Abstract
Background:
Leishmaniases are parasitic diseases caused by Leishmania species and transmitted by the bite of sand flies. The genus Lutzomyia and Phlebotomus of sand flies are known to be the responsible vector for transmitting almost all Leishmania species to humans. The detection of Leishmania DNA in species of the genus Sergentomyia, in different regions, suggests their likely role in Leishmania transmission.
Methods:
Our objective was to determine the potential geographical distribution of Sergentomyia minuta, the most dominant Sergentomyia species in Morocco, using ecological niche modeling.
Results:
The results showed the widespread geographical distribution of S. minuta in Morocco, specifically in northern and central Morocco where visceral and cutaneous leishmaniasis foci occur. There were six abiotic factors affecting the distribution of S. minuta whose annual precipitation, precipitation seasonality and precipitation of driest month as the most important ecological variables of the model.
Conclusion:
A positive statistical correlation between human leishmaniasis cases and S. minuta abundance was noted suggesting the potential involvement of S. minuta in local Leishmania transmission cycles.
Keywords: Sergentomyia minuta, Ecological niche modeling, MaxEnt, Morocco
Introduction
Phlebotomine sand flies (Diptera: Psychodidae) are small nocturnal insects of which only the female is a hematophagous. Currently, about 1000 species of sand flies are distributed in almost all biogeographic regions of the world (1). These insects play a crucial role in the epidemiology of relevant diseases, some being of great veterinary and medical importance. Indeed, certain species of sand flies are vectors of various infectious and parasitic agents such as agents of canine and human leishmaniasis, bartonellosis and several arboviruses. Allergic reactions can also be caused by the exposure of sand fly bites, though is not linked to the spread of disease (2).
Leishmaniasis remains the most related diseases to sand flies. The genera Phlebotomus, Lutzomyia and Sergentomyia contain all hematophagous species of sand flies (3), with only the genus Lutzomyia in the New World, and Phlebotomus in the Old World responsible for transmitting almost all Leishmania species to humans (4, 5).
Like most countries around the Mediterranean, leishmaniases in Morocco are a serious public health problem. These affections are widely represented, from the Moroccan Rif Mountains to the palm groves of the Moroccan Anti-Atlas (6). Three epidemiological entities are known in Morocco: zoonotic cutaneous leishmaniasis due to Leishmania major, anthroponotic cutaneous leishmaniasis due to L. tropica and visceral and cutaneous leishmaniasis due to L. infantum (6).
Regarding disease transmission, only vectorial role of species of the genus Phlebotomus (namely, Phlebotomus papatasi, P. sergenti, P. ariasi, P. longicuspis and P. perniciosus) was investigated in Morocco (7–9); where sand fly fauna has 22 species including, nine species of the genus Sergentomyia (10): Sergentomyia christophersi, S. clydei, S. africana, S. minuta, S. dreyfussi, S. schwetzi, S. antennata, S. fallax and S. lewisi.
Considering the epidemiological status of leishmaniasis in Morocco, it is critically important to highlight the probable role as a vector for other sand fly species. Vector incrimination depends on the accumulation of evidence based mainly on its geographical and temporal abundance. According to many authors, Sergentomyia species are spread throughout Morocco, especially S. minuta and S. fallax (11, 12). Sergentomyia minuta is collected in urban as well as in rural area (13) up to 1,200m (14). It was considered as ubiquitous species in Morocco (15) with high density and a long activity period (16, 17).
In addition, Leishmania DNA was detected in species of the genus Sergentomyia in different regions suggesting their likely role in Leishmania transmission (18). Studies conducted in leishmaniasis foci in Iran, Mali and Portugal have reported the detection of L. major DNA in Sergentomyia sintoni (19), S. darlingi (20) and S. minuta (21). Earlier, it has also been isolated from S. garnhami in Kenya (22). Other reports also detected L. donovani DNA in S. babu in India (23) and more recently, L. siamensis detected in S. gemmea in Thailand (24). Moreover, several viruses have been isolated from sand flies of the genus Sergentomyia such as the Saboya virus, viruses RNA of ArD 95737 and ArD 111740 (25, 26), which has made it possible to suspect the vector role of Sergentomyia species (27).
Despite the presence and the abundance of Sergentomyia species in Morocco, no study has invested its potential involvement in leishmaniasis transmission cycles. Therefore, we used ecological niche modeling to identify the distribution of the most dominant species of Sergentomyia genus in Morocco and consequently discuss its potential involvement in local Leishmania transmission cycles.
Materials and Methods
Study area
Morocco is a northern African country, bordering the North Atlantic Ocean and the Mediterranean Sea. The current study covered sand fly sampling in 190 localities with altitude ranges between sea level and up to 2123m above sea (Fig. 1). These localities covered mainly northern and central Morocco, where human cutaneous and visceral leishmaniases are endemic (28).
Fig. 1.
Distribution of the Sergentomyia spp in Morocco according to bioclimate map
The climate in Morocco is mostly Mediterranean; however, seven bioclimatic regimes are occurred due to topographic differences across the country (Fig. 1). In 2014, the total human population in Morocco is about 33.7 million, with an urbanization rate of 60% (29).
Occurrence data and environmental variables
Occurrence records of sand flies were collected from our field survey (published and unpublished data) and literature using the keyword “species name, Morocco” as a search word to retrieve all data archived in PubMed database. Thus, about 70% of the 190 localities were investigated by the Ecology and Environment Laboratory (L2E) team between 2005 and 2016. The rest of the localities were published observations of the investigations in Morocco (20 publications on PUBMED). The coordinates of all localities were registered with a global positioning system (GPS).
For ecological niche modeling, 19 climatic variables (Bio1-Bio19) were used with elevation precision (ALT) for each locality (Table 1). Altitude and bioclimatic data were obtained from the WorldClim project (http://www.worldclim.org), with a spatial resolution of 30 arc seconds (about 1km2).
Table 1.
Description and sources of environmental variables collected for the model
| Environmental variables | Abbreviation | Unit | Source |
|---|---|---|---|
| Annual mean temperature | Bio 1 | °C | WorldClim |
| Mean diurnal range (mean of monthly (max temperature–min temperature)) | Bio 2 | °C | WorldClim |
| Isothermality (BIO2/BIO7) (×100) | Bio 3 | _ | WorldClim |
| Temperature seasonality (standard deviation ×100) | Bio 4 | °C | WorldClim |
| Max temperature of warmest month | Bio 5 | °C | WorldClim |
| Min temperature of coldest month | Bio 6 | °C | WorldClim |
| Temperature annual range (BIO5–BIO6) | Bio 7 | °C | WorldClim |
| Mean temperature of wettest quarter | Bio 8 | °C | WorldClim |
| Mean temperature of driest quarter | Bio 9 | °C | WorldClim |
| Mean temperature of warmest quarter | Bio 10 | °C | WorldClim |
| Mean temperature of coldest quarter | Bio 11 | °C | WorldClim |
| Annual precipitation | Bio 12 | mm | WorldClim |
| Precipitation of wettest month | Bio 13 | mm | WorldClim |
| Precipitation of driest month | Bio 14 | mm | WorldClim |
| Precipitation seasonality (coefficient of variation) | Bio 15 | mm | WorldClim |
| Precipitation of wettest quarter | Bio 16 | mm | WorldClim |
| Precipitation of driest quarter | Bio 17 | mm | WorldClim |
| Precipitation of warmest quarter | Bio 18 | mm | WorldClim |
| Precipitation of coldest quarter | Bio 19 | mm | WorldClim |
| Elevation | ALT | m | GTOPO30 |
Construction of the model
The modeling was performed using Maximum entropy (MaxEnt) version 3.3.3 (30), which uses an optimization procedure comparing the presence of the species with the characteristics of the environment, based on the principle of maximum entropy. This program allows for inference from incomplete information despite limited occurrence data (31–33).
Distribution data for the sand fly species and environmental data were imported into MaxEnt v. 3. 3. 3 (31), a total of 15 model replicates were run, with 30% of the points of presence used to test the model and 70% for model construction (34).
Model Evaluation
To evaluate the quality of model produced by MaxEnt, we analyzed the Operating Characteristics Curve Receiver Operating Characteristic (ROC) which assigns a unique value according to the model performance Area Under the Curve (AUC).
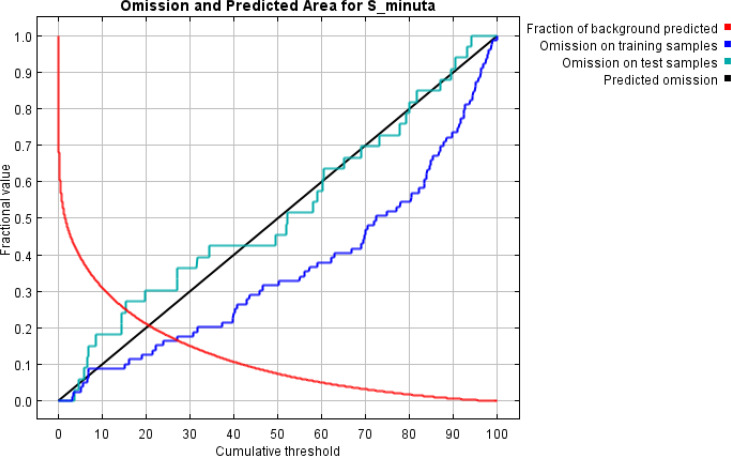
ROC analysis is a measure of sensitivity, which corresponds to the true positive rate (no error of omission), compared to the false positive rate (superfluous forecast error). Also, the ROC analysis evaluates the ability of the model to correctly predict the occurrence of the species. The closer the AUC value is to 1, the closer the correlation of the model (31) (Figs. 2, 3). The importance of the variables in explaining the potential geographic distribution of S. minuta was estimated using the Jackknife Environmental Variables Importance Test (31) (Table 2), which assesses the relative contribution (%) of the variables used to generate the distribution model produced by MaxEnt.
Fig. 2.
Analysis of the omission / commission of Sergentomyia minuta
Fig. 3.
Sensitivity and performance of the model for Sergentomyia minuta species
Table 2.
Analysis of the contribution of the most important ecological variables of the model
| Variable | Percent contribution (%) | AUC without the variable | AUC with only the variable |
|---|---|---|---|
| Bio 12 | 41.2 | 0.917 | 0.867 |
| Bio 15 | 23.9 | 0.922 | 0.892 |
| Bio 14 | 9.4 | 0.916 | 0.859 |
| Bio 4 | 5.9 | 0.914 | 0.890 |
| Bio 19 | 5.5 | 0.914 | 0.906 |
| Bio 3 | 4.8 | 0.914 | 0.868 |
Statistical analysis
The Chi-squared test (χ2) was used to analyze the correlation between variation of S. minuta abundance and the presence/absence of human leishmaniasis cases (cutaneous leishmaniasis by L. tropica and visceral leishmaniasis by L. infantum) in all sampling sites.
Epidemiological data (human cutaneous and visceral leishmaniasis cases) were obtained from official Moroccan Ministry of Health reports (28).
Results
In this study, we assembled 190 sampling sites for sand fly species. Five Sergentomyia species were noted: Sergentomyia minuta (N= 150), S. fallax (N= 110), S. dreyfussi (N= 57), S. africana (N= 10) and S. christophersi (N= 15). Phlebotomus species were also recorded, specifically Phlebotomus sergenti (N= 102), P. papatasi (N= 82), P. longicuspis (N= 78), P. perniciosus (N= 76) and P. ariasi (N= 43), while P. alexandri (N= 09), P. kazeruni (N= 03), P. langeroni (N= 02), and P. mariae (N= 02) were noted with very low reporting.
Sergentomyia minuta was the most predominant species and it was collected in the 150/190 sampling sites between 14 and 2123m (Table 3). To identify habitat suitability and potential limiting factor of S. minuta, all ecological variables were analyzed. There were 6/19 ecological variables (Bio 3, Bio 4, Bio 12, Bio 14, Bio 15, Bio 19), which were the most important factors affecting the distribution of S. minuta in the study area.
Table 3.
Description of Sergentomyia species on sampling sites
| Sergentomyia spp. | Total of specimens | Number of stations occupied | Altitude range (m) |
|---|---|---|---|
| S. minuta | 6746 | 150 | [14–2123] |
| S. fallax | 5582 | 110 | [14–1351] |
| S. dreyfussi | 474 | 57 | [282–1309] |
| S. africana | 43 | 10 | [402–1340] |
| S. christophersi | 99 | 15 | [282–1500] |
Figure 4 shows a representation of the MaxEnt model for the S. minuta. Warmer colors show areas with better predicted conditions. The white dots indicate the presence locations used for training, while the purple dots show the locations of the tests.
Fig. 4.

Estimation of the distribution of Sergentomyia minuta species in Morocco by MaxEnt
Since 1995, leishmaniases are notifiable diseases in Morocco (Ministerial decree n°683-95). All human cases are listed in leishmaniasis epidemiological information collection system and published yearly. We used data recorded of CL and VL between 2005 and 2016 (28) corresponding to 190 localities.
Statistical analysis of the presence and absence of leishmaniasis cases (cutaneous CL and visceral leishmaniasis VL) and the distribution of S. minuta in Morocco showed a positive correlation between VL by L. infantum and S. minuta abundance (r= 0.211) and between CL by L. tropica and S. minuta abundance (r= 0.166).
According to Chi-squared test (χ2) test, S. minuta was more associated to VL distribution (χ2 = 7,166, ddl= 2, P= 0,028) compared to CL (χ2 = 3,256, ddl= 2, P= 0,196).
Discussion
Morocco is located in the subtropical zone. It benefits from a Mediterranean climate, characterized by a dry warm season, nuanced by three essential influences: the progressive remoteness of the Atlantic coast, the altitude and the approach of the southern desert (36). The geographical location of Morocco is a major factor of its bioclimatic diversity which favors the abundance and the diversity of phlebotomine species; including Sergentomyia species that can tolerate the aridity (37–39). Thus, a considerable specific richness in Morocco for Sergentomyia genus counter to the others Mediterranean’s regions such as Tunisia, where only six species of Sergentomyia have been reported (40–42).
Sergentomyia species were collected in different environments but are rather subservient to the extradomestic environment (43). This is related to their trophic preferences, and their adaptability to dry and open habitats. Thus, they find suitable conditions in wild environments.
According to WHO (43), sand flies of the genus Sergentomyia have long been known to feed on reptiles. They are involved in the transmission of Sauroleishmania, of reptiles in the Old World. They do not present an epidemiological risk for humans (44), although some species can feed on humans (45). Recent work on Sergentomyia spp shows that these species can be infected by human Leishmania spp. and the blood meal analysis shows that it is a mammalian blood, including humans (18). Leishmania tropica DNA was detected from S. ingrami and S. hamoni collected in Ghana (46). All of these results raise questions about the role of Sergentomyia spp. as a possible vector of Leishmania spp. which has a great impact on human and animal health.
Among all Sergentomyia species, S. minuta is widely distributed around the Mediterranean area. It is the most abundant Sergentomyia species in the present study (Table 3) and early in Morocco (12).
In Greece, the Sergentomyia genus represented 84% of the total sand flies fauna with 38% of S. minuta (47). In France, Rioux et al. (48) reported a percentage of 73.7% for both genus and species. In Tunisia, it was noted that Sergentomyia accounted for 70% of sand flies, of which 41.5% belonged to the species S. minuta (49). In Algeria, Belazzoug et al. (50) found this species with a rate of 39.75% of total specimens.
Regarding its potential vector role, Periera et al. (51) have isolated L. infantum from S. minuta, and blood meal analysis has shown that it is human blood. In Italy, recently, Latrofa et al. (52) detected L. infantum DNA in S. minuta from endemic area of canine leishmaniasis. S. minuta was also naturally infected with L. major in Portugal (21) and in Tunisia (53). In addition, Maia et al. (54) detected Leishmania spp. in S. minuta collected in southern Portugal; while Bravo-Barriga et al. (55) identified Leishmania DNA in S. minuta of Spain.
In Morocco, though the well-known role of Phlebotomus papatasi, P. sergenti and Larroussius species (P. perniciosus, P. longicuspis and P. ariasi), as competent vector of L. major, L. tropica and L. infantum, respectively (56), that of S. minuta needs further investigation.
Ecological niche modelling has been widely used in order to identify the probability of sand fly species occurrence (57). To the best of our knowledge, the present study constitutes a first modeling of Sergentomyia species. AUC values of our model were greater than 0.9 (Table 2) indicating that the model performed has a good robustness (31).
In the 190 localities used for the model, we noted the coexistence of Sergentomyia species and Phlebotomus species including the proven vectors of leishmaniasis forms in Morocco: P. papatasi, P. sergenti, P. perniciosus, P. ariasi and P. longicuspis. According to the region, P. sergenti and P. papatasi were more abundant in the central Morocco, while P. perniciosus was more recorded in northern Morocco.
The presence and the abundance of S. minuta in the endemic area of leishmaniasis in Morocco can be the most important criteria for its possible vectorial incrimination. Our results showed the widespread geographical distribution of S. minuta in Morocco, specifically in northern and central Morocco (Fig. 4) where VL and CL foci occur (16, 58).
In addition, a positive correlation between visceral leishmaniasis (r= 0.211) and cutaneous leishmaniasis (r= 0.166) with S. minuta abundance was noted and confirm this S. minuta-Leishmania spatial overlapping.
Sand fly behaviors can also determine its vector implication. S. minuta presented an anthropophilic behavior in Portugal (54). In Morocco, S. minuta is a ubiquitous species (13) which shows an adaptation and an important ecological tolerance. It was collected in the different bioclimatic floors with preference to a semiarid climate and altitudes between 800 to 1,000m (12). In addition, the seasonal distribution of S. minuta showed abundance from May to September in sub-humid and semi-arid stages in Morocco (15). In arid bioclimate, it was active during two periods, October–November and April–May–June (59). This long period of S. minuta activity favors the S. minuta-Leishmania temporal overlapping in these areas.
Sand flies of Morocco, including Sergentomyia species, were found highly dependent on environmental conditions; and their abundance and geographical distribution were affected by several ecological variables (59–62). According to our model, annual precipitation (Bio 12) and precipitation seasonality (Bio 15) were significantly more important in the distribution of S. minuta in Morocco (Table 2). Precipitation plays an important role in sand flies’ life cycle (63). Modeling approach of Chargaff et al. (57), found that annual precipitation has a significant role in distribution of Leishmania vector species in Mediterranean basin (57).
S. minuta is recorded in arid region of Tunisia with an annual precipitation between 88 and 157mm (53), also in Portugal with an annual precipitation equal to 730mm (64). In the present study, it prefers regions with an annual precipitation (Bio 12) between: 50–1100mm, and precipitation seasonality (Bio 15) between: 30–100mm (Supporting Information S1).
Conclusion
Effective vector control requires accurate vector identification. The spatial and temporal overlapping of S. minuta with Leishmania species seemed to present the important criteria necessary to be incriminated as a potential vector of the Leishmania species in Morocco. Hence, the present study opened a debate on the potential role of Sergentomyia species, especially S. minuta in the transmission of leishmaniasis in Morocco. Hypothesis deserves further researches including, but not limited to, protozoan isolation from engorged specimens as well as experimental transmission.
Acknowledgements
The authors would like to thank Elouise Gaylard for linguistic consultation. The authors declare that there is no conflict of interest.
References
- 1.Hazratian T, Vatandoost H, Oshaghi MA, Yaghoobi-Ershadi MR, Fallah E, Rafizadeh S, Shirzadi MR, Shayeghi M, Akbarzadeh K, Rassi Y. (2016) Diversity of sand flies (Diptera: Psychodidae) in endemic focus of visceral leishmaniasis in Azar Shahr District, East Azarbaijan Province, north west of Iran. J Arthropod Borne Dis. 10(3): 328–334. [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid Y, Valenzuela JG, Kamhawi S, Rowton E, Sacks DL, Ribeiro JM. (2000) Delayed-type hypersensitivity to Phlebotomus papatasi sand fly bite: An adaptive response induced by the fly?. Proc Natl Acad Sci USA. 97(12): 6704–6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killick-Kendrick R, Killick-Kendrick M. (1999) Biology of sand fly vectors of Mediterranean canine leishmaniasis. In: Canine Leishmaniasis: an update. The First International Canine Leishmaniasis Forum, 1999 February, Barcelona, Spain, pp. 28–31. [Google Scholar]
- 4.Mukherjee S, Hassan MQ, Ghosh A, Ghosh KN, Bhattacharya A, Adhya S. (1997) Leishmania DNA in Phlebotomus and Sergentomyia species during a kala-azar epidemic. Am J Trop Med Hyg. 57: 423–425. [DOI] [PubMed] [Google Scholar]
- 5.Berdjane-Brouk Z, Koné AK, Djimde AA, Charrel RN, Ravel C, Delaunay P, del Giudice P, Diarra AZ, Doumbo S, Goita S, Thera MA, Depaquit J, Marty P, Doumbo OK, Izri A. (2012) First detection of Leishmania major DNA in Sergentomyia (Spelaeomyia) darlingi from cutaneous leishmaniasis foci in Mali. PLoS One. 7: e28266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahime K, Boussaa S, Bounoua L, Ouanaimi F, Messouli M, Boumezzough A. (2014) Leishmaniasis in Morocco: diseases and vectors. Asian Pac J Trop Dis. 4: 530–534. [Google Scholar]
- 7.Rioux JA, Guilvard E, Dereure J, Lanotte G, Denial M, Pratlong F, Serres E, Belmonte A. (1986) Infestation naturelle de Phlebotomus papatasi (Scopoli, 1786) par Leishmania major MON-25. A propos de 28 souches isolées dans un foyer du Sud Marocain. In Rioux J.A. (ed.) Leishmania. Taxinomie et phylogenèse. Applications écoépidémiologiques. International Colloquium CNRS/INSERM, 1984 July 2–6. Institut Méditerranéen d’Etudes Épidémiologiques et Ecologiques, Montpellier, France, pp. 471–480. [Google Scholar]
- 8.Ajaoud M, Es-sette N, Charrel RN, laamrani-Idrissi A, Nhammi H, Riyad M, Lemrani M. (2015) Phlebotomus sergenti in a cutaneous leishmaniasis focus in Azilal Province (High Atlas, Morocco): Molecular detection and genotyping of Leishmania tropica and feeding behavior. PLoS Negl Trop Dis. 9: e0003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarrouk A, Kahime K, Boussaa S, Belqat B. (2015) Ecological and epidemiological status of species of the Phlebotomus perniciosus complex (Diptera: Psychodidae, Phlebotominae) in Morocco. Parasitol Res. 115(3): 1045–1051. [DOI] [PubMed] [Google Scholar]
- 10.Moroccan Ministry of Health (2010) Fight against Leishmaniasis. Activity Guide. Directorate of Epidemiology and Disease Control, Parasitic Diseases Service, Ministry of Health. Morocco. Available at: http://www.sante.gov.ma
- 11.Ouanaimi F, Boussaa S, Boumezzough (2015) Phlebotomine sand flies (Diptera: Psychodidae) of Morocco: results of an entomological survey along three transects from northern to southern country. Asian Pac J Trop Dis. 5(4): 299–306. [Google Scholar]
- 12.Kahime K, Boussaa S, El Mazabi A, Boumezzough A. (2015) Spatial relations between Environmental factors and Phlebotomine sand fly populations (Diptera: Psychodidae) in central and southern Morocco. J Vector Ecol. 40: 342–54. [DOI] [PubMed] [Google Scholar]
- 13.Boussaa S, Boumezzough A, Sibold B, Alves-Pires C, Morillas Marquez F, Glasser N, Pesson B. (2009) Phlebotomine sandflies (Diptera: Psychodidae) of the genus Sergentomyia in Marrakech region, Morocco. Parasitol Res. 104: 1027–1033. [DOI] [PubMed] [Google Scholar]
- 14.Guernaoui S, Boumezzough A, Laamrani A. (2006) Altitudinal structuring of sandflies (Diptera: Psychodidae) in the High-Atlas mountains (Morocco) and its relation to the risk of leishmaniasis transmission. Acta Trop. 97: 346–351. [DOI] [PubMed] [Google Scholar]
- 15.Bailly-Choumara H, Abonnenc E, Pastre J. (1971) Contribution à l’étude des phlébotomes du Maroc. (Diptera: Psychodidae): données faunistiques et écologiques. Cah ORSTOM. Ser Entomol Med Parasitol. 9: 431–460. [Google Scholar]
- 16.Boussaa S, Guernaoui S, Pesson B, Boumezzough A. (2005) Seasonal fluctuations of phlebotomine sand fly populations (Diptera: Psychodidae) in the urban area of Marrakech, Morocco. Acta Trop. 95: 86–91. [DOI] [PubMed] [Google Scholar]
- 17.El Miri H, Rhajaoui M, Himmi O, Ouahabi S, Benhoussa A, Faraj C. (2013) Entomological study of five cutaneous leishmaniasis foci in the Sidi Kacem Province, north Morocco. Ann Soc Entomol Fr. 49(2): 154–159. [Google Scholar]
- 18.Maia C, Depaquit J. (2016) Can Sergentomyia (Diptera: Psychodidae) play a role in the transmission of mammal-infecting Leishmania? Parasite. 23: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parvizi P, Amirkhani A. (2008) Mitochondrial DNA characterization of Sergentomyia sintoni populations and finding mammalian Leishmania infections in this sand fly by using ITSrDNA. Iran J Vet Res. 22: 9–18. [Google Scholar]
- 20.Berdjane-Brouk Z, Koné AK, Djimde AA, Charrel RN, Ravel C, Delaunay P, del Giudice P, Diarra AZ, Doumbo S, Goita S, Thera MA, Depaquit J, Marty P, Doumbo OK, Izri A. (2012) First detection of Leishmania major DNA in Sergentomyia (Spelaeomyia) darlingi from cutaneous leishmaniasis foci in Mali. PLoS One. 7: e28266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campino L, Cortes S, Dionísio L, Neto L, Afonso MO, Maia C. (2013) The first detection of Leishmania major in naturally infected Sergentomyia minuta in Portugal. Mem Inst Oswaldo Cruz. 108: 516–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutinga MJ, Massamba NN, Basimike M, Kamau CC, Amimo FA, Onyido AE, Omo-go DM, Kyai FM, Wachira DW. (1994) Cutaneous leishmaniasis in Kenya: Sergentomyia garnhami (Diptera: Psychodidae), a possible vector of Leishmania major in Kitui District: a new focus of the disease. East Afr Med J. 71: 424–428. [PubMed] [Google Scholar]
- 23.Mukherjee S, Hassan MQ, Ghosh A, Ghosh KN, Bhattacharya A, Adhya S. (1997) Leishmania DNA in Phlebotomus and Sergentomyia species during a kala-azar epidemic. Am J Trop Med Hyg. 57: 423–425. [DOI] [PubMed] [Google Scholar]
- 24.Kanjanopas K, Siripattanapipong S, Ninsaeng U, Hitakarun A, Jitkaew S, Kaewtaphaya P, Tan-ariya P, Mungthin M, Charoenwong C, Leelayoova S. (2013) Sergentomyia (Neophlebotomus) gemmea, a potential vector of Leishmania siamensis in southern Thailand. BMC Infect Dis. 13: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ba Y, Trouillet J, Thonnon J, Fontenille D. (1999) Phlebotomus of Senegal: survey of the fauna in the region of Kedougou. Isolation of arbovirus. Bull Soc Pathol Exot. 92(2): 131–135. [PubMed] [Google Scholar]
- 26.Charrel RN, Izri A, Temmam S, de Lamballerie X, Parola P. (2006) Toscana Virus RNA in Sergentomyia minuta Flies. Emerg Infect Dis. 12(8): 1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geevarghese G, Arankalle VA, Jadi R, Kanojia PC, Joshi MV, Mishra AC. (2005) Detection of Chandipura virus from sand flies in the genus Sergentomyia (Diptera: Phlebotomidae) at Karimnagar District, Andhra Pradesh, India. J Med Entomol. 42: 495–496. [DOI] [PubMed] [Google Scholar]
- 28.Kholoud K, Denis S, Lahouari B, Abdelmonaim M, Hidan EJ, Soua B. (2018) Management of Leishmaniases in the Era of Climate Change in Morocco. Int J Environ Res Public Health . 15(7): 1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.High Planning Commission of Morocco (2014) Report of General Census of Population and Housing. Morocco. Available at: http://www.hcp.ma
- 30.Hijmans R, Cameron S, Parra J, Jones P, Jarvis A, Richardson K. (2005) WorldClim, Version 1.3. University of California, Berkeley. [Google Scholar]
- 31.Phillips SJ, Anderson RP, Schapire RE. (2006) Maximum entropy modeling of species geographic distributions. Ecol Modell. 190: 231–259. [Google Scholar]
- 32.Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, McC J, Overton M, Townsend Peterson A, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberón J, Williams S, Wisz MS, Zimmermann NE. (2006) Novel methods improve prediction of species distributions from occurrence data. Ecography. 29: 129–151. [Google Scholar]
- 33.Pearson TRH, Brown SL, Birdsey RA. (2007) Measurement guidelines for the sequestration of forest carbon Gen. Tech. Rep. NRS-18. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station; p. 42. [Google Scholar]
- 34.Nogués-Bravo D. (2009) Predicting the past distribution of species climatic niches. Glob Ecol Biogeogr. pp. 521–531. [Google Scholar]
- 35.Mokhtari N, Mrabet R, Lebailly P, Bock L. (2014) Spatialisation des bioclimats, de l’aridité et des étages de végétation du Maroc. Rev Mar Sci Agron Vét. 2(1): 50–66. [Google Scholar]
- 36.Sebbar A. (2013) Etude de la variabilité et de l’évolution de la pluviométrie au Maroc (1935–2005): Réactualisation de la carte des précipitations. [PhD dissertation]. Faculté des Sciences Ben M’Sik Casablanca, Morocco. [Google Scholar]
- 37.Adler S, Theodor O. (1929) The distribution of sandflies and leishmaniasis in Palestine, Syria and Mesopotamia. Ann Trop Med Parasitol. 23: 269–306. [Google Scholar]
- 38.Lupasco G, Duport M, Dancesco P, Cristesco A. (1965) Recherches sur les espèces de Phlébotomes sauvages de Roumanie. Arch Roum Pathol Exp Microbiol. 24: 195–202. [PubMed] [Google Scholar]
- 39.Newstead R, Sinton JA. (1921) On a collection of Pappataci flies (Phlebotomus) from India. Ann Trop Med Parasitol. 15: 103–106. [Google Scholar]
- 40.Depaquit J, Léger N, Killick-Kendrick R. (1998) Description de Phlebotomus (Paraphlebotomus) riouxi n. sp (Diptera: Psychodidae) d’Afrique du Nord. Parasite. 151–158. [DOI] [PubMed] [Google Scholar]
- 41.Ghrab J, Rhim A, Bach-Hamba D, Chahed MK, Aoun K, Nouira S, Bouratbine A. (2006) Phlebotominae (Diptera: Psychodidae) of human leishmaniosis sites in Tunisia. Parasite. 13 : 23–33. [DOI] [PubMed] [Google Scholar]
- 42.Chamkhi J, Guerbouj S, Ben Ismail R, Guizani I. (2006) Description of the hitherto unknown female of Phlebotomus (Larroussius) Chadlii, Rioux, Juminer and Gibily, 1966 (Diptera: Psychodidae). Parasite. 13 (4): 299–303. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization (2002) Urbanization: an increasing risk factor for leishmaniasis. Wkly Epidemiol Rec. 77: 365–372. [PubMed] [Google Scholar]
- 44.Lane RP. (1993) Sandflies (Phlebotominae). In: Lane RP, Crosskey RW. (Eds.), Medical insects and arachids. (1st. Edn), London, Chapman and Hall; pp. 78–119. [Google Scholar]
- 45.Hoogstraal H, Dietlein DR, Heyneman D. (1962) Leishmaniasis in the Sudan Republic. Preliminary observations on man-biting sandflies (Psychodiae: Phlebotomus) in certain Upper Nile endemic Nile endemic areas. Trans R Soc Trop Med Hyg. 411–422. [DOI] [PubMed] [Google Scholar]
- 46.Nzelu CO, Kato H, Puplampu N, Desewu K, Odoom S, Wilson MD, Sakurai T, Katakura K, Boakye DA. (2014) First detection of Leishmania tropica DNA and Trypanosoma species in Sergentomyia sand flies (Diptera: Psychodidae) from an outbreak area of cutaneous leishmaniasis in Ghana. PLoS Negl Trop Dis. 8(2): e2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madulo-leblond G. (1983) Les phlébotomes (Diptera: Phlebotomidae) des îles Ioniennes. [PhD dissertation]. Université de Reins, France. [Google Scholar]
- 48.Rioux JA, Lanotte G, Petter F, Dereure J, Akalay O, Pratlong F, Velez ID, Fikri NB, Maazoum R, Denial M, Jarry DM, Zahaf A, Ashford RW, Cadi-Soussi M, Killick-Kendrick R, Benmansour N, Moreno G, Perieres J, Guilvard E, Zribi M, Kennou MF, Rispail P, Knechtli E, Serres E. (1986) Les leishmanioses cutanées du bassin méditerranéen occidental: de l’identification enzymatique à l’analyse éco -épidemiologique, l’exemple de trois ‘foyers’, tunisien, marocain et français. In: Leishmania. Taxonomie et Phylogenèse. Applications Écoépidémiologiques, ed. Rioux J.A. Montpellier, France: Institut Méditerranéen d’Etudes Épidémiologiques et Ecologiques; pp. 365–395. [Google Scholar]
- 49.Haddad N. (1998) Les phlébotomes du Liban-Inventaire et corollaire éco- épidémiologique. Université de Pharmacie Reims, France. [Google Scholar]
- 50.Belazzoug S, Mahzoul D, Rioux JA. (1986) [The Phlebotomus Sandflies (Diptera, Psychodidae) of M'Sila and Bou-Saada]. Arch Inst Pasteur Alger. 55: 117–124. [PubMed] [Google Scholar]
- 51.Peira S, Pita-Pereira D, Araujo-Pereira T, Britto C, Costa-Rego T, Ferrolho J, Vilhena M, Rangel EF, Vilela ML, Afonso MO. (2017) First molecular detection of Leishmania infantum in Sergentomyia minuta (Diptera: Psychodidae) in Alentejo, southern Portugal. Acta Trop. 174 : 45–48. [DOI] [PubMed] [Google Scholar]
- 52.Latrofa MS, Iatta R, Dantas-Torres F, Annoscia G, Gabrielli S, Pombi M, Gradoni L, Otranto D. (2018) Detection of Leishmania infantum DNA in phlebotomine sand flies from an area where canine leishmaniosis is endemic in southern Italy. Vet Parasitol. 253: 39–42. [DOI] [PubMed] [Google Scholar]
- 53.Jaouadi K, Ghawar W, Salem S, Gharbi M, Bettaieb J, Yazidi R, Harrabi M, Hamarsheh O, Ben Salah A. (2015) First report of naturally infected Sergentomyia minuta with Leishmania major in Tunisia. Parasit Vectors. 8: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maia C, Parreira R, Cristóvão JM, Freitas FB, Afonso MO, Campino L. (2015) Molecular detection of Leishmania DNA and identification of blood meals in wild caught phlebotomine sand flies (Diptera: Psychodidae) from southern Portugal. Parasit Vectors. 8: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bravo-Barriga D, Parreira R, Maia C, Blanco-Ciudad J, Odete AM, Frontera E, Campino L, Pérez-Martín JE, Serrano Aguilera FJ, Reina D. (2016) First molecular detection of Leishmania tarentolae-like DNA in Sergentomyia minuta in Spain. Parasitol Res. 115: 1339. [DOI] [PubMed] [Google Scholar]
- 56.Mhaidi I, El Kacem S, Ait Kbaich M, El Hamouchi A, Sarih M, Akarid K, Lemrani M. (2018) Molecular identification of Leishmania infection in the most relevant sand fly species and in patient skin samples from a cutaneous leishmaniasis focus, in Morocco. PLoS Negl Trop Dis. 12(3): e0006315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chalghaf B, Chemkhi J, Mayala B, Harrabi M, Benie GB, Michael E, Ben Salah A. (2018) Ecological niche modeling predicting the potential distribution of Leishmania vectors in the Mediterranean basin: impact of climate change. Parasit Vectors. 11(1): 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhajaoui M. (2011) Human leishmaniases in Morocco. A nosogeographical diversity. Pathol Bio. 59(4): 226–229. [DOI] [PubMed] [Google Scholar]
- 59.Rioux JA, Rispail P, Lanotte G, Lepart J. (1984) Relations Phlébotomes-bioclimats en écologie des leishmanioses Corollaires épidémiologiques. L’exemple du Maroc. Bull Soc Bot. France. 131(2–4): 549–557. [Google Scholar]
- 60.Rispail P, Dereure J, Jarry D. (2002) Risk zones of human Leishmaniases in the Western Mediterranean basin: correlations between vector sand flies, bioclimatology and phytosociology. Mem Inst Oswaldo Cruz. 97(4): 477–483. [DOI] [PubMed] [Google Scholar]
- 61.Bounoua L, Kahime K, Houti L, Blakey T, Ebi KL, Zhang P, Imhoff ML, Thome KJ, Dudek C, Sahabi SA, Messouli M, Makhlouf B, El Laamrani A, Boumezzough A. (2013) Linking climate to incidence of zoonotic cutaneous leishmaniasis (L. major) in pre-Saharan North Africa. Int J Environ Res Public Health. 10(8): 3172–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boussaa S, Kahime K, Samy AM, Salem AB, Boumezzough A. (2016) Species composition of sand flies and bionomics of Phlebotomus papatasi and P. sergenti (Diptera: Psychodidae) in cutaneous leishmaniasis endemic foci, Morocco. Parasit Vectors. 9: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kasap OE, Alten B. (2006) Comparative demography of the sand fly Phlebotomus papatasi (Diptera: Psychodidae) at constant temperatures. J Vector Ecol. 31: 378–385. [DOI] [PubMed] [Google Scholar]
- 64.Branco S, Alves-Pires C, Maia C, Cortes S, Cristovão JMS, Gonçalves L, Campino L, Afonso MO. (2013) Entomological and ecological studies in a new potential zoonotic leishmaniasis focus in Torres Novas municipality, Central Region, Portugal. Acta Trop. 125(3): 339–348. [DOI] [PubMed] [Google Scholar]