Abstract
Background
Hyperuricemia has long been associated with increased cardiovascular risk, and arterial stiffness is proposed as a mediator. The present study is aimed at examining the associations of uric acid (UA) in blood and urine with arterial stiffness in a Chinese cohort.
Methods
A total of 2296 participants (mean age: 43.0 years) from our previously established cohort of Hanzhong Adolescent Hypertension Study were included. The participants were classified as subjects with or without arterial stiffness, which was defined as brachial-ankle pulse wave velocity (baPWV) ≥ 1400 cm/s and/or carotid intima-media thickness (CIMT) ≥ 0.9 mm. Multivariate regression analyses were used to examine the relationship between serum and urinary UA and the risk of arterial stiffness after adjusting for age, gender, systolic blood pressure, fasting glucose, BMI, heart rate, total cholesterol, and triglycerides.
Results
baPWV was positively correlated with urinary uric acid/creatinine ratio (uUA/Cre) (β = 0.061, P < 0.001), while CIMT was correlated with uUA/Cre (β = 0.085, P < 0.001) and fractional excretion of uric acid (FEUA) (β = 0.044, P = 0.033) in all subjects. In addition, uUA/Cre was significantly associated with the risk of high baPWV [1.032 (1.019-1.045)] and arterial stiffness [1.028 (1.016-1.040)].
Conclusion
Our study showed that urinary UA excretion was significantly associated with the risk of arterial stiffness in Chinese adults. These findings suggest that UA, especially urinary UA, may be used as a simple, noninvasive marker for early detection of arterial stiffness in otherwise healthy subjects.
1. Introduction
Arterial stiffness is a strong predictor of cerebrovascular and cardiovascular diseases not only in patients with high risk but also in the general population [1–3]. Several methods for quantifying arterial stiffness have been proposed. The most commonly used technique is measurement of the pulse-wave velocity (PWV), which provides a noninvasive method of assessing stiffness along an arterial section in clinical settings [4, 5]. Brachial-ankle pulse wave velocity (baPWV), which reflects the function of the artery, is a classic artery elasticity measure and an independent predictor for cardiovascular events [4–6]. Carotid intima-media thickness (CIMT) reflects early structural changes in artery walls and is considered a window to systemic vascular arterial stiffness [7, 8]. BaPWV has also been shown to correlate positively with CIMT [9]. BaPWV and CIMT, especially when combined, are useful in predicting future vascular events [10].
Uric acid (UA) is the final oxidation product of purine metabolism. For decades, it has been hypothesized that the antioxidant properties of UA might be protective against oxidative stress, inflammation, and cell injury [11]. Recent studies now suggest that serum uric acid (SUA) is an independent risk factor for cardiovascular and renal disease, particularly in patients with diabetes, hypertension, or heart failure [12]. In addition, although a few studies have investigated the relationship between SUA and arterial stiffness, the results were inconsistent [13–15]. The majority of them investigated primarily on a basic disease, and only baPWV or CIMT was used to represent arterial stiffness. However, there are few reports in a general population regarding these associations.
The kidney plays a key role in regulation of circulating UA levels. Approximately 70% of UA is easily filtered into the renal tubule, and 90% of filtered UA is reabsorbed by the S1 segment of the proximal convoluted tubule. Approximately 10% of filtered UA is finally excreted [16]. Previous evidence demonstrated that elevated urinary UA was a suspected risk factor for calcium oxalate kidney stones in subjects with calcium nephrolithiasis [17, 18]. Recent studies have shown that the urinary UA may be used as a simple, noninvasive marker of the severity of disease and mortality. For example, the urinary uric acid/creatinine ratio (uUA/Cre) has been found to be remarkably higher in hypoxic premature infants or in neonates with birth asphyxia, and this ratio was correlated significantly with the clinical severity of the disease [19–21]. In addition, we recently show that uUA/Cre was significantly associated with the risk of subclinical renal damage (SRD) and its progression in Chinese adults [22]. However, no study has investigated the relationship between urinary UA and arterial stiffness.
In the present study, based on our previously established cohort [23–25], we aimed to investigate the associations of UA in blood and urine with arterial stiffness as measured by increased baPWV and CIMT.
2. Materials and Methods
2.1. Study Sample
In March and April 1987, we established the cohort of Hanzhong Adolescent Hypertension Study based on a baseline survey of 4623 adolescents aged 6–15 years in over 20 schools of three towns (Qili, Laojun, and Shayan) in Hanzhong, Shaanxi, China. This is an ongoing prospective, population-based cohort study of children and adolescents who regularly undergo follow-ups for investigating the development of cardiovascular risk factors originating in children and young adults. Details of the study protocol have been published elsewhere [23–25].
In this study, we followed up this cohort from April to July 2017, and a total of 2780 were followed up this time. The total rate of this follow-up was 60.3%, which was very rare for such a long-term follow-up. The participant selection process is described in Figure 1. Participants were excluded if they had missing data on serum or urinary biochemistry (n = 17 and 390, resp.), baPWV and CIMT (n = 44), blood pressure (n = 28), weight and height (n = 1), and if they had a self-identified history of stroke, coronary heart disease, or renal failure (n = 4), leaving 2296 subjects for the primary analyses.
Figure 1.
Flow diagram for recruitment of participants.
Written informed consent was obtained from all participants, and the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University approved the study protocol (code: 2015–128). The study conducted in according with principles of the Helsinki Declaration (2008). This trial is registered with NCT02734472 (https://www.clinicaltrials.gov).
2.2. Anthropometric Measurements
Participants completed standardized questionnaires that inquired about medical history, current medications, alcohol and tobacco use, physical activity, and family history. Body weight and height were measured. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Participants who reported continuous or cumulative smoking for 6 months or more were defined as cigarette smokers [26]. Alcohol consumption was defined as subjects who claimed that they consumed alcohol (liquor, beer, or wine) every day and that consumption lasted for 6 months [27]. Blood pressure (BP) was measured in the sitting position using a standard mercury sphygmomanometer as previously described [28–31]. Hypertension was defined as a systolic BP of ≥140 mm Hg, a diastolic BP ≥ 90 mm Hg, or as the use of antihypertensive agents according to subjects' self-report or pharmacy data.
2.3. Laboratory Investigations
All subjects were instructed to avoid alcohol, animal offal, seafood, coffee/tea, beans, and heavy physical activity prior to their sample collection a week ago. Venous blood samples were obtained from all participants after overnight fasting for the determination of serum uric acid (SUA), serum creatinine, serum glucose, and lipid profile (total cholesterol, triglycerides, low-density lipoprotein (LDL), and high-density lipoprotein (HDL)), according to established methods [23, 25, 31].
The first-void and mid-stream urine was collected, followed by venous blood sampling. Urinary concentrations of UA, creatinine, and albumin were measured by an automatic biochemical analyser (Hitachi, Tokyo, Japan). Details of these assays were described previously [22, 23, 31]. UA is transported in the proximal tubule by secretory and reabsorbing transporters, and its handling is a useful marker of proximal tubular function [32, 33]. Fractional excretion of uric acid (FEUA) can be calculated after a simultaneous collection of blood and urine using the standard formula: ([urine uric acid] × [serum creatinine])/([urine creatinine] × [serum uric acid]) × 100, expressed as percentage. eGFR was calculated using the formula adapted from the Modification of Diet in Renal Disease equation on the basis of data from Chinese subjects with CKD [34]. The specific formula is as follows: eGFR = 175 × serum creatinine−1.234 × age−0.179(×0.79 for women), where serum creatinine concentration is in milligrams per deciliter and age is in years.
2.4. Markers of Arterial Stiffness
BaPWV was measured using a volume-plethysmographic device (BP-203RPEII, Nihon Colin, Japan) as previously reported [25, 30, 35–37]. The average value of baPWV values on both sides was used for analysis. BaPWV ≥ 1400 cm/s is an independent variable for risk stratification by Framingham score and for discrimination of subjects with cardiovascular disease. Therefore, high baPWV was defined as baPWV ≥ 1,400 cm/s [30, 36].
The CIMT, defined as the distance the distance from the intima-luminal interface to the media-adventitial interface, was measured as described elsewhere [25, 38]. CIMT was bilaterally visualized and measured at the plaque-free area of the common carotid arteries (10–20mm proximal to the tip of the flow divider). Three images were obtained for both common carotid arteries, and the average of the six measured was used for analysis. The same sonographer who was blinded to the subjects' clinical status carried out all the measurements. According to 2018 Chinese guidelines for the management of hypertension, individuals with early stages of arterial stiffness were defined as those with a nonatherosclerotic CIMT ≥ 0.9 mm [12]. In the current study, we defined arterial stiffness as baPWV ≥ 1400 cm/s and/or CIMT ≥ 0.9 mm as previously reported [25, 38].
2.5. Statistical Analysis
Data are expressed as the means ± standard deviations for normally distributed values, as medians (inter-quartile range) for nonnormally distributed values, and as percentages. Significant differences between the groups were calculated using Student's t-test, the Mann–Whitney test, and χ2 test as appropriate. The partial correlation coefficient R was measured to assess the relationship between two variables. We performed regression analyses to test associations of UA in serum and urine with arterial stiffness with linear and logistic regression analyses. These analyses were multivariate, adjusting for traditional cardiovascular risk factors and potential confounders. All statistical analyses were conducted using SPSS 16.0 (SPSS, Inc., Chicago, IL). P < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of Participants
Table 1 presents the characteristics of all subjects according to arterial stiffness status. The prevalence of men, alcohol drinking, smoking, diabetes, and hypertension and age, SBP, DBP, total cholesterol, blood glucose, triglycerides, LDL, SUA, serum creatinine, baPWV, and CIMT were higher in participants with arterial stiffness than in those without arterial stiffness, but HDL-C and FEUA were higher in those without arterial stiffness.
Table 1.
Characteristics of participants categorized by arterial stiffness (AS) status.
| Characteristics | All | Subjects with AS | Subjects without AS | P value |
|---|---|---|---|---|
| No. of subjects (%) | 2296 (100.0) | 581 (25.3) | 1715 (74.7) | — |
| Gender (male, %) | 55.3 | 73.3 | 49.2 | <0.001 |
| Age (years) | 43.0 (40.0-45.0) | 43.0 (40.0-45.0) | 43.0 (40.0-45.0) | <0.001 |
| Alcohol consumption (%) | 29.2 | 37.3 | 26.5 | <0.001 |
| Current smoking (%) | 43.0 | 55.4 | 38.8 | <0.001 |
| Diabetes mellitus (%) | 4.0 | 7.4 | 2.9 | <0.001 |
| Hypertension (%) | 20.6 | 50.3 | 10.5 | <0.001 |
| BMI (kg/m2) | 23.8 (21.9-26.0) | 24.7 (22.7-26.8) | 23.5 (21.6-25.6) | 0.368 |
| Heart rate (beats/min) | 69.0 (63.5-75.5) | 71.5 (65.8-78.3) | 68.5 (63.0-74.5) | <0.001 |
| SBP (mmHg) | 121.3 (112.-131.3) | 133.3 (124.3-146.7) | 110.0 (118.0-126.3) | <0.001 |
| DBP (mmHg) | 76.0 (69.0-84.0) | 86.0 (78.7-93.3) | 73.7 (67.7-79.7) | <0.001 |
| SUA (μmol/L) | 279.2 (225.0-335.3) | 305.8 (263.0-360.1) | 269.8 (218.6-324.2) | <0.001 |
| Fasting glucose (mmol/L) | 4.57 (4.28-4.91) | 4.67 (4.34-5.01) | 4.54 (4.26-4.86) | <0.001 |
| Total cholesterol (mmol/L) | 4.51 (4.04-5.01) | 4.64 (4.17-5.15) | 4.47 (4.01-4.97) | <0.001 |
| Triglycerides (mmol/L) | 1.33 (0.96-1.95) | 1.55 (1.01-2.23) | 1.26 (0.92-1.84) | <0.001 |
| LDL (mmol/L) | 2.50 (2.13-2.91) | 2.58 (2.22-3.01) | 2.46 (2.10-2.86) | <0.001 |
| HDL (mmol/L) | 1.15 (0.99-1.33) | 1.10 (0.97-1.28) | 1.16 (1.00-1.35) | <0.001 |
| Serum creatinine (μmol/L) | 76.7 ± 14.2 | 79.6 ± 14.8 | 75.7 ± 13.9 | <0.001 |
| eGFR (mL/min/1.73 m2) | 97.2 (86.9-110.0) | 95.6 (85.9-108.7) | 97.6 (87.2-110.3) | 0.150 |
| uUA/Cre | 0.20 (0.12-0.33) | 0.20 (0.11-0.32) | 0.20 (0.12-0.33) | 0.566 |
| FEUA | 5.19 (3.04-9.17) | 5.07 (2.76-8.58) | 5.44 (3.13-9.37) | 0.025 |
| CIMT (mm) | 0.62 (0.53-0.75) | 0.68 (0.57-0.83) | 0.60 (0.53-0.70) | <0.001 |
| baPWV (cm/s) | 1219.0 (1093.6-1374.1) | 1498.0 (1428.0-1624.8) | 1163.5 (1061.5-1265.0) | <0.001 |
AS: arterial stiffness; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; SUA: serum uric acid; LDL: low-density lipoprotein; HDL: high-density lipoprotein; uUA/Cre: urinary uric acid/creatinine ratio; FEUA: fraction excretion of uric acid; baPWV: brachial-ankle pulse wave velocity; CIMT: carotid intima-media thickness. Nonnormally distributed variables are expressed as the median (interquartile range). All other values are expressed as mean ± SD or n, %.
3.2. Correlations of Serum and Urinary UA Levels with baPWV and CIMT
Partial correlation analyses showed that baPWV was positively correlated with SUA (r = 0.05, P = 0.017) and uUA/Cre (r = 0.085, P < 0.001, Figure 2). CIMT was correlated with uUA/Cre (r = 0.088, P < 0.001) and FEUA (r = 0.049, P = 0.02) (Figure 2).
Figure 2.
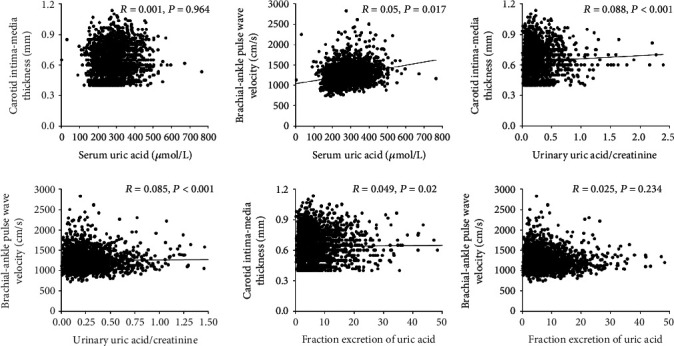
Correlations among baPWV and CIMT with SUA, uUA/Cre and FEUA in all subjects. Partial correlation analyses were used after adjusting for age, gender, hypertension, diabetes, BMI, heart rate, total cholesterol, and triglycerides.
Multiple linear regression analysis found that baPWV was closely correlated with age, BMI, SBP, fasting glucose, heart rate, total cholesterol, SUA, and uUA/Cre (β = 0.061, P < 0.001), while it was inversely correlated with sex (R2 = 0.463). In addition, CIMT was positively correlated with age, BMI, fasting glucose, total cholesterol, uUA/Cre (β = 0.085, P < 0.001), and FEUA (β = 0.044, P = 0.033) but negatively correlated with sex in all subjects (R2 = 0.047) (Table 2).
Table 2.
Relationship between various characteristics and baPWV and CIMT.
| Characteristics | baPWV | CIMT | ||
|---|---|---|---|---|
| β | P value | β | P value | |
| Gender (male) | -0.119 | <0.001 | -0.135 | <0.001 |
| Age (years) | 0.102 | <0.001 | 0.082 | <0.001 |
| BMI (kg/m2) | -0.073 | <0.001 | 0.094 | <0.001 |
| SBP (mmHg) | 0.596 | <0.001 | 0.005 | 0.828 |
| Fasting glucose (mmol/L) | 0.044 | 0.006 | 0.059 | 0.006 |
| Heart rate (beats/min) | 0.092 | <0.001 | 0.024 | 0.261 |
| Total cholesterol (mmol/L) | 0.039 | 0.014 | 0.046 | 0.032 |
| Triglycerides (mmol/L) | 0.003 | 0.846 | -0.027 | 0.238 |
| SUA (μmol/L) | 0.056 | 0.005 | 0.021 | 0.422 |
| uUA/Cre | 0.061 | <0.001 | 0.085 | <0.001 |
| FEUA | 0.014 | 0.370 | 0.044 | 0.033 |
BaPWV: brachial-ankle pulse wave velocity; CIMT: carotid intima-media thickness; BMI: body mass index; SBP: systolic blood pressure; SUA: serum uric acid; uUA/Cre: urinary uric acid/creatinine ratio; FEUA: fraction excretion of uric acid.
3.3. Associations of Serum and Urinary UA Levels with Arterial Stiffness
To extend this finding to clinical practice, we further investigated the association between UA and high baPWV (baPWV ≥ 1400 cm/s) or increased CIMT (CIMT ≥ 0.9 mm) based on multiple logistic regressions. uUA/Cre was significantly associated with the risk of high baPWV [1.032 (1.019-1.045), P < 0.001] but not increased CIMT [1.546 (0.680-3.515), P = 0.299] after adjusting for confounding factors. However, no significant association was found in either SUA or FEUA (Table 3).
Table 3.
Association between various characteristics and the risk of high baPWV or increased CIMT.
| Characteristics | High baPWV (≥1400 cm/s) | Increased CIMT (≥0.9 mm) | ||
|---|---|---|---|---|
| Odds ratios (CI) | P value | Odds ratios (CI) | P value | |
| Gender (male) | 0.458 (0.334-0.629) | <0.001 | 0.405 (0.240-0.684) | 0.001 |
| Age (years) | 1.063 (1.025-1.103) | 0.001 | 1.137 (1.078-1.198) | <0.001 |
| BMI (kg/m2) | 0.938 (0.898-0.979) | 0.004 | 1.074 (1.004-1.150) | 0.038 |
| SBP (mmHg) | 1.097 (1.085-1.108) | <0.001 | 1.005 (0.992-1.017) | 0.470 |
| Fasting glucose (mmol/L) | 1.018 (0.922-1.125) | 0.721 | 1.131 (1.000-1.280) | 0.050 |
| Heart rate (beats/min) | 0.997 (0.922-1.125) | 0.746 | 0.997 (0.976-1.018) | 0.746 |
| Total cholesterol (mmol/L) | 1.202 (1.028-1.405) | 0.021 | 1.135 (0.883-1.458) | 0.322 |
| Triglycerides (mmol/L) | 1.007 (0.918-1.105) | 0.877 | 0.897 (0.742-1.084) | 0.260 |
| SUA (μmol/L) | 1.001 (0.999-1.003) | 0.165 | 0.999 (0.996-1.002) | 0.680 |
| FEUA | 0.997 (0.987-1.008) | 0.624 | 1.001 (0.990-1.013) | 0.820 |
| uUA/Cre | 1.032 (1.019-1.045) | <0.001 | 1.546 (0.680-3.515) | 0.299 |
Logistic regression analyses were used to test the risk of high baPWV and increased CIMT, after adjustment for age, gender, SBP, fasting glucose, BMI, heart rate, total cholesterol, and triglycerides. CI: confidence interval; BaPWV: brachial-ankle pulse wave velocity; CIMT: carotid intima-media thickness; BMI; body mass index; SBP: systolic blood pressure; SUA: serum uric acid; FEUA: fraction excretion of uric acid; uUA/Cre: urinary uric acid/creatinine ratio.
To further comprehensively and accurately evaluate arterial stiffness, we combined CIMT and baPWV and defined arterial stiffness as baPWV ≥ 1400 cm/s and/or CIMT ≥ 0.9 mm. We found that after adjusting for multiple confounders, uUA/Cre [1.028 (1.016-1.040)] was significantly associated with the risk of arterial stiffness (Table 4). SUA (P = 0.268) and FEUA (P = 0.719) did not remain in the final model.
Table 4.
Association between various characteristics and the risk of arterial stiffness.
| Characteristics | Odds ratios (CI) | P value |
|---|---|---|
| Gender (male) | 0.451 (0.338-0.601) | <0.001 |
| Age (years) | 1.186 (1.049-1.124) | <0.001 |
| BMI (kg/m2) | 0.964 (0.927-1.003) | 0.070 |
| SBP (mmHg) | 1.078 (1.069-1.088) | <0.001 |
| Fasting glucose (mmol/L) | 1.051 (0.962-1.149) | 0.273 |
| Heart rate (beats/min) | 1.445 (0.864-2.418) | 0.161 |
| Total cholesterol (mmol/L) | 1.175 (1.018-1.356) | 0.027 |
| Triglycerides (mmol/L) | 0.987 (0.904-1.077) | 0.765 |
| SUA (μmol/L) | 1.001 (0.999-1.003) | 0.268 |
| FEUA | 0.998 (0.990-1.007) | 0.719 |
| uUA/Cre | 1.028 (1.016-1.040) | <0.001 |
Logistic regression analyses were used to test the risk of arterial stiffness, after adjustment for age, gender, SBP, fasting glucose, BMI, heart rate, total cholesterol, and triglycerides. CI: confidence interval; BMI: body mass index; SUA: serum uric acid; FEUA: fraction excretion of uric acid; uUA/Cre: urinary uric acid/creatinine ratio.
3.4. Sensitivity Analysis
To further exclude the potential influence of medication use, including antihypertensive drugs, we removed subjects with hypertension, diabetes, or hyperuricemia under treatment (n = 91). As shown in Supplemental Tables 1-2, all the results remained similar after adjustment for potential confounders.
4. Discussion
To the best of our knowledge, the present study is the first to evaluate the relationship between urinary UA excretion and the risk of arterial stiffness. Interestingly, we showed that uUA/Cre was significantly associated with baPWV and CIMT. In addition, when baPWV and CIMT are combined to comprehensively evaluate arterial stiffness, we found that uUA/Cre is significantly associated with an increased risk of arterial stiffness. These data indicate that the urinary UA may act as a simple noninvasive, cost-effective, single biochemical marker for assessing the severity of arterial stiffness.
Uric acid homeostasis is determined by the balance between production, intestinal secretion, and renal excretion [39]. The renal proximal tubule is responsible for almost all renal urate transport and is the primary site of urate reabsorption [32]. Several previous studies indicated that urinary UA may be associated with the disease incidence and development. Li et al. [40] reported that urinary UA was negatively associated with albuminuria in a cross-sectional study of 200 Chinese patients with chronic kidney disease. Ozanturk et al. [41] revealed significant correlation of nocturnal hypoxemia with UA excretion and uUA/Cre in a group of patients including obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) patients. Moreover, recent studies suggest that uUA/Cre is simple, noninvasive, painless, and economical in investigation for the diagnosis of perinatal asphyxia. For example, Chen et al. [42] showed that the uUA/Cre was remarkably higher in hypoxic premature infants than in hypoxic-term infants. Bader et al. [19] reported that uUA/Cre was elevated in infants with perinatal asphyxia compared with the control group, and that this ratio was correlated significantly with the clinical severity of the disease. Nariman et al. [43] found that uUA/Cre increased with the severity of disease and was associated with longer duration of stay and adverse outcome in all NICU-admitted neonates. The essence of neonatal asphyxia is hypoxia. Hypoxia can lead to energy metabolism disorders, excessive production of oxygen free radicals, accumulation of adenosine diphosphate (ADP), and adenosine monophosphate (AMP) caused by incompletely oxidization, which makes excessive adenosine, inosine, and hypoxanthine. After these substances are catabolized, uric acid in the blood increases, which leads to increased excretion of uric acid in the urine [44]. Therefore, the detection of UA in urine may help measure the degree of oxidative damage. To the best of our knowledge, this is the first study to investigate the relationship between urinary UA and baPWV, and we showed that urinary excretion of UA was significantly associated with baPWV and a risk of high baPWV in the general population. Cao and Wang [45] indicated that TAS and arterial elasticity are decreased while arterial stiffness is increased in elderly hypertensive patients. The decline in antioxidant capacity may be responsible for vascular damage and arterial elasticity decrease in elderly hypertensive patients. Therefore, we assume that oxidative damage may be the bridge between uric acid excretion and arterial stiffness.
Subclinical arterial stiffening may already exist in healthy individuals, and a high UA level may be useful in predicting the incidence of arterial stiffening. Many studies have investigated the relationship between SUA and arterial stiffness in various populations, with inconsistent findings [13–15]. However, majority of the studies have been conducted on patients with various disorders, such as hypertension, diabetes, and CKD. Moreover, only baPWV or CIMT was used to evaluate arterial stiffness in previous studies [13–15]. BaPWV can be evaluated as an arterial functional distensibility, whereas CIMT is considered to be a parameter of arterial structural change. In fact, structural and functional alterations of the arteries can suggest a functional impairment long before the appearance of clinical lesions. Nagai et al. [10] reported that baPWV and CIMT, especially when combined, are useful in predicting future vascular events. In the present study, when baPWV and CIMT are combined, we failed to show any association between SUA and arterial stiffness. Interestingly, our data show that urinary excretion of UA was significantly associated with the risk of arterial stiffness in the general population. Very recently, we also showed that urinary UA excretion was significantly associated with the risk of subclinical renal damage, defined as slightly increased albuminuria or decreased eGFR in Chinese adults [22]. In fact, UA per se can be detrimental to the kidneys, as shown in basic studies. UA can induce vascular smooth muscle cell proliferation, chronic inflammation, endothelial dysfunction, and activation of the renin angiotensin system (RAS) in the kidney, which may be possible mechanisms for renal damage [46, 47].
Some limitations of our study merit consideration. First, our results were obtained from northern-Chinese individuals and consequently may not be generalizable to other ethnic groups with different demographics. In addition, all subjects in this study were youth and middle aged between 36 and 45 years during the follow-up at 2017, and thus, the findings may not be generalizable to other age groups. Finally, a single measurement may be insufficient to assess the urinary UA excretion of individuals. Urinary UA excretions may change according to the urine sampling time because UA excretion may be influenced by the time at which food is consumed or the drugs they take. However, all subjects were instructed to avoid alcohol, animal offal, seafood, coffee/tea, beans, and heavy physical activity prior to their sample collection a week ago. Also, participants under medication were excluded to minimize the confounders in our sensitivity analysis, and the results were similar.
In summary, our study shows that urinary UA excretion was significantly associated with the risk of arterial stiffness in Chinese adults. However, we failed to find a significant relationship between serum UA and arterial stiffness. These findings suggest that UA, especially urinary UA, may be used as a simple, noninvasive marker for early detection of arterial stiffness in otherwise healthy subjects. Future studies are necessary to confirm the relationship between urinary UA and subclinical arterial stiffness and elucidate the potential mechanisms underlying this association.
Acknowledgments
We are sincerely indebted to all subjects for their sustained cooperation and commitment. We would particularly like to thank Drs. Rui-Hai Yang and Jun Yang (Institute of Cardiovascular Sciences, Hanzhong People's Hospital, Hanzhong, China) for providing assistance during follow-up. This work was supported by the National Natural Science Foundation of China No. 81600327 (Y.W.), No. 81870319 (J.-J.M.), No. 81700368 (C.C.), and No. 81600574 (H.L.); Fundamental Research Funds for the Central Universities (No. xjj2018103); Institutional Foundation of the First Affiliated Hospital of Xi'an Jiaotong University No. 2019QN-06; the Clinical Research Award of the First Affiliated Hospital of Xi'an Jiaotong University of China No. XJTU1AF-CRF-2017-021 (Y.W.) and XJTU1AF-CRF-2019-004 (J.-J.M.); Grants from China Postdoctoral Science Foundation funded project (No. 2018T111075 and 2018M631177); Special Financial Grant from Shaanxi Postdoctoral Science Foundation; Grants from the Major Chronic Non-communicable Disease Prevention and Control Research Key Project of the Ministry of Science and Technology of China (2017YFC1307604 and 2016YFC1300104); Grant 2017ZDXM-SF-107 from the Key Research Project of Shaanxi Province; and Grant from Key Laboratory of Molecular Cardiology of First Affiliated Hospital of Xi'an Jiaotong University (KLMC-2018-06).
Contributor Information
Yang Wang, Email: wangyangxxk@126.com.
Jian-Jun Mu, Email: mujjun@163.com.
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Authors' Contributions
Yang Wang, Xiao-Yu Zhang, and Wei-Hua Gao contributed equally to this work.
Supplementary Materials
Table S1: association between various characteristics and the risk of baPWV and CIMT in subjects without medication use (n = 2205). Table S2: association between various characteristics and the risk of arterial stiffness in subjects without medication use (n = 2205).
References
- 1.Mattace-Raso F. U. S., van der Cammen T. J. M., Hofman A., et al. Arterial stiffness and risk of coronary heart disease and stroke:the Rotterdam study. Circulation. 2006;113(5):657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 2.Dijk J. M., van der Graaf Y., Grobbee D. E., Banga J. D., Bots M. L., SMART Study Group Increased arterial stiffness is independently related to cerebrovascular disease and aneurysms of the abdominal aorta: the Second Manifestations of Arterial Disease (SMART) Study. Stroke. 2004;35(7):1642–1646. doi: 10.1161/01.STR.0000130513.77186.26. [DOI] [PubMed] [Google Scholar]
- 3.Hatanaka R., Obara T., Watabe D., et al. Association of Arterial Stiffness with Silent Cerebrovascular Lesions: The Ohasama Study. Cerebrovascular Diseases. 2011;31(4):329–337. doi: 10.1159/000322599. [DOI] [PubMed] [Google Scholar]
- 4.Munakata M. Brachial-ankle pulse wave velocity: background, method, and clinical evidence. Pulse. 2015;3(3-4):195–204. doi: 10.1159/000443740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munakata M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Current Hypertension Reviews. 2014;10(1):49–57. doi: 10.2174/157340211001141111160957. [DOI] [PubMed] [Google Scholar]
- 6.Ohkuma T., Ninomiya T., Tomiyama H., et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. 2017;69(6):1045–1052. doi: 10.1161/HYPERTENSIONAHA.117.09097. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter M., Sinclair H., Kunadian V. Carotid intima media thickness and its utility as a predictor of cardiovascular disease: a review of evidence. Cardiology in Review. 2016;24(2):70–75. doi: 10.1097/CRD.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 8.Nezu T., Hosomi N., Aoki S., Matsumoto M. Carotid intima-media thickness for atherosclerosis. Journal of Atherosclerosis and Thrombosis. 2016;23(1):18–31. doi: 10.5551/jat.31989. [DOI] [PubMed] [Google Scholar]
- 9.Rho Y. H., Chung C. P., Oeser A., et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis and Rheumatism. 2009;61(11):1580–1585. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagai K., Shibata S., Akishita M., et al. Efficacy of combined use of three non-invasive atherosclerosis tests to predict vascular events in the elderly; carotid intima-media thickness, flow- mediated dilation of brachial artery and pulse wave velocity. Atherosclerosis. 2013;231(2):365–370. doi: 10.1016/j.atherosclerosis.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Lippi G., Montagnana M., Franchini M., Favaloro E. J., Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clinica Chimica Acta. 2008;392(1-2):1–7. doi: 10.1016/j.cca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Chinese guidelines for the management of hypertension Writing Group. 2018 Chinese guidelines for the management of hypertension. Chinese Journal Of Cardiovascular Medicine. 2019;24(1):24–56. doi: 10.3969/j.issn.1007-5410.2019.01.002. [DOI] [Google Scholar]
- 13.Zhang J., Xiang G., Xiang L., Sun H. Serum uric acid is associated with arterial stiffness in men with newly diagnosed type 2 diabetes mellitus. Journal of Endocrinological Investigation. 2014;37(5):441–447. doi: 10.1007/s40618-013-0034-9. [DOI] [PubMed] [Google Scholar]
- 14.Cicero A. F., Salvi P., D'Addato S., Rosticci M., Borghi C., Brisighella Heart Study group Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. Journal of Hypertension. 2014;32(1):57–64. doi: 10.1097/HJH.0b013e328365b916. [DOI] [PubMed] [Google Scholar]
- 15.Liu X., Wu J., Wu H., et al. Association of serum uric acid with arterial stiffness in peritoneal dialysis patients. Kidney and Blood Pressure Research. 2018;43(5):1451–1458. doi: 10.1159/000493659. [DOI] [PubMed] [Google Scholar]
- 16.Lytvyn Y., Perkins B. A., Cherney D. Z. Uric Acid as a Biomarker and a Therapeutic Target in Diabetes. Canadian Journal of Diabetes. 2015;39(3):239–246. doi: 10.1016/j.jcjd.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Coe F. L., Kavalach A. G. Hypercalciuria and hyperuricosuria in patients with calcium nephrolithiasis. The New England Journal of Medicine. 1974;291(25):1344–1350. doi: 10.1056/NEJM197412192912510. [DOI] [PubMed] [Google Scholar]
- 18.Coe F. L. Treated and Untreated Recurrent Calcium Nephrolithiasis in Patients with Idiopathic Hypercalciuria, Hyperuricosuria, or No Metabolic Disorder. Annals of Internal Medicine. 1977;87(4):404–410. doi: 10.7326/0003-4819-87-4-404. [DOI] [PubMed] [Google Scholar]
- 19.Bader D., Gozal D., Weinger-Abend M., Berger A., Lanir A. Neonatal urinary uric acid/ceratinine ratio as an additional marker of perinatal asphyxia. European Journal of Pediatrics. 1995;154(9):747–749. doi: 10.1007/BF02276720. [DOI] [PubMed] [Google Scholar]
- 20.Patel K. P., Makadia M. G., Patel V. I., Nilayangode H. N., Nimbalkar S. M. Urinary uric acid/creatinine ratio - a marker for perinatal asphyxia. Journal of Clinical and Diagnostic Research. 2017;11(1):SC08–SC10. doi: 10.7860/JCDR/2017/22697.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhongir A. V., Yakama A. V., Saha S., Radia S. B., Pabbati J. The urinary uric acid/creatinine ratio is an adjuvant marker for perinatal asphyxia. European Journal of Pharmaceutical and Medical Research. 2015;2(5):520–528. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Chen C., Yan Y., et al. Association of uric acid in serum and urine with subclinical renal damage: Hanzhong Adolescent Hypertension Study. PloS one. 2019;14(11, article e0224680) doi: 10.1371/journal.pone.0224680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Hu J.-W., Qu P.-F., et al. Association between urinary sodium excretion and uric acid, and its interaction on the risk of prehypertension among Chinese young adults. Scientific reports. 2018;8(1) doi: 10.1038/s41598-018-26148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng W., Mu J., Chu C., et al. Association of blood pressure trajectories in early life with subclinical renal damage in middle age. Journal of the American Society of Nephrology. 2018;29(12):2835–2846. doi: 10.1681/ASN.2018030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Yuan Y., Gao W. H., et al. Predictors for progressions of brachial-ankle pulse wave velocity and carotid intima-media thickness over a 12-year follow-up: Hanzhong Adolescent Hypertension Study. Journal of Hypertension. 2019;37(6):1167–1175. doi: 10.1097/HJH.0000000000002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu C., Dai Y., Mu J., et al. Associations of risk factors in childhood with arterial stiffness 26 years later. Journal of Hypertension. 2017;35(Supplementary 1):S10–S15. doi: 10.1097/HJH.0000000000001242. [DOI] [PubMed] [Google Scholar]
- 27.Li X. X., Zhao Y., Huang L. X., et al. Effects of smoking and alcohol consumption on lipid profile in male adults in northwest rural China. Public Health. 2018;157:7–13. doi: 10.1016/j.puhe.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Wang D., Chu C., et al. Effect of salt intake and potassium supplementation on urinary renalase and serum dopamine levels in Chinese adults. Cardiology. 2015;130(4):242–248. doi: 10.1159/000371794. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Liu F.-Q., Wang D., et al. Effect of salt intake and potassium supplementation on serum renalase levels in Chinese adults: a randomized trial. Medicine. 2014;93(6) doi: 10.1097/MD.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Lv Y. B., Chu C., et al. Plasma renalase is not associated with blood pressure and brachial-ankle pulse wave velocity in Chinese adults with normal renal function. Kidney & Blood Pressure Research. 2016;41(6):837–847. doi: 10.1159/000452587. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Chu C., Wang K.-K., et al. Effect of salt intake on plasma and urinary uric acid levels in Chinese adults: an interventional trial. Scientific reports. 2018;8(1) doi: 10.1038/s41598-018-20048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipkowitz M. S. Regulation of uric acid excretion by the kidney. Current Rheumatology Reports. 2012;14(2):179–188. doi: 10.1007/s11926-012-0240-z. [DOI] [PubMed] [Google Scholar]
- 33.Maesaka J. K., Fishbane S. Regulation of renal urate excretion: A critical review. American Journal of Kidney Diseases. 1998;32(6):917–933. doi: 10.1016/S0272-6386(98)70067-8. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y. C., Zuo L., Chen J. H., et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. Journal of the American Society of Nephrology. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Mu J. J., Geng L. K., et al. Effect of salt intake and potassium supplementation on brachial-ankle pulse wave velocity in Chinese subjects: an interventional study. Brazilian Journal of Medical and Biological Research. 2015;48(1):83–90. doi: 10.1590/1414-431x20144213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J. W., Wang Y., Chu C., et al. The relationships of the fractional excretion of uric acid with brachial-ankle pulse wave velocity and ankle brachial index in Chinese young adults. Kidney & Blood Pressure Research. 2018;43(1):234–245. doi: 10.1159/000487677. [DOI] [PubMed] [Google Scholar]
- 37.Liao Y. Y., Ma Q., Chu C., et al. The predictive value of repeated blood pressure measurements in childhood for cardiovascular risk in adults: the Hanzhong Adolescent Hypertension Study. Hypertension Research. 2020 doi: 10.1038/s41440-020-0480-7. [DOI] [PubMed] [Google Scholar]
- 38.Yuan Y., Chu C., Zheng W. L., et al. Body mass index trajectories in early life is predictive of cardiometabolic risk. The Journal of Pediatrics. 2020;219:31–37.e6. doi: 10.1016/j.jpeds.2019.12.060. [DOI] [PubMed] [Google Scholar]
- 39.Bobulescu I. A., Moe O. W. Renal transport of uric acid: evolving concepts and uncertainties. Advances in Chronic Kidney Disease. 2012;19(6):358–371. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F., Guo H., Zou J., et al. Urinary excretion of uric acid is negatively associated with albuminuria in patients with chronic kidney disease: a cross-sectional study. BMC nephrology. 2018;19(1) doi: 10.1186/s12882-018-0892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozanturk E., Ucar Z. Z., Varol Y., et al. Urinary uric acid excretion as an indicator of severe hypoxia and mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease. Revista Portuguesa de Pneumologia (English Edition) 2016;22(1):18–26. doi: 10.1016/j.rppnen.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Chen H. J., Yau K. I., Tsai K. S. Urinary uric acid/creatinine ratio as an additional marker of perinatal asphyxia. Journal of the Formosan Medical Association. 2000;99(10):771–774. [PubMed] [Google Scholar]
- 43.Nariman S., Mosayebi Z., Sagheb S., Rastad H., Hosseininodeh S. S. Urinary uric acid/creatinine ratio as a marker of mortality and unfavorable outcome in NICU-admitted neonates. Iranian Journal of Pediatrics. 2016;26(4, article e5739) doi: 10.5812/ijp.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drury J. A., Nycyk J. A., Cooke R. W. I. Comparison of urinary and plasma malondialdehyde in preterm infants. Clinica Chimica Acta. 1997;263(2):177–185. doi: 10.1016/S0009-8981(97)00051-X. [DOI] [PubMed] [Google Scholar]
- 45.Cao J., Wang H. Y. Association between total antioxidant status and atherosclerosis in elderly patients with essential hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41(10):857–861. [PubMed] [Google Scholar]
- 46.Prasad S. O. S., Qing Y. X. Associations between hyperuricemia and chronic kidney disease: a review. Nephro-Urology Monthly. 2015;7(3, article e27233) doi: 10.5812/numonthly.7(3)2015.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Hu J. W., Lv Y. B., et al. The role of uric acid in hypertension of adolescents, prehypertension and salt sensitivity of blood pressure. Medical Science Monitor. 2017;23:790–795. doi: 10.12659/MSM.899563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: association between various characteristics and the risk of baPWV and CIMT in subjects without medication use (n = 2205). Table S2: association between various characteristics and the risk of arterial stiffness in subjects without medication use (n = 2205).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.


