelling older adults: a systematic review and meta-analysis of cohort studies. Osteoporos Int 29:1253–1262 [DOI] [PubMed] [Google Scholar]
- 20.Frisoli A Jr., Martin FG, Carvalho ACC, Borges J, Paes AT, Ingham SJM (2018) Sex effects on the association between sarcopenia EWGSOP and osteoporosis in outpatient older adults: data from the SARCOS study. Arch Endocrinol Metab 62:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu R, Leung J, Woo J (2014) Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc 15:918–923 [DOI] [PubMed] [Google Scholar]
- 22.Masud T, Binkley N, Boonen S, Hannan MT (2011) Official Positions for FRAX(R) clinical regarding falls and frailty: can falls and frailty be used in FRAX(R)? From Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX(R). J Clin Densitom 14:194–204 [DOI] [PubMed] [Google Scholar]
- 23.Su Y, Leung J, Kwok T (2018) The role of previous falls in major osteoporotic fracture prediction in conjunction with FRAX in older Chinese men and women: the Mr. OS and Ms. OS cohort study in Hong Kong. Osteoporos Int 29:355–363 [DOI] [PubMed] [Google Scholar]
- 24.Harvey NC, Oden A, Orwoll E, et al. (2018) Falls Predict Fractures Independently of FRAX Probability: A Meta-Analysis of the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res 33:510–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonafede M, Shi N, Barron R, Li X, Crittenden DB, Chandler D (2016) Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch Osteoporos 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weycker D, Edelsberg J, Barron R, Atwood M, Oster G, Crittenden DB, Grauer A (2017) Predictors of near-term fracture in osteoporotic women aged >/=65 years, based on data from the study of osteoporotic fractures. Osteoporos Int 28:2565–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller PD, Barlas S, Brenneman SK, Abbott TA, Chen YT, Barrett-Connor E, Siris ES (2004) An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med 164:1113–1120 [DOI] [PubMed] [Google Scholar]
- 28.Chen LK, Liu LK, Woo J, et al. (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101 [DOI] [PubMed] [Google Scholar]
- 29.Kwok AW, Gong JS, Wang YX, Leung JC, Kwok T, Griffith JF, Leung PC (2013) Prevalence and risk factors of radiographic vertebral fractures in elderly Chinese men and women: results of Mr. OS (Hong Kong) and Ms. OS (Hong Kong) studies. Osteoporos Int 24:877–885 [DOI] [PubMed] [Google Scholar]
- 30.Orwoll ES, Lapidus J, Wang PY, et al. (2017) The Limited Clinical Utility of Testosterone, Estradiol, and Sex Hormone Binding Globulin Measurements in the Prediction of Fracture Risk and Bone Loss in Older Men. J Bone Miner Res 32:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN Jr. (1990) Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr 52:214–218 [DOI] [PubMed] [Google Scholar]
- 32.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42:467–475 [DOI] [PubMed] [Google Scholar]
- 33.Su Y, Woo JW, Kwok TCY (2019) The Added Value of SARC-F to Prescreening Using FRAX for Hip Fracture Prevention in Older Community Adults. J Am Med Dir Assoc 20:83–89 [DOI] [PubMed] [Google Scholar]
- 34.Schabenberger O Introducing the GLIMMIX procedure for generalized linear mixed models. Cary, NC: SAS Institute, 2005. [Google Scholar]
- 35.Zhu LY, Chan R, Kwok T, Cheng KC, Ha A, Woo J (2019) Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: a randomized controlled trial. Age Ageing 48:220–228 [DOI] [PubMed] [Google Scholar]
- 36.Trajanoska K, Schoufour JD, Darweesh SK, et al. (2018) Sarcopenia and Its Clinical Correlates in the General Population: The Rotterdam Study. J Bone Miner Res 33:1209–1218 [DOI] [PubMed] [Google Scholar]
- 37.Chalhoub D, Cawthon PM, Ensrud KE, et al. (2015) Risk of Nonspine Fractures in Older Adults with Sarcopenia, Low Bone Mass, or Both. J Am Geriatr Soc 63:1733–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris R, Chang Y, Beavers K, et al. (2017) Risk of Fracture in Women with Sarcopenia, Low Bone Mass, or Both. J Am Geriatr Soc 65:2673–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cawthon PM (2015) Assessment of Lean Mass and Physical Performance in Sarcopenia. J Clin Densitom 18:467–471 [DOI] [PubMed] [Google Scholar]
- 40.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 41.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB (2010) Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 25:513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB (2001) Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 90:2157–2165 [DOI] [PubMed] [Google Scholar]
- 43.Liu D, Sartor MA, Nader GA, et al. (2013) Microarray analysis reveals novel features of the muscle aging process in men and women. J Gerontol A Biol Sci Med Sci 68:1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vellas B, Fielding RA, Bens C, et al. (2018) Implications of ICD-10 for Sarcopenia Clinical Practice and Clinical Trials: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging 7:2–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


