Abstract
Influenza is a serious respiratory disease responsible for significant morbidity and mortality due to both annual epidemics and pandemics; its treatment involves the use of neuraminidase inhibitors. (−)-Oseltamivir phosphate (Tamiflu) approved in 1999, is one of the most potent oral anti-influenza neuraminidase inhibitors. Consequently, more than 70 Tamiflu synthetic procedures have been developed to date. Herein, we highlight the evolution of Tamiflu synthesis since its discovery over 20 years ago in the quest for a truly efficient, safe, cost-effective and environmentally benign synthetic procedure. We have selected a few representative routes to give a clear account of the past, present and the future with the advent of enabling technologies.
Keywords: Enabling technologies, Evolution, Influenza, Tamiflu synthesis
Graphical abstract
1. Introduction
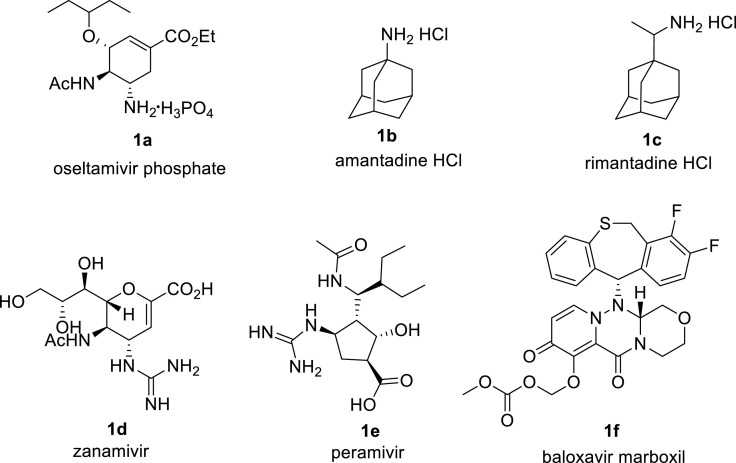
Tamiflu, also known as oseltamivir phosphate 1 is a potent chemotherapeutic agent for influenza treatment [[1], [2], [3]] Influenza is a severe viral infection of the respiratory system regarded as the most serious respiratory disease, which is responsible for significant morbidity and mortality due to both annual epidemics and predictable pandemics [[1], [2], [3]] More specifically, the avian influenza H5N1 has a mortality rate of about 60% [4] Unfortunately, little has been done to change the influenza infection patterns in past decades despite influenza being the most studied viral infection before the arrival of the human immunodeficiency virus (HIV) [5,6] Now in this global society, a highly aggressive strains of the influenza virus such as the H5N1 can mutate to become easily transmitted from human to human and spark another deadly pandemic just as with current Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection (COVID19) pandemic, [7,8] which has brought the world to its knees. The surge in drug-resistant influenza strains resulting from naturally occurring mutations reminds us of the need for continued research to discover more potent neuraminidase inhibitors [[9], [10], [11], [12], [13]] The current anti-influenza drugs are useful templates in the development of new neuraminidase inhibitors through structure-activity relationship studies. As the research towards new and better treatment remains a top priority, it is equally important to improve the availability of the current anti-influenza drugs by developing better synthetic procedures to guard the world against influenza. Drugs such as oseltamivir phosphate (Tamiflu) 1a, amantadine HCl 1b, rimantadine HCl 1c, zanamivir 1d and peramivir 1e have been developed for the treatment of influenza over the years [[1], [2], [3]] and more recently, baloxavir marboxil 1f was developed (Fig. 1 ) [14] The last four are FDA approved and are currently the recommended influenza drugs by Centres for Disease Control and Prevention (CDC) [15] Of all the drugs, Tamiflu is the most commonly used since its FDA approval in 1999 making its synthesis an important research area (see Fig. 2).
Fig. 1.
Commercially available drugs for the treatment of influenza.
Fig. 2.
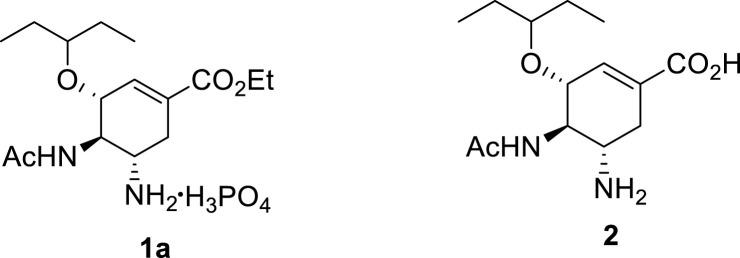
Structures of oseltamivir carboxylate 2.
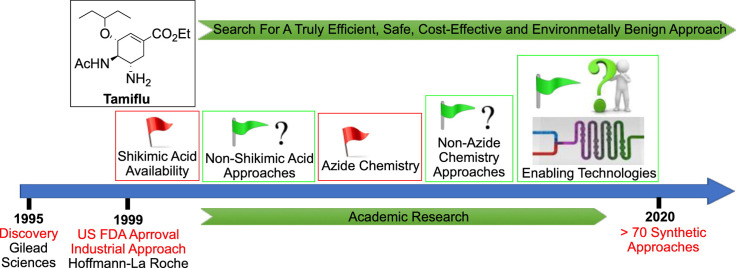
Tamiflu was discovered by Gilead Sciences in 1995, patented in 1996, co-developed with F. Hoffmann-La Roche Ltd and marketed by F. Hoffmann-La Roche and commercially launched in November 1999 [[16], [17], [18], [19], [20], [21]] In the early years of its discovery, (−)-shikimic acid was used as starting material for the synthesis of Tamiflu and furthermore, the current and only industrial synthetic route still uses (−)-shikimic acid. In response to an increasing threat of an influenza pandemic, diverse synthetic approaches have been developed and very insightful reviews of their relative merits have been published [[1], [2], [3],[22], [23], [24], [25], [26], [27]] However, there were legitimate (−)-shikimic acid availability concerns in the early years of the development of this drug. Shikimic acid, which is a natural product isolated from a plant of Chinese star anise was unavailable in consistent purity and enough quantity, which prompted extensive studies into (−)-shikimic acid free synthetic routes in both industry and academia. Fortunately, this has been addressed by the development of more efficient extraction and purification processes or alternatively by fermentation using a genetically engineered E.coli bacteria [25,[28], [29], [30]] Furthermore, Yoshida and Ogasawara demonstrated an enantioconvergent synthesis of shikimic acid via a palladium mediated elimination reaction [31] Although more studies utilising alternative starting materials are still ongoing, they have not been as efficient as the current shikimic acid based production route. The use of the potentially hazardous azide chemistry for the introduction of amino and acetomido groups to the ring was, and is still, a major concern [1,2,32] Azide chemistry poses many safety concerns because of its hazardous and highly exothermic nature, which become more pronounced at a large scale [2,[33], [34], [35], [36], [37]] As a result, numerous studies towards azide-free synthetic routes were done, which unfortunately have not been as good as the current production route. Herein, this review highlights the evolution towards efficient and safe synthetic routes of Tamiflu since its first approval 20 years ago. Since there has been over 70 published synthetic routes and some review articles, [[1], [2], [3],[22], [23], [24], [25], [26]] a few selected representative routes will be used to give a clear account of the past, present and the future with the arrival of enabling technologies [38] such as flow chemistry.
2. Discovery and synthesis by Gilead sciences
Oseltamivir carboxylate 2 was the first molecule identified by Gilead scientists for development, but the ethyl ester prodrug oseltamivir phosphate (Tamiflu) 1a was ultimately chosen as the clinical candidate based on its potent in vitro and in vivo activities and its good oral bioavailability after extensive diversity-oriented discovery chemistry studies by Kim et al. [[16], [17], [18], [19], [20], [21]].
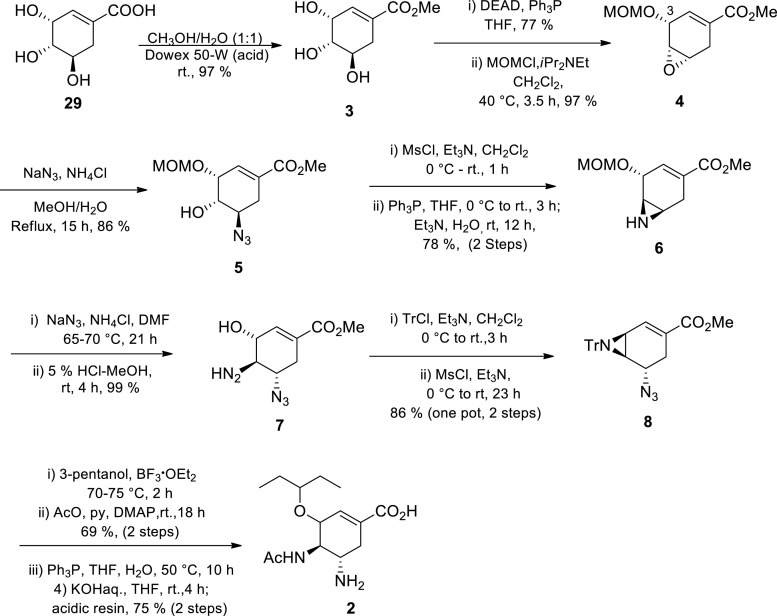
Gilead Sciences researchers first synthesised the oseltamivir carboxylate 2 from a natural product, (−)-shikimic acid 29, as the starting material (Scheme 1 ) [20] (−)-Shikimic acid derivative 3 was treated under Mitsunobu conditions resulting in selective activation of the least selectively hindered OH-group at C-5 whilst the C-3 OH is MOM protected, affording epoxide 4 [20] Epoxide 4 was subsequently opened regio-and stereospecifically using azide chemistry, selective azidating the C-5 to afford azido alcohol 5. Mesylation of 5, followed by azide reduction afforded aziridine 6. Once more, azide chemistry was utilised in regioselective aziridine-opening at C-5 followed by MOM group cleavage affording amino alcohol 7 [20] Aziridine 8 was synthesised from 7 by a two-step, one-pot process: (1) protection of the amino functionality with a trityl group, and (2) mesylation of the hydroxyl group. Regio-selective ring-opening of aziridine 8 with 3-pentanol in the presence of Lewis acid catalyst BF3 ·OEt2 subsequently followed by acetylation of the resulting amine afforded the corresponding amido ether. The azide group on the resulting amido ether was reduced, followed by hydrolysis of the methyl ester under basic conditions affording oseltamivir carboxylate 2 in 15% overall yield over the 14 steps despite using protecting group chemistry [20] The choice of their starting material (−)-shikimic acid was justified; it has the carbocyclic system with chirality which is also present in the target compound 2 or which can be used to handle the introduction of the desired stereochemistry. However, at that time, (−)-shikimic acid availability was one of the major drawbacks since effective extraction and purification methods had not been developed. The use of potentially explosive azide-containing intermediates is another drawback associated with this synthetic route, which restricted the synthesis to milligram scale.
Scheme 1.
Gilead Sciences’ synthetic route of the first candidate 2 for development [20].
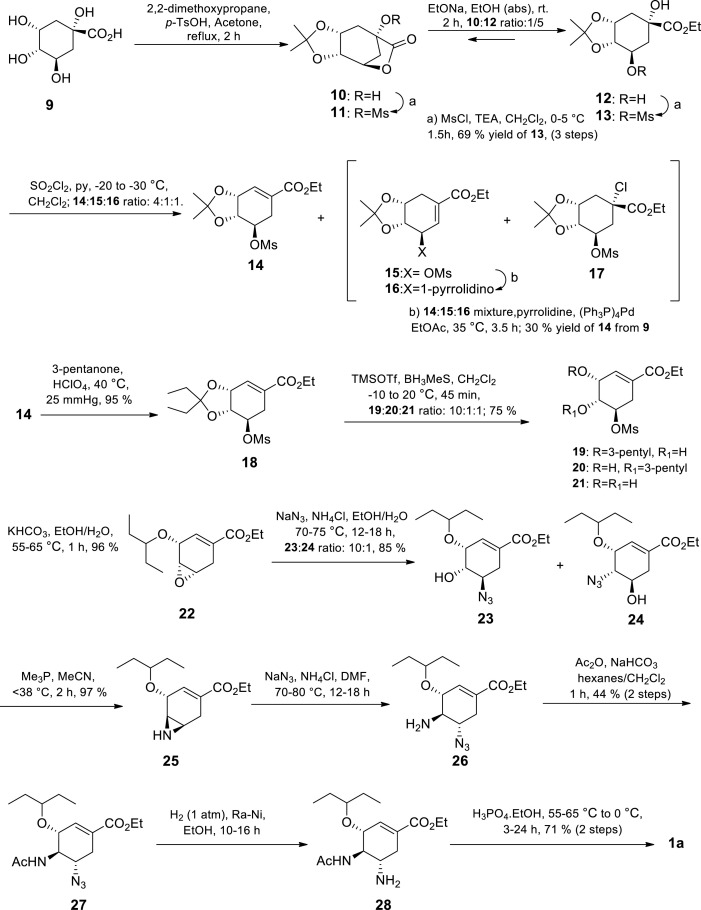
Due to scarcity of (−)-shikimic acid in large quantities at the time, [17,39] Gilead scientists went on to prepare Tamiflu 1a at multi-gram scale from more available (−)-quinic acid 9 (Scheme 2 ) [39] The first large scale route by Gilead sciences from (−)-quinic acid consisted of 12 steps and afforded an overall yield of 4.4% [39] Despite the relative low yield, it was successfully implemented in a standard pilot plant producing kilogram quantities of Tamiflu 1a and the potentially hazardous azide chemistry was safely handled. Furthermore, minimal protecting group manipulations were employed and no chromatography was required for isolation.
Scheme 2.
First large scale synthesis of Tamiflu 1a by Gilead Sciences [39].
3. Roche industrial approach
There has been extensive research by Gilead Sciences, [[16], [17], [18], [19], [20],39] Roche [30,[40], [41], [42], [43], [44], [45], [46]] and other numerous scientific laboratories to develop an efficient, safe large-scale route towards Tamiflu since 1995 [[1], [2], [3],[22], [23], [24], [25], [26]] The efforts led to the 12-steps Roche industrial route starting from (−)-shikimic acid affording an overall yield of ∼35% (Scheme 3 ) [46] after (−)-shikimic acid availability had been improved by the development of more efficient extraction and purification processes or alternatively by fermentation using a genetically engineered E.coli bacteria [25,28,29].
Scheme 3.
Roche’s industrial synthesis [46].
As with Gilead Sciences approaches (Scheme 1, Scheme 2), [20,39] the Roche industrial route utilised azide chemistry in structuring the 1,2-diamine moiety in 28 [46] Contrary to the Gilead Sciences’ routes, where the pentyloxy group is introduced in the latest stage (Scheme 1, Scheme 2), [20,39] Roche introduced the 3-pentyloxy moiety early at C-3 by regio-selective reduction of acetal 18 [46] However as with the Gilead routes (Scheme 1, Scheme 2), the Roche approach has drawbacks that include the challenge to safely handle the thermally unstable azide reagents and intermediates on large scale. Another drawback was associated with the utilisation of shikimic acid which was scarce at that time. These drawbacks prompted the scientific community to extensively develop numerous alternative routes.
4. Alternative synthetic approaches
Although the Roche industrial route is currently supplying the world with enough tonnes of Tamiflu, the route raised three concerns as aforementioned: a) the use of shikimic acid, which had limited availability in the early days of development b) the use of potentially explosive azide chemistry and c) long synthetic route with low overall yield. Consequently, this prompted the development of numerous alternative synthetic approaches to address the concerns [[1], [2], [3],[22], [23], [24], [25], [26],47] These approaches can generally be categorised into two main classes: shikimic acid-dependent and shikimic acid-independent approaches in which both the azide-dependent and azide-independent approaches can be subclasses. Herein, a few selected and representative alternative synthetic strategies that can potentially be scaled-up after minimal modifications are highlighted in each class.
4.1. Shikimic acid dependent approaches
After (−)-shikimic acid availability improvement with time, researchers embarked on the development of shorter and higher yielding synthetic routes. These routes can be classified into two groups: namely azide chemistry dependent and azide-free routes.
4.1.1. Azide-dependent routes
Azide-dependent routes utilize potentially hazardous azide chemistry to introduce two amino groups on the cyclohexene ring system. In 2009, Karpf and Trussardi at Hoffman-La Roche reported an efficient nine-step synthetic route towards Tamiflu 1a from (−)-shikimic acid 29 (Scheme 4 ) [30,44].
Scheme 4.
Eight-step synthesis of Tamiflu by Karpf and Trussardi at Hoffmann-La Roche [30].
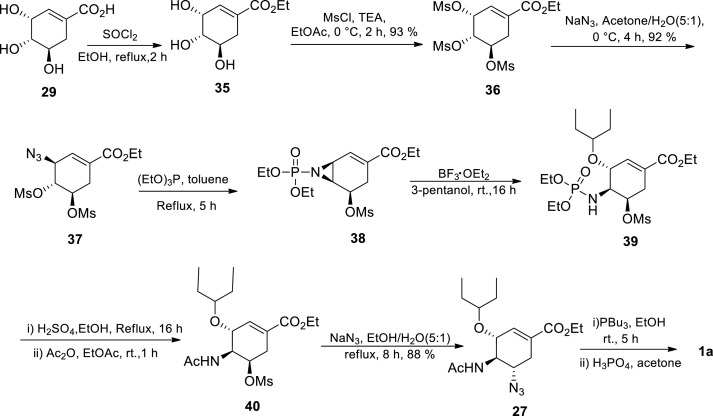
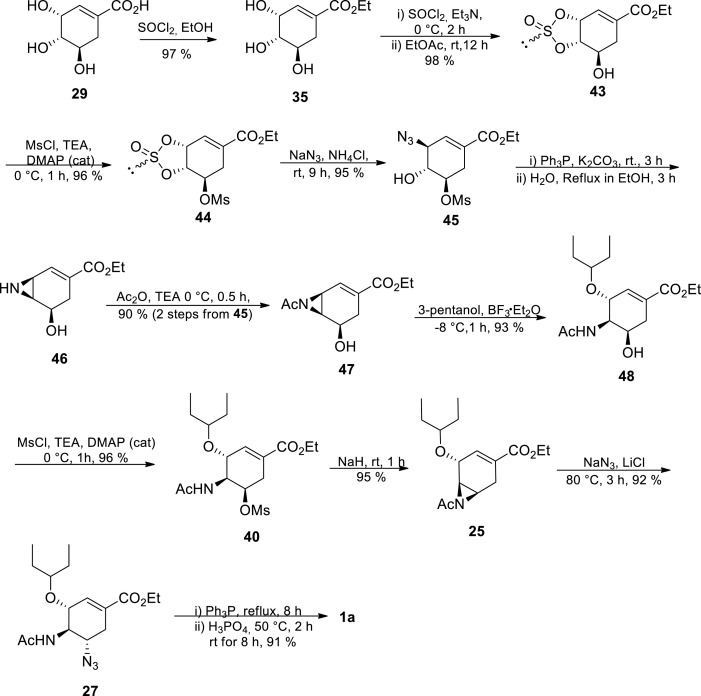
The authors started with (−)-shikimic acid 29 esterification to afford ethyl shikimate 35 according to a reported procedure [46] The synthesis proceeded via the O-trimesylate 36 followed by regio- and stereoselective nucleophilic substitution of the allylic O-mesylate at C-3 by an azide group resulting in azide 37. In the presence of triethyl phosphite (P(EtO)3) and under reflux, aziridine 38 was formed from the azide 37. This was followed by regio- and stereoselective aziridine ring opening at the allylic position with 3-pentanol in the presence of a Lewis acid catalyst providing 39. N–P bond cleavage in 39 followed by N-acetylation afforded mesylate 40, which went on to react with sodium azide to afford 27. The azide group was reduced to the amine group followed by the addition of phosphoric acid (H3PO4) affording the drug Tamiflu 1a. Protecting group manipulations, chromatographic separations and tedious purifications were not required in this route. It proceeded with an unoptimised overall yield of 20%, utilising cheap and commercially available chemicals [30] Although the route could neither avoid azide chemistry nor improve the overall yield, it presents a potentially scalable, elegant and shorter synthetic route. In addition to Karpf and Trussardi [30] procedure, Trussardi [44] disclosed a similar process in which compound 39 could alternatively be azidated first before acetylation.
In 2013, Kalashnikov et al. [28] reported almost a similar synthetic route to that of Karpf and Trussardi [30] The route afforded an optimised overall yield of 27%. Although this 10-step procedure utilises azide chemistry and is accompanied by lower overall yield, it uses minimum amount of expensive reagents making it attractive.
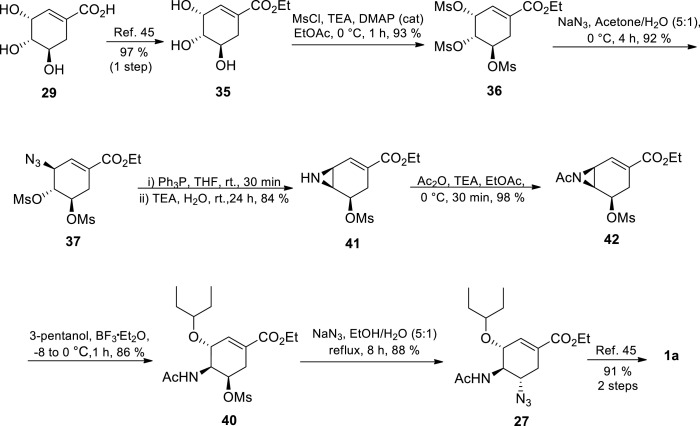
Utilising the experience accumulated from their first 13 steps approach (44% overall yield) from (−)-shikimic acid, [48] Shi’s group developed an optimised 8 steps route (Scheme 5 ) [49] The route is almost similar to Karpf’s approach, [30] but mainly differs on the Staudinger reaction conditions in the aziridination step. Shi and coworkers [49] uses triphenylphosphine (PPh3) in the presence of triethyl amine and large excess of water to afford aziridine 41, whereas Karpf utilises triethyl phosphite under anhydrous conditions to afford aziridine 38 (Scheme 4, Scheme 5) [30,49] The use of triphenyl phosphate involves tedious purification due to the triphenylphosphine oxide by-product. N-Acetyl aziridine 42 was formed after a simple acetylation of aziridine 41. Stereoselective ring opening of N-acetyl aziridine 42 was excellently done in the presence of boron trifluoride etherate in 3-pentanol to yield 86% of acetamide 40. The (S)-configuration of C-1 in compound 42 was inverted to the (R)-configuration of C-3 in acetamide 44 according to the Walden-type inversion. The allylic C-1 position of compound 42 is more reactive than the C-6 position of compound 42, thus resulting in a regioselective ring opening reaction. This was followed by a nucleophilic replacement of OMs at C-5 with the N3 group and it was accomplished by treating acetamide 40 with NaN3 affording azide 27 in 88% yield, and in the process reversing the (R)-configuration of C-5 to the (S)-configuration. The azide 27 was finally transformed to Tamiflu 1a according to reported procedure [46,49].
Scheme 5.
Optimised synthesis of Tamiflu 1a from shikimic acid 29 by Shi’s group [49].
A short and practical approach towards Tamiflu 1a with an impressive 47% overall yield was developed by Shi et al. [49] The overall yield improved slightly (35–47%) and the number of transformations were considerably reduced relative to the Roche industrial approach (12 steps to 8 steps) [46,49] This approach represents a model of atom economy since no protecting group manipulations were needed. Unfortunately, the researchers resorted to the potentially hazardous azide chemistry on two occasions after other safer nitrogen-containing nucleophiles such as ammonia, benzylamine and allylamine failed to afford the desired products [49] This drawback is however more than compensated by the elegance, simplicity and efficiency of the approach. This synthetic route is evidently a major player in the goal to develop an efficient scalable process towards oseltamivir phosphate synthesis. However, there is need to find ways of dealing with the potentially hazardous steps involved to guarantee a truly scalable and safe process, which is applicable in industry.
Building on their earlier work in which they synthesised Tamiflu 1a from shikimic acid via cyclic sulfite intermediates, [50] Shi and coworkers reported an improved 11-step route starting from shikimic acid via a 3,4-cyclic sulfite intermediate 43 affording Tamiflu in 55% overall yield (Scheme 6 ), [51] which is significantly better than the industrial route (35% overall yield). All the transformations were clean with each step affording yields greater than 90%, meaning that subsequent steps could be performed without purification of crude products. This approach generally shares some similarities with other shikimic acid dependent routes such as the use of azide chemistry and can be scaled-up easily.
Scheme 6.
Shi’s 11-step Tamiflu 1a synthetic route via a 3,4-cyclic sulfite intermediate 43 [51].
Generally, the shikimic acid-azide chemistry dependent procedures proceeded in good yields. They can potentially be performed at large scale if the azide chemistry safety is guaranteed, as with the current industrial route. This can be achieved by performing holistic process calorimetric studies before scale-up, use of highly skilled personals and working under very strict conditions. This is usually not easy to achieve. However, the use of either enabling technologies such as continuous flow technology that are known to enhance process safety or alternative safe chemistry to introduce the two amino groups on the cyclohexene ring system can be considered.
Most recently, Watts group reported a 8-step total flow synthesis of Tamiflu starting from ethyl shikimate 35 derived from shikimic acid (Scheme 7 ) [52,53] Taking lessons from the previously reported shikimic acid-based routes, [28,30,48,49,51,54] the authors aimed to ensure azide chemistry safety, processing time reduction and process overall yield improvement by taking advantage of continuous flow chemistry technology. Flow chemistry technology is an enabling technology, which has attracted considerable attention in synthetic chemistry and pharmaceutical industry owing its efficiency, easy scale-up, safety and reproducibility; industry is now using the technology up to 2000 tonnes per annum [[55], [56], [57], [58], [59]] This has seen numerous approaches for pharmaceutical drugs being redesigned into continuous flow synthesis [56,58,[60], [61], [62], [63], [64], [65]] The technology allows for in situ generation and consumption of dangerous intermediates, preventing their accumulation thus enhancing process safety [55,[66], [67], [68]] Additionally, microreactors can handle exotherms extremely well, due to the inherent high surface area to volume ratio and rapid heat dissipation unlike the conventional batch process [55,69].
Scheme 7.
Continuous flow synthesis towards Tamiflu 1a by Sagandira and Watts.
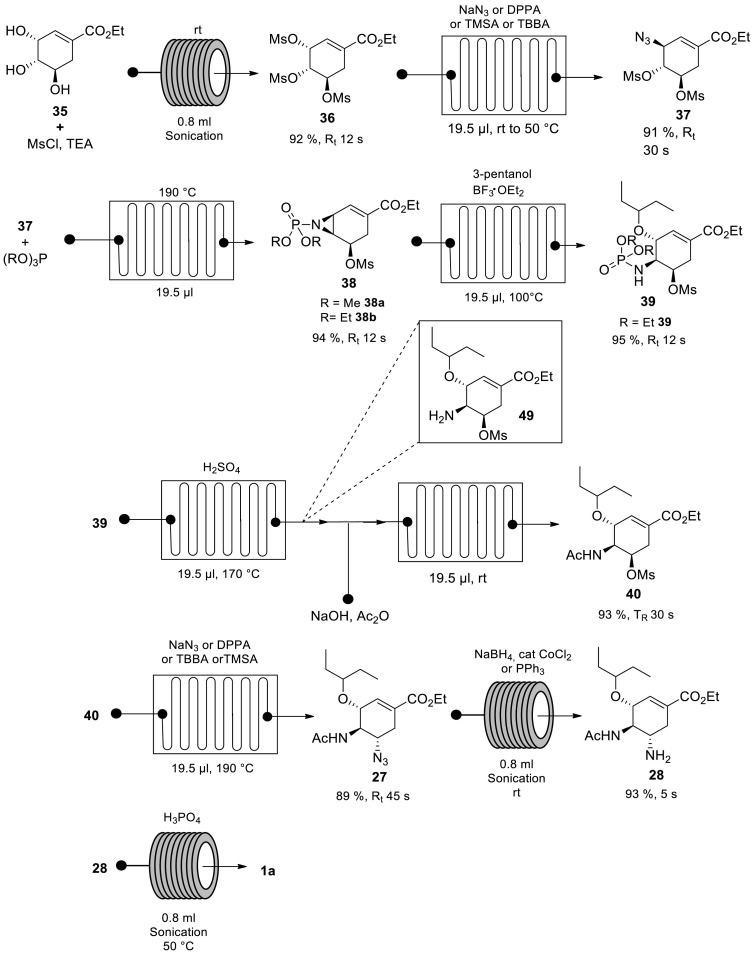
The authors started by treating ethyl shikimate 35 with MsCl in the presence of TEA at room temperature under sonication to afford mesyl shikimate 36 (Scheme 7) [53] Subsequent treatment of mesyl shikimate 36 with NaN3 afforded azide 37. Other azidating agents such as DPPA, TMSA and TBBA gave comparable results although they are accompanied by poor atom efficiency [53,68] The treatment of azide 37 with either (MeO)3P or (EtO)3P at 190 °C afforded the desired aziridines 38a and 38b respectively. Aziridine 38b ring opening was accomplished in the presence of 3-pentanol and BF3·Et2O at 100 °C to afford 3-pentyl ether 39. Acetamide 40 was subsequently afforded via a tandem of reactions; N–P bond cleavage of 3-pentyl ether 39 using H2SO4 at 170 °C forming intermediate 49 in situ, subsequently followed by acetylation with Ac2O at room temperature to afford acetamide 40. Acetamide 40 was then treated with NaN3 to afford azide 27 [53,68] Subsequent azide 27 reduction at room temperature under sonication using NaBH4 in the presence of catalytic CoCl2 afforded oseltamivir 28. In the final step, Tamiflu 1a was afforded by treating oseltamivir 28 with H3PO4 at 50 °C under sonication.
The authors demonstrated an efficient synthetic route for Tamiflu 1a with 58% overall yield and 3.5 min total residence time starting from ethyl shikimate 35. This process elegantly handled the hazardous azide chemistry involved in this procedure by taking advantage of flow chemistry technology. The overall yield of the process is literature comparable, however, processing time is significantly shorter than all the reported procedures, which are mostly greater than 30 h [1,2] Without doubt, this presents a safe, efficient and scalable procedure for the synthesis of the drug.
4.1.2. Shikimic acid dependent azide-free routes
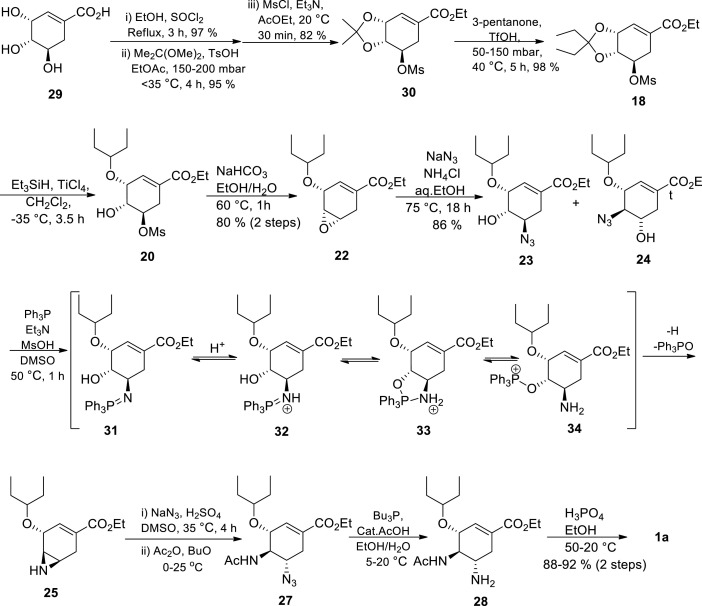
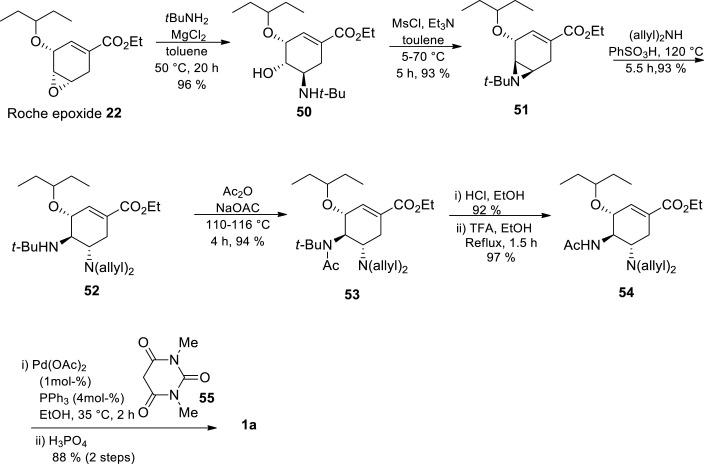
Due to azide safety concerns, azide-free routes were developed to introduce the two amino groups on the cyclohexene ring system [39,70,71] The goal was to identify a non-azide nucleophile that is compatible with the rest of the functional groups on the molecule and with the strong tendency of the cyclohexene intermediates towards aromatization. In 2000, Karpf and Trussardi [41] at Roche reported an azide free transformation of Roche epoxide 22 to Tamiflu 1a in 35% overall yield over 6 steps at a multi-gram scale without the use of chromatography purification. The authors used allyl amine as the nitrogen nucleophile instead of azide chemistry. Hughes and coworkers developed a 9-step azide free route starting from shikimic acid via the so called Roche epoxide 22 (Scheme 8 ) [45] The authors used tBuNH2 as the non-azide nitrogen nucleophile. The epoxide 22 opening was regio-selective and it was treated with tBuNH2-MgCl2 complex affording amino alcohol 50 in 96% yield. The presence of a bulky tert-butyl group on the nitrogen atom enabled selective mesylation at the oxygen atom resulting in aziridine 51 formation. Aziridine 51 opening was done using diallylamine in the presence of PhSO3H and then the secondary amine 52 was acetylated to give 54. There was only one purification in the sequence between epoxide 22 to Tamiflu 1a where 54 was purified by precipitation of the corresponding HCl salt. Finally, acidic conditions were used in cleavage of the N-tert-butyl group and deallylation through Pd-catalyzed allyl transfer to 1,3-dimethylbarbituric acid, followed by phosphate salt formation to afford Tamiflu 1a. The overall yield for this azide-free approach was 35–38%, which is comparable to the industrial route [45,46] This approach represented the first example that avoids the utilisation of potentially hazardous azide chemistry, the convectional way of introducing nitrogen functionality on the ring.
Scheme 8.
Hughes and coworkers azide free synthesis of Tamiflu 1a [45].
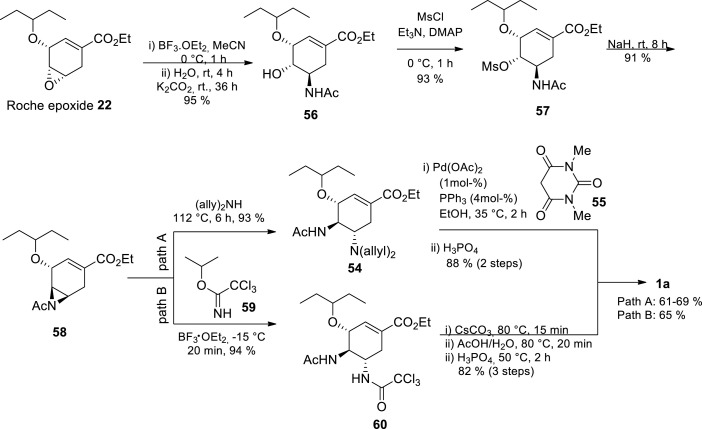
In 2013, Shi and coworkers reported a high yielding novel azide-free asymmetric synthesis of Tamiflu starting from the Roche’s epoxide 22 affording Tamiflu 1a in 61–69% overall yield (Scheme 9 ) [54] Compared with Roche azide-free syntheses, this route from Roche’s epoxide 22 has been shortened from 9 to 6 steps accompanied by a yield increase from 35–38% to 61–69% [45,54] Furthermore, the process is high yielding and shorter compared to the current industrial approach (35%, 12 steps). This presents a truly efficient and safe approach which can potentially be performed at large scale.
Scheme 9.
Azide-free asymmetric synthesis of Tamiflu starting from the Roche’s epoxide 22 by Shi and coworkers [54].
4.2. Shikimic acid-independent approaches
The legitimate (−)-shikimic acid availability concerns in the early years of the development of Tamiflu led to the development of shikimic acid-free routes. Although the shikimic acid availability improved with time, alternative routes are still being explored to date. Unlike the shikimic acid-dependent approaches which take advantage of the already present chiral cyclohexene backbone to introduce the groups at C3, C4, and C5 with the desired stereochemistry, the shikimic acid independent approaches construct the cyclohexene backbone through various strategies such as Diels-Alder reaction, Horner-Wadsworth-Emmons reaction, aldol condensation, Michael addition, sugars, nitroalkenes by Curtius rearrangement. These approaches display ingenuity in the construction of the cyclohexene ring system, the induction of the three stereogenic centres, the introduction of the two amino groups, the introduction of the 3-pentylether side chain and the regioselective introduction of the 1,2-double bound on the cyclohexene ring of the drug starting from readily available and affordable starting materials.
4.2.1. Tamiflu via Diels-Alder approach
Approaches in this class utilize the Diels-Alder reaction to construct the cyclohexene ring system of the drug based on the investigations by Brion [72] The chemo-enzymatic enantioconvergent synthesis of Tamiflu 1a by Roche group used furan 61 and ethyl acrylate 62 as starting materials (Scheme 10 ) [25] The Diels-Alder reaction and [3 + 2] cycloaddition to afford endo-aziridine (−)-63 were the key steps of this route. The main advantages of this approach are the use of very inexpensive starting materials and reagents, minimal protecting group manipulations, and the fact that resolution of the material was carried out very early in the synthesis, which should considerably increase the throughput. Conversely, the low overall yield of 3.2% and use of azide are detrimental. In 2013, Yamashita et al. [73] reported an enantioconvergent approach to an important Tamiflu 1a intermediate (ethyl shikimate 29) via Diels-Alder reaction and subsequent lipase-mediated kinetic resolution.
Scheme 10.
Diels-Alder approach towards Tamiflu 1a by Roche group [25].
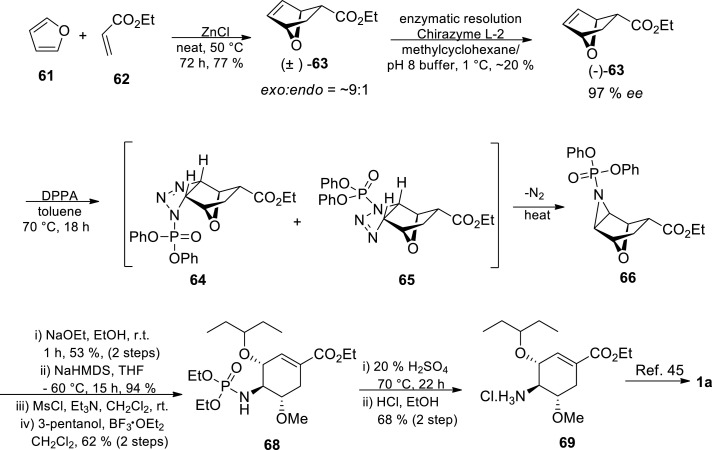
Shibasaki and coworkers [47,[74], [75], [76], [77], [78]] have been very active in Tamiflu synthesis research since their first procedure in 2006 which afforded Tamiflu in 1.4% overall yield over 17 steps [78] This procedure was characterised by extensive protection group chemistry, very low yield as well as the use of azide chemistry [78] In 2007, Shibasaki and coworkers reported a 12-step approach towards Tamiflu 1a via the Diels-Alder reaction and Curtius rearrangement reaction as the key steps [77] In this approach, chirality is introduced with the help of a chiral ligand, and relies on the preparative chiral HPLC to obtain enantiomerically pure material (Scheme 11 ) [77].
Scheme 11.
Shibasaki’s group third-generation synthesis of Tamiflu 1a [77].
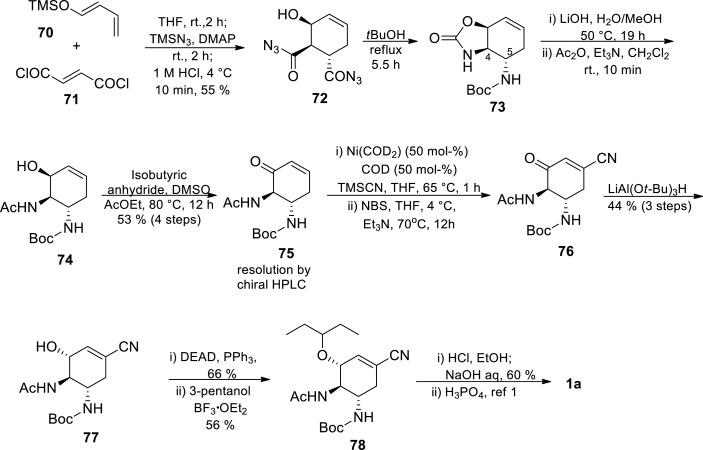
The synthetic route started with a Diels-Alder reaction between diene 70 and dienophile 71, in the presence of an azidating agent, affording an appropriately functionalised cyclohexene azidated skeleton 72 [77] The Curtius rearrangement of the acyl azide 72 and subsequent intramolecular trapping of the resulting isocyanate by t-BuOH gave the unsymmetrically protected 1,2-trans-diamine 73 exclusively [77] Selective hydrolysis of cyclic carbamate moiety of 73 with LiOH and subsequent N-acetylation afforded derivative 74, which was then oxidised to enone 75 under modified Moffat conditions with isobutyric anhydride as an activator for DMSO [77] Chiral HPLC was then used to separate the enantiomers at this stage, and enantiomerically pure 75 was obtained. The Michael addition of cyanide was carried out by treating 75 with TMSCN in the presence of Ni(COD)2 and 1,5-cyclooctadiene (COD) affording silyl enol ether followed by α-bromination with NBS and subsequent HBr elimination with trimethylamine resulting in β-cyanoenone 76. This was then followed by the introduction of the ethoxy carbonyl group at the β-position of the enone and then the introduction of the pentyloxy group. Stereoselective reduction of the ketone 76 with LiAl(Ot-Bu)3H afforded 77 in 44% yield in three steps. The aziridine formed under Mitsunobu conditions went through a ring-opening reaction with 3-pentanol affording 78. In the final stages, ethanolysis of the cyanide and cleavage of the Boc group proceeded in one pot using acidic ethanol. The free amine form of 2 was formed after basification, which was then followed by treatment with H3PO4 to give Tamiflu 1a [77].
This 12 steps route has advantages over the previous routes because it utilises commercially available starting materials and requires minimal protection group manipulations. It however still employs potentially hazardous azide chemistry as a means to prepare the substrate for a Curtius rearrangement, which would most likely make this approach be ruled out on scale for safety reasons, unless other processing techniques such as flow chemistry are utilised to address these safety concerns [2,79] The resolution of (±)-75 via chiral HPLC was a major contributing factor to the very low overall yield (2.8%). Just as the commercial route, the hazardous azide chemistry is employed, which was unfortunately accompanied by very low yield. To address this low yield drawback, Shibasaki’s group later developed a barium-catalyzed asymmetric Diels-Alder route that would increase the throughput (Scheme 12 ) [47].
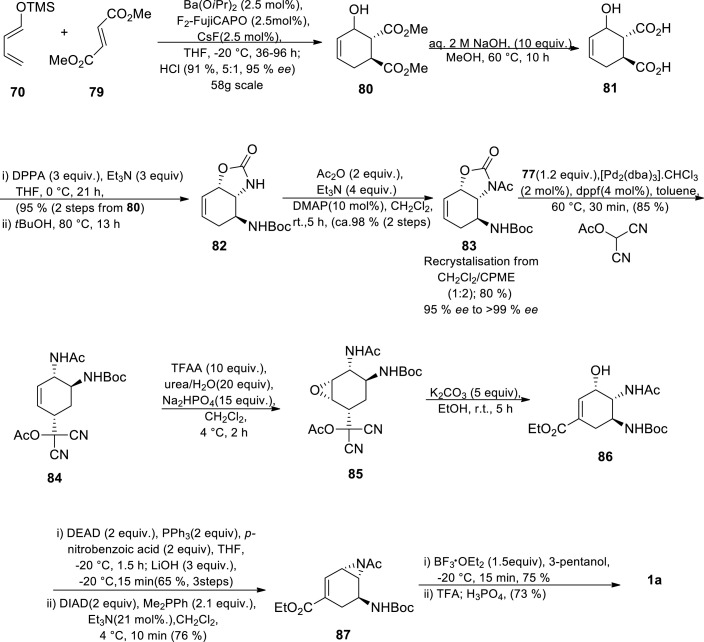
Scheme 12.
Catalytic asymmetric Diels-Alder approach towards synthesis of oseltamivir phosphate by Shibasaki group (Fourth generation approach) [47].
The cyclohexene skeleton was synthesised through a Diels-Alder reaction between diene 70 and dienophile 79 affording 80 on a 58g scale [47] This was followed by the hydrolysis of the methyl esters to afford 81. Azidation of 81 yielded diacyl azide in situ which was subsequently converted to cyclic carbamate 82 via Curtius rearrangement in the presence of anhydrous t-BuOH. The carbamate 82 was acetylated to provide enantiomerically pure intermediate 83 was obtained in 80% yield (>99% ee) after recrystallization of the crude product from CH2Cl2/cyclopentyl methyl ether (1:2). Enantiomerically pure intermediate 83 was subsequently treated with protected hydroxyl malononitrile in the presence of [Pd2(dba)3].CHCl3 to generate alkene 84 after regioselective allylic substitution. The alkene 84 was epoxidised with TFAA to exclusively afford epoxide 85. Alcohol 86 was generated by the conversion of the acetoxydicyanomethyl group to an ethoxycarbonyl group and subsequent E2 epoxide opening with ethanolic K2CO3. The stereochemistry of the hydroxyl group on 86 was inverted under Mitsunobu conditions and the second Mitsunobu conditions generated aziridine 87. The aziridine 87 was treated with 3-pentanol in the presence of BF3·Et2O followed by Boc-protecting group removal and treatment with H3PO4 to give Tamiflu 1a [47].
This approach is unique in its chirality introduction as it is introduced in the first step through asymmetric Diels-Alder reaction. This considerably increased the overall yield (16%) of the approach compared to their previous approach [47,77] The synthetic route is also characterised by low catalytic loading which is advantageous. An interesting way of introducing an ester group on the cyclohexene using malononitrile was unearthed. Although the procedure is low yielding compared to the current industrial, it has the potential to become a scalable process if necessary optimisation and safety measures for dealing with potentially hazardous azides intermediates are developed.
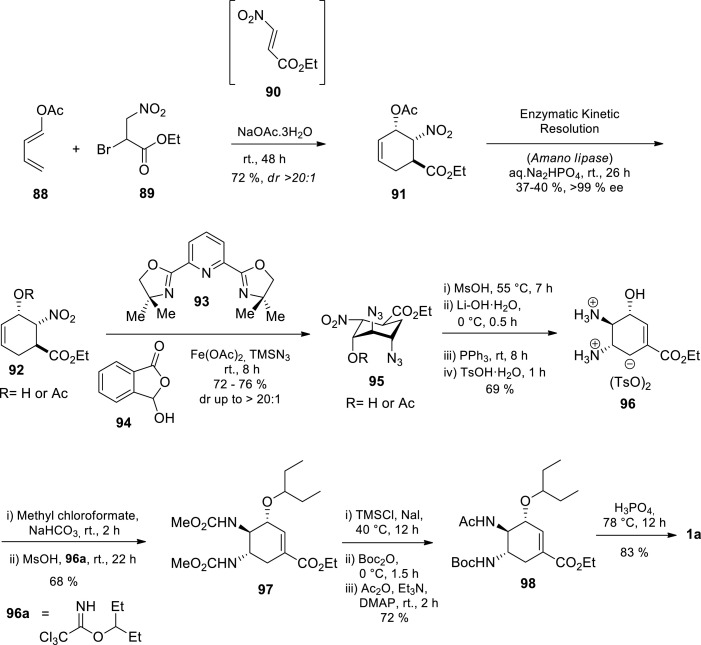
Most recently, Li and coworkers demonstrated enantioselective synthesis of Tamiflu 1a on a gram scale, in which the key trans-diamino moiety was efficiently installed via an iron-catalyzed stereo-selective olefin diazidation (Scheme 13 ) [80] Based on Danishefsky and coworkers’s work, the authors prepared racemic 91 via a Diels–Alder reaction of diene 88 with nitroacrylate 90 generated in situ from bromo-3-nitropropanoate 89 [80,81] Nitroacrylates decompose under the reaction conditions, therefore efficient cycloaddition was achieved by in situ generation of the nitroacrylate 90 from its precursor, bromo-3-nitropropanoate 89. Subsequent enzymatic kinetic resolution of 91 using Amano Lipase afforded 92 in 44% yield. Due to known safety concerns associated with azide chemistry, a chemical hazard assessment of the olefin diazidation to investigate the feasibility of Tamiflu 1a production on a larger scale was conducted. To avoid the risk associated with the azidation process, the authors performed an iron-catalyzed olefin diazidation with TMSN3 in the presence of Fe(OAc)2 93 and benziodoxole 94 to afford diazide 95 in 72% yield. A straightforward hydrolysis-elimination procedure converted 95 to intermediate trans, trans-diazido alcohol, which was converted to trans, trans-hydroxyl diaminium tosylates 96 via a standard reduction-protonation procedure. Subsequently, 96 acylation afforded a carbamate which underwent selective alkylation to afford 97. The crystalline solid 97 was further converted to 98, the penultimate synthetic target, via a gram-scale procedure that involves TMSCl–NaI-mediated carbamate deprotection and selective N-acylation of both Boc and Ac groups. Finally, N-Boc deprotection of 98 using H3PO4 in hot EtOH afforded Tamiflu 1a.
Scheme 13.
Enantioselective synthesis of Tamiflu 1a via the iron-catalyzed stereoselective olefin diazidation [80].
Although the authors demonstrated an improved and effective iron-catalyzed procedure for highly functionalised yet electronically deactivated substrates that have been otherwise problematic and an oligomeric iron-azide catalyst was uniquely effective for the stereoselective diazidation, the procedure was accompanied by an unimpressively low overall yield (5%). Although the chemical hazard assessment of olefin diazidation using both differential scanning calorimetry (DSC) and the drop weight test (DWT) demonstrated the feasibility of performing this olefin diazidation reaction for large scale synthesis of Tamiflu 1a, an excess of TMSN3 (5 equiv.) was necessary for the reactivity and the reaction was only scaled up to 5 g scale.
In 2006, Corey and coworkers demonstrated a 11-steps procedure starting from a Diels- Alder reaction to afford Tamiflu 1a in 27% yield [82] Building on Corey et al. [82] work, other researchers reported various interesting synthetic routes towards Tamiflu 1a via Corey’s intermediate [74,75,[83], [84], [85]] Fukuyama coworkers [86,87] have also reported procedures which involved Diels–Alder reaction and avoids azide chemistry in which Tamiflu was synthesised in 22% and 5.6% overall yield respectively. More recently, Fang et al. [88] reported the synthesis of various substituted cyclohexenes which can be useful for building Tamiflu scaffolds starting from a Morita–Baylis–Hillman reaction and goes via a Diels– Alder reaction. Savoia and coworkers [89] also reported the synthesis of 1,2-diamine moiety, a valuable building block and precursor for Tamiflu starting from glyoxal through the corresponding 1,2-diimine.
4.2.2. Tamiflu via a Horner-Wadsworth-Emmons (H–W-E) reaction/aldol condensation/Michael addition
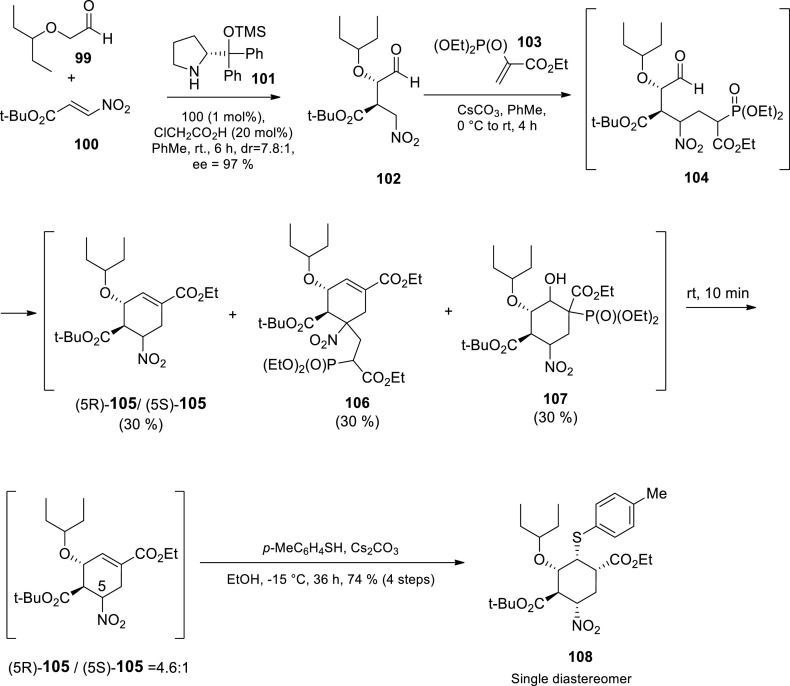
Hayashi and coworkers have been immensely involved in oseltamivir research since its discovery. They have published at least five different synthetic routes towards Tamiflu 1a to date [32,[90], [91], [92], [93], [94]] Some of their most recent and interesting approaches are reviewed herein. The authors designed a highly efficient two ‘one-pot’ sequences approach towards oseltamivir starting from aldehyde 99 and nitroolefin 100 (Scheme 14 )91 after their initial three ‘one-pot’ sequences procedure [93] The first ‘one-pot’ sequence started with the asymmetric addition of aldehyde 99 to nitroolefin 100 in the presence of the catalyst 101 to afford Michael adduct 102 in 7.8:1 diastereomeric ratio (dr) and 97% ee. Michael addition of the nitroaldehyde 102 to vinylphosphonate 103 subsequently followed by an intra-molecular H–W-E reaction via transient intermediate 104 created the cyclohexene ring. In the presence of Cs2CO3 as a base, an equimolecular mixture of (5R)-105/(5S)-105, 106 and 107 (30% each) was obtained. Treating this mixture with ethanol resulted in a 4.6:1 diastereomeric mixture of (5R)-105 (undesired) and (5S)-105 (desired). The (5R)-105/(5S)-105 mixture was then treated with 4-methylthiophenol and Cs2CO3 in ethanol afforded a Michael-addition product 108 as a single diastereomer in 74% yield [91] This first ‘one-pot’ sequence product 108 was used as the starting material in the second ‘one-pot’ sequence (Scheme 15 ) [91].
Scheme 14.
Hayashi’s group first ’one-pot’ sequence towards oseltamivir 28 [91].
Scheme 15.
Hayashi’s group second ’one-pot’ sequence towards oseltamivir 28 [91].
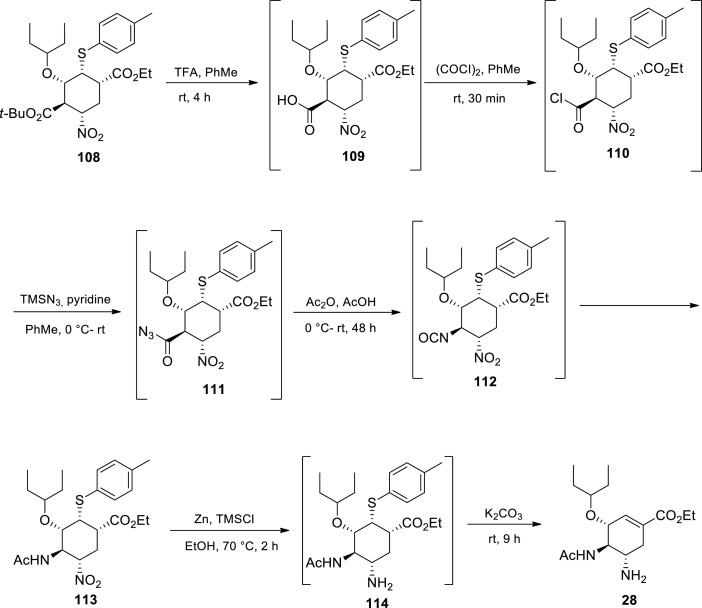
Hayashi’s second ‘one-pot’ sequence towards oseltamivir starts with the treatment of compound 108 (made in the first ‘one-pot’ sequence) with TFA, thus cleaving the tert-butyl ester, to afford carboxylic acid 109 which was subsequently converted to acid chloride 110 via oxalyl chloride and catalytic DMF treatment [91] The acid chloride 110 was converted to acyl azide 111 by TMSN3 and pyridine treatment in toluene. Without isolation, the compound underwent a Curtius rearrangement reaction at room temperature to give an isocyanate 112, which was then trapped with AcOH in the presence of Ac2O affording acetamide 113. Zinc powder was used for the nitro group reduction on compound 113 to afford amine 114. In the presence of K2CO3 in EtOH, oseltamivir 28 (free base) was afforded [91].
According to Hayashi and coworkers, [91] Tamiflu’s synthesis was accomplished by two ‘one-pot’ reaction sequences, with excellent overall yield (60%) and required only one purification by column chromatography. The approach required five isolations only. Unlike Ma and coworkers, [95] the authors could not avoid the use of the potentially explosive azide chemistry. The azide intermediate was not isolated to address the safety concerns posed by azides. Positively, their approach was characterised by low catalyst loading, no protecting group chemistry and the absence of halogenated solvents [91] Though, this approach is attractive for large scale manufacturing, the safety concerns posed by the use of azide chemistry needs to be addressed especially at large scale where the risk is very high.
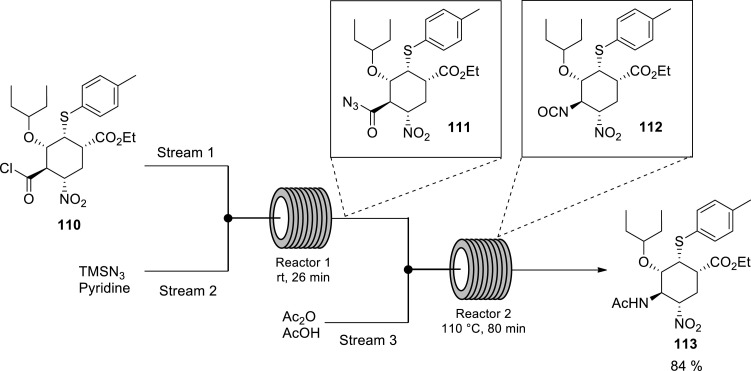
To address the aforementioned safety concerns associated with the use of potentially explosive acyl azide 111, [91] Hayashi and coworkers [32] demonstrated the handling of the Curtius rearrangement reaction of acyl azide 111 to isocyanate 112 by taking advantage of continuous flow technology (Scheme 16 ). Acyl azide 111 is a potentially explosive compound owing to its nitro and azide moieties [32] As aforementioned, continuous flow technology allows for in situ generation and consumption of dangerous intermediates, preventing their accumulation thus enhancing process safety [55,[66], [67], [68]] Additionally, microreactors can handle exotherms extremely well, due to the inherent high surface area to volume ratio and rapid heat dissipation unlike the conventional batch process [55,69] With this in mind, the authors treated acyl chloride 110 with TMSN3 and pyridine in the first reactor at room temperature for 26 min to afford acyl azide 111. Acyl azide 111 formed in situ underwent Curtius rearrangement to isocyanate 112 which is trapped with AcOH in the second reactor at 110 °C for 80 min residence time to afford acetamide 113 in 84% yield and the same yield was obtained at 10 g scale (Scheme 16) [32] The reaction was easily scaled-up in this system using parallel experiments.
Scheme 16.
Synthesis of acetamide 113 from acyl chloride 110via Curtius rearrangement of acyl azide 111 using a continuous flow system [32].
Evidently, the authors safely performed the hazardous Curtius rearrangement of azide 111 to isocyanate 112 in continuous flow at high temperature 110 °C accompanied by significantly shorter reaction time 80 min compared to the batch process (room temperature and 48 h) [32,91] They successfully demonstrated the possibilities of using continuous flow systems as a way of solving the problems associated with handling hazardous intermediates and products in the synthesis of Tamiflu. This technique has the potential of being utilised for good synthetic approaches towards Tamiflu, which were previously ruled out for large scale synthesis in batch systems on the basis of safety concerns poised by the use of the potentially explosive azide chemistry and other hazardous chemistry. Therefore, problems inherent in scale-up are effectively reduced, making microreactor technology an enabling tool in the synthesis of Tamiflu.
In 2016, Hayashi and Ogasawara published a time economical synthetic approach of (−)-oseltamivir (Scheme 17 ) [90] This 60 min total synthesis was accomplished in a single vessel over 5 steps.
Scheme 17.
Hayashi’s group 60 min time economical synthesis of (−)-oseltamivir 28 [90].
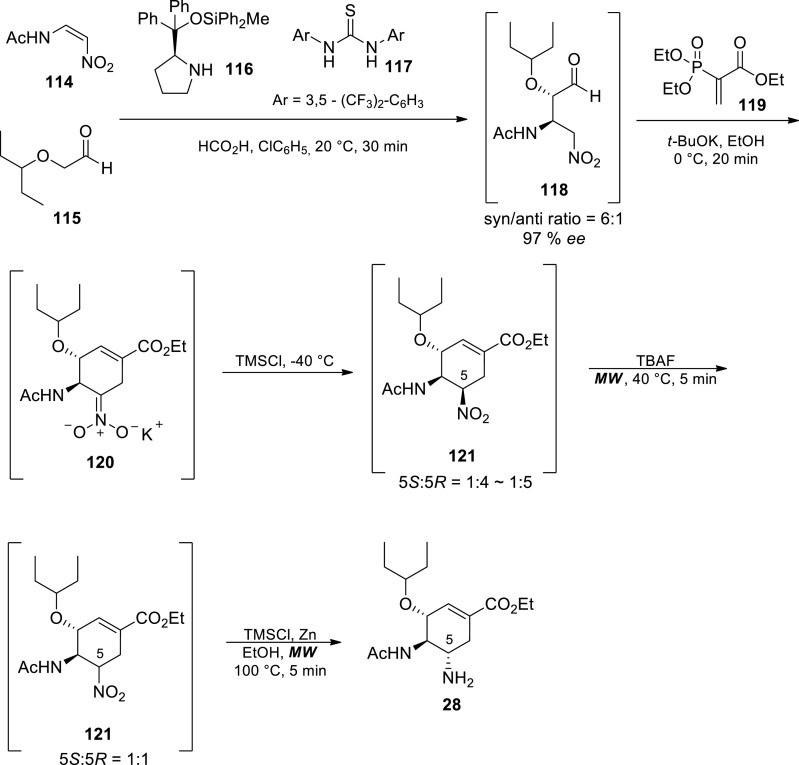
This 60 min economical total synthesis approach involved an asymmetric Michael addition reaction of nitroalkene 114 and α-alkoxyaldehyde 115 in the presence catalytic amount of diphenylprolinol silyl ether 116, Schreiner’s thiourea 117 and formic acid affording a Michael adduct 118 [90] Diphenylprolinol silyl ether 116 is key for the generation of a reactive intermediate enamine, thiourea 117 activates nitroalkene 114 via hydrogen bonding and formic acid suppresses side reactions. Cyclohexene 120 was produced via domino Michael addition and H–W-E reactions in the presence of ethyl acrylate derivative 119, t-BuOK, EtOH and Cs2CO3 in chlorobenzene. Protonation instantly occurred on the nitronate ion by a reaction with HCl generated in situ from trimethylsilyl chloride. In the presence of TMSCl, a mixture of 5(R) and 5(S) isomers of nitrocyclohexene 121 was obtained. The undesired 5(R) isomer was predominantly formed in this reaction [5(S)/5(R) = 1:4–5]. This was subsequently followed by epimerisation from the 5(R) to 5(S) isomer in the presence of TBAF and microwave (MW) irradiation to afford a mixture of isomers of 5(R) and 5(S) of nitrocyclohexene 121 [5S/5R = 1:1]. Lastly, the nitro group on compound 121 was reduced to an amine using Zn and MW irradiation to afford base free oseltamivir 28 [90].
This efficient 5-step approach was accomplished in just 60 min reaction time with an overall yield of 15% (Scheme 17). Although there are many economies in syntheses such as atom economy, redox economy, and step economy, [90] this synthetic approach serves as a perfect example of time economy as some of the previous syntheses are more than 30 h long [91] The synthetic design also satisfies step economy and pot economy as few steps (5 steps) were used and only one vessel was used throughout. An effective catalyst cocktail was developed for rapid asymmetric Michael addition reaction in excellent yield with excellent diastereo- and enantioselectivities [90] Time economy should also be considered in synthesis along with other efficiency factors in ideal organic synthesis such as greenness, yield and selectivity. The use of hazardous azide chemistry and protection group chemistry was avoided however no information was provided on scale. Without doubt, the approach is a major player in the goal to develop a safe and an efficient scalable process towards oseltamivir 28.
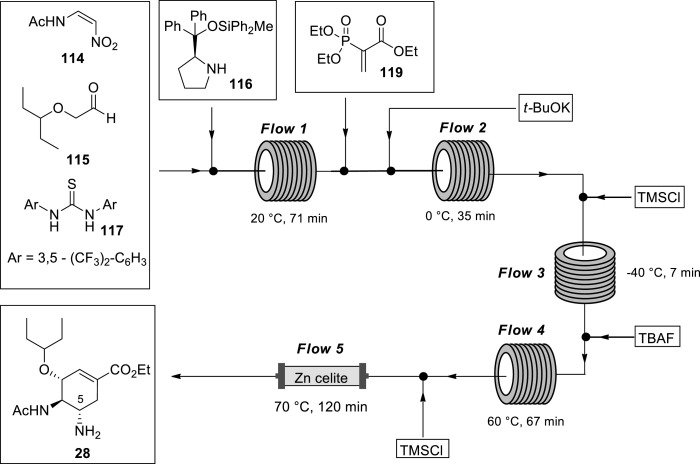
After successful accomplishment of the 60 min synthetic approach towards oseltamivir 28 in batch, Hayashi and Ogasawara [92] subsequently transferred the approach to continuous flow technology (Scheme 18 ). To successfully transfer the 60 min synthetic approach (Scheme 17) from the batch system to the flow system, the authors had to address the following problems: 1) In a flow system, generally all reagents have to be soluble in the solvent except for polymer-supported reagents, but nitroalkene 114 was barely soluble in the reaction solvent, 2) Zn was employed in the reduction of the nitro group to an amine which is a problematic transformation to conduct in continuous flow systems. The authors first optimised the 60 min approach in the batch system before the successfully development of a continuous-flow synthesis of (−)-oseltamivir summarised (Scheme 18) [92].
Scheme 18.
Continuous-flow synthesis of (−)-oseltamivir 28 by Hayashi and Ogasawara [92].
The (−)-oseltamivir 28 continuous flow synthesis system consisted of five flow units (Scheme 18) [92] Asymmetric Michael addition reaction was performed in the flow unit 1. A toluene solution of nitroalkene 114, α-alkoxyaldehyde 115, theourea derivative 117 and ClCH2CO2H was mixed with a toluene solution of catalyst 116 using a Comet-X-01 micro-mixing device at 20 °C (Scheme 19 ). The resulting mixture reacted in the tube for 71 min affording a Michael adduct 118. When the reaction was quenched at this stage, the conversion (91%, dr = 10:1, 97 ee) was almost the same as in the batch system ((95%, dr = 14:1, 97 ee). Domino Michael reaction and intermolecular Horner-Wadsworth-Emmons reactions occurred in the flow unit 2. Phosphoryl acrylate 119 was added into the system at a T-mixer followed by the addition of t-BuOK in EtOH at the next mixer (Comet-X-01) resulting in cyclohexene 121 (Scheme 17, 60% yield) at 0 °C after 35 min residence time. When the reaction was quenched, the undesired 5R-isomer was found to be predominant (5S/5R = 1:5). Flow unit 3 was used to protonate the nitronate ion. Potassium nitronate 120 (Scheme 17) was formed at the end of the domino reaction by the addition of TMSCl in EtOH at - 40 °C, which produced HCl in situ, with a residence time of 7 min. Epimerisation from 5R-isomer to the 5S isomer occurred in the flow unit 4 where potassium nitronate 120 was treated with a solution TBAF in EtOH at the Comet-X-01 mixer at 60 °C and residence time of 67 min to afford nitrocyclohexene 121 (5S/5R = 1:1). Flow unit 5 effected reduction of the nitro group to amine using Zn. Since Zn is a solid, a column reactor was used. The column reactor was packed with Zn (5 g) and Celite (8 g) in the presence of TMSCl in EtOH (0.6 M). This was done at 70 °C and 120 min residence time. Zn activity gradually decreased with time thus it was necessary to replace the column after every 5h. Oseltamivir 28 was isolated using an acid-base extraction and purified by column chromatography to afford (−)-oseltamivir 28 in 13% overall yield which is comparable to the batch approach (15%). Noteworthy, continuous flow synthesis can easily be scaled-up compared to batch making the flow procedure more attractive [57,65,67,79,[96], [97], [98], [99], [100]] The authors easily increased continuous flow productivity by long-time operation or directly scaling-up to a larger scale after optimisation on a small scale. However, batch scale-up is flawed at each stage of the scale-up, modifications made to the reactor vessel result in changes to the surface to volume ratio, process will need process re-optimisation at every stage of scale up due change is mass transfer ratios, which in turn have a profound effect on the thermal and mass-transport properties of the reaction. Evidently, this continuous flow synthesis technology is efficient and convenient. This work unearthed valuable insights towards the goal of developing efficient and safe processes for (−)-oseltamivir 28 continuous flow manufacturing.
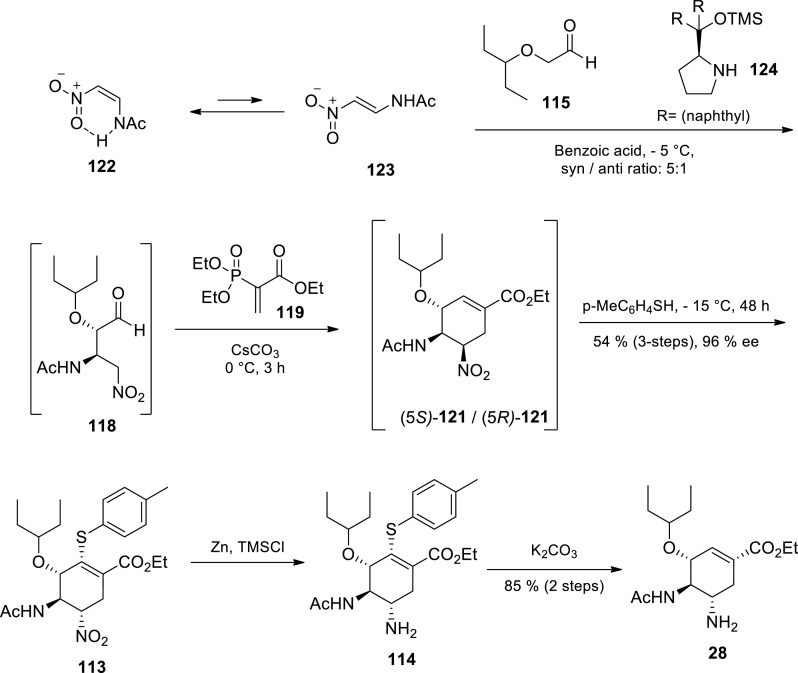
Scheme 19.
Ma’s group oseltamivir 28 synthesis via organocatalytic Michael addition [95].
Ma and coworkers developed an approach towards oseltamivir via organocatalytic Michael addition of an aldehyde to 2-amino-1-nitroethene (Scheme 19) [95] Nitroolefin 122 was prepared by (Z)-2-nitroethanamine acetylation due to the intra-molecular hydrogen bonding. Nitroolefin 122 was subsequently subjected to Michael addition with aldehyde 115 in the presence of an organic catalyst 124 and benzoic acid to afford a Michael-addition product 118 in 80% yield and 5:1 syn/anti ratio. Going forth, Ma et al. utilised Hayashi and coworkers’ [90,91] strategy to transform aldehyde 118 to (−)-oseltamivir 28. Aldehyde 118 was treated with vinyl-phosphonate 119 and Cs2CO3 to give cyclohexane 121 as a mixture of epimers at C-5. Cyclohexane 121 was subsequently treated with 4-methylthiophenol affording intermediate 113 [95] The combined yield for the 3 steps was 54% and 113 was obtained in 96% ee. Compound 113 nitro reduction was accomplished in the presence of Zn and TMSCl to afford 114. Elimination of 4-methylthiophenol on compound 114 with K2CO3 in MeOH regenerated the double bond to afford oseltamivir 28 in 85% yield. This 5 steps approach had an excellent overall yield of 46% from aldehyde 115 and nitroolefin 122. Furthermore, procedure required only two intermediate isolation operations which made the approach practically feasible in the preparation of the drug molecule. The authors utilised an asymmetric Michael addition to incorporate amino groups on the carbon skeleton as nitro and acetomido groups, thus avoiding the use of azide chemistry. This route was only carried out at 10 mmol scale and no protection group chemistry was required [95] This route is a potential industrial candidate, however extensive scale up studies, optimisation and safety concerns associated with nitro compounds need to be assessed.
4.2.3. Tamiflu synthesis from sugars
Affordable and abundantly available sugars such as d-xylose, [101] d-ribose, [102] D-mannitol, [103] D-glucal, [104] and d-glucose [105] have also been used as starting material for Tamiflu 1a synthesis [1,2,24,26] However, sugar dependent synthetic procedures are characteristically long (12–22 steps) accompanied with extensive chromatographic purification (9–16 operations) and low yields (2.6–15%). Herein, Liu and coworkers’ D-glucal procedure, [104] Wong et al. d-xylose procedure [101] and Kongkathip et al. [105] d-glucose procedure towards Tamiflu 1a are reviewed in detail.
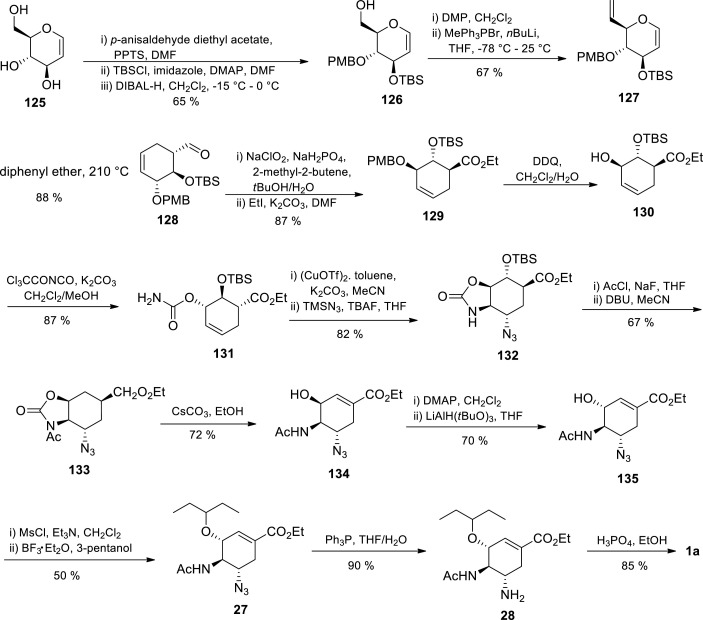
Just as with Ko et al. D-mannitol procedure, [103] Liu and coworkers [104] explored a Claisen rearrangement strategy in the construction of oseltamivir’s cyclohexene backbone from D-glucal 125 and the addition of diamino groups onto the cyclohexene backbone was accomplished via tandem intramolecular aziridination and ring opening (Scheme 20 ) [104] The D-glucal 125 was protected via conversion to 4,6-benzylidene acetal with p-anisaldehyde diethyl acetal in the presence of catalytic pyridinium p-toluene sulphonate (PPTS) and silylation of the 3-hydroxy group as the tert-butyldimethylsilyl ether. Subsequent reductive cleavage of an acetal followed by treatment with diisobutylaluminium hydride (DIBAL-H) afforded free alcohol 126 in 65% yield. The primary hydroxyl group of the alcohol was subjected to Desse-Martin periodinane oxidation yielding an aldehyde which underwent Wittig methylenation in the presence of methyltriphenylphosphonium bromide to afford alkene 127 in 67% yield. Claisen rearrangement of alkene 127 in diphenyl ether affording the cyclohexene core was accomplished at 210 °C in a sealed environment forming diastereoselective aldehyde 128 in 88% yield. Aldehyde 128 was subsequently oxidised to an acid using NaClO2/NaH2PO4 in the presence of 2-methyl-2-butene followed by esterification with ethyl iodide to afford ester 129 in 87% yield. Selective removal of p-methhoxybenzyl (PMB) protecting group was achieved with 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) to afford 130 in 92% yield. Treatment of compound 130 with trichloroacetyl isocyanate and potassium carbonate afforded carbamate 131 in 87% yield which was subsequently treated with Cu(OTf)2-toluene and TMSN3. This mechanistic step introduced a nitrogen functionality through stereo and regioselective aziridine opening afforded by TMSN3. Subsequent addition of TBAF in THF in stoichiometry amounts prompted formation of compound 132 in 82% yield. To avoid racemisation at C1 and aromatization owing to directly treatment of 132 with DBU, the authors first acetylated 132 with AcCl/NaH then followed by treatment with DBU to afford 133 in 67% yield. Subsequent hydrolysis of 133 with Cs2CO3 in ethanol afforded alcohol 134 in 72% yield. Alcohol 134 was oxidised with Dess-Martin periodinane, inverting the configuration at C3 and affording a ketone which was subsequently reduced with LiAlH(t-BuO)3 to afford a 70% yield of stereospecific 135. Treatment of compound 135 with MsCl/Et3N led to an aziridine intermediate and aziridine ring opening with 3-pentanol/BF3 .Et2O afforded azide 27 in 50% yield. Azide 27 reduction with Ph3P in (THF)/H2O afforded oseltamivir 28 in 90% yield, which upon treatment with H3PO4 in EtOH afforded Tamiflu 1a in 85% yield.
Scheme 20.
Tamiflu 1a synthesis via Claisen rearrangement starting from D-glucal by Liu and coworkers [104].
Although Liu et al. D-glucal approach started from an affordable, commercially and abundantly available D-glucal, it involves 22 steps accompanied by 16 tedious intermediate column chromatography purification operations and very low overall yield (2.6%) [104] Furthermore, the synthesis had a mechanistic step which required reaction at high temperature in a sealed tube in addition to protection group chemistry. These limitations make it difficult for the synthesis process to be scaled up to industrial scale.
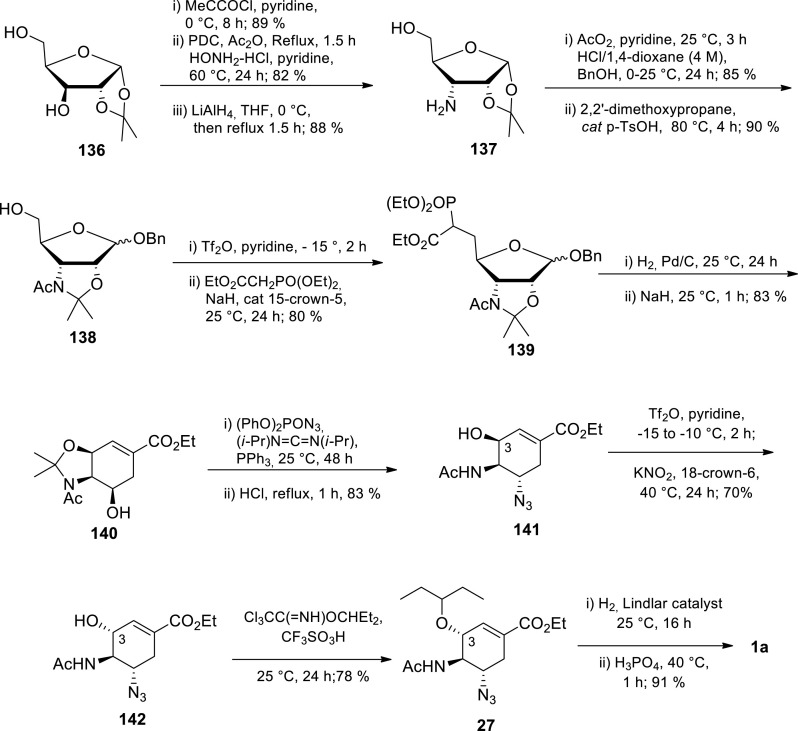
Wong and coworkers’ d-xylose approach is the highest yielding procedure (15% overall yield) [104] in the Tamiflu 1a ‘sugar-depended’ synthesis procedure class (Scheme 21 ) [1,2] The authors started from 1,2-O-isopropylidene-α-d-xylofuranose 136, prepared from d-xylose. The 1,2-O-isopropylidene-α-d-xylofuranose 136 was transformed to aminoalcohol 137 sequentially using pivaloyl chloride followed by alcohol oxidation with PDC affording ketone intermediate and reduction of the oxime derived from ketone with LiAlH4. Aminoalcohol 137 amino group was acetylated to afford acetamide which was sequentially treated with benzyl alcohol under acidic conditions to afford ribofuranoside intermediate as a mixture of anomers (α/β) = 7:3) and then with 2,2-dimethoxypropane to afford N,O-ketal 138 in the same anomeric ratio. N,O-Ketal 138 mixture was converted to triflate and the triflate group was subsequently displaced with triethyl phosphonoacetate to afford phosphoryl ester 139. Subsequent intramolecular Horner-Wadsworth-Emmons reaction using NaH and catalytic 15-crown-5 afforded cyclohexene 140. Azide 141 was prepared from cyclohexene 140 via a Mitsunobu reaction followed by deprotection of the amino and hydroxy groups using HCl. Subsequent stereo-chemistry inversion of the hydroxy group on Azide 141 using Tf2O, pyridine and KNO2 and 18-crown-6 afforded alcohol 142 which was then treated with 3-pentyl trichloroacetimidate to introduce the 3-pentyl ether functionality on azide 27. Hydrogenation of azide 27 in the presence of Lindlar catalyst followed by the addition of H3PO4 afforded Tamiflu 1a in 15% overall yield over the 16 steps. As with all sugar-dependent approaches, this procedure has extensive column chromatographic purification operations. Furthermore, protection group chemistry and hazardous azide chemistry was used.
Scheme 21.
Wong and coworkers’ enantioselective synthesis of Tamiflu 1a from d-xylose [104].
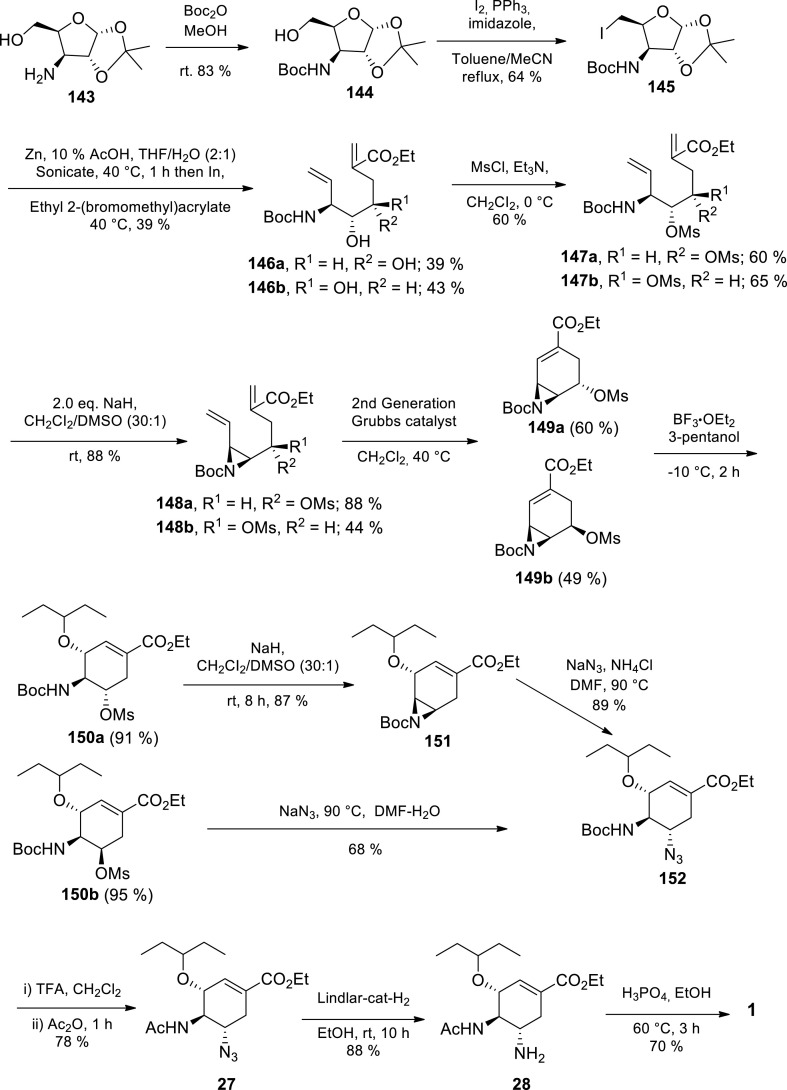
More recently, Kongkathip et al. demonstrated a 13-step procedure for Tamiflu starting from d-glucose (Scheme 22 ) [105] The 3-amino-3-deoxy-1,2-O-(1-methylethylidene)-α-d-xylofuranose 143 derived from d-glucose, underwent N-Boc protection in the presence of Boc2O to afford alcohol 144 in 83% yield. Subsequent alcohol 144 iodination by I2 in the presence of PPh3 and imidazole afforded iodide 145 in 64% yield. Zn-mediated fragmentation of iodide 145 afforded aldehyde intermediate, which subsequently underwent indium-mediated coupling with ethyl 2-(bromomethyl)acrylate to afford corresponding diene 146a and 146b in 39% and 43% yield, respectively. Protection of the dihydroxyl group of both 146a and 146b was achieved upon treatment with MsCl affording mesylate 147a and 147b in 60% and 65% yield, respectively. Subsequent treatment of 147a and 147b with NaH afforded aziridine 148a and 148b in 88% and 44%, respectively. Heating 148a and 148b to 40 °C with the Hoveyda-Grubbs 2nd generation catalyst resulted in ring closing metathesis, afforded cyclohexene aziridine 149a and 149b in 60% and 49% yield, respectively. Regio-and stereospecific N-Boc aziridine 149a and 149b ring opening with 3-pentanol and BF3.OEt2 afforded compounds 150a and 150b in 91% and 95% yield respectively, which can both be transformed to Tamiflu 1a. Compound 150a underwent intermolecular SN2 type when treated with NaH affording N-Boc aziridine 151a (87% yield) and subsequent azidation with NaN3 afforded azide 152 in 89% yield. Compound 150b was directly azidated to azide 152 in 68% yield. Treatment of azide 152 with TFA and subsequent acetylation with Ac2O afforded N–Ac azido 27 in 78% yield. N–Ac azido 27 reduction with H2 and Lindlar catalyst afforded oseltamivir 28 in 88% yield. Lastly, oseltamivir 28 was treated with H2PO4 to afford Tamiflu 1a in 70% yield. The key transformations in this approach are the three consecutive organometallic reactions: Zn-mediated fragmentation of 145, indium-mediated coupling between the aldehyde intermediate and ethyl 2-(bromomethyl)acrylate and the Hoveyda-Grubbs 2nd generation catalyzed ring closing metathesis of 148. Typical of sugar-dependent approaches, this procedure was characterised with low yield (3.8% over 13 steps) and extensive column chromatographic purification (12 operations). Furthermore, the authors could not avoid azide chemistry and protection group chemistry.
Scheme 22.
Kongkathip et al. d-glucose procedure towards Tamiflu 1a [105].
5. Conclusions
As with the current Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection (COVID19) pandemic, a highly aggressive strain of the influenza virus such as the H5N1 can mutate and spark another deadly pandemic. This reminds us of the need for continued research to discover more potent neuraminidase inhibitors. As the research towards new and better treatment remains a top priority, it is equally important to improve the availability of the current anti-influenza drugs by developing better synthetic procedures to guard the world against influenza. Though other influenza antiviral drugs such as peramivir, zanamivir and baloxavir marboxil are on the market, Tamiflu remains the most used anti-influenza drug 20 years after its approval. Consequently, Tamiflu synthesis remains an important research area. Although the Roche industrial route is currently supplying the world with enough tonnes of Tamiflu but it remains to be seen if will be suffice in the face of a pandemic, the route raised three concerns; a) the use of shikimic acid, which had limited availability in the early days of development b) the use of potentially explosive azide chemistry and c) long synthetic route with low overall yield. To address these concerns, academic and industrial researchers have made tremendous efforts in the quest for a truly efficient, safe, cost-effective and environmentally benign synthetic procedure resulting in more than 70 synthetic procedures to date since its discovery.
As reflected in this review and complimented by the published reviews, [[1], [2], [3],[22], [23], [24], [25], [26], [27],47] both the shikimic acid-free and azide chemistry-free approaches are mostly low yielding compared to their competitors despite their ingenuity in Tamiflu assembling. Having solved the shikimic acid availability concerns over the years by developing more efficient extraction and purification processes or alternatively by fermentation using genetically engineered E.coli bacteria, [25,[28], [29], [30]] the use of hazardous azide chemistry needs more attention. The application of continuous flow technology in Tamiflu synthesis proved to be a potential enabling tool for safe handling of the hazardous azide chemistry as well as improving efficiency [32,53,92] Continuous flow synthesis has attracted considerable attention in synthetic chemistry and pharmaceutical industry in the last decade owing to its well-documented advantages, [[55], [56], [57], [58], [59],65,67,79,[96], [97], [98], [99], [100]] resulting in numerous pharmaceutical drugs approaches being redesigned into continuous flow synthesis [56,58,[60], [61], [62], [63], [64], [65],106] In this light, we envisage that Tamiflu synthesis can hugely benefit from continuous flow technology application to afford truly efficient synthetic procedures. Furthermore, the promising Tamiflu synthetic approaches which were previously ruled out for large scale synthesis in batch based on either safety concerns or poor efficiency can be reconsidered in flow. We envisage that the incorporation of other enabling technologies such as artificial intelligence, machine learning for “Big data” analysis, can challenge the dogma of the past and come up with a truly efficient, safe, cost-effective and environmentally benign Tamiflu synthetic procedure. With this in mind, we are looking forward to see what the future of Tamiflu synthesis holds.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the National Research Foundation (NRF SARChI Grant), Council for Scientific and Industrial Research- Department of Science and Technology, South Africa (CSIR-DST Grant) and Nelson Mandela University for financial support.
Biographies

Cloudius R. Sagandira graduated with a BSc in Biochemistry and Chemistry from the Nelson Mandela University in 2014 where he went on to receive his Honours degree in Formulation Science (Cum Laude) in 2015. In the same year, he joined Professor Paul Watts’ group as a Master of Science candidate at the same University where he worked on potentially explosive azide chemistry in continuous flow systems and graduated in 2017 with distinction. Working in the same research group, he received his chemistry PhD degree in 2019 where he developed a continuous flow synthetic procedure for oseltamivir phosphate an anti-influenza drug. Currently, he is a postdoctoral fellow in the same research group working on the synthesis of numerous drugs in flow.

Francis M. Mathe graduated with a BSc in Chemistry and Physics from the Nelson Mandela University in 2015. In 2016, graduated with an Honours degree in Formulation Science (Cum Laude). In the same year, he joined Professor Paul Watts’ Group where worked on the development of an integrated continuous flow system for the synthesis of biodiesel from waste cooking oil and graduated in 2018 with a Msc Chemistry degree. Currently, he’s in the same group working towards attaining his PhD on the flow synthesis of silicon compounds from low-grade silicon.

Upenyu Guyo graduated with a BSc Honours degree in Chemical Technology from Midlands State University, Gweru, Zimbabwe in 2006. He obtained his MSc degree in Analytical Chemistry from University of Zimbabwe in 2008. He received his PhD degree in Chemistry from Nelson Mandela University in 2013 where he worked on batch and process flow synthesis of entirely new range of bio-derived plasticizer molecules for use in so-called sensitive applications. He joined North West University in 2013 for is postdoctoral research working on synthesis of functionalised polyolefins using aluminium triflate as a cocatalyst. He is currently a lecturer in the Department of Chemical Technology, Midlands State University, Gweru, Zimbabwe and a research associate in Professor Paul Watts’ Flow Chemistry Research Group.

Paul Watts started his career as a lecturer at the University of Hull (UK) in 2002, being promoted to full professor in 2011. At the University of Hull he led the micro reactor and flow technology group. In February 2013, he moved to Nelson Mandela University to hold the distinguished position of ‘SARChI Research Chair in Microfluidic Bio/Chemical Processing’. He has published over 120 highly cited papers. He strongly believes that scientists should conduct research that impacts society; the biggest project underway involves the local production of key drugs as the morbidity and mortality from major diseases are much more devastating in Africa than in other regions of the world. The vision is that new technology will be used within South Africa to manufacture generic drugs; this could create jobs and a new manufacturing industry within the country.
References
- 1.Magano J. Tetrahedron. 2011;67:7875–7899. [Google Scholar]
- 2.Magano J. Chem. Rev. 2009;109:4398–4438. doi: 10.1021/cr800449m. [DOI] [PubMed] [Google Scholar]
- 3.Laborda P., Wang S.-Y., Voglmeir J. Molecules. 2016;21:1513. doi: 10.3390/molecules21111513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Report . 2017. Standard Guidelines for the Clinical Management of Severe Influenza Virus Infections. [Google Scholar]
- 5.Saunders-Hastings P.R., Krewski D. Pathogens. 2016;5:66. doi: 10.3390/pathogens5040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taubenberger J.K. Influenza : The Once and Future Pandemic. 2010;125 [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y., Hao Z., Gao Y., Ping W., Wang Q., Peng S., Zhao B., Sun W., Zhu M., Li K., Han Y., Kuang D., Chu Q., Fu X., Zhang N. J. Thorac. Oncol. 2020;15:1065–1072. doi: 10.1016/j.jtho.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding Q., Lu P., Fan Y., Xia Y., Liu M. J. Med. Virol. 2020:1–7. doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warfield K.L., Schaaf K.R., DeWald L.E., Spurgers K.B., Wang W., Stavale E., Mendenhall M., Shilts M.H., Stockwell T.B., Barnard D.L., Ramstedt U., Das S.R. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-43030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrovolny H.M., Beauchemin C.A.A. PloS One. 2017;12:1–26. doi: 10.1371/journal.pone.0180582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato D., Kurebayashi Y., Takahashi T., Otsubo T., Otake H., Yamazaki M., Tamoto C., Minami A., Ikeda K., Suzuki T. PloS One. 2018;13:1–22. doi: 10.1371/journal.pone.0200761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizzorno A., Abed Y., Boivin G. Semin. Respir. Crit. Care Med. 2011;32:409–422. doi: 10.1055/s-0031-1283281. [DOI] [PubMed] [Google Scholar]
- 13.Hussain M., Galvin H.D., Haw T.Y., Nutsford A.N., Husain M. Infect. Drug Resist. 2017;10:121–134. doi: 10.2147/IDR.S105473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omoto S., Speranzini V., Hashimoto T., Nosh T., Yamaguchi H., Kawai M., Kawaguchi K., Uehara T., Shishido T. Nature. 2018;1–15 doi: 10.1038/s41598-018-27890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.https://www.cdc.gov/flu/treatment/index.html (accessed May 10, 2020).
- 16.Kim C.U., Lew W., Williams M.A., Wu H., Zhang L., Chen X., Escarpe P.A., Mendel D.B., Laver W.G., Stevens R.C. J. Med. Chem. 1998;41:2451–2460. doi: 10.1021/jm980162u. [DOI] [PubMed] [Google Scholar]
- 17.Kent, K. M., Kim, C. U., McGee, L. R., Munger, J. D., Prisbe, E. J., Postich, M. J., Rohloff, J. C., Kelly, D. E., Williams, M. A., Zhang, L. US5886213, 1999.
- 18.Kent, K. M., Kim, C. U., Mcgee, L. R., Munger, J. D., Prisbe, E. J., Postich, M. J., Rohloff, J. C., Kelly, D. E., Williams, M. A., Zhang, L. WO1998007685, 1998.
- 19.Bischofberger, N. W., Dahl, T. C., Hitchcock, M. J. M., Kim, C. U., Lew, W., Liu, H., Mills, R. G., Williams, M. A. WO1999014185, 1999.
- 20.Kim C.U., Lew W., Williams M.A., Liu H., Zhang L., Swaminathan S., Bischofberger N., Chen M.S., Mendel D.B., Tai C.Y., Laver W.G., Stevens R.C. J. Am. Chem. Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 21.Bischofberger, N., Kim, C. U., Lew, W., Liu, H., Williams, M. A. US5763483, 2008.
- 22.Li N., Shi Z., Tang Y., Shi Q., Zhang W., Zhang P. Curr. Org. Chem. 2014;18:2125–2138. [Google Scholar]
- 23.Abrecht S., Federspiel M.C., Estermann H., Fischer R., Karpf M., Mair H.-J., Oberhauser T., Rimmler G., Trussardi R., Zutter U. Chim Int J Chem. 2007;61:93–99. [Google Scholar]
- 24.Shibasaki M., Kanai M. Eur. J. Org Chem. 2008:1839–1850. [Google Scholar]
- 25.Abrecht S., Harrington P., Iding H., Karpf M., Wirz B., Zutter U. Chim Int J Chem. 2004;58:621–629. [Google Scholar]
- 26.Gong J., Xu W. Curr. Med. Chem. 2008;15:3145–3159. doi: 10.2174/092986708786848497. [DOI] [PubMed] [Google Scholar]
- 27.Limbani B., Bera S., Mondal D. Chemistry. 2020;5:6083–6122. [Google Scholar]
- 28.Kalashnikov A.I., Sysolyatin S.V., Sakovich G.V., Sonina IAS E.G. Russ Chem Bull Int Ed. 2013;62:163–170. [Google Scholar]
- 29.Draths K.M., Knop D.R., Frost J.W. J. Am. Chem. Soc. 1999;121:1603–1604. [Google Scholar]
- 30.Karpf M., Trussardi R. Angew. Chem. Int. Ed. 2009;48:5760–5762. doi: 10.1002/anie.200901561. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida N., Ogasawara K. Org. Lett. 2000;2:1461–1463. doi: 10.1021/ol005805q. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa H., Bondzic B.P., Hayashi Y. Eur. J. Org Chem. 2011:6020–6031. [Google Scholar]
- 33.Kanai Motomu, Shin Kamijo L.Y.C. Angew. Chem. Int. Ed. 2009;48:1070–1076. doi: 10.1002/anie.200804777. [DOI] [PubMed] [Google Scholar]
- 34.Werner L., Machara A., Hudlicky T. Adv. Synth. Catal. 2010;352:195–200. [Google Scholar]
- 35.rgen Haase J ü. 2010. Organic Azides: Syntheses and Applications. [Google Scholar]
- 36.Chuang T., Chen Y., Pola S. J. Org. Chem. 2010;75:6625–6630. doi: 10.1021/jo101394c. [DOI] [PubMed] [Google Scholar]
- 37.Smith C.J., Smith C.D., Nikbin N., Ley S.V. Baxendale IR. Org. Biomol. Chem. 2011;9:1927–1937. doi: 10.1039/c0ob00813c. [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick D.E., Battilocchio C., Ley S.V. ACS Cent. Sci. 2016;2:131–138. doi: 10.1021/acscentsci.6b00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohloff J.C., Kent K.M., Postich M.J., Becker M.W., Chapman H.H., Kelly D.E., Lew W., Louie M.S., McGee L.R., Prisbe E.J., Schultze L.M., Yu R.H., Zhang L. J. Org. Chem. 1998;63:4545–4550. [Google Scholar]
- 40.Zutter U., Iding H., Spurr P., Wirz B. J. Org. Chem. 2008;73:4895–4902. doi: 10.1021/jo800264d. [DOI] [PubMed] [Google Scholar]
- 41.Karpf M., Trussardi R. J. Org. Chem. 2001;66:2044–2051. doi: 10.1021/jo005702l. [DOI] [PubMed] [Google Scholar]
- 42.Abrecht, S., Karpf, M., Trussardi, R., Wirz, B. EP1127 872A1, 2001.
- 43.Federspiel M., Fischer R., Hennig M., Mair H.-J., Oberhauser T., Rimmler G., Albiez T., Bruhin J., Estermann H., Gandert C., Göckel V., Götzö S., Hoffmann U., Huber G., Janatsch G., Lauper S., Röckel-Stäbler O., Trussardi R., Zwahlen A.G. Org. Process Res. Dev. 1999;3:266–274. [Google Scholar]
- 44.Trussardi R. WO2009/037137A3. 2009 [Google Scholar]
- 45.Harrington P.J., Brown J.D., Foderaro T., Hughes R.C. Org. Process Res. Dev. 2004;8:86–91. [Google Scholar]
- 46.Federspiel M., Fischer R., Hennig M., Mair H.-J., Oberhauser T., Rimmler G., Albiez T., Bruhin J., Estermann H., Gandert C., Gockel V., Gotzo S., Hoffmann U., Huber G., Janatsch G., Lauper S., Rockel-Stabler O., Trussardi R., Zwahlen A.G. Org. Process Res. Dev. 1999;3:266–274. [Google Scholar]
- 47.Yamatsugu K., Yin L., Kamijo S., Kimura Y., Kanai M., Shibasaki M. Angew. Chem. Int. Ed. 2009;48:1070–1076. doi: 10.1002/anie.200804777. [DOI] [PubMed] [Google Scholar]
- 48.Nie L., Shi X. Tetrahedron: Asymmetry. 2009;20:124–129. [Google Scholar]
- 49.Nie L., Shi X., Ko K.H., Lu W. J. Org. Chem. 2009;74:3970–3973. doi: 10.1021/jo900218k. [DOI] [PubMed] [Google Scholar]
- 50.Nie L.D., Shi X.X., Quan N., Wang F.F., Lu X. Tetrahedron Asymmetry. 2011;22:1692–1699. [Google Scholar]
- 51.Nie L., Ding W., Shi X., Quan N., Lu X. Tetrahedron: Asymmetry. 2012;23:742–747. [Google Scholar]
- 52.Sagandira C.R., Watts P. J Flow Chem. 2019;9:79–87. [Google Scholar]
- 53.Sagandira C.R., Watts P. Synlett. 2020 doi: 10.1055/s-0039-1690878. [DOI] [Google Scholar]
- 54.Nie L., Wang F., Ding W., Shi X., Lu X. Tetrahedron: Asymmetry. 2013;24:638–642. [Google Scholar]
- 55.Akwi F.M., Watts P. Chem. Commun. 2018;54:13894–13928. doi: 10.1039/c8cc07427e. [DOI] [PubMed] [Google Scholar]
- 56.Porta R., Benaglia M., Puglisi A. Org. Process Res. Dev. 2016;20:2–25. [Google Scholar]
- 57.Diab S., McQuade D.T., Gupton B.F., Gerogiorgis D.I. Org. Process Res. Dev. 2019;23:320–333. doi: 10.1021/acs.oprd.8b00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hughes D.L. Org. Process Res. Dev. 2018;22:13–20. [Google Scholar]
- 59.de Souza R.O.M.A., Watts P. J Flow Chem. 2017;7:146–150. [Google Scholar]
- 60.de Souza J.M., Galaverna R., de Souza A.A.N., Brocksom T.J., Pastre J.C., de Souza R.O.M.A., de Oliveira K.T. An. Acad. Bras. Cienc. 2018;90:1131–1174. doi: 10.1590/0001-3765201820170778. [DOI] [PubMed] [Google Scholar]
- 61.Suveges N.S., Rodriguez A.A., Diederichs C.C., de Souza S.P., Leão R.A.C., Miranda L.S.M., Horta B.A.C., Pedraza S.F., de Carvalho O.V., Pais K.C., Terra J.H.C., de Souza R.O.M.A. Eur. J. Org Chem. 2018;2018:2931–2938. [Google Scholar]
- 62.Verghese J., Kong C.J., Rivalti D., Yu E.C., Krack R., Alcázar J., Manley J.B., McQuade D.T., Ahmad S., Belecki K., Gupton B.F. Green Chem. 2017;19:2986–2991. [Google Scholar]
- 63.Ziegler R.E., Desai B.K., Jee J.A., Gupton B.F., Roper T.D., Jamison T.F. Angew. Chem. Int. Ed. 2018;57:7181–7185. doi: 10.1002/anie.201802256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bogdan A.R., Dombrowski A.W. J. Med. Chem. 2019;62:6422–6468. doi: 10.1021/acs.jmedchem.8b01760. [DOI] [PubMed] [Google Scholar]
- 65.Brocklehurst C.E., Vecchia L.L.A., Lehmann H. Chim Oggi - Chem Today. 2014;32:30–35. [Google Scholar]
- 66.Sagandira C.R., Watts P. Eur. J. Org Chem. 2017;2017:6554–6565. [Google Scholar]
- 67.Sagandira C.R., Watts P. J Flow Chem. 2018;8:69–79. [Google Scholar]
- 68.Sagandira C.R., Watts P. Beilstein J. Org. Chem. 2019;15:2577–2589. doi: 10.3762/bjoc.15.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ehrfeld W., Hessel V., Löwe H. WILEY-VCH Verlag GmbH; Weinheim: 2001. Microreactors: New Technology for Modern Chemistry. [Google Scholar]
- 70.Carr R., Ciccone F., Gabel R., Guinn M., Johnston D., Mastriona J., Vandermeer T., Groaning M. Green Chem. 2008;10:743–745. [Google Scholar]
- 71.Zheng Q., Huang A. CN103304437A. 2013 [Google Scholar]
- 72.Brion F. Tetrahedron Lett. 1982;23:5299–5302. [Google Scholar]
- 73.Yamashita Y., Hanaya K., Sugai T., Mizushima T., Shoji M. Tetrahedron. 2013;69:6527–6532. [Google Scholar]
- 74.Alagiri K., Furutachi M., Yamatsugu K., Kumagai N., Watanabe T., Shibasaki M. J. Org. Chem. 2013;78:4019–4026. doi: 10.1021/jo400360j. [DOI] [PubMed] [Google Scholar]
- 75.Furutachi M., Kumagai N., Watanabe T., Shibasaki M. Tetrahedron. 2014;70:9113–9117. [Google Scholar]
- 76.Morita M., Sone T., Yamatsugu K., Sohtome Y., Matsunaga S., Kanai M., Watanabe Y., Shibasaki M. Bioorg. Med. Chem. Lett. 2008;18:600–602. doi: 10.1016/j.bmcl.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 77.Yamatsugu K., Kamijo S., Suto Y., Kanai M., Shibasaki M. Tetrahedron Lett. 2007;48:1403–1406. [Google Scholar]
- 78.Fukuta Y., Mita T., Fukuda N., Kanai M., Shibasaki M. J. Am. Chem. Soc. 2006;128:6312–6313. doi: 10.1021/ja061696k. [DOI] [PubMed] [Google Scholar]
- 79.Becker R., Koch K., Nieuwland P.J., FPJT Rutjes. Chim Oggi/Chemistry Today. 2012;30:6–8. [Google Scholar]
- 80.Li H., Shen S.J., Zhu C.L., Xu H. J. Am. Chem. Soc. 2018;140:10619–10626. doi: 10.1021/jacs.8b06900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Danishefsky S., Prisbylla M.P., Hiner S.S. J. Am. Chem. Soc. 1978;100:2918. [Google Scholar]
- 82.Yeung Y.Y., Hong S., Corey E.J. J. Am. Chem. Soc. 2006;128:6310–6311. doi: 10.1021/ja0616433. [DOI] [PubMed] [Google Scholar]
- 83.Kipassa N.T., Okamura H., Kina K., Hamada T., Iwagawa T. Org. Lett. 2008;10:815–816. doi: 10.1021/ol7029646. [DOI] [PubMed] [Google Scholar]
- 84.Trajkovic M., Ferjancic Z., Saicic R.N. Synth. Met. 2013;45:389–395. [Google Scholar]
- 85.Bhowmik S., Batra S. Eur. J. Org Chem. 2013:7145–7151. [Google Scholar]
- 86.Satoh N., Akiba T., Yokoshima S., Fukuyama T. Tetrahedron. 2009;65:3239–3245. [Google Scholar]
- 87.Satoh N., Akiba T., Yokoshima S., Fukuyama T. Angew. Chem. Int. Ed. 2007;46:5734–5736. doi: 10.1002/anie.200701754. [DOI] [PubMed] [Google Scholar]
- 88.Chen S.H., Chang C.H., Fang J.M. Tetrahedron Lett. 2016;57:4293–4296. [Google Scholar]
- 89.Balestri D., Grilli S., Romano C., Savoia D. Eur. J. Org Chem. 2014;2014:8021–8025. [Google Scholar]
- 90.Hayashi Y., Ogasawara S. Org. Lett. 2016:3426–3429. doi: 10.1021/acs.orglett.6b01595. [DOI] [PubMed] [Google Scholar]
- 91.Hayato I., Takaki S., Orita H., Uchimaru T., Hayashi Y. Chem. Eur J. 2010;16:12616–12626. doi: 10.1002/chem.201001108. [DOI] [PubMed] [Google Scholar]
- 92.Ogasawara S., Hayashi Y. Synthesis. 2017;49:424–428. [Google Scholar]
- 93.Ishikawa H., Suzuki T., Hayashi Y. Angew. Chem. Int. Ed. 2009;48:1304–1307. doi: 10.1002/anie.200804883. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka T., Tan Q., Kawakubo H., Hayashi M. J. Org. Chem. 2011;76(13):5477–5479. doi: 10.1021/jo200698g. 2011; 76. [DOI] [PubMed] [Google Scholar]
- 95.Zhu S., Yu S., Wang Y., Ma D. Angew. Chem. Int. Ed. 2010;49:4656–4660. doi: 10.1002/anie.201001644. [DOI] [PubMed] [Google Scholar]
- 96.Kockmann N., Roberge D.M. Chem Eng Process Process Intensif. 2011;50:1017–1026. [Google Scholar]
- 97.Peshkovsky A.S., Bystryak S. Chem Eng Process Process Intensif. 2014;82:132–136. [Google Scholar]
- 98.Roberge D.M., Gottsponer M., Eyholzer M., Kockmann N. Chim. Oggi. 2009;27:8–11. [Google Scholar]
- 99.Kockmann N., Gottsponer M., Roberge D.M. Chem. Eng. J. 2011;167:718–726. [Google Scholar]
- 100.Saber M., Commenge J.M., Falk L. Chem. Eng. Sci. 2010;65:372–379. [Google Scholar]
- 101.Shie J.J., Fang J.M., Wang S.Y., Tsai K.C., Cheng Y.S.E., Yang A.S., Hsiao S.C., Su C.Y., Wong C.H. J. Am. Chem. Soc. 2007;129:11892–11893. doi: 10.1021/ja073992i. [DOI] [PubMed] [Google Scholar]
- 102.Osato H., Jones I.L., Chen A., Chai C.L.L. Org. Lett. 2010;12:60–63. doi: 10.1021/ol9024716. [DOI] [PubMed] [Google Scholar]
- 103.Ko J.S., Keum J.E., Ko S.Y. J. Org. Chem. 2010;75:7006–7009. doi: 10.1021/jo101517g. [DOI] [PubMed] [Google Scholar]
- 104.Ma J., Zhao Y., Ng S., Zhang J., Zeng J., Than A., Chen P., Liu X.W. Chem. Eur J. 2010;16:4533–4540. doi: 10.1002/chem.200902048. [DOI] [PubMed] [Google Scholar]
- 105.Kongkathip B., Akkarasamiyo S., Kongkathip N. Tetrahedron. 2015;71:2393–2399. [Google Scholar]
- 106.Baumann M., Moody T.S., Smyth M., Wharry S. Org. Process Res. Dev. 2020 doi: 10.1021/acs.oprd.9b00524. [DOI] [PMC free article] [PubMed] [Google Scholar]