Abstract
Background
Phototesting studies in cutaneous lupus erythematosus have yielded variable results, with most trials reporting photo-induction of lesions by both UVA and UVB in substantial numbers of patients.
Objectives
To determine the minimal erythema dose in patients with subacute cutaneous lupus erythematosus (SCLE) and controls.
Patients/methods
We phototested nine patients with SCLE and 14 skin type-matched controls, using repetitive dosing of UVA1 and UVB, but with filters that removed most of the shorter UVC and longer infrared and visible light. In addition, DNA was isolated from anticoagulated blood to genotype the TNF-α 308 region in each patient and control.
Results
We were unable to demonstrate a difference in minimal erythema dose (MED) between patients and controls, or any correlation of MED with either TNF genotype or systemic drug therapy for SCLE. In addition, no SCLE skin lesions were induced in the nine patients with either UVA or UVB, and one patient cleared a skin lesion after low-dose UVA1 irradiation.
Conclusions
The potential role of wavelengths outside the UVA and UVB range in the photo-induction of cutaneous lupus skin lesions needs to be investigated, and there is a need to standardize phototesting equipment and procedures for patients with cutaneous lupus erythematous.
Keywords: lupus erythematosus, phototesting, ultraviolet
Ultraviolet (UV) radiation is a well-established trigger of cutaneous lupus erythematosus (1–5), and subacute cutaneous lupus erythematosus (SCLE) is one of the more photosensitive subsets of cutaneous lupus erythematous (LE) (6). Skin lesions often occur in a photodistributed pattern and are more common in the summer months (7, 8). There are clearly both environmental and genetic factors in the pathogenesis of SCLE (9–11). Sunlight exposure, the anti-Ro antibody, HLA type, and polymorphisms in complement molecules and TNF-α have all been correlated with the presence of disease (12–16).
Photoprovocation studies using artificial sources of UVA (320–400 nm), UVB (290–320 nm), and visible light are a common way to examine photosensitivity in SCLE patients (17–24). Of concern, there is large variability in the reported results, with the percentages of SCLE patients showing photoprovocation ranging from 50% to 100% in response to UV radiation. The variation can be partially explained by differences in light radiation sources and filters, ultraviolet doses and dosing schedules, size of the testing area, and site of testing (21).
Recently, Gasparro and Brown reported that the radiation sources typically used in phototesting protocols are often contaminated with non-physiologic wavelengths of light. Of particular concern is UVC radiation, which accounts for between 3% and 11% of the light produced by common, unfiltered fluorescent sunlamps (25, 26). UVC is absorbed by the ozone layer in the atmosphere and never reaches the earth’s surface. This subtype of radiation has no physiologic impact on the formation of SCLE lesion in vivo and may be one explanation for the discrepancies in the lesional induction rates found in the photoprovocation literature. Similarly, Gasparro and Brown have noted the lack of routine use of filters to remove visible light and heat from UVA testing sources. Complicating matters further, many studies fail to report properly the action spectrum of UV radiation emitted from their sources. This compounds the variability between the studies and makes an accurate assessment of the impact of pure UVA or UVB radiation difficult to evaluate.
In the present study, we sought to examine the effects of physiologically relevant doses of UV radiation. Using controlled irradiation devices with filters to block unwanted wavelengths, we phototested a cohort of nine patients with SCLE. We assessed the minimal erythema dose (MED) and whether lesions could be provoked with physiologically relevant doses of ultraviolet radiation. Spot sizes were standardized by using a spot size that works for MED testing. We compared the MED of patients with SCLE with skin-type matched controls and correlated these results with the TNF-α promoter polymorphism. Finally, we used low-dose UVA1 to see whether we could clear lesional SCLE skin.
Methods
Patients
RB approval was attained from the University of Pennsylvania and the VA Medical Center of Philadelphia. Nine patients with clinically and histologically diagnosed SCLE were recruited from the autoimmune skin disease clinic at the Hospital of the University of Pennsylvania. All patients were classified as having skin type I or II skin (27). Medication at the time of the study was carefully recorded. Age-matched healthy controls with similar skin types were recruited from the surrounding population. Exclusion criteria included use of photosensitizing drugs, recent sun exposure, and topical treatment to the areas being irradiated (Table 1).
Table 1.
Subacute cutaneous lupus erythematous patient information
| Patient no. | Gender | Skin type | TNF genotype | Therapy | MED (mJ/cm2) |
|---|---|---|---|---|---|
| 1 | F | 2 | GA | – | 120 |
| 2 | F | 1 | AA | Prednisone 20 mg, dapsone, *HC | 90 |
| 3 | F | 2 | GG | *HC | 120 |
| 4 | M | 2 | GA | Prednisone 30 mg | 90 |
| 5 | F | 2 | GA | – | 90 |
| 6 | F | 1 | GA | – | 110 |
| 7 | F | 2 | GA | – | 70 |
| 8 | F | 1 | GG | – | 90 |
| 9 | F | 2 | GG | – | 110 |
| Control no. | Gender | Skin type | TNF genotype | MED (mJ/cm2) |
|---|---|---|---|---|
| 1 | M | 2 | GA | 100 |
| 2 | M | 2 | GG | 70 |
| 3 | M | 2 | GG | 120 |
| 4 | M | 2 | GG | 120 |
| 5 | F | 2 | GG | 90 |
| 6 | F | 1 | GG | 50 |
| 7 | F | 2 | GG | 90 |
| 8 | F | 2 | GG | 90 |
| 9 | F | 2 | GG | 90 |
| 10 | M | 1 | GG | 100 |
| 11 | M | 1 | GG | 140 |
| 12 | M | 2 | GA | 90 |
| 13 | M | 2 | GA | 100 |
| 14 | M | 2 | GA | 120 |
*HC is hydroxychloroquine 200 mg twice a day.
MED, minimal erythema dose.
Radiation sources
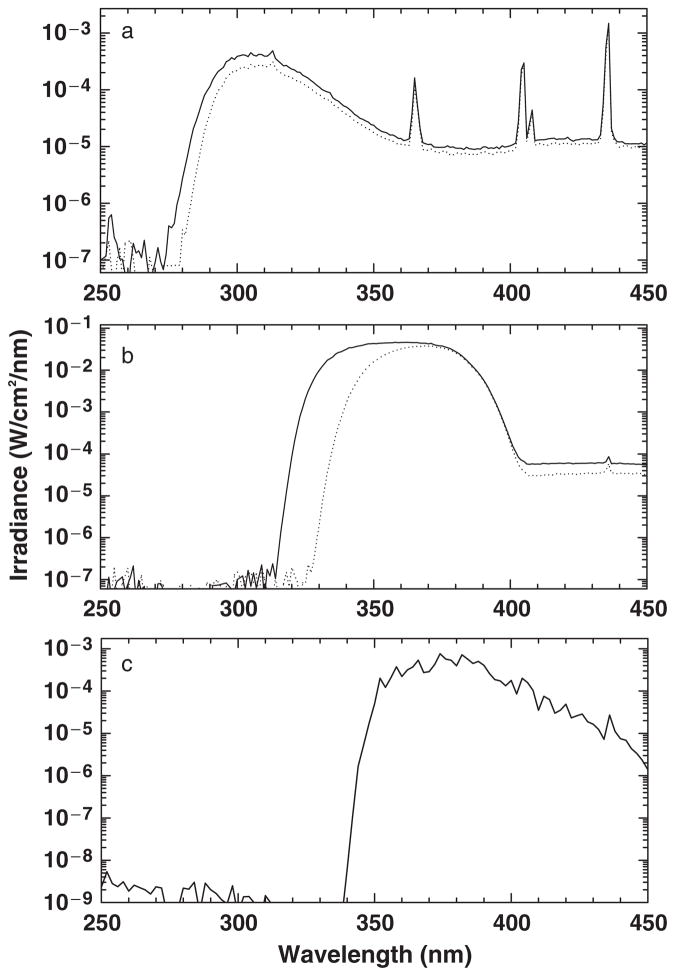
The UVB source was a Westinghouse FS-72/T12 broadband UVB light box (Ultralight, Laurenceville, GA, USA), with the filtered UVB light source measured by spectroradiometric measurement at the time of the experiments showing 0.64% UVC, 40.55% UVB, 17.36% UVA, and 41.47% visible and near infrared (Vis+NIR). A kodacel filter (Eastman Kodac Co., Rochester, NY, USA) was used to block UVC radiation (Fig. 1a). UVB doses were measured with an International Light UV IL-443 UVB meter (Newburyport, MA, USA) after a 15-min warm-up period of the bulbs.
Fig. 1.
(a) Spectrum from Westinghouse FS-72/12 lamps without (solid line) and with (dotted line) a Kodacel filter to block UVC. (b) Spectrum from a xenon arc solar simulator (Solar Light Company, Philadelphia, PA, USA) with a 3mm 335nm Schott filter (solid line) was used to block all wavelengths shorter than UVA, and a 3mm 345 Schott filter (dotted line) was used for lesional spot irradiation. A UG11 filter was used to block infrared and visible wavelengths. (c) Spectrum of Mutzhas lamp.
The UVA source consisted of a Berger xenon arc solar simulator (Solar Light Company, Philadelphia, PA, USA). A 3mm 335nm Schott filter (Schott Glass Technologies Inc., Duryea, PA, USA) was used to block all wavelengths shorter than UVA, and a UG11 filter was used to block the majority of infrared and visible light. A 3mm 345 Schott filter was used for lesional spot irradiation. The solar simulator with a WG335 Schott filter showed 0.000083% UVC, 0.0049% UVB, 99.84% UVA (12.0% UVA2 and 88% UVA1), and 0.15% Vis+NIR (Fig. 1b). The solar simulator with a WG345 Schott filter showed 0.00018% UVC, 0.00021% UVB, and 99.84% UVA (0.003% UVA2 and 99.7% UVA1). The irradiance was measured with an IL 1400A Research Radiometer (International Light, Newburyport, MA, USA) after a 15 min warm-up period of the solar simulator.
The spectrum of each UV source was measured using a scanning double monochromator spectroradiometer (model OL 754, Optronic Laboratories, Orlando, FL, USA) configured with 0.25/1.0/0.25mm slits and a 4-in. diameter integrating sphere with a 6mm entrance aperture, scanning at 1 nm increments from 250 to 450 nm. The spectroradiometer was calibrated by scanning an NIST traceable tungsten–halogen spectral irradiance standard (model 752-10E, Optronic Laboratories) powered by a precision current source (model 65, Optronic Laboratories) at 1nm increments using procedures established by the manufacturer of the instrument. This system also used a small portable dual source calibration module (model 752–150, Optronic Laboratories) to check both the photometric gain, using a small tungsten-halogen source, and the wavelength accuracy, using Hg lines from a small fluorescent source. Before each calibration or measurement, the wavelength calibration and gain are established or verified.
Phototesting procedures
Each patient and control subject was given a panel of eight 1 cm2 spots of UVB radiation in increasing increments of 25% to determine the MED, the smallest amount of UVB radiation needed to produce confluent erythema. MED testing was performed on photoprotected areas of the mid to lower back. Patients were given one MED of UVB radiation and 20 J/cm2 of UVA1 radiation to separate 1 cm2 areas of non-lesional skin on their lower backs for 5 consecutive days. These spots were deliberately placed in areas not receiving daily exposure to the sun. In addition, each patient also received 5 J/cm2 of UVA1 radiation to a pre-existing skin lesion for 5 consecutive days to see whether this would affect resolution of lesions. Lesions were occasionally in photo-distributed areas. Each patient was examined 1, 2, 3, 7, 14, and 21 days after irradiation to determine the presence of photo-induced lesions. The criteria for a positive photoprovocation test were the slow development of a lesion over several days or weeks, a characteristic appearance of these lesions resembling clinical SCLE, and confirmation of clinical diagnosis by histology. Clearance involved the complete resolution of a clinical lesion in the absence of any additional medication.
TNF-α genotyping
DNA was isolated from anticoagulated blood according to standard protocols (Gentra systems, Minneapolis, MN, USA). An allele-specific polymerase chain reaction was used to genotype the TNF-α 308 region in each patient and control, as previously described in detail (28).
Statistics
Comparisons between patients and controls, and within patient subgroups were performed using the unpaired, two-tailed t-test.
Results
MED
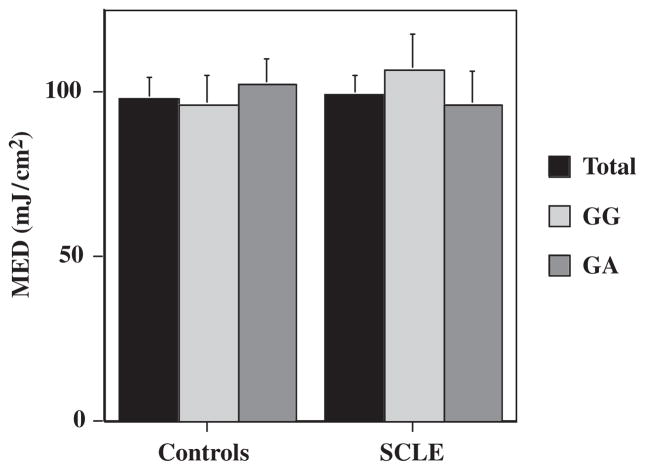
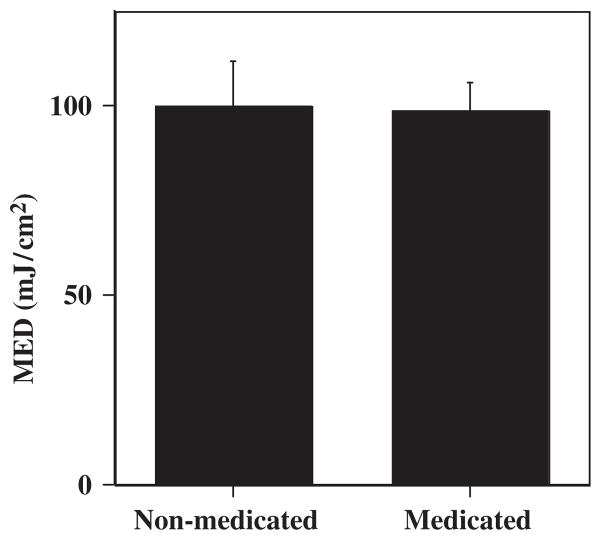
There was no significant difference in the MED of UVB radiation between the −308 TNF promoter polymorphism GA and GG SCLE patients (P=0.5; Table 1, Fig. 2). The mean MED in our SCLE cohort was 98.89 mJ/cm2, not significantly different from the mean MED of the control population: 97.86 mJ/cm2 (P=0.91; Table 1, Fig. 2). There was also no significant difference between the MED of the treated patients, 98.33 mJ/cm2, compared with those not receiving treatment: 100 mJ/cm2 (P=0.93; Fig. 3).
Fig. 2.
Comparison of the minimal erythema dose (MED) of subacute cutaneous lupus erythematosus (SCLE) patients with type II skin with skin type-matched controls, stratified for the −308 TNF-α promoter polymorphism genotype.
Fig. 3.
Comparison of the minimal erythema dose (MED) of treated vs. untreated subacute cutaneous lupus erythematosus patients.
Photoprovocation
Utilizing our phototesting protocol, we did not induce lesions in any of our SCLE patients in response to either UVA or UVB radiation. We saw clearance of a pre-existing lesion in response to 5 J/cm2 of UVA1 radiation in one patient (patient 2, Table 1).
Discussion
Consistent with previously published results, we found no significant difference between the MED of our patient population and our control population (18, 20, 21). Treatment also appeared to have no effect on the MED in our SCLE cohort. Interestingly, we found that the MED in our patient and control populations using a UVB radiation source with a kodacel filter to minimize UVC contamination was similar to those found in previous studies using unfiltered UVB radiation (18, 20–22). Several authors have postulated that the UVC component of the UVB radiation sources commonly used in phototesting studies makes a significant contribution to the erythema response (25, 26, 29). After minimizing UVC, we expected to see slightly higher MED values than those previously reported in SCLE populations. One explanation for this may be our inclusion of subjects with only phenotype I or II skin. MEDs are known to vary with skin type, with progressively increasing amounts of radiation needed to induce erythema in darker skin (30). The phototesting literature does not typically distinguish between skin type in either MED determination or lesional induction (Table 2). Skin type may influence the MED values listed in photoprovocation studies, and may also be one factor in the highly variable photoprovocation rates.
Table 2.
Published photoprovocation studies
| First author | UVB source | UVB spectrum | UVA source | UVA spectrum | UVB dose | UVA dose | % Photo- provocation
|
|
|---|---|---|---|---|---|---|---|---|
| UVB | UVA | |||||||
| Wolska (24) | Westinghouse FS-20 | 290–320 nm | F20T12-BL (Sylvania Light Company) | 320–400 nm | 1–2 MED × 1 day | 3 J/cm × 1 day | 62.50 | 0 |
| van Weelden (22) | Philips TL 20 W/12 | 280–360 nm | UVASUN 3000 | 330–460 nm with filters | 2 × MED (variable based on response) × 6 days | 2 × MED (variable based on response) × 6 days | 87.50 | 75 |
| Lehmann (20) | UV-800 Philips TL 20 W/12 | 285–350 nm | UVASUN 3000 | 330–460 nm | 1.5 MED × 3 days | 100 mJ × 3 days | 27 | 9 |
| Hasan (17) |
|
|
UVASUN 3000 | 340–400 nm | 1–3 MED for 1–3 days | 20–100 J/cm × 3 days | 100 | 86 |
| Walchner (23) | ? | ? | ? | ? | 1.5 MED × 3 days | 100 J/cm2 × 3 days | 35 | 5 |
| Kuhn (18) | UV-800 Lamp with Philipps TL 20 W/12 | 285–350 nm | UVASUN 3000 or Sellas 2000 | 330–460 nm or 310–330 | 1.5 MED × 3 days | 100 J/cm2 × 3 days | 52 | 41 |
| Sanders (21) | Philips TF 20 W/12 | 280–360 nm | UVASUN 3000 | 330–460 nm | 2 × MED × 1 day (variable based on response) × 6 days | 2 × MED × 1 (variable based on response) × 6 days | 100 | 100 |
In contrast to the high rates of photoprovocation generally seen in SCLE patients, we saw no induction of skin lesions in our SCLE cohort in response to either UVA or UVB irradiation (Table 2). This may be secondary in part to our use of standardized physiologic doses of UVA and UVB radiation. A thorough search of the literature reveals that phototesting protocols use differing doses of radiation, often at supra-physiologic levels (Table 2). Some studies utilized variable doses of UVB radiation decided on a perpatient basis in their protocols (17, 21, 22). This practice makes it difficult to infer the total doses of radiation received by each patient and consequently difficult to infer overall UVB-induced photosensitivity. Many groups also administered doses of UVA radiation approaching four to five times the physiologic levels (18, 20, 23). In contrast to the significant photo-induction rates seen in studies using up to 100 J/cm2 of UVA radiation, Wolska et al. (24) saw no photo-induction in response to one application of 3 J/cm2 of UVA radiation, a dose that they had found to provoke erythema in patients with actinic reticuloid and wheal in UVA-sensitive PMLE. We saw no induction of lesions in response to a repeated dose of 20 J/cm2 of UVA radiation, suggesting that while large doses of UVA may be able to induce SCLE-type lesions, physiologic doses of UVA radiation may not be wholly responsible for the photosensitivity of SCLE or that variables like the size of the phototesting area and location are important aspects of standardizing photoirradiation protocols. These other variables may contribute to the varying results reported in different studies. Clearly, the approach to phototesting should be standardized with regard to the radiation sources as well as these other variables.
Previous phototesting studies have used larger spot sizes than the 1 cm2 area used in our study (Table 3) (17, 18, 20–22, 24). The recommended spot size for standardized studies is 5 × 8 cm2 (31, 20, 23). A review of the literature did not yield any controlled studies comparing spot sizes and photoprovocation, or explaining why larger spot size was necessary for photoprovocation. Rather, the use of larger spot size seems based on personal experience (22). Phototesting studies use a variety of spot sizes (Table 3). Wolska et al. (24) documented photo-induction of lesions using spot sizes as small as 2 cm2, suggesting that the 5 × 8 cm2 spot size is not a necessary condition for photoprovocation.
Table 3.
Spot Sizes used in published phototesting studies
| First author | Spot size |
|---|---|
| Wolska (24) | 2 cm2 sites on the lower back |
| van Weelden (22) | 6 × 8 cm2 on extensor forearms; 5 × 8 cm2 on the trunk |
| Lehmann (20) | 5 × 8 cm2 on the back or extensor surfaces of the arms |
| Hasan (17) | 5 × 8 cm2 to 2 × 2 cm2 on upper back, upper arm or forearm |
| Kuhn (18) | 4 × 5 cm2 on upper back or extensor arms |
| Sanders (21) | 6 × 8 cm2 on the extensor surface of the forearms or trunk |
Photosensitivity in LE is thought to be caused by a combination of ultraviolet-induced keratinocyte apoptosis, leading to translocation of intracellular antigens to the cell surface where they are exposed to the immune system, increased production of UV-induced cytokines, such as TNF-α, IL-1, IL-3, and TGF-β, and an increased production of reactive oxygen species (6, 23). More recent models suggest that either increased apoptosis or reduced clearance of ultraviolet-induced apoptotic keratinocytes, in the correct genetic background, leads to uptake of self-antigens and processing by dendritic cells (32). Neither theory of photosensitivity would explain why a large spot size is needed for photoprovocation. The fact that larger spot sizes are required for photo-induction may suggest that there are non-physiologic or external factors contributing to provocation of lesions, including heat generated from a large spot size, or physiologic factors, such as systemic effects of cytokine production that may result from larger spot size irradiation.
In addition to administering physiologic doses of radiation, we also sought to define accurately the spectra of our radiation sources and to minimize contamination of our sources with heat, infrared light, and UVC radiation. Recently, several authors have argued for the routine inclusion of spectral data in every phototesting study (33, 34, 29). Only a few phototesting cutaneous LE studies give the percentage of UV radiation emitted from the bulbs (17, 22). Light sources differ dramatically in the relative amount of wavelengths they produce, and the inclusion of non-physiologic wavelengths of light may greatly affect an individual’s response to this artificial radiation. The minimization of these non-physiologic wavelengths may explain in part the lack of photoinduction seen in our SCLE cohort.
We used a UG5 and UG11 filter to remove much of the heat and infrared radiation from the solar simulator used for UVA irradiation. These longer wavelengths outside of the ultraviolet range can have effects on the skin and may be relevant in induction of skin lesions (21). Most studies utilize the Mutzhas UVASUN 3000 lamp, which includes wavelengths well above the UVA range (Fig. 1c, Table 2).
Kodacel filters were used to minimize UVC radiation in our UVB-testing protocol. These filters have previously been shown to decrease significantly the transmittance of UVC radiation, allowing for less than 0.5% transmission of wavelengths under 290nm (25, 34). While UVC radiation is only 3–11% of the output from unfiltered commonly used fluorescent sunlamps, several authors have suggested that UVC can cause greater than 15% of the erythema response to unfiltered broad-band UVB radiation (25, 34, 26, 29, 35). When transmitted by artificial light sources, UVC wavelengths are absorbed by DNA, RNA, and cellular proteins, and can influence cellular functioning (29). Physiologically, UVC is absorbed in the atmosphere and does not contribute to the photosensitivity of SCLE patients in vivo. None of the commonly cited phototesting studies reviewed in Table 2 utilized filters to block UVC radiation. The lack of photo-induction seen in our SCLE cohort in response to purer UVB radiation may indicate that UVC contributes to the photosensitivity induced by artificial light sources.
Several authors have suggested that low-dose UVA1 radiation (340–400 nm) may also be useful as a treatment modality in cutaneous lupus, leading to decreased disease activity, decreased photosensitivity, and amelioration of pre-existing cutaneous lesions (36, 37). To discern the effects of low-dose UVA1 in our SCLE cohort, we included a treatment arm in our protocol in which each patient was given five daily doses of 5 J/cm2 of UVA1 radiation to a pre-existing lesion. Clearance of the lesion was observed in one patient 1 week after cessation of the treatments. While our study duration was short and our sample size was small, UVA1 as a treatment modality deserves exploration in future phototesting studies.
In summary, the MED was no different in SCLE patients vs. controls, and there was no difference in MED within the SCLE group when controlled for active treatment or TNF promoter polymorphism genotype. Using repetitive doses of one MED of UVB or 20 J/cm2 of UVA to 1 cm2 areas on the lower back in SCLE patients, no lesions were induced. In one patient, a lesion cleared with spot UVA1 treatment. The variability of phototesting results in various published studies suggests a need to evaluate the role of the many factors that vary between these studies, including spot size, location, and radiation sources.
Acknowledgments
This work was presented at the Society of Investigative Dermatology in May 2004, and is published in abstract form in the Journal of Investigative Dermatology 122:A48, 2004. This work is supported in part by grants from the NIH K24-AR 02207 and VA Merit Review. This work is supported in part by grants from the Lupus Research Institute, a V.A. Merit Review Grant, and the National Institutes of Health (1K24AR002207).
References
- 1.Amit M, Molad Y, Kiss S, Wysenbeek AJ. Seasonal variations in manifestations and activity of systemic lupus erythematosus. Br J Rheumatol. 1997;36:449–452. doi: 10.1093/rheumatology/36.4.449. [DOI] [PubMed] [Google Scholar]
- 2.Cripps DJ, Rankin J. Action spectra of lupus erythematosus and experimental immunofluorescence. Arch Dermatol. 1973;107:563–567. [PubMed] [Google Scholar]
- 3.Epstein JH, Tuffanelli D, Dubois EL. Light sensitivity and lupus erythematosus. Arch Dermatol. 1965;91:483–485. doi: 10.1001/archderm.1965.01600110069013. [DOI] [PubMed] [Google Scholar]
- 4.Haga HJ, Brun JG, Rekvig OP, Wetterberg L. Seasonal variations in activity of systemic lupus erythematosus in a subarctic region. Lupus. 1999;8:269–273. doi: 10.1191/096120399678847858. [DOI] [PubMed] [Google Scholar]
- 5.Wysenbeek AJ, Block DA, Fries JF. Prevalence and expression of photosensitivity in systemic lupus erythematosus. Ann Rheum Dis. 1989;48:461–463. doi: 10.1136/ard.48.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee LA, Farris AD. Photosensitivity diseases: cutaneous lupus erythematosus. J Investig Dermatol Symp Proc. 1999;4:73–78. doi: 10.1038/sj.jidsp.5640186. [DOI] [PubMed] [Google Scholar]
- 7.Parodi A, Caproni M, Cardinali C, et al. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. A review by the Italian group of immunodermatology. Dermatology. 2004;200:6–10. doi: 10.1159/000018307. [DOI] [PubMed] [Google Scholar]
- 8.Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115:1409–1415. [PubMed] [Google Scholar]
- 9.Millard TP, Kondeatis E, Cox A, et al. A candidate gene analysis of three related photosensitivity disorders: cutaneous lupus erythematosus, polymorphic light eruption and actinic prurigo. Br J Dermatol. 2001;145:229–236. doi: 10.1046/j.1365-2133.2001.04339.x. [DOI] [PubMed] [Google Scholar]
- 10.Werth VP, Bashir M, Zhang W. Photosensitivity in rheumatic diseases. J Investig Dermatol Symp Proc. 2004;9:54–63. doi: 10.1111/j.1087-0024.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 11.Werth VP, Zhang W, Dortzbach K, Sullivan K. Association of a promoter polymorphism of TNFalpha with subacute cutaneous lupus erythematosus and distinct photoregulation of transcription. J Invest Dermatol. 2000;115:726–730. doi: 10.1046/j.1523-1747.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 12.Lacour JP. Lupus and sun. Revue de Medecine Interne. 1996;17:196–199. doi: 10.1016/0248-8663(96)81245-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee LA, Roberts CM, Frank MB, McCubbin VR, Reichlin M. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262–1268. [PubMed] [Google Scholar]
- 14.Racila DM, Sontheimer CJ, Sheffield A, et al. Homozygous single nucleotide polymorphism of the complement C1QA gene is associated with decreased levels of C1q in patients with subacute cutaneous lupus erythematosus. Lupus. 2003;12:124– 132. doi: 10.1191/0961203303lu329oa. [DOI] [PubMed] [Google Scholar]
- 15.Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664–671. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- 16.Watson RM, Talwar P, Alexander E, Bias WB, Provost TT. Subacute cutaneous lupus erythematosus-immunogenetic associations. J Autoimmun. 1991;4:73–85. doi: 10.1016/0896-8411(91)90008-z. [DOI] [PubMed] [Google Scholar]
- 17.Hasan T, Nyberg F, Stephansson E, et al. Photosensitivity in lupus erythematosus, UV photoprovocation results compared with history of photosensitivity and clinical findings. Br J Dermatol. 1997;136:699–705. [PubMed] [Google Scholar]
- 18.Kuhn A, Sonntag M, Richter-Hintz D, et al. Phototesting in lupus erythematosus: a 15-year experience. J Am Acad Dermatol. 2001;45:86–95. doi: 10.1067/mjd.2001.114589. [DOI] [PubMed] [Google Scholar]
- 19.Leenutaphong V, Boonchai W. Phototesting in oriental patients with lupus erythematosus. Photodermatol Photoimmunol Photomed. 1999;15:7–12. doi: 10.1111/j.1600-0781.1999.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann P, Holzle E, Kind P, Goerz G, Plewig G. Experimental reproduction of skin lesions in lupus erythematosus by UVA and UVB radiation. J Am Acad Dermatol. 1990;22:181–187. doi: 10.1016/0190-9622(90)70020-i. [DOI] [PubMed] [Google Scholar]
- 21.Sanders CJG, van Weelden H, Kazzaz GAA, et al. Photosensitivity in patients with lupus erythematosus: a clinical and photobiological study of 100 patients using a prolonged phototest protocol. Br J Dermatol. 2003;149:131–137. doi: 10.1046/j.1365-2133.2003.05379.x. [DOI] [PubMed] [Google Scholar]
- 22.van Weelden H, Velthuis PJ, Baart de la Faille H. Light-induced skin lesions in lupus erythematosus: photobiological studies. Arch Dermatol Res. 1989;281:470–474. doi: 10.1007/BF00510082. [DOI] [PubMed] [Google Scholar]
- 23.Walchner M, Messer G, Kind P. Phototesting and photoprotection in LE. Lupus. 1997;6:167–174. doi: 10.1177/096120339700600212. [DOI] [PubMed] [Google Scholar]
- 24.Wolska H, Blaszczyk M, Jablonska S. Phototests in patients with various forms of lupus erythematosus. Int J Dermatol. 1989;28:98–103. doi: 10.1111/j.1365-4362.1989.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown DB, Peritz AE, Mitchell DL, et al. Common fluorescent sunlamps are an inappropriate substitute for sunlight. Photochem Photobiol. 2000;72:340–344. [PubMed] [Google Scholar]
- 26.Learn DB, Beard J, Moloney SJ. The ultraviolet C energy emitted from FS lamps contributes significantly to the induction of human erythema and murine ear edema. Photodermatol Photoimmunol Photomed. 1993;9:147–153. [PubMed] [Google Scholar]
- 27.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:3146–3154. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan KE, Wooten C, Schmeckpeper BJ, Goldman D, Petri MA. A promoter polymorphism of tumor necrosis factor alpha associated with systemic lupus erythematosus in African-Americans. Arthr Rheum. 1997;40:2207–2211. doi: 10.1002/art.1780401215. [DOI] [PubMed] [Google Scholar]
- 29.Learn DB, Beasley DG, Giddens LD, et al. Minimum doses of ultraviolet radiation required to induce murine edema and immunosuppression are different and depend on the ultraviolet emission spectrum of the source. Photochem Photobiol. 1995;62:1066–1075. doi: 10.1111/j.1751-1097.1995.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 30.Baron ED. Correlating skin type and minimum erythema dose. Arch Dermatol. 1999;135:1278–1279. doi: 10.1001/archderm.135.10.1278. [DOI] [PubMed] [Google Scholar]
- 31.Kind P, Lehmann P, Plewig G. Phototesting in lupus erythematosus. J Invest Dermatol. 1993;100:53S–57S. doi: 10.1111/1523-1747.ep12355594. [DOI] [PubMed] [Google Scholar]
- 32.Orteu CH, Sontheimer RD, Dutz JP. The pathophysiology of photosensitivity in lupus erythematosus. Photodermatol Photoimmunol Photomed. 2001;17:95–113. doi: 10.1034/j.1600-0781.2001.170301.x. [DOI] [PubMed] [Google Scholar]
- 33.Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002;28:4–13. doi: 10.1016/s1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- 34.Gasparro FP, Brown DB. Photobiology 102: UV sources and disemetry-the proper use and measurement of “photons as a reagent”. J Invest Dermatol. 2000;114:613–615. doi: 10.1046/j.1523-1747.2000.00940.x. [DOI] [PubMed] [Google Scholar]
- 35.McKinlay A, Diffey B. A reference spectrum for ultraviolet-induced erythema in human skin. CIE. 1987;6:17–22. [Google Scholar]
- 36.McGrath H., Jr Ultraviolet-A1 irradiation decreases clinical disease activity and autoantibodies in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 1994;12:129–135. [PubMed] [Google Scholar]
- 37.McGrath H., Jr Ultraviolet A1 (340–400 nm) irradiation and systemic lupus erythematosus. J Investig Dermatol Symp Proc. 1999;4:79–84. doi: 10.1038/sj.jidsp.5640187. [DOI] [PubMed] [Google Scholar]