Abstract
Background
The risk factors contributing to embolism in cardiac myxoma (CM) are yet controversial. This systematic review and meta-analysis aimed to clarify the risk factors of embolism for the CM patients.
Methods
PubMed, Embase, Cochrane library, Web of Science, China National Knowledge Infrastructure, Wan Fang, and Wei Pu databases were searched from inception to June 2019. Statistical analysis was conducted using Stata version 14.0. The pooled odds ratio or mean difference with 95% confidence interval was estimated for each risk factor.
Results
Herein, 12 studies, encompassing 1814 patients, were included. The pooled results suggested that New York Heart Association (NYHA) class I/II (P < 0.01), hypertension (P = 0.03), irregular tumor surface (P < 0.01), tumor in atypical location (P = 0.01), narrow base of tumor (P < 0.01), and increased fibrinogen (FIB) (P < 0.01) are significant risk factors of embolism in CM patients. However, sex, age, body mass index, smoking, left ventricular ejection fraction, diabetes, hyperlipidemia, atrial fibrillation, valvular heart disease, coronary heart disease, tumor size, platelet count, white blood cells, and hemoglobin were not associated with embolism (all P > 0.05).
Conclusions
NYHA class (I/II), hypertension, irregular tumor surface, atypical tumor location, the narrow base of tumor, and increased FIB were significant risk factors of embolism in CM patients. For CM patients with these factors, early surgery might be beneficial to prevent embolism.
Keywords: Cardiac myxoma, Embolism, Risk factors, Meta-analysis
Background
Cardiac myxomas (CMs) are the most prevalent type of primary cardiac tumors in adults [1] that account for approximately half of all benign lesions. The manifestations of CM patients include obstruction, preoperative embolization, and constitutional symptoms. Embolization is a major and fatal complication that occurs in 20–45% of CM patients [2]; it includes cerebral embolism and peripheral embolism. Cerebral embolism accounts for about 50% of the embolic events, mainly acute stroke symptoms. Peripheral embolism may involve extremity, visceral, and coronary arteries [3]. The right-sided CMs are associated with pulmonary embolism. These embolic events are significant causes of mortality in CM patients [4]; however, the exact factors related to the occurrence of embolism are not yet clearly identified.
Although, several risk factors of embolism are recently reported [5–16], some are controversial. For instance, a previous study [17] showed that the small size of the tumor was an independent risk factor for embolism, whereas other studies neither found any association [14] nor presented a contrary conclusion [13]. Some studies reported that the male gender was associated with an increased risk of embolism [18], while others failed to find this association [6, 7]. Therefore, we conducted this systematic review with meta-analysis to clarify the risk factors of embolism in CM patients.
Methods
Data sources and search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was followed [19]. The PubMed, Embase, Cochrane library, Web of Science, China National Knowledge Infrastructure, Wan Fang, and Wei Pu databases were used for further studies. The search strings are reported in Additional file 1. Studies published up to June 30, 2019, were eligible. No language restriction was applied. Reference lists of selected studies were checked to ensure complete coverage.
Eligibility criteria
The studies that fulfilled the following criteria were eligible for inclusion in this meta-analysis: 1) All CM patients who underwent surgical resection and were confirmed by pathological examination; 2) Patients in each study were classified into two groups (embolic and non-embolic); 3) Embolic events were diagnosed by clinical symptoms or imaging examination. If the same population was presented in more than one publication, the study with the largest sample was included.
We excluded the studies that met at least one of the following criteria: 1) Sample size < 50; 2) Abstracts from conferences, letters to the editor, and reviews; 3) Incomplete data.
Data extraction and quality assessment
Two authors (L.P and J.W) reviewed all retrieved articles and extracted data independently. The titles and abstracts were first screened to identify potentially eligible articles, and then, full texts were read to confirm their eligibility for inclusion in this meta-analysis. The extracted data included the following information: first author, year of publication, country, study type, sample size, age, incidence of embolism, and risk factors that include patient characteristics (sex, age, New York Heart Association (NYHA) class and atrial fibrillation (AF)), tumor characteristics (tumor surface and tumor location), and hematological parameters (white blood cell (WBC) and platelet count (PLT)). The NYHA class was divided into two groups (I/II vs. III/IV). The tumor surface was classified as irregular and regular [7]. The tumor location was classified into “typical location” (tumor arise from the interatrial septum at the border of the fossa ovalis in the left atrium) and “atypical location” (tumor arise from other sites of the left atrium or in the other cardiac chambers) [20]. The extracted data were cross-checked, and any disagreements were resolved by discussion or consultation with the third author (L.G).
The quality of the included studies was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist [21] for Case Series. It contains ten items, encompassing clear criteria for inclusion, the information of participants, and the statistical methods used. Each item was determined by yes, no, unclear, and not applicable.
Statistical analysis
Stata 14.0 was used for the statistical analysis of all data. The odds ratio/mean difference (OR/MD) and 95% confidence interval (CI) were calculated using the fixed-effect model or the random effect model. The heterogeneity among studies was quantified using the Cochran’s Q test and chi-square (I2) test. I2 = 25% was considered low, 50% was moderate, and 75% was high. When I2 > 50% or P-value < 0.05 was identified for substantial heterogeneity, we used the random effect model; otherwise, a fixed-effect model was adopted. A sensitivity analysis was conducted when substantial heterogeneity was presented. Publication bias was assessed by the Egger’s test. All tests were two-sided, and the results were considered statistically significant at P < 0.05.
Results
Search results and characteristics of included studies
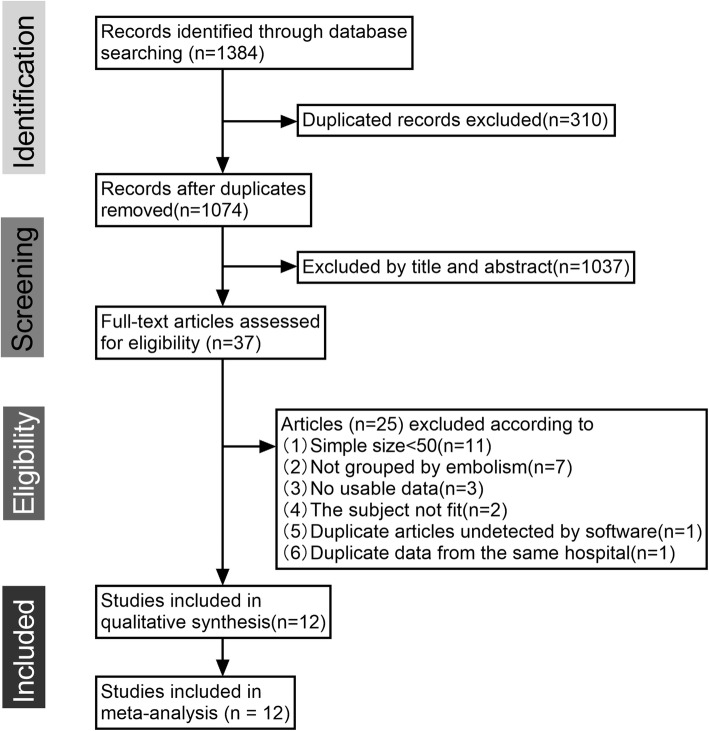
A total of 12 studies [5–16] encompassing 1814 patients who underwent surgery with CM were selected for the current analysis. The detailed study selection progress is shown in Fig. 1, and the main characteristics of included studies are summarized in Table 1.
Fig. 1.
Flowchart of study selection
Table 1.
Characteristics of included studies
| study | country | type of study | sample size | age(Mean ± SD or median(IQR)) | incidence of embolism | risk factors | the JBI Appraisal Checklist | ||
|---|---|---|---|---|---|---|---|---|---|
| embolisim group | non-embolism group | embolisim group | non-embolism group | ||||||
| Cao 2017 [5] | China | Retrospective case series | 24 | 87 | 36.0 ± 8.1 | 36.4 ± 6.5 | 21.62% | tumor size | yes(6)no(3)unclear(1) not applicable(0) |
| Deng 2015 [6] | China | Retrospective case series | 33 | 129 | 48(38–61) | 54(46–63) | 20.37% | tumor location, tumor surface, MPV, PLT | yes(8)no(1)unclear(1) not applicable(0) |
| Lee 2012 [7] | Korea | Retrospective case series | 13 | 46 | 59.2 ± 12.6 | 58.3 ± 12.6 | 22.03% | tumor surface | yes(7)no(2)unclear(1) not applicable(0) |
| Wang 2016 [8] | China | Retrospective case series | 32 | 175 | 39.7 ± 16.6 | 45.0 ± 15.5 | 15.46% | tumor size, tumor surface | yes(7)no(2)unclear(1) not applicable(0) |
| Yin 2016 [9] | China | Retrospective case series | 143 | 322 | 59.4 ± 10.9 | 52.3 ± 12.4 | 44.41% | age, BMI, tumor size, tumor surface, tumor location, LAD CHA2DS2–VASc score | yes(8)no(1)unclear(1) not applicable(0) |
| Li 2018 [10] | China | Retrospective case series | 27 | 158 | 48.19 ± 13.11 | 49.39 ± 14.97 | 14.59% | tumor location,tumor base size, FIB | yes(7)no(2)unclear(1) not applicable(0) |
| Xu 2016 [11] | China | Retrospective case series | 26 | 156 | 58.54 ± 12.65 | 57.29 ± 11.11 | 14.29% | tumor surface, tumor base size, FIB | yes(8)no(1)unclear(1) not applicable(0) |
| Boyacıoğlu 2017 [12] | Turkey | Retrospective case series | 25 | 74 | 49 ± 16.89 | 50.12 ± 15.71 | 25.26% | AF, tumor surface, tumor size | yes(7)no(2)unclear(1) not applicable(0) |
| Kalçık 2019 [13] | Turkey | Retrospective case series | 13 | 80 | 50(36–62) | 56(45–65) | 13.98% | LAD, AF, tumor size, tumor surface | yes(7)no(2)unclear(1) not applicable(0) |
| Ping 2019 [14] | China | Retrospective case series | 32 | 75 | 54.66 ± 13.21 | 51.72 ± 13.76 | 29.91% | tumor surface, tumor location | yes(7)no(2)unclear(1) not applicable(0) |
| Canga 2017 [15] | Turkey | Retrospective case series | 13 | 53 | 51.1 ± 11.4 | 55.9 ± 12.4 | 19.70% | sex,tumor location,tumor surface | yes(7)no(2)unclear(1) not applicable(0) |
| Zheng 2014 [16] | China | Retrospective case series | 15 | 63 | 49 ± 9 | 52 ± 6 | 19.23% | tumor surface | yes(7)no(2)unclear(1) not applicable(0) |
SD: standard deviation IQR: interquartile range LAD: left atrium diameter the CHA2DS2-VASc: congestive heart failure, hypertension, age ≥ 75 (doubled), diabetes, stroke(doubled), vascular disease, age 65–74, and sex category (female) recommended by European Society of Cardiology (ESC) is an easy-to-remember means of assessing stroke risk of patients with AF
Meta-analysis of risk factors
Based on the data available from the included studies, the risk factors were classified as patient characteristics, tumor characteristics, and hematological parameters in the current review. A total of 20 risk factors were individually analyzed using a fixed-effect or a random effect model to estimate the association with embolism in CM patients. The main characteristics of each risk factor are summarized in Table 2.
Table 2.
Main characteristics of each risk factor
| Risk Factors | No. Studies | Effect Model | I2, % | Ph | MD/OR | Effect Size (95% CI) | P |
|---|---|---|---|---|---|---|---|
| sex(male) | 12 | F | 16.8 | 0.28 | OR | 1.21(0.96–1.53) | 0.11 |
| age | 9 | R | 76.8 | < 0.01 | MD | -0.04(−3.64–3.56) | 0.94 |
| BMI | 4 | R | 83.7 | < 0.01 | MD | 1.21(−0.43–2.84) | 0.18 |
| NYHA class(I/II) | 4 | F | 0.0 | 0.61 | OR | 2.98(1.66–5.33) | < 0.01* |
| smoking | 6 | F | 0.0 | 0.44 | OR | 0.90(0.64–1.28) | 0.56 |
| LVEF | 6 | F | 7.0 | 0.37 | MD | 0.59(−0.20–1.38) | 0.14 |
| hypertension | 8 | F | 0.1 | 0.43 | OR | 1.41(1.04–1.92) | 0.03* |
| diabetes | 8 | F | 0.0 | 0.73 | OR | 1.32(0.89–1.94) | 0.16 |
| hyperlipidemia | 6 | F | 0.0 | 0.79 | OR | 0.99(0.53–1.85) | 0.96 |
| atrial fibrillation | 7 | F | 48.5 | 0.07 | OR | 1.25(0.88–1.80) | 0.22 |
| valvular heart disease | 3 | F | 0.0 | 0.62 | OR | 0.76(0.41–1.40) | 0.38 |
| coronary heart disease | 3 | R | 84.2 | < 0.01 | OR | 0.99(−0.87–2.85) | 0.32 |
| tumor surface(irregular) | 11 | F | 40.4 | 0.08 | OR | 3.99(3.04–5.25) | < 0.01* |
| tumor size | 8 | R | 83.3 | < 0.01 | MD | -0.10(−0.76–0.57) | 0.78 |
| tumor location(atypical) | 4 | F | 15.9 | 0.31 | OR | 1.81(1.13–2.88) | 0.01* |
| tumor base size | 2 | F | 0.0 | 0.66 | MD | −0.36(−0.51--0.22) | < 0.01* |
| PLT | 3 | F | 0.0 | 0.83 | MD | 9.95(−6.02–25.91) | 0.22 |
| WBC | 4 | F | 34.6 | 0.21 | MD | 0.18(−0.33–0.68) | 0.49 |
| HB | 4 | F | 0.0 | 0.40 | MD | 1.65(−2.91–6.21) | 0.48 |
| FIB | 2 | F | 0.0 | 0.57 | MD | 0.62(0.28–0.95) | < 0.01* |
F: fixed-effects model R: random-effects model Ph: P value of heterogeneity *P < 0.05
Patient characteristics
A meta-analysis was performed to assess the 12 risk factors, of which NYHA class (I/II) (OR = 2.98, 95% CI = 1.66–5.33, P < 0.01) (Fig. 2) and hypertension (OR = 1.41, 95% CI = 1.04–1.92, P = 0.03) (Fig. 3) significantly increased the risk of embolism in CM patients. However, no statistically significant difference was detected in the meta-analysis with respect to sex (OR = 1.21, 95% CI = 0.96–1.53, P = 0.11), age (MD = -0.04, 95% CI = -3.64–3.56, P = 0.94), body mass index (BMI) (MD = 1.21, 95% CI = -0.43–2.84, P = 0.18), smoking (OR = 0.90, 95% CI = 0.64–1.28, P = 0.56), left ventricular ejection fraction (LVEF) (MD = 0.59, 95% CI = -0.20–1.38, P = 0.14), diabetes (OR = 1.32, 95% CI = 0.89–1.94, P = 0.16), hyperlipidemia (OR = 0.99, 95% CI = 0.53–1.85, P = 0.96), AF (OR = 1.25, 95% CI = 0.88–1.80, P = 0.22), valvular heart disease (OR = 0.76, 95% CI = 0.41–1.40, P = 0.38), and coronary heart disease (OR = 0.99, 95% CI = -0.87–2.85, P = 0.32).
Fig. 2.
Forest plot for NYHA class between embolism: 4 studies were included, I2 = 0.0%, fixed-effect model was adopted; the result showed NYHA I/II is a risk factor
Fig. 3.
Forest plot for hypertension between embolism: 8 studies were included, I2 = 0.1%, fixed-effect model was adopted; the result showed hypertension a risk factor
Tumor characteristics
A meta-analysis was performed for 4 risk factors. Of these, irregular tumor surface (OR = 3.99, 95% CI = 3.04–5.25, P < 0.01) (Fig. 4), atypical location (OR = 1.81, 95% CI = 1.13–2.88, P = 0.01) (Fig. 5), and narrow base (MD = -0.36, 95% CI = -0.51–-0.22, P < 0.01) (Fig. 6) significantly increased the risk of embolism in CM patients, while tumor size (MD = -0.10, 95% CI = -0.76–0.57, P = 0.78) was not associated with the condition.
Fig. 4.
Forest plot for tumor surface between embolism: 11 studies were included, I2 = 40.4%, fixed-effect model was adopted; the result showed irregular surface is a risk factor
Fig. 5.
Forest plot for tumor location between embolism: 4 studies were included, I2 = 15.9%, fixed-effect model was adopted; the result showed atypical location is a risk factor
Fig. 6.
Forest plot for tumor base size between embolism: 2 studies were included, I2 = 0.0%, fixed-effect model was adopted; the result showed narrow base is a risk factor
Hematological parameters
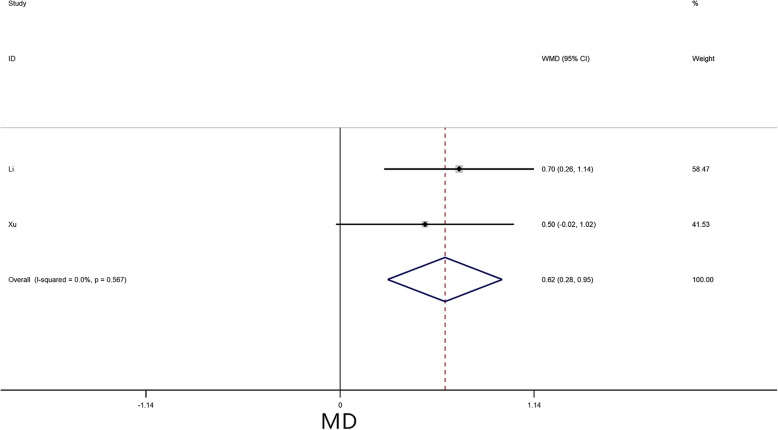
A meta-analysis was performed for 4 risk factors. Of these, increased fibrinogen (FIB) (MD = 0.62, 95% CI = 0.28–0.95, P < 0.01) (Fig. 7) significantly increased the risk of embolism in CM patients, while no statistically significant difference was detected in the meta-analysis with respect to PLT (MD = 9.95, 95% CI = -6.02–25.91, P = 0.22), WBC (MD = 0.18, 95% CI = -0.33–0.68, P = 0.49), and hemoglobin (HB) (MD = 1.65, 95% CI = -2.91–6.21, P = 0.48).
Fig. 7.
Forest plot for FIB between embolism: 2 studies were included, I2 = 0.0%, fixed-effect model was adopted; the result showed increased FIB is a risk factor
Sensitivity analysis
High heterogeneity across studies was detected regarding age (I2 = 76.8%), BMI (I2 = 83.7%), and coronary heart disease (I2 = 84.2%). Sensitivity analysis showed a similar trend among various studies except for the study by Yin et al. [9]. After the exclusion of Yin’s study, the among-study heterogeneity was not detected (all I2 = 0.00%, all P > 0.05); also, the overall effect did not differ significantly (all P > 0.05).
In addition, tumor size had high heterogeneity (I2 = 83.3%), and sensitivity analysis showed a similar trend among studies except for the study by Kalçık et al. [13]. After this study was excluded, the among-study heterogeneity was found to be moderate (I2 = 58.6%, P = 0.03), while the overall effect did not differ significantly (P = 0.19).
Publication bias
General considerations suggest that use of the Egger’s test with substantially fewer 10 studies would be unwise [22]; hence, the publication bias was assessed for sex and tumor surface. The Egger’s test not detect any publication bias on sex (P = 0.096) and tumor surface (P = 0.051).
Discussion
In the current meta-analysis, a total of 20 potential factors were studied. The results suggested that the NYHA class, hypertension, tumor surface, tumor location, tumor base size, and FIB were independent risk factors associated with embolism.
Among patient characteristics, we found that 2/12 factors (NYHA class and hypertension) were significantly associated with embolism. The NYHA class is the most commonly used indicator of clinical response to cardiac function. The current study showed that better cardiac function (class I/II) was at a significantly higher risk of embolism than the worse function (class III/IV). This finding might be attributed to the fact that most embolic group patients were diagnosed after an acute embolic event; also, the tumor blockage symptoms were relatively less, and the overall cardiac function was better than that of non-embolic group. Conversely, the non-embolic group patients have a prolonged course of the disease and are likely to show hemodynamic changes; this phenomenon was associated with obstructive heart failure. However, the LVEF was not associated with embolism. We think this difference may attribute to the fact that some patients exist heart failure with preserved ejection fraction, and they also could reach NYHA class III/IV [23]. Hence, additional quantitative research about cardiac function is imperative. Some studies reported that hypertension might be a major risk factor of deep venous thromboembolism [24, 25]. Interestingly, the present study found that hypertension also was a risk factor of embolism in CM patients, which could be because high blood pressure increases the activity of PLT, making the blood hypercoagulable [26].
Regarding tumor characteristics, we found that three factors are related to embolism: tumor surface, location, and base. Macroscopically, the surface of the tumor is classified into two types [27, 28] as follows: Type 1 is characterized by an irregular surface and soft consistency, while type 2 presents a regular surface and compact consistency. Consistent with the previous studies [29–31], the current study found that embolic events are often associated with type 1 myxoma. This correlation might occur because the type 1 myxoma is prone to be friable, which leads to the shedding of tumor fragments into the bloodstream [13]. In addition, we found that atypical location is a risk factor of embolism. This conclusion is consistent with the result of the study by Deng et al. [6]. Thus, we hypothesized that the atypical location plays a significant role in hemodynamics than typical location. Lastly, the narrow tumor base was also a risk factor in this study owing to its great mobility, which tends to generate fragments [11].
In terms of hematological parameters, the present study designated increased FIB as a risk factor of embolism. It is a substrate for thrombin and is directly involved in the clotting process. In addition, FIB promotes the aggregation of PLT, increases blood viscosity, and aggravates red blood cell adhesion, all of which promote thrombosis [32]. Thus, we inferred that increased FIB promotes thrombosis on the surface of CM, which contributes to embolism.
To the best of our knowledge, this is the first meta-analysis to investigate the risk factors of embolism in patients with CM, with some practical clinical implications. Nevertheless, this meta-analysis also has some limitations. First, since all enrolled studies were retrospective case series, residual confounders and unidentified factors were inevitable in observational studies. Second, all the included studies originated from Asia, which might lead to selection bias. Third, the number of overlapped risk factors in each of the studies was small for a comprehensive statistical analysis. Finally, with respect to the tumor size, the data of our meta-analysis showed high heterogeneity. Although one major source of heterogeneity was detected by sensitivity analysis, other differences between the studies should be considered.
Conclusions
This systematic review and meta-analysis identified the following significant risk factors of embolism for CM patients: NYHA class I/II, hypertension, irregular tumor surface, atypical tumor location, the narrow base of tumor, and increased FIB. For CM patients with these factors, early surgery may be beneficial for preventing embolism.
Supplementary information
Acknowledgments
Not applicable
Abbreviations
- CM
Cardiac myxoma
- OR
Odds ratio
- MD
Mean difference
- CI
Confidence interval
- NYHA
New York heart association
- FIB
Fibrinogen
- BMI
Body mass index
- LVEF
Left ventricular ejection fraction
- AF
Atrial fibrillation
- PLT
Platelet count
- WBC
White blood cells
- HB
Hemoglobin
Authors’ contributions
L.G gave his substantial contribution to the conception of the work. L. P, J. W, L. G and Y. L contributed to the study design. L. P wrote the draft of the manuscript. J. W and Y. L revised it critically for intellectual content. Y. L revised the manuscript. All authors approved the final version of the manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12872-020-01631-w.
References
- 1.Dias RR, Fernandes F, Ramires FJA, Mady C, Albuquerque CP, Jatene FB. Mortality and embolic potential of cardiac tumors. Arq Bras Cardiol. 2014;103(1):13–18. doi: 10.5935/abc.20140096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan SM, Humuruola G. Stroke of a cardiac myxoma origin. Rev Bras Cir Cardiovasc. 2015;30(2):225–234. doi: 10.5935/1678-9741.20150022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang QS, Yang FF, Zhu F, Yao CS. A case report of left atrial myxoma-induced acute myocardial infarction and successive stroke. Medicine (Baltimore) 2018;97(51):e13451. doi: 10.1097/MD.0000000000013451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cianciulli TF, Cozzarin A, Soumoulou JB, Saccheri MC, Mendeze RJ, Beck MA, et al. Twenty years of clinical experience with cardiac myxomas: diagnosis, treatment, and follow up. J Cardiovasc Imaging. 2019;27(1):37–47. doi: 10.4250/jcvi.2019.27.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao GF, Bi Q, Cao L, Wang C. The clinical characteristics of stroke in young patients with cardiac myxoma. Chin J Intern Med. 2017;56(4):263–267. doi: 10.3760/cma.j.issn.0578-1426.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 6.He DK, Zhang YF, Liang Y, Ye SX, Wang C, Kang B, et al. Risk factors for embolism in cardiac myxoma: a retrospective analysis. Med Sci Monit. 2015;21:1146–1154. doi: 10.12659/MSM.895027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Kim JH, Na CY, Oh SS. Eleven years’ experience with Korean cardiac myxoma patients: focus on embolic complications. Cerebrovascular Dis. 2012;33(5):471–479. doi: 10.1159/000335830. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZJ, Chen SQ, Zhu M, Zhang H, Li HX, Yuan GD, et al. Risk prediction for emboli and recurrence of primary cardiac myxomas after resection. J Cardiothorac Surg. 2016;11:22. doi: 10.1186/s13019-016-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin L, Wang J, Li W, Ling XY, Xue Q, Zhang YF, et al. Usefulness of CHA2DS2-VASc scoring systems for predicting risk of perioperative embolism in patients of cardiac myxomas underwent surgical treatment. Sci Rep. 2016;6:39323. doi: 10.1038/srep39323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu R. Analysis of risk factors for vascular embolization of cardiac myxoma [master’s thesis] Nanning (GX): Guangxi Medical University; 2018. p. 45. [Google Scholar]
- 11.Xu GX. Risk assessment of vascular embolization of cardiac myxoma [dissertation] Hangzhou (ZJ): Zhejiang University; 2016. p. 80. [Google Scholar]
- 12.Boyacıoğlu K, Kalender M, Dönmez AA, Çayhan B, Tuncer MA. Outcomes following embolization in patients with cardiac myxoma. J Card Surg. 2017;32(10):621–626. doi: 10.1111/jocs.13220. [DOI] [PubMed] [Google Scholar]
- 13.Kalçık M, Bayam E, Güner A, Küp A, Kalkan S, Yesin M, et al. Evaluation of the potential predictors of embolism in patients with left atrial myxoma. Echocardiography. 2019;36(5):837–843. doi: 10.1111/echo.14331. [DOI] [PubMed] [Google Scholar]
- 14.Ping LY, Ying K, Xiong YP, Zhang CQ, Liu YN, Guo LY. Echocardiography in predicting the risk of embolism in atrial myxoma. Chin J Ultrasound Med. 2019;35(9):799–802. [Google Scholar]
- 15.Canga Y, Karataş MB, Calık AN, Tanik VO. Cardiac myxoma: fourteen-year experience of a tertiary reference center. Kosuyolu Heart J. 2017;20(3):210–216. doi: 10.5578/khj.46500. [DOI] [Google Scholar]
- 16.Zheng Z, Guo GJ, Xu L, Lei L, Wei X, Pan YM. Left atrial myxoma with versus without cerebral embolism: length of symptoms, morphologic characteristics, and outcomes. Tex Heart Inst J. 2014;41(6):592–595. doi: 10.14503/THIJ-13-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, et al. Embolic potential of cardiac tumors and outcome after resection. Stroke. 2009;40(1):156–162. doi: 10.1161/STROKEAHA.108.525709. [DOI] [PubMed] [Google Scholar]
- 18.Pinede L, Duhaut P, Lorie R. Clinical presentation of left atrial cardiac myxoma a series of 112 consecutive cases. Medicine (Baltimore) 2001;80(3):159–172. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(2):123–130. [PMC free article] [PubMed] [Google Scholar]
- 20.Vaideeswar P, Gupta R, Mishra P, Lanjewar C, Raut A. Atypical cardiac myxomas: a clinicopathologic analysis and their comparison to 64 typical myxomas. Cardiovasc Pathol. 2012;21(3):180–187. doi: 10.1016/j.carpath.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer's Manual: The Joanna Briggs Institute; 2017. Available from https://reviewersmanual.joannabriggs.org/.
- 22.Sterne JAC, Sutton AJ, Loannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 23.Dalos D, Mascherbauer J, Zotter-Tufaro C, Duca F, Kammerlander AA, Aschauer S, et al. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68(2):189–198. doi: 10.1016/j.jacc.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 24.Yang SD, Liu H, Sun YP, Yang DL, Shen Y, Feng SQ, et al. Prevalence and risk factors of deep vein thrombosis in patients after spine surgery: a retrospective case-cohort study. Sci Rep. 2015;5:11834. doi: 10.1038/srep11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Li J, Jiang Y. Association between hypertension and deep vein thrombosis after orthopedic surgery: a meta-analysis. Eur J Med Res. 2016;21:13. doi: 10.1186/s40001-016-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long JJ, Zhao AT, Liu J, Luo BQ. Relationship between hypercoagulability and insulin resistance in patients with essential hypertension. Chin J Hemorh. 2004;14(2):186–188. [Google Scholar]
- 27.Swartz MF, Lutz CJ, Chandan VS, Landas S, Fink GW. Atrial myxomas: pathologic types, tumor location, and presenting symptoms. J Card Surg. 2006;21(4):435–440. doi: 10.1111/j.1540-8191.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 28.Jain S, Maleszewski JJ, Stephenson CR, Klarich KW. Current diagnosis and management of cardiac myxomas. Expert Rev Cardiovasc Ther. 2015;13(4):369–375. doi: 10.1586/14779072.2015.1024108. [DOI] [PubMed] [Google Scholar]
- 29.Ha JW, Kang WC, Chung N, Chang BC, Rim SJ, Kwon JW, et al. Echocardiographic and morphologic characteristics of left atrial myxoma and their relation to systemic embolism. Am J Cardiol. 1999;83(11):1579–1582. doi: 10.1016/S0002-9149(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 30.Orlandi A, Ciucci A, Ferlosio A, Pellegrino A, Chiariello LG, Spagnoli LG, et al. Increased expression and activity of matrix metalloproteinases characterize embolic cardiac myxomas. Am J Pathol. 2005;166(6):1619–1628. doi: 10.1016/S0002-9440(10)62472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L, Gu TX, Shi EY. Echocardiographic findings and clinical correlation with cardiac myxoma. JACC Cardiovasc Imaging. 2016;9(5):618–621. doi: 10.1016/j.jcmg.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Drouet L, Sollier CBD. Is fibrinogen a predictor or a marker of the risk of cardiovascular events. Therapie. 2005;60(2):125–136. doi: 10.2515/therapie:2005017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.