Abstract
Background
The pharmacokinetics of proton pump inhibitors (PPIs) may be affected by food intake. We aimed to evaluate the effect of food on the pharmacokinetics of omeprazole, rabeprazole, and pantoprazole.
Setting
The study population comprised 186 healthy volunteers participating in 6 bioequivalence clinical trials.
Method
Subjects were evaluated to determine the effect of a high-fat breakfast on the pharmacokinetics of omeprazole (n = 36), rabeprazole (n = 69), and pantoprazole (n = 81).
Main outcome measure
Drug plasma concentrations were measured using high-performance liquid chromatography coupled to mass spectrometry.
Results
Food affected the pharmacokinetics of omeprazole (increased Tmax and decreased AUC and Cmax), pantoprazole (increased Tmax and decreased AUC), and rabeprazole (increased Tmax, Cmax and half-life). Food increased variability in Tmax for all 3 drugs, delaying absorption around 3 to 4 h and until 20 h in some subjects.
Conclusion
As food delays the absorption of PPIs and increases their variability, it would be better to administer these drugs under fasting conditions.
Trial registration
European Union Drug Regulating Authorities Clinical Trials Database: EudraCT: 2004–003863-59 (registration date 05/MAR/2004), EudraCT 2006–001162-17 (registration date 17-MAR-2006), EudraCT: 2007–002489-37 (registration date 12-JUN-2007), EudraCT: 2007–002490-31 (registration date 12-JUN-2007), EudraCT: 2010–024029-19 (registration date 23-NOV-2010).
Keywords: Proton pump inhibitors, Food, Pharmacokinetics, Omeprazole, Rabeprazole, Pantoprazole
Bulleted statements on the impact of the research findings on patients, pharmacy or clinical practice
To be more effective, it is recommended to administer PPIs better under fasting conditions.
More effective administration could improve patient outcomes.
Background
Proton pump inhibitors (PPIs) are used for the treatment of acid-related diseases such as gastric and duodenal ulcers, gastroesophageal reflux disease, non-erosive reflux disease, and Zollinger-Ellison syndrome. They are also used in combination with antibiotics for the eradication of Helicobacter pylori [1].
PPIs inhibit gastric H+/K+-ATPase pump activity, resulting in potent acid inhibition. However, the ability of PPIs to suppress gastric acid varies widely between individuals. Several factors contribute to this phenomenon, including the considerable variation in the oral bioavailability of PPIs, the need for the ATPase pumps to be activated by food, the influence of Helicobacter pylori–associated gastritis, and genetic variation in enzyme activity [2]. Indeed, drug formulation, food intake, and single-nucleotide polymorphisms in cytochrome P450 (CYP) 2C19 enzyme also influence PPIs pharmacokinetic parameters [3–5]. Thus, the time to maximum plasma concentration (Tmax) varies from 1 h to 5 h depending on the drug formulation and/or food effect [3, 4].
Tytgat et al. reported that the bioavailability of PPIs was markedly affected by food intake [6]; consequently, PPIs should be taken under fasting conditions. However, PPIs prescribing information is controversial, since some authors state that in case of omeprazole and rabeprazole the concomitant ingestion of food does not affect their bioavailability [7, 8], whereas others state that omeprazole should be taken at least 1 h before a meal [9]. The recommendations for pantoprazole depend on the formulation: tablets could be taken regardless of meal timing, but oral suspension should be taken 30 min before a meal [10]. To date, there was some evidence that taking PPIs with food affected their absorption. However, until now it has not been rigorously evaluated. This study provides the necessary evidence.
Aim of the study
This study aimed to evaluate the influence of food on the pharmacokinetic parameters of three PPIs (omeprazole, rabeprazole and pantoprazole) and to determine whether food is more relevant for any of them.
Methods
Study design
The study population comprised 186 healthy Caucasian adult volunteers from 6 single-dose bioequivalence clinical trials with pantoprazole (40 mg, enteric-coated tablets), rabeprazole (20 mg, enteric-coated tablets), and omeprazole (40 mg, oral capsules). The bioequivalence clinical trials were phase I, randomized, open-label, crossover, single-centre, with two periods separated by a 7-day washout stage. Subjects were randomised to one of the two treatment sequences, RT or TR where R was the reference formulation and T was the test formulation. Each of the clinical trial was crossover for evaluation of a test formulation compared to a reference formulation either under fed or fasting conditions. Different subjects participated in each trial, but in case of omeprazole, that 35 of 36 volunteers were the same for the trials under fed and fasting conditions. The treatments were allocated in a balanced manner on every inclusion day (blocks of 4 subjects). Our study adheres to CONSORT guidelines.
Considering the expected intrasubject variability for each study, using a multiplicative model, sample size was calculated to reject a difference between both formulations of 20%, with a power of 80% and an alpha error of 0.05, according to the bioequivalence approaches habitually accepted by the Health Authorities (acceptance limits of 0.8–1.25).
From those 186 healthy volunteers, 81 subjects received pantoprazole (36 under fasting conditions, EudraCT: 2004–003863-59; and 45 with food, EudraCT 2006–001162-17), 69 received rabeprazole (35 under fasting conditions, EudraCT: 2007–002489-37; and 34 with food, EudraCT: 2007–002490-31) and 36 received omeprazole (35 under fasting conditions and 36 with food; EudraCT: 2010–024029-19). In case of omeprazole, 35 volunteers were the same for both trials. In the trials with food, volunteers fasted for 10 h, the breakfast was taken in 20 min (between 30 and 10 min previous to dosing) and the drug was administered 10 min after the end of breakfast. The meal had a high fat content (50–60% of total caloric content of the meal) and high calorie count (approximately 800 to 1000 kcal) according to EMA [11] and FDA [12] guidelines.
The inclusion criteria were as follows: non-smoking male and female volunteers, age 18 to 55 years, body mass index (BMI) within the 18.5–30.0 range, free from any organic or psychiatric conditions, no taking any drug and with normal vital signs, electrocardiogram (ECG), medical records and physical examination. It was not allowed to take other drugs during the study.
Sample processing, quantification, and pharmacokinetic analysis
Sampling was extended for 12 h in the clinical trials under fasting conditions and for 24 h under fed conditions, as follows: (i) Fasting omeprazole – 21 samples: predose, 0.33, 0.67, 1, 1.33, 1.67, 2, 2.33, 2.67, 3, 3.33, 3.67, 4, 4.5, 5, 5.5, 6, 7, 8, 10 and 12 h; (ii) fed omeprazole - 26 samples: predose, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9, 10, 1 h, 12, 14, 17, 20 and 24 h; (iii) fasting pantoprazole – 14 samples: predose, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10 and 12 h; (iv) fed pantoprazole – 37 samples: predose, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9, 9.5, 10, 10.5, 11, 11.5, 12, 12.5, 13, 13.5, 14, 15, 16, 17, 18, 19, 20, 21, 22 and 24 h; (v) fasting rabeprazole – 15 samples: predose, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 7, 8, 10 and 12 h; (vi) fed rabeprazole – 37 samples: predose, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9, 9.5, 10, 10.5, 11, 11.5, 12, 12.5, 13, 13.5, 14, 15, 16, 17, 18, 19, 20, 21, 22 and 24 h.
Sample processing, genotyping for CYP2C19 and drug quantification were performed according to Román et al. [5]. As the formulations were bioequivalent, we used the mean concentrations obtained after receiving both test and reference formulations to calculate the pharmacokinetic parameters of each subject. Pharmacokinetic parameters were estimated from the plasma concentration–time data by non-compartmental analysis (WinNonlin Professional, version 2.0., Pharsight Corporation, USA) as reported by Román et al. [5].
Data analysis
WinNonLin Professional software version 2.0 was used for the statistical analysis. Pharmacokinetic parameters were log-transformed, and AUC and Cmax were adjusted for dose and weight. The values of pharmacokinetic parameters were expressed as mean ± standard deviation. An analysis of the variance (ANOVA) test was applied to calculate the statistical significance of the differences in pharmacokinetic parameters considering the factors sex and food condition; in the model for omeprazole the factor subject was also included. The 90% confidence interval of the ratio of geometric means between fed and fast conditions were calculated. To avoid the influence of CYP2C19 polymorphisms, this analysis was repeated in CYP2C19*1/*1 subjects. p ≤ 0.05 was considered significant.
Results
Study population
We analyzed 186 volunteers (95 men and 91 women). Average age was higher in the omeprazole study (26.73 ± 5.74 years) than in the pantoprazole study (23.81 ± 3.18 years, p ≤ 0.001) and the rabeprazole study (24.62 ± 3.78 years, p ≤ 0.05). Weight was similar in the three drugs clinical trials (66.25 ± 11.27 kg for pantoprazole, 68.21 ± 13.07 kg for rabeprazole, and 67.69 ± 12.78 kg for omeprazole).
Thirty five subjects from omeprazole study accepted CYP2C19 genotyping, being 16 *1/*1, 7 *1/*2, 1 *2/*2, 10 *1/*17 and 1 *2/*17. In the case of pantopazole, 33 subjects were genotyped in the fasting trial (14 *1/*1, 6 *1/*2, 11 *1/*17 and 2 *17*17) and 36 in the fed trial (14 *1/*1, 6 *1/*2, 14 *1/*17, 1 *17/*17 and 1 *2/*17). In the rabeprazole studies, 30 subjects were genotyped in the fasting trial (14 *1/*1, 5 *1/*2, 9 *1/*17 and 2 *2/*17) and 22 in the fed trial (9 *1/*1, 4 *1/*2, 8 *1/*17 and 1 *2/*17).
Role of food in the pharmacokinetics of PPIs
The effect of food on the pharmacokinetics of omeprazole, pantoprazole, and rabeprazole is shown in Table 1. Mean plasma concentration-time profiles are depicted in Fig. 1.
Table 1.
Pharmacokinetic parameters for pantoprazole, rabeprazole, and omeprazole under fasting and fed conditions. Among 71 volunteers receiving omeprazole, 35 were the same subjects. Data are expressed as mean ± standard deviation. *p ≤ 0.05 vs. men
| n | AUC0-∞ (ng·h/ml) | Cmax (ng/ml) | Tmax (h) | t1/2 (h) | Vd/F (l/kg) | Cl/F (L/h·kg) | Ke | |
|---|---|---|---|---|---|---|---|---|
| Pantoprazole (n = 81) | ||||||||
| Fast | 36 | 6951.4 ± 3293.2 | 2779.1 ± 861.6 | 3.0 ± 0.8 | 1.3 ± 0.6 | 0.17 ± 0.03 | 0.11 ± 0.04 | 0.61 ± 0.21 |
| Men | 18 | 6584.5 ± 3832.8 | 2316.4 ± 580.9 | 3.1 ± 0.8 | 1.5 ± 0.7 | 0.18 ± 0.03 | 0.11 ± 0.03 | 0.56 ± 0.23 |
| Women | 18 | 7318.4 ± 2711.6 | 3241.8 ± 858.8 | 2.9 ± 0.8 | 1.1 ± 0.3 | 0.16 ± 0.03 | 0.11 ± 0.05 | 0.65 ± 0.18 |
| Fed | 45 | 4633.5 ± 2022.7 | 2646.2 ± 603.1 | 7.0 ± 3.0 | 1.3 ± 0.6 | 0.26 ± 0.10 | 0.16 ± 0.06 | 0.63 ± 0.21 |
| Men | 23 | 4607.8 ± 2265.1 | 2590.7 ± 601.2 | 5.6 ± 1.1 | 1.3 ± 0.5 | 0.25 ± 0.08 | 0.15 ± 0.07 | 0.61 ± 0.23 |
| Women | 22 | 4660.4 ± 1787.8 | 2704.2 ± 613.6 | 8.4 ± 3.7* | 1.2 ± 0.7 | 0.28 ± 0.12 | 0.17 ± 0.05 | 0.65 ± 0.19 |
|
Fed vs. Fast ratio; 90% CI; p value |
65.6; 55.7–77.2 p = 0.0001 |
93.7; 85.5–102.7 p = 0.2412 |
227.2; 203.2–254.1 p = 0.0001 |
95.3; 82.8–109.8 p = 0.5749 |
147.1; 134.3–161.0 p = 0.0001 |
151.0; 128.7–177.2 p = 0.0001 |
104.9; 91.1–120.7 p = 0.5749 |
|
| Rabeprazole (n = 69) | ||||||||
| Fast | 35 | 971.9 ± 361.2 | 556.4 ± 176.2 | 3.7 ± 0.9 | 1.3 ± 0.5 | 0.60 ± 0.17 | 0.35 ± 0.11 | 0.60 ± 0.20 |
| Men | 18 | 836.3 ± 313.0 | 479.1 ± 139.7 | 3.6 ± 0.9 | 1.2 ± 0.5 | 0.55 ± 0.11 | 0.35 ± 0.13 | 0.64 ± 0.20 |
| Women | 17 | 1115.5 ± 361.4 | 638.3 ± 177.2 | 3.7 ± 0.9 | 1.4 ± 0.5 | 0.65 ± 0.20 | 0.35 ± 0.10 | 0.56 ± 0.20 |
| Fed | 34 | 1047.3 ± 382.3 | 675.8 ± 218.8 | 7.2 ± 2.6 | 2.2 ± 1.3 | 0.91 ± 0.45 | 0.34 ± 0.18 | 0.42 ± 0.22 |
| Men | 19 | 1045.8 ± 361.49 | 634.6 ± 144.8 | 6.9 ± 3.0 | 2.3 ± 1.3 | 0.91 ± 0.52 | 0.31 ± 0.18 | 0.38 ± 0.17 |
| Women | 15 | 1049.2 ± 420.3 | 727.9 ± 284.0 | 7.6 ± 2.0 | 2.0 ± 1.3 | 0.92 ± 0.37 | 0.38 ± 0.18 | 0.48 ± 0.27 |
|
Fed vs. Fast ratio; 90% CI; p value |
106.7; 91.3–124.8 p = 0.4886 |
121.3; 106.9–137.6 p = 0.0132 |
191.5; 172.2–212.9 p = 0.0001 |
153.1; 126.9–184.7 p = 0.0003 |
144.3; 125.6–165.9 p = 0.0001 |
93.1; 79.5–109.1 p = 0.4531 |
65.3; 54.1–78.8 p = 0.0003 |
|
| Omeprazole (n = 36) | ||||||||
| Fast | 35 | 2190.8 ± 2011.5 | 930.9 ± 434.6 | 2.0 ± 0.7 | 1.1 ± 0.6 | 0.64 ± 0.34 | 0.53 ± 0.38 | 0.76 ± 0.29 |
| Men | 17 | 2375.9 ± 2480.1 | 858.8 ± 432.6 | 2.1 ± 0.7 | 1.3 ± 0.8 | 0.65 ± 0.38 | 0.50 ± 0.44 | 0.70 ± 0.29 |
| Women | 18 | 2016.0 ± 1495.4 | 999.1 ± 437.7 | 1.9 ± 0.6 | 1.0 ± 0.4 | 0.63 ± 0.31 | 0.56 ± 0.34 | 0.82 ± 0.29 |
| Fed | 36# | 1928.3 ± 1878.5 | 682.6 ± 394.1 | 4.9 ± 1.1 | 1.3 ± 0.6 | 1.02 ± 1.07 | 0.64 ± 0.55 | 0.67 ± 0.32 |
| Men | 18 | 2135.8 ± 2282.7 | 725.0 ± 447.6 | 4.5 ± 0.9 | 1.3 ± 0.7 | 0.75 ± 0.52 | 0.57 ± 0.49 | 0.69 ± 0.35 |
| Women | 18 | 1720.9 ± 1402.0 | 640.2 ± 640.1 | 5.4 ± 1.0 | 1.3 ± 0.5 | 1.30 ± 1.38 | 0.73 ± 0.61 | 0.65 ± 0.30 |
|
Fed vs. Fast ratio; 90% CI; p value |
86.2; 81.2–91.6 p = 0.0002 |
63.0; 55.0–72.2 p = 0.0001 |
262.5; 232.6–296.2 p = 0.0001 |
117.6; 105.6–130.9 p = 0.0156 |
136.1; 105.6–130.9 p = 0.0029 |
116.1; 108.7–124.1 p = 0.0006 |
85.0; 76.4–94.7 p = 0.0156 |
|
#35 of the subjects participated in both fast and fed omeprazole clinical trials
Fig. 1.
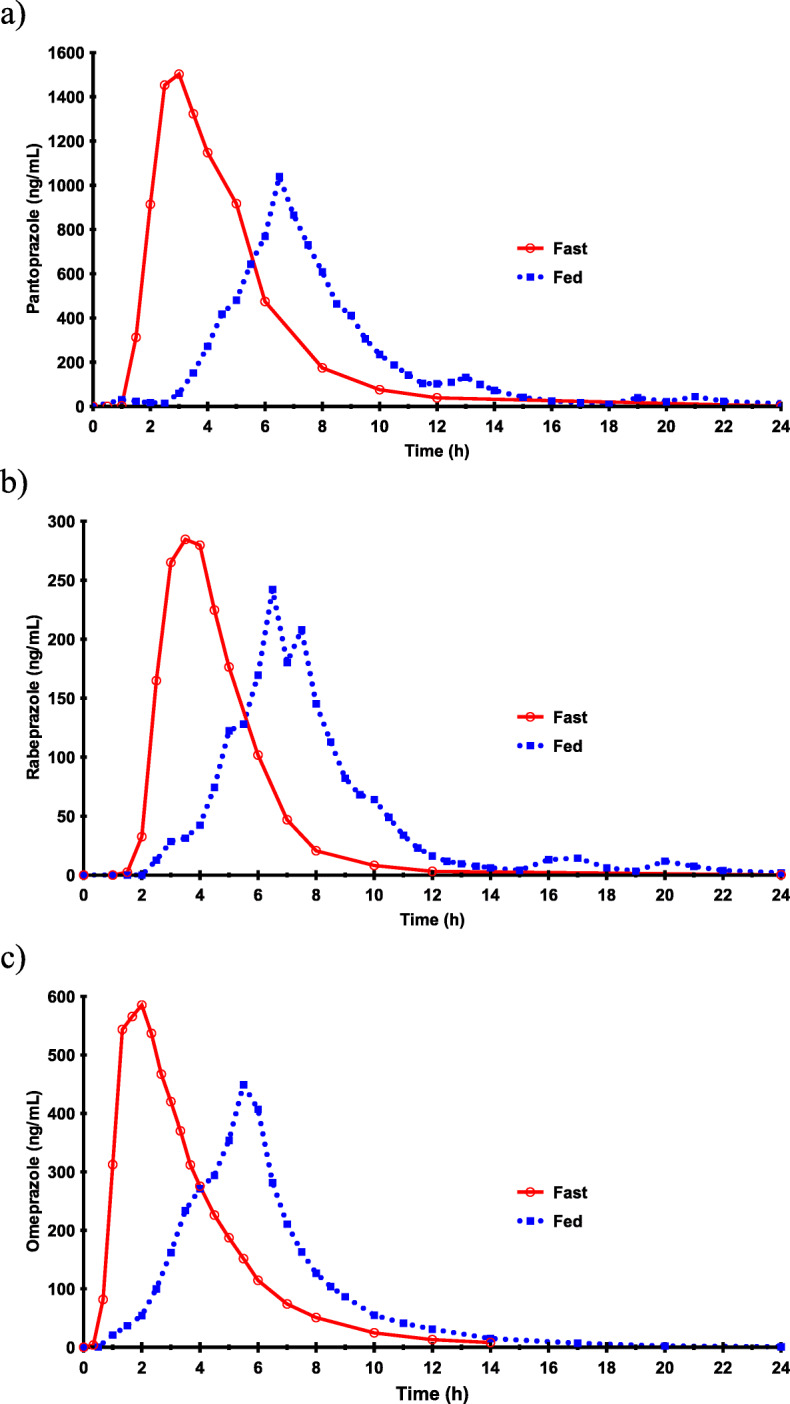
Mean plasma concentration-time profiles of (a) pantoprazole, (b) rabeprazole and (c) omeprazole, when administered under fed and fasting conditions
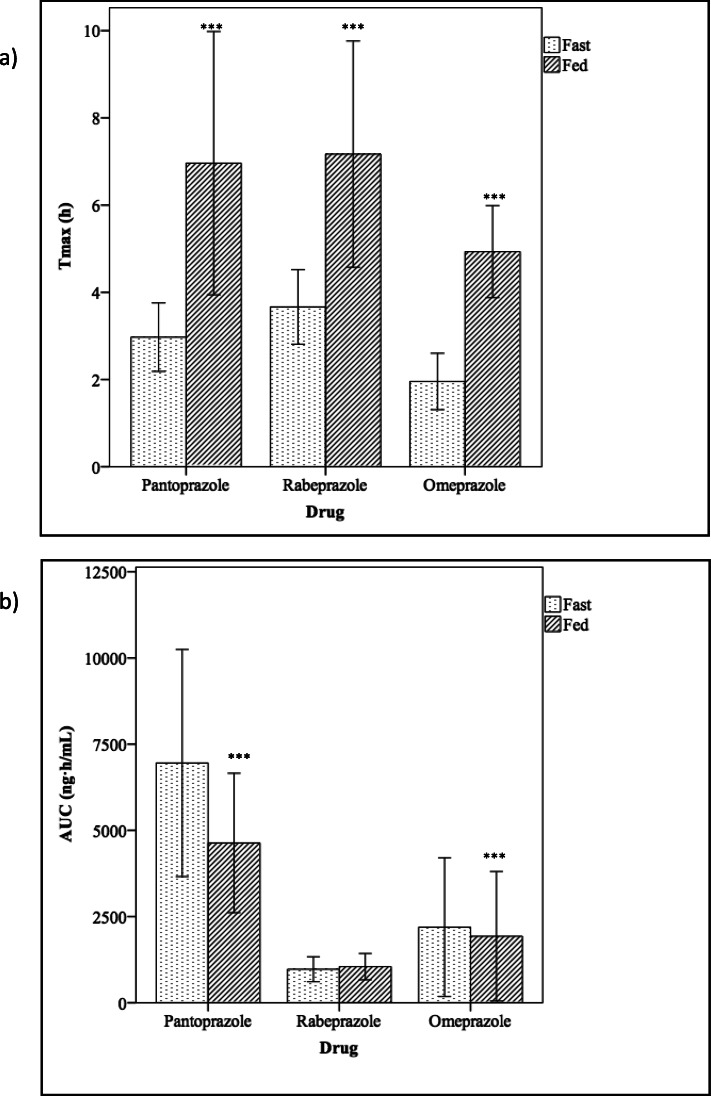
Under fasting conditions, Tmax was significantly reached earlier for omeprazole (2 h) than for pantoprazole (3 h) and rabeprazole (3.7 h) (p ≤ 0.001) (Fig. 2a). In addition, Tmax was also significantly reached earlier for pantoprazole than for rabeprazole (p ≤ 0.001).
Fig. 2.
Differences in PPI Tmax (a) and AUC (b) when administered under fed and fasting conditions. ***p < 0.001. Bars represented as mean and standard deviation
Tmax was delayed when only pharmacokinetic data after food intake were taken into consideration: Tmax for omeprazole (4.9 h) was earlier than for pantoprazole (7 h) and rabeprazole (7.2 h) (p ≤ 0.001), but no differences were found between pantoprazole and rabeprazole (Fig. 2a).
The administration of omeprazole with food delayed its mean Tmax by about 3 h (p ≤ 0.001) and increased the variability of Tmax, with a range of 1–3.5 h under fasting conditions and a range of 1–17 h under fed conditions. Under fed conditions, omeprazole AUC and Cmax were 12 and 27% lower, respectively (p ≤ 0.001), and half-life was 15% higher (p ≤ 0.05 and p ≤ 0.001, respectively).
Food delayed the pantoprazole Tmax by around 4 h (p ≤ 0.001), with a range of 1.5–5 h under fasting conditions and 1–21 h under fed conditions. In addition, pantoprazole AUC was 33% lower (p ≤ 0.001) under fed conditions (Fig. 2b).
Food also increased the rabeprazole Tmax by about 3.5 h (p ≤ 0.001), with a range of 2–7 h under fasting conditions and 3–20 h under fed conditions. The rabeprazole Cmax and half-life were 21 and 66% higher under fed conditions (p ≤ 0.05 and p ≤ 0.001, respectively).
When only subjects with CYP2C19*1/*1 were taken into account (n = 82; 31 omeprazole, 28 pantoprazole, and 23 rabeprazole) similar results were obtained: food increased variability and delayed Tmax by 2.7 h for pantoprazole (from 3 to 5.7 h, p ≤ 0.001), 3 h for omeprazole (from 1.8 to 4.9 h, p ≤ 0.001), and 5.2 h for rabeprazole (from 3.9 h to 9.1 h, p ≤ 0.001) (Table 2). In addition, under fed conditions, the rabeprazole half-life was higher (p ≤ 0.05); and the omeprazole AUC and Cmax were lower (p ≤ 0.05 and p ≤ 0.01, respectively).
Table 2.
Pharmacokinetic parameters for pantoprazole, rabeprazole, and omeprazole administered under fasting and fed conditions, considering only CYP2C19*1/*1 subjects. Data are expressed as mean ± standard deviation. *p ≤ 0.05 vs. men
| n | AUC0-∞ (ng·h/ml) | Cmax (ng/ml) | Tmax (h) | t1/2 (h) | Vd/F (l/kg) | Cl/F (L/h·kg) | Ke | |
|---|---|---|---|---|---|---|---|---|
| Pantoprazole (n = 28) | ||||||||
| Fast | 14 | 6085.3 ± 2922.6 | 2539.7 ± 836.3 | 3.0 ± 0.7 | 1.2 ± 0.4 | 0.18 ± 0.03 | 0.12 ± 0.05 | 0.64 ± 0.19 |
| Men | 8 | 4780.9 ± 2471.8 | 2019.8 ± 492.8 | 3.0 ± 0.7 | 1.3 ± 0.5 | 0.20 ± 0.02 | 0.14 ± 0.06 | 0.64 ± 0.23 |
| Women | 6 | 7824.6 ± 2702.2 | 3233.0 ± 685.0 | 2.9 ± 0.8 | 1.1 ± 0.2 | 0.16 ± 0.04 | 0.11 ± 0.04 | 0.63 ± 0.12 |
| Fed | 14 | 4689.0 ± 2303.3 | 2660.7 ± 531.2 | 5.7 ± 1.2 | 1.2 ± 0.5 | 0.24 ± 0.05 | 0.16 ± 0.07 | 0.67 ± 0.20 |
| Men | 8 | 4520.4 ± 2518.4 | 2564.3 ± 581.1 | 5.3 ± 1.1 | 1.2 ± 0.6 | 0.23 ± 0.03 | 0.15 ± 0.07 | 0.67 ± 0.25 |
| Women | 6 | 4913.8 ± 2192.7 | 2789.3 ± 475.5 | 6.2 ± 1.1 | 1.1 ± 0.3 | 0.25 ± 0.06 | 0.17 ± 0.08 | 0.68 ± 0.14 |
|
Fed vs. Fast ratio; 90% CI; p value |
74.8; 55.8–100.1 p = 0.1010 |
103.5; 91.3–117.2 p = 0.6431 |
192.2; 166.5–221.9 p = 0.001 |
95.6; 76.7–119.0 p = 0.7262 |
127.5; 113.2–143.6 p = 0.0018 |
129.1; 96.7–172.3 p = 0.1439 |
104.6; 84.0–130.3 p = 0.7262 |
|
| Rabeprazole (n = 23) | ||||||||
| Fast | 14 | 901.2 ± 234.6 | 536.3 ± 138.2 | 3.9 ± 0.8 | 1.3 ± 0.6 | 0.60 ± 0.11 | 0.37 ± 0.09 | 0.63 ± 0.20 |
| Men | 7 | 870.2 ± 295.6 | 512.2 ± 146.8 | 3.9 ± 1.0 | 1.5 ± 0.7 | 0.59 ± 0.10 | 0.33 ± 0.10 | 0.57 ± 0.25 |
| Women | 7 | 932.2 ± 172.0 | 560.4 ± 135.8 | 3.8 ± 0.7 | 1.1 ± 0.2 | 0.61 ± 0.12 | 0.41 ± 0.07 | 0.68 ± 0.13 |
| Fed | 9 | 968.9 ± 387.5 | 606.3 ± 206.8 | 9.1 ± 3.3 | 2.0 ± 0.9 | 0.92 ± 0.34 | 0.38 ± 0.18 | 0.45 ± 0.23 |
| Men | 4 | 999.7 ± 322.7 | 572.2 ± 215.1 | 10.3 ± 5.1 | 2.5 ± 0.7 | 1.02 ± 0.39 | 0.30 ± 0.11 | 0.30 ± 0.11 |
| Women | 5 | 944.2 ± 469.6* | 633.6 ± 220.7 | 8.2 ± 0.6 | 1.5 ± 0.9* | 0.85 ± 0.32 | 0.45 ± 0.20* | 0.57 ± 0.25* |
| Fed vs. Fast |
104.5; 82.7–131.9 p = 0.7488 |
111.7; 89.5–139.2 p = 0.3987 |
228.8; 187.0–278.9 p = 0.0001 |
152.0; 113.4–203.7 p = 0.023 |
147.6; 121.6–179.3 p = 0.0025 |
94.7; 75.1–119.5 p = 0.6913 |
65.8; 49.1–88.2 p = 0.023 |
|
| Omeprazole (n = 36) | ||||||||
| Fast | 15 | 1709.0 ± 1299.6 | 859.5 ± 394.9 | 1.8 ± 0.6 | 1.0 ± 0.4 | 0.69 ± 0.38 | 0.57 ± 0.39 | 0.81 ± 0.23 |
| Men | 8 | 1474.9 ± 1403.1 | 654.1 ± 305.6 | 2.0 ± 0.7 | 0.9 ± 0.4 | 0.68 ± 0.46 | 0.62 ± 0.44 | 0.82 ± 0.25 |
| Women | 7 | 1976.5 ± 1219.8 | 1094.3 ± 366.6 | 1.5 ± 0.3 | 1.0 ± 0.3 | 0.69 ± 0.46 | 0.53 ± 0.36 | 0.79 ± 0.23 |
| Fed | 16# | 1523.3 ± 1290.5 | 604.4 ± 362.6 | 4.7 ± 1.0 | 1.0 ± 0.4 | 0.77 ± 0.43 | 0.62 ± 0.41 | 0.80 ± 0.36 |
| Men | 10 | 1409.7 ± 1162.1 | 582.8 ± 419.1 | 4.3 ± 1.0 | 1.0 ± 0.4 | 0.73 ± 0.38 | 0.63 ± 0.49 | 0.84 ± 0.39 |
| Women | 6 | 1712.7 ± 1250.2 | 640.4 ± 275.1 | 5.4 ± 0.4 | 1.1 ± 0.4 | 0.84 ± 0.54 | 0.57 ± 0.29 | 0.72 ± 0.32 |
|
Fed vs. Fast ratio; 90% CI; p value |
88.5; 82.4–95.1 p = 0.0101 |
64.3; 52.5–78.7 p = 0.0019 |
278.3; 227.7–340.1 p = 0.0001 |
104.8; 87.7–125.3 p = 0.6457 |
118.9; 93.3–151.5 p = 0.2271 |
115.3; 105.3–126.2 p = 0.0159 |
95.4; 79.8–114.0 p = 0.6457 |
|
#15 of the subjects participated in both fast and fed omeprazole clinical trials
Role of sex in the pharmacokinetics of PPIs
There were no differences in the pharmacokinetic parameters between men and women for the three drugs, but sex affected the pantoprazole Tmax under fed conditions that was higher in women (p ≤ 0.05) (Table 1).
When only CYP2C19*1/*1 subjects are taken into account, under fed conditions rabeprazole AUC was higher (p ≤ 0.05) and half-life was lower in women (p ≤ 0.05).
Discussion
Our data showed that food delays the absorption of all PPIs by a mean of 3–4 h. This finding agrees with those of previous studies for esomeprazole [13], tenatoprazole [14], and rabeprazole [15].
The effectiveness of the initial antisecretory action of a PPI depends on factors such as timing in relation to meals [16]. Because of their short half-lives, PPIs are best administered before a meal or ideally after a period of fasting [17] to ensure that the proton pumps are maximally activated when the drug is available in plasma [16]. Nevertheless, as shown in a study on physician prescription patterns, there is much confusion about the optimal time to take a PPI in relation to meals [17].
AUC is the primary pharmacokinetic parameter to determine the antisecretory effect on omeprazole that is independent on Cmax [18]. Meals with a high fat content slow gastric emptying [19], thus delaying absorption of the drug and lowering AUC and Cmax values [20]. In our opinion, a low-fat breakfast would also influence PPI absorption, however, the effect might be less noticeable and maybe not clinically relevant. In this regard, the presence of food has been reported to reduce the Cmax and AUC of omeprazole [21] and esomeprazole [13]. Thomson et al. found that the evening meal reduced the tenatoprazole AUC compared with evening administration of the drug under fasting conditions [14]. Our data are in accordance with this finding, since the pantoprazole AUC and omeprazole AUC and Cmax were significantly lower under fed conditions. A decrease in AUC higher than 20% may be clinically relevant and can influence the effect on acid secretion. However, it should be further evaluated in chronic PPI treatment, since a single-dose study is not the best approach to established the actual correlation.
However, food increased rabeprazole Cmax and half-life. The metabolism of omeprazole, pantoprazole, and rabeprazole is mediated mainly by CYP2C19 and CYP3A4 [22], but the involvement of CYP3A4 is higher for rabeprazole. The inhibition of CYP3A4 by food may be associate to a lower first-pass metabolism that may explain the increase in Cmax and half-life when rabeprazole is administered under fed conditions. Although the meals given to the volunteers did not contain grapefruit or other known CYP3A4 inhibitors, other components of food could also inhibit in some extent CYP3A4. Moreover, the difference in Cmax can also be due to different genetic profile in subjects participating in the fed and fast studies, since it disappears when considering only CYP2C19*1/*1 subjects (see Table 2).
Clearance and volume of distribution cannot be properly calculated when the drug is administered by oral route and we can only calculate these parameters adjusted for bioavailability. In this way, Cl/F is calculated as dose/AUC, and Vd/F as Cl/Ke. So, the differences found in these parameters may reflect the differences in bioavailability when the drug is administered with food.
Food increased variability in Tmax for all 3 drugs, delaying absorption around 3 to 4 h and until 20 h in some subjects. This can be related with the different effect of a high-fat meal on stomach empting and CYP3A4 inhibition in each subject.
Food intake increases the gastric pH that activates ATPase molecules, thus resulting in acid secretion [2]. PPIs require secretion of acid for activation and binding to ATPase molecules [13]; therefore, food may affect the pharmacokinetics and pharmacodynamics of PPIs [3, 4].
Because of the direct relationship between plasma AUC and the antisecretory effects of PPIs [23, 24], it might be expected that administration of PPIs with food would decrease acid suppression. In this respect, Andersson et al. reported that inhibition of intragastric acid secretion by esomeprazole increases with higher exposure (AUC) [23]. Therefore, a higher AUC correlates with higher efficacy. For this reason, in the case of pantoprazole and omeprazole, since food decreases the AUC, it would be better to administer these drugs under fasting conditions. However, Iwata et al. observed that pre-dinner administration of PPIs could increase their efficacy in patients with gastroesophageal reflux disease [25].
Since findings are contradictory, some authors state that food did not affect PPIs. In their review, Swan et al. reported that the bioavailability of rabeprazole was not influenced by co-ingestion of food [26]. Junghard et al. found that food decreased AUC and Cmax but had no effect on the percentage of time that intragastric pH was > 4.0, because of the more extended plasma concentration profile (longer duration with esomeprazole) [24]. Huber et al. observed that concomitant intake of a standard breakfast with pantoprazole (40 mg) had no effect on bioavailability [27]. In this regard, our study sheds light to this controversy, since we found a clear influence of food intake in omeprazole, rabeprazole and pantoprazole pharmacokinetic parameters.
In our opinion, based on our results, omeprazole and pantoprazole drug label should include the following sentence: “As food delays the absorption of PPIs around 3 to 4 hours and decreases their bioavailability, it would be better to administer these drugs under fasting conditions.”
Finally, the effect of sex was analysed because all factors that may influence pharmacokinetics must be taken into account. However, the differences found in AUC for rabeprazole are very small (around 5%), so it is not expected to be related to a different clinical effect.
Conclusion
In conclusion, administration of PPIs with food delays absorption around 3 to 4 h and increases their variability. Food also decreases oral exposure of omeprazole and pantoprazole. Consequently, it would be better to administer PPIs under fasting conditions to improve their efficacy.
Acknowledgements
This study would not have been possible without the cooperation of the volunteers. We are grateful to Mr. Thomas O’Boyle for writing assistance, which was financed by Fundación de Investigación Biomédica del Hospital Universitario de La Princesa.
Abbreviations
- AUC
Area under the concentration-time curve
- BMI
Body Mass Index
- Cmax
Maximum concentration
- CYP
Cytochrome P450
- ECG
Electrocardiogram
- EMA
European Medicines Agency
- FDA
US Food and Drug Administration
- PPI
Proton pump inhibitor
- Tmax
Time to reach the maximum concentration
Authors’ contributions
D.O., M.S.R. and T.C. wrote the manuscript; D.O., M. R. and F.A.S designed the research; D.O., M.R, T.C., M.S.R., G.M. and F.A.S performed research; D.O, T.C. and F.A.S analyzed the data; M.S.R and G.M. contributed with analytical tools. The authors read and approved the final manuscript.
Funding
The analysis, interpretation of data and the manuscript writing was partially funded by Fundación Teófilo Hernando, a nonprofit foundation linked to Universidad Autónoma de Madrid.
Availability of data and materials
The dataset supporting the conclusions of this article is available under the petition to the corresponding author.
Ethics approval and consent to participate
The protocols complied with current Spanish legislation on clinical research in humans and were approved by the Research Ethics Committee from Hospital Universitario de La Princesa, duly authorized by the Spanish Drug Agency and under the guidelines of Good Clinical Practice. All participants gave their written informed consent for genotyping and were free to withdraw from the study at any time. The bioequivalence single-dose clinical trials were performed at Hospital Universitario de La Princesa (Madrid, Spain) between 2006 and 2010.
Consent for publication
Not Applicable.
Competing interests
F.A.S. and D.O. have been consultants or investigators in clinical trials sponsored by the following pharmaceutical companies: Abbott, Alter, Chemo, Cinfa, FAES, Farmalíder, Ferrer, GlaxoSmithKline, Galenicum, Gilead, Italfarmaco, Janssen-Cilag, Kern, Normon, Novartis, Servier, Silverpharma, Teva and Zambon. The remaining authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–731. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatlebakk JG, Katz PO, Castell DO. Medical therapy: management of the refractory patients. Gastroenterol Clin North Am. 1999;28:847–860. doi: 10.1016/S0889-8553(05)70093-5. [DOI] [PubMed] [Google Scholar]
- 3.Pan WJ, Goldwater DR, Zhang Y, Pilmer BL, Hunt RH. Lack of a pharmacokinetic interaction between lansoprazole or pantoprazole and theophylline. Aliment Pharmacol Ther. 2000;14:345–352. doi: 10.1046/j.1365-2036.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- 4.Rhim SY, Park JH, Park YS, Lee MH, Hwang KG, Kim YS, et al. Pharmacokinetics and bioequivalence of 20 mg omeprazole capsule in 24 healthy Korean male volunteers. Int J Clin Pharmacol Ther. 2009;47:23–29. doi: 10.5414/CPP47023. [DOI] [PubMed] [Google Scholar]
- 5.Román M, Ochoa D, Sánchez-Rojas SD, Talegón M, Prieto-Pérez R, Rivas Á, et al. Evaluation of the relationship between polymorphisms in CYP2C19 and the pharmacokinetics of omeprazole, pantoprazole and rabeprazole. Pharmacogenomics. 2014;15:1893–1901. doi: 10.2217/pgs.14.141. [DOI] [PubMed] [Google Scholar]
- 6.Tytgat GN. Shortcomings of the first-generation proton pump inhibitors. Eur J Gastroenterol Hepatol. 2001;13(Suppl 1):S29–S33. [PubMed] [Google Scholar]
- 7.AEMPS. Full prescribing information for omeprazole. Available at: http://www.aemps.gob.es/cima/pdfs/es/ft/65687/FT_65687.pdf. Accessed 6 July 2020.
- 8.FDA full prescribing information for rabeprazole: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM214745.pdf. Accessed 6 July 2020.
- 9.FDA. Omeprazole label. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019810s096lbl.pdf. Accessed 6 July 2020.
- 10.FDA full prescribing information for pantoprazole: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020987s045lbl.pdf. Accessed 6 July 2020.
- 11.EMA (European Medicines Agency). Committee for Human Medicinal Products (CHMP). CHMP efficacy working party therapeutic subgroup on pharmacokinetics (EWP-PK). Questions & Answers: Positions on specific questions addressed to the EWP therapeutic subgroup on Pharmacokinetics. 2010. Doc. Ref.: EMA/618604/2008 Rev. 2.
- 12.FDA (Food and Drugs Administration) US Department of Health and Human Services. Center for Drug Evaluation and Research (CDER). Food-effect bioavailability and fed bioequivalence studies. Rockville: Guidance for Industry; 2002. [Google Scholar]
- 13.Sostek MB, Chen Y, Andersson T. Effect of timing of dosing in relation to food intake on the pharmacokinetics of esomeprazole. Br J Clin Pharmacol. 2007;64:386–390. doi: 10.1111/j.1365-2125.2007.02889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson AB, Cohen P, Ficheux H, Fiorentini P, Domagala F, Homerin M, et al. Comparison of the effects of fasting morning, fasting evening and fed bedtime administration of tenatoprazole on intragastric pH in healthy volunteers: a randomized three-way crossover study. Aliment Pharmacol Ther. 2006;23:1179–1187. doi: 10.1111/j.1365-2036.2006.02781.x. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda S, Ohnishi A, Ogawa T, Tomono Y, Hasegawa J, Nakai H, et al. Pharmacokinetic properties of E3810, a new proton pump inhibitor, in healthy male volunteers. Int J Clin Pharmacol Ther. 1994;32:466–473. [PubMed] [Google Scholar]
- 16.Sachs G, Shin JM, Hunt R. Novel approaches to inhibition of gastric acid secretion. Curr Gastroenterol Rep. 2010;12:437–447. doi: 10.1007/s11894-010-0149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrison AF, Jarboe LA, Weinberg BM, Nimmagadda K, Sullivan LM, Wolfe MM. Patterns of proton pump inhibitor use in clinical practice. Am J Med. 2001;111:469–473. doi: 10.1016/S0002-9343(01)00901-9. [DOI] [PubMed] [Google Scholar]
- 18.Lind T, Cederberg C, Ekenved G, Haglund U, Olbe L. Effect of omeprazole - a gastric proton pump inhibitor - on pentagastrin stimulated acid secretion in man. Gut. 1983;24:270–276. doi: 10.1136/gut.24.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer EA. The physiology of gastric storage and emptying. In: Johnson LR, Alpers DH, Jacobson ED, Christensen J, Walsh JH, editors. Physiology of the gastrointestinal tract. 3. New York: Raven Press; 1994. pp. 929–976. [Google Scholar]
- 20.Benet LZ, Kroetz DL, Sheiner LB. Pharmacokinetics. The dynamics of drug absorption, distribution, and elimination. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s: the pharmacological basis of therapeutics. 9. New York: McGraw-Hill; 1996. pp. 3–28. [Google Scholar]
- 21.Liu Z, Ding L, Zhong S, Cao X, Jiang L, Duan H. Pharmacokinetics of a new immediate-release compound omeprazole capsule and its comparison with the enteric-coated formulation under fasting and fed conditions. Drug Res (Stuttg) 2013;63:370–375. doi: 10.1055/s-0033-1341477. [DOI] [PubMed] [Google Scholar]
- 22.Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors--emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13(Suppl 3):27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 23.Andersson T, Röhss K, Bredberg E, Hassan-Alin M. Pharmacokinetics and pharmacodynamics of esomeprazole, the S-isomer of omeprazole. Aliment Pharmacol Ther. 2001;15:1563–1569. doi: 10.1046/j.1365-2036.2001.01087.x. [DOI] [PubMed] [Google Scholar]
- 24.Junghard O, Hassan-Alin M, Hasselgren G. The effect of the area under the plasma concentration vs time curve and the maximum plasma concentration of esomeprazole on intragastric pH. Eur J Clin Pharmacol. 2002;58:453–458. doi: 10.1007/s00228-002-0502-1. [DOI] [PubMed] [Google Scholar]
- 25.Iwata A, Ikeda K, Hirose K, Takatori H, Takahashi K, Sanayama Y, et al. Pre-dinner administration increases the efficacy of proton pump inhibitors on refractory GERD symptoms in connective tissue disease patients. Mod Rheumatol. 2013;23:357–364. doi: 10.3109/s10165-012-0662-5. [DOI] [PubMed] [Google Scholar]
- 26.Swan SK, Hoyumpa AM, Merritt GJ. Review article: the pharmacokinetics of rabeprazole in health and disease. Aliment Pharmacol Ther. 1999;13(Suppl 3):11–17. doi: 10.1046/j.1365-2036.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- 27.Huber R, Hartmann M, Bliesath H, Lühmann R, Steinijans VW, Zech K. Pharmacokinetics of pantoprazole in man. Int J Clin Pharmacol Ther. 1996;34:185–194. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is available under the petition to the corresponding author.