Abstract
Introduction:
A machine learning technique that imitates neural system and brain can provide better than traditional methods like logistic regression for survival prediction and create an algorithm by determining influential factors.
Aim:
To determine the influential factors on survival time of palliative care cancer patients and to compare two statistical methods for better prediction of survival.
Methods:
One-year data is gathered from the patients that we followed in the palliative care clinic of our hospital (2017-2018) (n = 189). All data were retrospectively evaluated. After descriptive statistics, we used Pearson and Spearman correlations for parametric and non-parametric variables. The Artificial Neural Networks (ANN) and logistic regression model were applied to parameters which have a significant correlation with short survival.
Results:
Significantly correlated variables with short survival were Palliative Performance Scale (PPS), Edmonton Symptom Assessment System (ESAS), Karnofsky Performance Scale (KPS), brain, liver, and distant metastasis, hemogram parameters, cero-reactive protein (CRP) and albumin (ALB). ANN model showed 89.3% prediction accuracy while the logistic regression model showed 73.0%. ANN model achieved a better AUC value of 0.86 than logistic regression model (0.76).
Discussion:
There are several prognostic evaluation tools such as PPS, KPS, CRP, albumin, leukocytes, neutrophil were reported several studies as survival-related parameters in logistic regression models, also. Many studies compare ANN with logistic regression. When we evaluated these parameters totally, we observed the same relations with survival then we used the same parameters in the ANN model. The effectivity of the survival prediction models can be improved with the use of ANN.
Conclusion:
ANN provides a more accurate estimation than logistic regression. ANN model is an important statistical method for survival prediction of cancer patients.
Keywords: palliative care, prognostic estimates, logistic regression, artificial neural networks, survival prediction, classification
1. INTRODUCTION
In recent years, the increasing numbers of cancer patients and long-term oncological treatments have escalated the emergency admission rates due to both the side effects of the drugs and the clinical symptoms caused by cancer itself in our daily practice. In addition, these patients are concentrated on in-hospital services. Current evaluation criteria are not sufficient in deciding the transition from curative cancer treatments to palliative treatments. Accurate 30-days survival predictions, directing patients to palliative care at the right time will preventboth patients and caregivers from suffering and ensure correct treatments. Survival analyzes should be performed as accurately as possible. The effect of patient data such as clinical, laboratory results, KPS, PPS on survival estimation need to be carefully analyzed.
Prediction of survival is one of the trending research areas for data mining techniques (1, 2). Traditional survival models were used such as the Cox proportional hazard model in many studies carried out survival analysis of patients. The logistic regression is widely used to observe the risk conditions among exposure and disease (3). Logistic regression is one of the machine learning techniques with statistical background which can be used in binary decisions in medicine such as classification with two classes.
ANN (Artificial Neural Networks) is also a machine learning technique, which imitates the neural system and the design of the brain with neurons and synapses. ANN was used in many outcomes and survival studies. Rughani et al. demonstrated the usefulness of ANN in the survival prediction of patients after brain injury (4). Gohari et al. compared the survival prediction performance of the Cox model and ANN in patients with colorectal cancer (5). Parsaeian et al. obtained better results using ANN comparing to logistic regression (6). Faradmal et al. in predicting breast cancer relapse compared the performance of ANN and log-logistic regression (7).
2. AIM
The purpose of this study is twofold; the first is to determine the predictors of 30-days survival of palliative care cancer patients via ANN and logistic regression models and the second is to predict 30-days survival using the patient data. Performances of two prediction models are compared with the area under the receiver operating characteristic (ROC) curve (AUC).
3. METHODS
3.1 Data
This investigation was planned based on one-year patient data (n=189) that we followed in the palliative care clinic of our hospital (2017-2018). All data was retrospectively used for this study. The scientific study committee of our hospital reviewed and approved the database for using this study. At the beginning of their hospitalization, all patients are informed that their data could be tracked and their written consent is received. In our palliative care unit of the chest diseases referral center, the patients with advanced COPD (Chronic Obstructive Pulmonary Disease) and interstitial lung diseases are excluded from the study; only cancer cases are included. Most of them were patients with lung cancer who had stopped their advanced oncologic treatment. All patients received supportive treatment regarding their symptom characteristics.
Patient data contain information such as gender, age, symptoms, and signs causing the admission to PCU, the condition of metastasis, biochemical analysis (ALB, CRP, CRP/ALB ratio), hemogram parameters (white, red blood cells, hemoglobin, hematocrit, neutrophil (NE), monocytes, lymphocytes, eosinophil, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), mean platelet volume (MPV), platelets (PLT), Neutrophil/Lymphocyte ratio). The patient data of prognostic and symptoms measurements are also available. The tools used are PPS and ESAS. PPS score was modified from the Karnofsky Performance Scale’s functional status of ambulation, activity level, evidence of disease, and amounts of self-care oral intake, level of consciousness (8-10). The ESAS is a validated self-reported tool to measure the nine prevalent symptoms of cancer (11). All patient data has been recorded on the first 1-3 days of hospitalization.
Some of the patients died when they were hospitalized. Mortality data of the discharged patients were obtained from the national death notification system after one year (2019). We determined that all of our discharged patients died at the exact time recorded by the national death notification system. All the patients are classified according to their survival time (hospital admission to death) being less or more than thirty days.
3.2 Data Analysis
SPSS 23.0 for Windows is used for statistical analysis. Descriptive statistics are revealed as number and percentage for categorical variables and mean, standard deviation, minimum, and maximum for scale variables. Shapiro-Wilk test is used to test the normality assumption. Comparisons of the two independent patient groups are made by Student’s t-test when scale variables are normally distributed and when the normality condition is not provided, by the Mann Whitney U test. Comparisons of ratios in independent groups are performed with the Chi-square test. The significance level alpha is accepted as p<0.05. To examine the relationships, Pearson and Spearman’s correlations are used for parametric and non-parametric variables, respectively. All variables with significant correlations are taken as input variables.
For 30-days survival prediction between admission and death, using the significantly correlated factors as input variables, we compared the performances of two classification techniques; logistic regression and ANN. Logistic regression is a linear machine learning method that is used in binary classification problems. It calculates the logarithmic probability of the target variable using a linear function of input variables (independent factors) (3, 12).
Another machine learning algorithm is ANN that imitates the synaptic design of the brain. This computational tool enables transferring knowledge or rules concealed in data to the network structure by processing experimental data. An ANN is constructed with three layers: the input layer, hidden layers, and the output layer. All of the input layer nodes are transferred to the output layer in a stratified way. Input layers can be output for the other layer or raw data in the first layer. The main task of the hidden layer is to extract classified information from existing data. The output layer shows the final output of the network. The outputs from the nodes in one layer consist of a weighted linear combination that was transformed by a nonlinear function. This nonlinear function allows the neural network to grasp sophisticated relations between the independent variables and enhance the performance of data-driven machine learning technique (12-14).
The total dataset is split into a training set, a cross-validation set, and a test set. The training set is used for deriving the parameters related to survivability prediction, the cross-validation set for preventing overfitting, and the test set for evaluating these derived predictors. All significantly correlated variables are used as input data for both ANN and logistic regression classification models. The parameters that affect survival are evaluated by logistic regression and ANN models with a small dataset of patients who live less or more than thirty days. To compare ANN and logistic regression models in our setting, we used the estimated AUC of ROC.
4. RESULTS
The symptom distributions and palliative prognostic measurements of 189 patients, the majority of which consisted of lung cancers and males, are given in Table 1.
Table 1. Characteristics of documented data of patients in PCU (n=189).
| Mean±SD | Min-Max | ||
|---|---|---|---|
| Age | 64.53±11.60 | 26-91 | |
| Karnofsky Performance Status | 32.86±17.63 | 10-90 | |
| Edmonton Symptom Assessment System | 55.51±15.78 | 2-90 | |
| Palliative Performance Scale | 33.81±17.81 | 10-90 | |
| n | % | ||
| Gender | Female | 36 | 19 |
| Male | 153 | 81 | |
| Symptom | Pain | 57 | 30.2 |
| Dyspnea | 153 | 81 | |
| Cough | 56 | 29.6 | |
| Hemoptysis | 11 | 5.8 | |
| Tiredness | 53 | 28 | |
| Lack of appetite | 68 | 36 | |
| Constipation | 8 | 4.2 | |
| Insomnia | 13 | 6.9 | |
| Nausea | 11 | 5.8 | |
| Lack of well-being | 14 | 7.4 | |
| Diagnosis | Lung Cancer | 156 | 82.5 |
| Brain Tumor | 1 | 0.5 | |
| Colon Cancer | 3 | 1.6 | |
| Laryngeal Cancer | 2 | 1.1 | |
| Malignant Melanoma | 1 | 0.5 | |
| Breast Cancer | 9 | 4.8 | |
| Bladder Cancer | 2 | 1.1 | |
| Malign Pleural Mesothelioma | 6 | 3.2 | |
| Gastric Cancer | 1 | 0.5 | |
| Ovarian Cancer | 1 | 0.5 | |
| Esophageal Cancer | 3 | 1.6 | |
| Pancreatic Cancer | 2 | 1.1 | |
| Prostate Cancer | 1 | 0.5 | |
| Renal Cancer | 1 | 0.5 |
The mean age was 64.53 ± 11.60. Statistically significant parameters that differ between the two patient groups, which are classified by their survival being less or more than thirty days, were determined. Distant metastasis, brain metastasis, liver metastasis, symptom burden (ESAS) were higher in patients with short survival than others. Whereas, the level of albumin and prognostic indices (KPS and PPS) were lower in patients with short survival as shown in Table 2.
Table 2. Differences in patients who died within 30-days and the others.
| Death within 30-days (n=125) | Others (n=64) | P | |||
|---|---|---|---|---|---|
| CRP | 152.49 | ±104.12 | 118.21 | ±90.57 | 0.027 |
| ALB | 2.8 | ±0.48 | 3.08 | ±0.59 | 0.001 |
| Leucocytes (AN) | 14.18 | ±8.39 | 12.24 | ±6.10 | 0.102 |
| MCHC | 31.70 | ±1.49 | 32.23 | ±1.23 | 0.016 |
| NE (AN) | 11.88 | ±8.05 | 10.02 | ±5.94 | 0.102 |
| Distant Metastasis | 35 | 28% | 7 | 10.9% | 0.009 |
| Brain Metastasis | 18 | 14.4% | 3 | 4.7% | 0.051 |
| Liver Metastasis | 19 | 15.2% | 3 | 4.7% | 0.033 |
| KPS | 26.88 | ±13.16 | 44.53 | ±19.43 | <0.001 |
| ESAS | 59.18 | ±13.77 | 48.34 | ±17.05 | <0.001 |
| PPS | 27.92 | ±13.81 | 45.31 | ±19.19 | <0.001 |
Significant correlations with short survival were PPS, ESAS, KPS, brain, liver, distant metastasis, leucocytes, neutrophil, MCHC, CRP, and ALB. The presence of the brain, liver, and distant metastasis, the level of CRP, leucocytes, neutrophil, the measurement of the symptom burden measurement (ESAS) were positively correlated with the short term of survival. However, the level of the albumin, MCHC and the prognostic measurements such as KPS and PPS were negatively correlated as shown in Table 3.
Table 3. The Correlations.
| Death within 30-days | Correlation | p |
|---|---|---|
| CRP | 0.151 | 0.038 |
| ALB | -0.223 | 0.002 |
| Leucocytes (AN) | 0.146 | 0.046 |
| MCHC | -0.183 | 0.012 |
| NE (AN) | 0.153 | 0.036 |
| Distant Metastasis | 0.194 | 0.007 |
| Brain Metastasis | 0.146 | 0.045 |
| Liver Metastasis | 0.155 | 0.033 |
| KPS | -0.460 | <0.001 |
| ESAS | 0.317 | <0.001 |
| PPS | -0.452 | <0.001 |
The logistic regression model and ANN model are both trained using correlated parameters as the input variables for survival prediction and for investigating their influence. The results of the classification are given in Table 4.
Table 4. True and Predicted Classes of Logistic Regression and ANN.
| True Class | ||||
|---|---|---|---|---|
| Predicted Class | Death within 30-days | Other | ||
| Artificial Neural Networks | Training | Death within 30-days | 77 | 25 |
| Other | 8 | 23 | ||
| Cross-Validation | Death within 30-days | 18 | 4 | |
| Other | 2 | 4 | ||
| Test | Death within 30-days | 20 | 3 | |
| Other | 0 | 5 | ||
| Total | Death within 30-days | 115 | 32 | |
| Other | 10 | 32 | ||
| Logistic Regression | Total | Death within 30-days | 105 | 31 |
| Other | 20 | 33 | ||
The logistic regression model had an AUC value of 0.76 as shown in Figure 1. However, the ANN model achieved a better AUC value of 0.86 than the logistic regression model as shown in Figure 2 for all three parts of the dataset. Additionally, the ANN model showed 89.3% prediction accuracy while logistic regression model showed 73.0%, as summarized in Table 5.
Figure 1. ROC Curve of Logistic Regression Classifier.
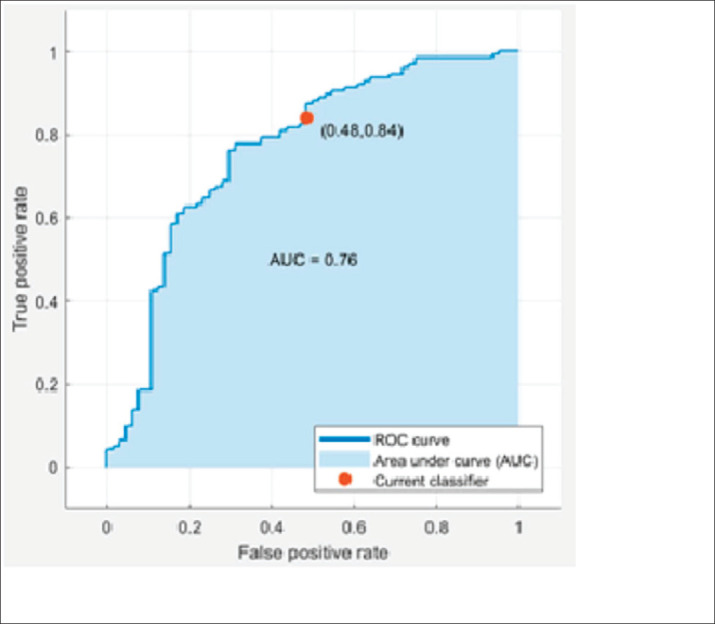
Figure 2. ROC Curve of ANN Classifier for Training, Cross-Validation and Testing Data Sets.
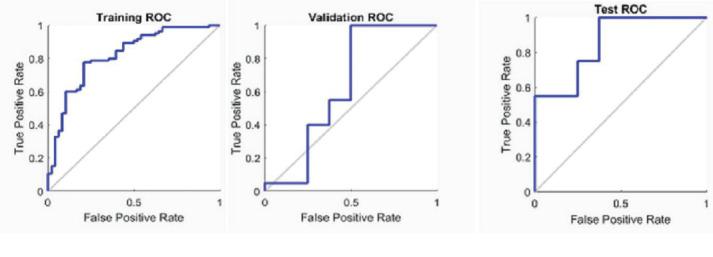
Table 5. Accuracy, Sensitivity, Specificity and AUC of LR and ANN results.
| Method | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|
| Artificial Neural Networks | 89.3% | 38% | 100% | 0.86 |
| Logistic Regression | 73.0% | 48% | 84% | 0.76 |
5. DISCUSSION
Accurate prediction of cancer patients’ survival is valuable for managing expectations, planning of care, and end-of-life. It is critical in the triangle of patients, caregivers/relatives, and clinicians (15, 16). A meta-analysis by White et al. reported that that clinicians’ predictions are frequently inaccurate in palliative care (17).
There are several prognostic evaluation tools such as PPS, KPS in a palliative care setting. Many studies compare ANN with other statistical models such as logistic regression. In a study comparing the logistic regression and the ANN model in predicting hospital mortality after hepatocellular carcinoma operation, it was shown that the ANN model provides a more accurate estimation (23). Additionally, it is reported that the ANN model was more accurate in predicting 5-year mortality than multiple logistic regression in breast cancer patients after surgery (24). In the study of Faradmal et al., the ANN model predicts breast cancer relapse better than the logistic regression model (7). However, over time, both methods showed reducing the performance of prediction.
Similarly, this study, which also used palliative care patient data for the ANN model, we found better AUC value (0.86) than the logistic regression to predict survival. However, there are some opposing studies. These studies reported that the ANN model was not superior to the traditional models for prediction, which would be related to the data structure (21). If the factor and response relationship in the data sample is unknown, the ANN model is better than traditional models to predict this relationship (1). ANN model may rapidly recognize non-linear patterns, linear patterns even probable effects (25).
The aim of our study was to compare two prediction models and to predict the short survival of the patients who were hospitalized in the palliative care service. For this reason, we determined the parameters that are correlated with survival from patient data. When we use the parameters that have a significant correlation with the survival as the ANN input parameters, we found that the model has determined the actual survival with high accuracy (89.3%).
These parameters used in our algorithm have been found to have relations with survival individually. Additionally, the ANN model showed a fairly good prediction ability. ANN classification model was used as a model to test and confirm the factors in the clinic and their effects on survival relationships in our study.
This algorithm contains data from laboratory parameters such as CRP, ALB, leucocytes, neutrophil, MCHC, and performance measurements such as KPS and PPS. Furthermore, the patient’s symptom load (ESAS) and metastasis status were also included. These algorithm components are already associated with shorter survival, regarding the results obtained from other studies. In our study, it has been used as a collective algorithm and it is composed of parameters which have been determined by traditional statistical methods.
MCHC, which is a component of our algorithm, has a role in predicting short survival. MCHC is the average hemoglobin level in the erythrocyte. A few studies are showing the relevance of MCHC to survival. MCHC was negatively correlated with survival in our study. Increased MCHC level in hospitalized patients with acute myocardial infarction is associated with decreased hospitalization time and one-year mortality. Continuing inflammatory response may lead to iron deficiency, which decreases MCHC (26-29).
Several studies have shown that a high level of CRP correlates with patients with poorer prognosis in patients with resectable lung cancer. Besides, the CRP level was associated with reduced serum albumin, resulting in progressive weight loss, poor performance, the higher mortality rate in cancer patients (30-32).
The level of albumin can reveal the nutritional status of patients and correlates with short survival in advanced cancer. Cancer is in inflammation state with a low level of albumin. The systemic inflammatory response was found related to poor survival in cancer patients (33, 34). In our study, CRP and albumin were selected as algorithm component in the ANN model due to the relationship with poor survival.
Neutrophil and leukocyte count were also included in our algorithm. Neutrophilia and leukocytosis were important factors for survival prediction for this study. It was reported that both neutropenia and leukocytosis (mainly due to neutrophils) have been related to a poor course in cancer patients by another study, also (35). Tumor related leukocytosis and neutrophilia may result from granulocyte colony-stimulating factors sourced from the solid tumor (36, 37). The relation between neutrophilia and lower performance score was shown (38). Neutrophils ease invasion-metastasis cascade, repressing natural killer cell activity and enhancing the extravasation of tumor cells (39).
We thought that patient’s symptom load was crucial and it is selected as another parameter of this study for ANN and logistic regression models. Symptom burden measurement (ESAS) was positively correlated with the short term of survival in our study, similar to another study. McGee et al. demonstrated that ESAS was as a prognostic tool and could complement ECOG (Eastern Cooperative Oncology Group performance status) in the estimation of survival in advanced lung cancer (40).
The association of survival with performance status measurements in terminally ill cancer patients was studied many times. The KPS and ECOG status were the two most frequent measurements (41). Even if, the performance status was accepted as a significant prognostic factor for survival, possible acute influences should be considered (35). The KFS and PPS that are used as the palliative performance tools, were correlated with short survival in this study.
Additionally, the presence of metastasis has also been determined in several studies as a prognostic factor in advanced cancer patients (42). This study showed the presence of distant metastasis, especially brain and liver involvement, is correlated with short survival.
Since the correlation of the above-mentioned factors with short survival was found in several studies, the correlations between these factors were also found in our study, and we developed modeling for ANN and logistic regression to create a predictive algorithm from these factors for survival. Thus, we have tested the accuracy of them or survival prediction by applying both ANN and logistic regression methods. We found that ANN provides a more accurate estimation than logistic regression.
A limitation of this study is that most of the cases are lung cancer patients because our center is in a respiratory reference hospital. The effectivity of the model can be improved with the use of artificial neural networks including more cases and covering all cancer types.
6. CONCLUSION
This study showed that ANN recognizes linear, non-linear even influence effect and can be used as a 30-days survival prediction algorithm for cancer patients. The usefulness of this algorithm can be determined by applying to a large number of palliative patients in future prospective studies. As suggested by the literature, our study also contributes to the validation of ANN for future prognostication (43). We think that the ANN model is an important statistical method for survival prediction that can include a considerable number of parameters and provides the opportunity to practice in very large patient populations.
Acknowledgments:
No.
Authors contribution:
All authors contributed equally to the preparation of this article.
Declaration of Conflicting Interests:
The authors declare that there is no conflict of interest.
Financial support and sponsorship:
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1.Kurt I, Ture M, Kurum A. Comparing performances of logistic regression, classification and regression tree, and neural networks for predicting coronary artery disease. Expert Systems with Applications. 2008;34:366–374. doi: 10.1016/j.eswa.2006.09.004. [DOI] [Google Scholar]
- 2.Faradmal J, Kazemnejad A, Khodabakhshi R, Gohari MR, Hajizadeh E. Comparison of three adjuvant chemotherapy regimes using an extended log-logistic model in women with operable breast cancer. Asian Pac J Cancer Prev. 2010;11(2):353–358. [PubMed] [Google Scholar]
- 3.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 4.Rughani AI, Dumont TM, Lu Z, Bongard J, Horgan MA, Penar PL, et al. Use of an artificial neural network to predict head injury outcome. J Neurosurg. 2010;113(3):585–590. doi: 10.3171/2009.11.JNS09857. [DOI] [PubMed] [Google Scholar]
- 5.Gohari MR, Biglarian A, Bakhshi E, Pourhoseingholi MA. Use of an artificial neural network to determine prognostic factors in colorectal cancer patients. Asian Pac J Cancer Prev. 2011;12(6):1469–1472. [PubMed] [Google Scholar]
- 6.Parsaeian M, Mohammad K, Mahmoudi M, Zeraati H. Comparison of logistic regression and artificial neural network in low back pain prediction: second national health survey. Iran J Public Health. 2012;41(6):86–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Faradmal J, Soltanian AR, Roshanaei G, Khodabakhshi R, Kasaeian A. Comparison of the performance of log-logistic regression and artificial neural networks for predicting breast cancer relapse. Asian Pac J Cancer Prev. 2014;15(14):5883–5888. doi: 10.7314/apjcp.2014.15.14.5883. [DOI] [PubMed] [Google Scholar]
- 8.Lau F, Downing GM, Lesperance M, Shaw J, Kuziemsky C. Use of Palliative Performance Scale in end-of-life prognostication. J Palliat Med. 2006;9(5):1066–1075. doi: 10.1089/jpm.2006.9.1066. [DOI] [PubMed] [Google Scholar]
- 9.Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 10.Maltoni M, Scarpi E, Pittureri C, Martini F, Montanari L, Amaducci E. Prospective comparison of prognostic scores in palliative care cancer populations. The Oncologist. 2012;17(3):446–454. doi: 10.1634/theoncologist.2011-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. doi: 10.1089/jpm.2006.9.296. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, You S, So S, Lee J, Yook S, Jang DP. A data-driven artificial intelligence model for remote triage in the prehospital environment. PloS One. 2018;13:e0206006. doi: 10.1371/journal.pone.0206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 14.Hornik K, Stinchcombe M, White H. Multilayer feedforward networks are universal approximators. Neural Networks. 1989;2:359–366. [Google Scholar]
- 15.Avati A, Jung K, Harman S, Downing L, Ng A, Shah NH. Improving palliative care with deep learning. BMC Med Inform Decis Mak. 2018;18(4):122. doi: 10.1186/s12911-018-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, Grambow S, Parker J, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727–737. doi: 10.1016/s0885-3924(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 17.White N, Reid F, Harris A, Stone P. A systematic review of predictions of survival in palliative care: how accurate are clinicians and who are the experts? PloS One. 2016;11:e0161407. doi: 10.1371/journal.pone.0161407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowen ME, Strawderman RL, Czerwinski JL, Smith MJ, Halasyamani LK. Mortality predictions on admission as a context for organizing care activities. J Hospit Med. 2013;8(5):229–235. doi: 10.1002/jhm.1998. [DOI] [PubMed] [Google Scholar]
- 19.Meffert C, Rücker G, Hatami I, Becker G. Identification of hospital patients in need of palliative care–a predictive score. BMC Palliat Care. 2016;15:21. doi: 10.1186/s12904-016-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramchandran KJ, Shega JW, Von Roenn J, Schumacher M, Szmuilowicz E, Redemaker A, et al. A predictive model to identify hospitalized cancer patients at risk for 30-day mortality based on admission criteria via the electronic medical record. Cancer. 2013;119(11):2074–2080. doi: 10.1002/cncr.27974. [DOI] [PubMed] [Google Scholar]
- 21.Xiang A, Lapuerta P, Ryutov A, Buckley j, Azen S. Comparison of the performance of neural network methods and Cox regression for censored survival data. Computational Statistics & Data Analysis. 2000;34(2):243–257. doi: 10.1016/S0167-9473(99)00098-5. [DOI] [Google Scholar]
- 22.Lancashire LJ, Rees RC, Ball GR. Identification of gene transcript signatures predictive for estrogen receptor and lymph node status using a stepwise forward selection artificial neural network modelling approach. Artif Intell Med. 2008;43:99–111. doi: 10.1016/j.artmed.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Shi HY, Lee KT, Lee HH, Ho WH, Sun DP, Wang JJ, et al. Comparison of artificial neural network and logistic regression models for predicting in-hospital mortality after primary liver cancer surgery. PloS One. 2012;7:e35781. doi: 10.1371/journal.pone.0035781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang SH, Loh JK, Tsai JT, Houg MF, Shi HY. Predictive model for 5-year mortality after breast cancer surgery in Taiwan residents. Chin J Cancer. 2017;36:23. doi: 10.1186/s40880-017-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Luo W, Su M, Wei H, Wei J, Zhang X, et al. Comparison between artificial neural network and Cox regression model in predicting the survival rate of gastric cancer patients. Biomed Rep. 2013;1:757–760. doi: 10.3892/br.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YL, Hu ZD. Lower mean corpuscular hemoglobin concentration is associated with poorer outcomes in intensive care unit admitted patients with acute myocardial infarction. Ann Transl Med. 2016;4:190. doi: 10.21037/atm.2016.03.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganz T, Nemeth E. Iron sequestration and anemia of inflammation, in. Semin Hematol. 2009;46:387–393. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClung JP, Karl JP. Iron deficiency and obesity: the contribution of inflammation and diminished iron absorption. Nutr Rev. 2009;67:100–104. doi: 10.1111/j.1753-4887.2008.00145.x. [DOI] [PubMed] [Google Scholar]
- 29.Archer NM, Brugnara C. Diagnosis of iron-deficient states. Crit Rev Clin Lab Sci. 2015;52:256–272. doi: 10.3109/10408363.2015.1038744. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Lian L, Qin H, Wang WJ, Ren R, Xu MD, et al. Prognostic evaluation of patients with resectable lung cancer using systemic inflammatory response parameters. Oncol Lett. 2019;17:2244–2256. doi: 10.3892/ol.2018.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara M, Matsuzaki Y, Shimuzu T, Tomita M, Ayabe T, Enomoto Y, et al. Preoperative serum C-reactive protein level in non-small cell lung cancer. Anticancer Res. 2007;27:3001–3004. [PubMed] [Google Scholar]
- 32.Lee JG, Cho BC, Bae MK, Lee CY, Park IK, Kim DJ, et al. Preoperative C-reactive protein levels are associated with tumor size and lymphovascular invasion in resected non-small cell lung cancer. Lung Cancer. 2009;63:106–110. doi: 10.1016/j.lungcan.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240–6248. doi: 10.1200/JCO.2005.06.866. [DOI] [PubMed] [Google Scholar]
- 34.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 35.Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14:999–1011. doi: 10.1007/s00520-006-0079-9. [DOI] [PubMed] [Google Scholar]
- 36.Wilcox RA. Cancer-associated myeloproliferation: old association, new therapeutic target. Mayo Clinic Proceedings. 2010;85:656–663. doi: 10.4065/mcp.2010.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mabuchi S, Matsumoto Y, Isohashi F, Yoshioka Y, Ohashi H, Morii E, et al. Pretreatment leukocytosis is an indicator of poor prognosis in patients with cervical cancer. Gynecologic Oncology. 2011;122:25–32. doi: 10.1016/j.ygyno.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 38.Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4:83–91. doi: 10.1158/2326-6066.CIR-15-0313. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, et al. Neutrophils suppress intraluminal NK cell–mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6:630–649. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGee SF, Zhang T, Jonker H, Laurie SA, Goss G, Nicholas G, et al. The Impact of Baseline Edmonton Symptom Assessment Scale Scores on Treatment and Survival in Patients With Advanced Non–small-cell Lung Cancer. Clin Lung Cancer. 2018;19:e91–e99. doi: 10.1016/j.cllc.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Ng T, Chew L, Yap CW. A clinical decision support tool to predict survival in cancer patients beyond 120 days after palliative chemotherapy. J Pallit Med. 2012;15:863–869. doi: 10.1089/jpm.2011.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachelot T, Ray-Coquard I, Catimel G, Guastalla JP, Dumortier A, Chauvin F, et al. Multivariable analysis of prognostic factors for toxicity and survival for patients enrolled in phase I clinical trials. Ann Oncol. 2000;11:151–156. doi: 10.1023/a:1008368319526. [DOI] [PubMed] [Google Scholar]
- 43.Hui D, Paiva CE, Del Fabbro EG, Steer C, Naberhuis J, van de Wetering M, et al. Prognostication in advanced cancer: update and directions for future research. Support Care Cancer. 2019;6:1973–1984. doi: 10.1007/s00520-019-04727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


