Abstract
Introduction
Emery-Dreifuss muscular dystrophy (EDMD) is a disease characterized by skeletal muscle wasting, major tendon contractures, and cardiac conduction defects. Mutations in the gene encoding emerin cause EDMD1. Our previous studies suggested that emerin activation of histone deacetylase 3 (HDAC3) to reduce histone 4-lysine 5 (H4K5) acetylation (ac) is important for myogenic differentiation.
Methods
Pharmacological inhibitors (Nu9056, L002) of histone acetyltransferases targeting acetylated H4K5 were used to test whether increased acetylated H4K5 was responsible for the impaired differentiation seen in emerin-deficient myogenic progenitors.
Results
Nu9056 and L002 rescued impaired differentiation in emerin deficiency. SRT1720, which inhibits the nicotinamide adenine dinucleotide (NAD)+-dependent deacetylase sirtuin 1 (SIRT1), failed to rescue myotube formation.
Discussion
We conclude that emerin regulation of HDAC3 activity to affect H4K5 acetylation dynamics is important for myogenic differentiation. Targeting H4K5ac dynamics represents a potential new strategy for ameliorating the skeletal muscle wasting seen in EDMD1.
Keywords: cell signaling, emerin, Emery-Dreifuss muscular dystrophy, myogenic differentiation
1 |. INTRODUCTION
The nuclear envelope is composed of two lipid bilayers, the outer nuclear membrane, which is contiguous with the endoplasmic reticulum, and the inner nuclear membrane.1 Although the outer and inner nuclear membranes arise from a common membrane, they are functionally distinct. Underlying the inner nuclear membrane is a network of type V intermediate filament proteins named lamins that provide nuclear rigidity and elasticity.2 The inner nuclear membrane contains a large number of unique integral inner nuclear membrane proteins,3 many of which show cell-type–specific expression.4–11 Inner nuclear membrane proteins function in diverse roles, including nuclear structure, genomic organization, chromatin architecture, gene expression, cell cycle regulation, and cytoskeletal organization.1,12 The nuclear lamins and their associated inner nuclear membrane proteins define the nuclear lamina.
Emerin is a lamin-binding, integral inner nuclear membrane protein. Mutations in the gene encoding emerin cause X-linked Emery-Dreifuss muscular dystrophy (EDMD1), an inherited disorder causing progressive skeletal muscle wasting, irregular heart rhythms, and contractures of major tendons.13–16 Mutations in LMNA, which encodes lamins A and C, cause an autosomal-dominant form of EDMD, EDMD2.1,2 Evidence suggests the skeletal muscle wasting seen in EDMD is not caused by increased damage to the myofiber, but by impaired differentiation of skeletal muscle stem cells. Supporting this hypothesis, skeletal muscle necrosis and increased skeletal muscle fiber permeability are rarely seen in EDMD patients.17 Further, emerin knockout mice (also commonly referred to as emerin-null or emerin-deficient mice) exhibit delayed skeletal muscle regeneration and repair, motor coordination defects, and mild atrioventricular conduction defects.18,19 Skeletal muscle from EDMD1 and EDMD2 patients and emerin-deficient mice both showed altered expression of muscle regeneration pathway components.18,20 Emerin-deficient myogenic progenitors and emerin-downregulated C2C12 myoblasts exhibit impaired differentiation and myotube formation21–23 due to aberrant temporal activation of myogenic differentiation genes24 and disruption of key signaling pathways,25 suggesting defective muscle regeneration contributes to the EDMD skeletal muscle phenotype.18,21,22
The coordinated temporal expression of MyoD, Myf5, Pax3, and Pax7, which are important for proper differentiation, is disrupted in emerin-deficient myogenic progenitors,26 due to the inability of the genome to properly reorganize during differentiation.18,20,25 This supports the hypothesis that emerin-deficient myogenic progenitors fail to undergo the transcriptional reprogramming required for myogenic differentiation. Furthermore, emerin was shown to bind directly to histone deacetylase 3 (HDAC3) and activate its deacetylase activity.27 HDAC3 activity is required for proper dynamic reorganization of MyoD, Myf5, Pax3, and Pax7.26 Thus, regulation of HDAC3 activity by emerin is critical for transcriptional reprogramming during myogenic differentiation.
We used histone acetyltransferase (HAT) inhibitors targeting HATs mediating H4K5 acetylation (eg, Tip60/KAT5) to further test the hypothesis that acetylation dynamics on lysine 5 of histone 4 (H4K5) were important for myogenic differentiation. Herein we show that increased H4K5 acetylation (H4K5ac) contributes to the impaired differentiation of emerin-deficient myogenic progenitors. Targeting H4K5ac dynamics represents a potential new strategy for ameliorating the skeletal muscle wasting seen in EDMD1.
2 |. METHODS
2.1 |. Pharmacological treatments
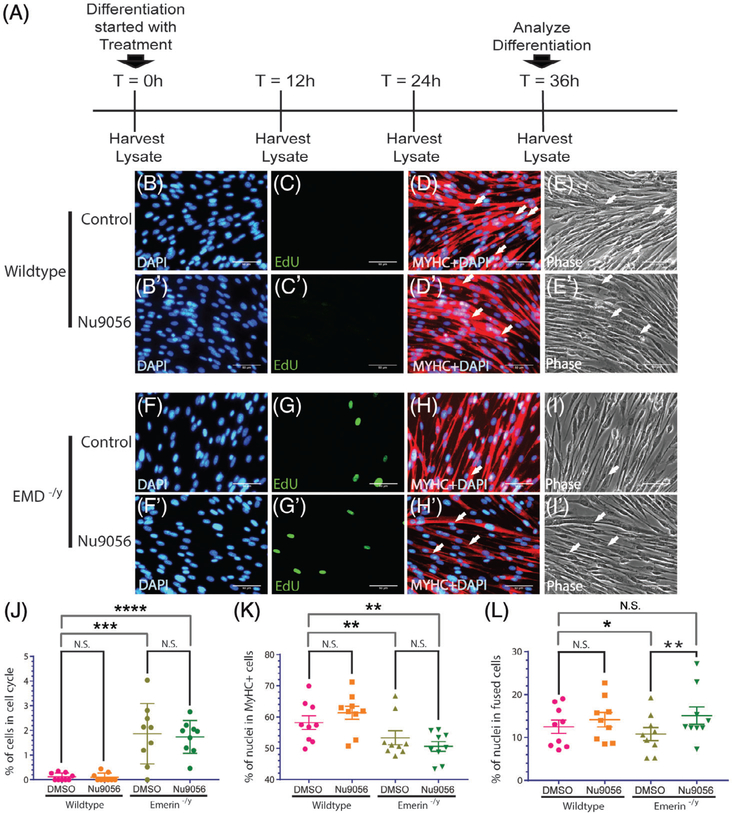
In previous work we showed that emerin-deficient myogenic progenitors had impaired differentiation and were rescued by activation of HDAC3.23 We chose to inhibit HATs to independently test whether altered H4K5 acetylation dynamics was responsible for the impaired differentiation of emerin-deficient progenitors. HAT inhibitors (HATi) selected for these studies were chosen because they preferentially inhibit acetylation of lysine residues targeted by HDAC3 (eg, H4K5).28 Cell cycle withdrawal, myosin heavy chain (MyHC) expression, and myotube formation were analyzed 36 hours after differentiation induction, as described previously.23 HAT inhibitor Nu9056 was selected because it is a highly specific inhibitor of histone acetyltransferase Tip60/KAT5.29 Tip60/KAT5 mediates the acetylation of H4K5, H4K8, H4K12, and H4K16 (see Table S1 online). A second HAT inhibitor, L002, was used to test whether inhibition of H4K5 acetylation rescued myogenic differentiation of emerin-deficient progenitors. L002 inhibits H4 acetylation in cells at low micromolar concentrations (see Table S1 online).30 A sirtuin 1 (SIRT1) activator (SRT1720) was used to confirm that the rescue of emerin-deficient progenitor differentiation was due to changes in acetylation states of HDAC3 target residues (eg, H4K5ac). Unlike HDAC3, SIRT1 is an nicotinamide adenine dinucleotide (NAD)+-dependent protein deacetylase.31 SIRT1 deacetylates H3K9ac, but does not affect H4K5, H4K8, or H4K12 acetylation.32 1.5 μmol/L of SRT1720 was added to wild-type or emerin-deficient myogenic progenitors upon differentiation induction and differentiation was analyzed after 36 hours (Figure 1A).
FIGURE 1.
Inhibition of HAT activity with Nu9056 treatment rescues myotube formation in emerin-deficient myogenic progenitors. A, Timeline showing the time-point Nu9056 was added and whole cell lysate collection for Western blot analysis. Representative images at 40× magnification of vehicle-treated wild-type (B-E) or emerin-deficient (F-I) and Nu9056-treated wild-type (B’-E’)or emerin-deficient (F’-I’) cells 36 hours after initiating differentiation. Arrows mark myotubes (eg, three myotubes in I0 vs one myotube in I). J-L, Quantification of >500 nuclei for each experimental treatment (n = 3) was carried out to determine the percentage of myogenic progenitors in the cell cycle (J), expressing MyHC (K), and formed tubes (L) 36 hours after inducing differentiation. Results are expressed as mean ± standard deviation (n = 3). Scale bars = 50 μm. HAT, histone acetyltransferase; MyHC, myosin heavy chain; N.S., not significant. *P < .05; **P < .01; ***P < .001; ****P < .0001 using paired, two-tailed t tests
Concentrations of compounds used in this study were based on the reported half-maximal inhibitory concentration (IC50) for each com pound29,30,33,34 and according to the manufacturer’s instructions (EMD Millipore). Next, 0.25–30 μmol/L of each compound was added to wild-type and emerin-deficient myogenic progenitors and tested for viability and impaired proliferation. The concentrations used in this study failed to inhibit cell proliferation and had no effect on cell viability. A 1.0-mmol/L stock solution of L002 in dimethylsulfoxide (DMSO) was added to a final concentration of 0.5 μmol/L in differentiation medium. A 1.0-mmol/L stock solution of Nu9056 in DMSO was added to a final concentration of 0.5 μmol/L in differentiation medium. A 3.0-mmol/L stock solution of SRT1720 in DMSO was added to a final concentration of 1.5 μmol/L in differentiation medium. Differentiation media containing each inhibitor or DMSO was added to induce differentiation of wild-type or emerin-deficient myogenic progenitors.
2.2 |. Cell culture
Wild-type and emerin-null H2K mouse myogenic progenitors were a generous gift from Tatiana Cohen and Terence Partridge (Children’s National Medical Center, Washington, DC). Emerin-null H2K mice were generated by Tatiana Cohen and Terence Partridge by breeding emerin-null (C57Bl/6) and H-2KbtsA58 mice to create emerin-deficient mice in the H-2KbtsA58 background.35,36 Myogenic progenitors were isolated and maintained as previously described.24,25,36 Briefly, extensor digitorum longus (EDL) muscles were isolated and placed into 2 mg/mL collagenase (Product #C0130, Sigma) in Dulbecco’s modified Eagle medium (DMEM; Product #11995–065, Invitrogen) for 1–2 hours at 35 C. Individual fibers were then isolated and each fiber was transferred serially through 2–4 Petri dishes containing DMEM to select for undamaged fibers. Fibers were placed into Matrigel-coated Petri dishes containing DMEM, 10% horse serum (Product #16050–098, Invitrogen), 0.5% chick embryo extract (Product #CE6507, Accurate Chemical), 2% L-glutamine (Product #25030–081, Invitrogen), and 1% penicillin/streptomycin (Product #15140–122, Invitrogen) for 3–4hoursat37 C. Myogenic progenitors were isolated from individual fibers by transferring each fiber into one Matrigel-coated well of a 24-well plate containing proliferation media consisting of DMEM, 20% heat-inactivated fetal bovine serum (FBS; Product #10082–147, Invitrogen,), 2% chick embryo extract, 2% L-glutamine, 1% penicillin/streptomycin, and 20 ng/mL γ-interferon (Product #IF005, Millipore). The fibers were incubated for 24–48 hours at 33 C and 10% CO2. Upon attachment of a single myogenic progenitor to the well, the fiber was removed and the myogenic progenitor was incubated in proliferation media for another 48 hours at 33 C and 10% CO2. Approximately 200 cells are expected after 48 hours and these were split and proliferated until enough cells were obtained for our analyses. H2K myogenic progenitors were maintained in proliferation media at 33 Cand10%CO2.Cellsbetweenpassages4and10wereusedfor these studies.
Cell cultures for proliferation and differentiation of H2Ks were done as described previously.23 Briefly, for proliferation, wild-type and emerin-deficient H2K myogenic progenitors were seeded onto tissue-culture plates (Falcon Catalog Nos. 353046 and 3530003) and maintained at 33°C and 10% CO2 in proliferation medium (high-glucose DMEM supplemented with 20% heat-inactivated FBS, 2% L-glutamine, 2% chick embryo extract, 1% penicillin/streptomycin, sodium pyruvate, and 20 U/mL γ-interferon; ThermoFisher Scientific). The plates were coated with 0.01% gelatin (Sigma-Aldrich) before seeding.
Wild-type and emerin-deficient H2K myogenic progenitors were seeded onto 12-well tissue-culture plates coated with 0.01% gelatin (Sigma-Aldrich) for differentiation induction. Cells were seeded at 23 500 cells/cm2 in proliferation media for 24 hours at 33°C and 10% CO2. Differentiation was stimulated by replacing the proliferation medium with differentiation medium (high-glucose DMEM with sodium pyruvate, 5% horse serum, and 2% L-glutamine; ThermoFisher Scientific). The cells were maintained at 37 C and 5% CO2 throughout differentiation.
2.3 |. 5-Ethynyl-2’-deoxyuridine assays and immunofluorescence microscopy
Cells were treated with 10 μmol/L 5-ethynyl-2’-deoxyuridine (EdU; ThermoFisher Scientific) in DMSO for 2 hours before fixing, while incubating at 37 C and 5% CO2. Cells were then fixed with 3.7% formaldehyde for 15 minutes and washed three times with phosphate-buffered saline (PBS). Fixed cells were then stored at 4°C with 0.1% sodium azide in PBS. The cells were permeabilized with 0.5% Triton X-100 in PBS for 20 minutes, washed twice with 3% bovine serum albumin (BSA) in PBS for 5 minutes per wash, and treated with the Click-IT EdU reaction cocktail for 25 minutes. Cells were washed with PBS and blocked for 1 hour at room temperature with 3% BSA with 0.1% Triton X-100. Myosin heavy chain (MyHC) antibodies (1:20 [Santa Cruz Biotechnologies], H-300 for L002 and Nu506 experiments; 1:50 [Santa Cruz Biotechnologies], B-5 for SRT1720 treatments) were added and the cells were incubated at room temperature for 1 hour. The cells were washed with PBS three times and treated with Alexa Fluor 594 secondary antibodies (1:200; C10637 and A11032, ThermoFisher Scientific) at room temperature for 1 hour, washed with PBS, and incubated with 4’,6- diamidino-2-phenylindole (DAPI) for 5 minutes.
Images were taken using the EVOS-FL imaging system (ThermoFisher Scientific) for experiments with L002. The remainder of the images were taken with the EVOS-FL Auto (ThermoFisher Scientific). All images were obtained using a long working distance 40× objective. At least three replicates, with each replicate containing three culture wells per group, were done for each drug treatment. Images from five different sections from each well were taken, with each section containing approximately 50–200 cells. The total number of cells analyzed for each experiment ranged between 500 and 1500.
The cell counter plugin on ImageJ was used to count proliferating cells. The percent of cells still in the cell cycle was determined by dividing the number of EdU-positive nuclei by the total number of nuclei. The DAPI and MyHC images were superimposed to calculate the percentage of cells expressing MyHC. Myotube formation was determined by superimposing the phase-contrast image, which allowed for monitoring nuclei within a shared cytoplasm, with DAPI and MyHC images. Myotube formation, or the differentiation index, was determined by counting the number of nuclei in MyHC-positive cells that contained three or more nuclei divided by the total number of nuclei in the field.
2.4 |. Western blotting
Differentiating H2K cells were resuspended directly in sample buffer and 50 000–100 000 cell equivalents were separated by sodium dodecylsulfate polyacrylamide–gel electrophoresis and transferred to a nitrocellulose membrane. The membranes were blocked either at room temperature for 2 hours or overnight at 4°C in 3% BSA in PBS with 0.1% Tween (PBST). Antibodies against H4 (1:50 000; 05–858, Millipore), H4K5ac (1:1000; 07–327, Millipore), H3K9ac (1:10 000; ab4441, Abcam), H3K18ac (1:1000; ab1191, Abcam), H3K27ac (1:1000; ab4729, Abcam), H4K16ac (1:2000; ab109463, Abcam), and MyHC (1:1000; B-5, Santa Cruz) were then incubated either at room temperature for 2 hours or overnight at 4°C. The membranes were washed three times in PBS and incubated with goat anti-rabbit horseradish peroxidase (HRP) or goat anti-mouse HRP secondary antibody (1:10 000; ThermoFisher Scientific) in PBST, either at room temperature for 2 hours or overnight at 4°C. The membranes were treated with electrochemiluminescence detection reagent (Products # RPN2106V1 and RPN2106V20, GE Healthcare; and imaged using the Chemidoc system, Bio-Rad Laboratories). Densitometry was done using ImageLab software (Bio-Rad Laboratories) according to the manufacturer’s instructions.
3 |. RESULTS
Wild-type and emerin-deficient myogenic progenitors were differentiated for 36 hours in the presence of the HAT inhibitor Nu9056 to confirm independently that HAT inhibition rescued emerin-deficient myogenic differentiation. Nu9056 0.5 μmol/L in DMSO or DMSO alone was incubated with wild-type or emerin-deficient myogenic progenitors upon differentiation induction (Figure 1A). Nu9056 treatment had no effect on cell cycle withdrawal of wild-type or emerin-deficient myogenic progenitors (Figure 1C,G,J). Nu9056 treatment failed to rescue myoblast commitment, as the number of MyHC-expressing cells was similar in Nu9056-treated (51.0%) and untreated emerin-deficient myogenic progenitors (50.3%; Figure 1H,K). Myotube formation in emerin-deficient progenitors was rescued by Nu9056 treatment, as 15.1% of Nu9056-treated emerin-deficient progenitors fused to form myotubes, compared with 10.8% of DMSO-treated emerin-deficient progenitors (Figure 1I,L). Myotube formation in Nu9056-treated emerin-deficient progenitors was statistically similar to that of wild-type progenitors (P = .11).
A second HAT inhibitor, L002, was used to test whether inhibition of H4K5 acetylation rescued myogenic differentiation of emerin-deficient progenitors. Wild-type and emerin-deficient myogenic progenitors were treated with 0.5 μmol/L L002 upon differentiation induction (Figure 1A). L002-treated wild-type progenitors exited the cell cycle normally (Figure 2B’,I). Not unexpectedly, 2.7% of emerin-deficient progenitors failed to exit the cell cycle after 36 hours (Figure 2F,I). Also, 2.1% of emerin-deficient progenitors treated with L002 remained in the cell cycle (Figure 2F’,I). L002 treatment significantly increased the percentage of differentiating emerin-deficient progenitors expressing MyHC (46%; Figure 2G,J; P = .015). The number of MyHC-positive cells in L002-treated differentiating emerin-deficient progenitors was statistically similar to that of untreated wild-type progenitors (47.8% in wild-type, P = 0.35; Figure 2C,G’,J), indicating rescue of myoblast commitment. L002 treatment increased myotube formation 1.8-fold in differentiating emerin-deficient progenitors (Figure 2H,K) completely rescuing myotube formation to wild-type levels (P = .97 for L002-treated emerin-deficient cells vs wild-type cells; Figure 2D,H’,K).
FIGURE 2.
Inhibition of HAT activity with L002 treatment rescues myotube formation and myosin heavy chain expression in emerin-deficient myogenic progenitors (A-D) or emerin-deficient (E-H) and L002-treated wild-type (A’-D’) or emerin-deficient (E’-H’) cells 36 hours after initiating differentiation. Arrows mark myotubes (eg, four myotubes in H’ vs 1 myotube in H). I-K, Quantification of >500 nuclei for each experimental treatment (n = 4) was carried out to determine the percentage of myogenic progenitors in the cell cycle (I), expressing MyHC (J), and formed tubes (K) 36 hours after inducing differentiation. Results are expressed as mean ± standard deviation (n = 4). HAT, histone acetyltransferase; MyHC, myosin heavy chain; N.S., not significant; *P < .05, **P < .01, ***P < .001, and ****P < .0001 using paired, two-tailed t tests. Scale bars = 50 μm
A SIRT1 activator (SRT1720) was used to confirm that the rescue of emerin-deficient progenitor differentiation was due to changes in acetylation states of HDAC3 target residues (eg, H4K5ac). Activation of SIRT1 by treatment with 1.5 μmol/L SRT1720 failed to rescue cell cycle withdrawal of differentiating emerin-deficient progenitors, as 7.0% of DMSO-treated and 5.4% of SRT1720-treated cells were cycling (Figure 3F,I; P = .09). Also, 41.1% of SRT1720-treated differentiating emerin-deficient progenitors expressed MyHC compared with 42.7% of DMSO-treated emerin-deficient progenitors (Figure 3G,J; P = .49). SRT1720 treatment also failed to rescue myotube formation in emerin-deficient progenitors (Figure 3H, K; P = .44).
FIGURE 3.
SIRT1 activation with SRT1720 treatment does not change cell cycle exit, myosin heavy chain expression, or myotube formation in emerin-deficient myogenic progenitors (A-D) or emerin-deficient (E-H) and SRT1720-treated wild-type (A’-D’)or emerin-deficient (E’-H’) cells 36 hours after initiating differentiation. Arrows indicate myotubes (eg, 1 myotube in H’ and H). I-K, Quantification of more than 500 nuclei for each experimental treatment (n = 3) was carried out to determine the percentage of myogenic progenitors in the cell cycle (I), expressing MyHC (J), and formed tubes (K) 36 hours after inducing differentiation. Results are expressed as mean ± standard deviation (n = 3). Scale bars = 50 μm. DMSO, dimethylsulfoxide; MyHC, myosin heavy chain; N.S., not significant; SIRT1, sirtuin 1. *P < .05 and **P < .01 using paired two-tailed t tests
Treatment of emerin-deficient progenitors with L002 during differentiation reduced H4K5ac by 3.8-fold (Figure 4A,B), comparable with H4K5ac levels in wild-type progenitors. Nu9056 treatment decreased H4K5ac 3.2-fold in emerin-deficient myogenic progenitors (Figure 4A,B). Decreased H4K5ac seen in emerin-deficient myogenic progenitors treated with Nu9056 is similar to the H4K5ac levels seen in differentiating wild-type progenitors. Western blotting confirmed the levels of H4K5ac were unchanged by treatment with SRT1720 (Figure 4C,D).
FIGURE 4.
H4K5 acetylation is decreased by treatment with L002 and Nu9056. Western blotting of whole cell lysates treated with: Nu9056, L002 (A) or SRT1720 (B) to analyze H4K5 acetylation during differentiation of wild-type and emerin-deficient progenitors. DMSO-only treatment was the control. Three biological replicates are shown for each treatment. B,D, Densitometry was performed, and acetylated H4K5 in each sample was normalized to total H4 protein in each sample. Levels of acetylated H4K5 for each condition were normalized to DMSO-treated cells. Results are expressed as mean ± standard deviation (n = 3 for each condition); DMSO, dimethylsulfoxide; H4K5, histone 4 lysine 5; N.S., not significant using paired two-tailed t tests
Acetylation of H3K9, H3K18, H3K27, and H4K16 were monitored during impaired differentiation in emerin deficiency in the presence of Nu9056, L002, and SRT1720. H3K9ac, H3K18ac, and H3K27ac were all increased in emerin-deficient myogenic progenitors and during emerin-deficient myogenic differentiation (Figure 5), including during the transition to myoblast commitment. Treatment of emerin-deficient progenitors with Nu9056 had no significant effect on acetylation of H3K9, H3K18, H3K27, or H4K16 (Figure 5). L002 treatment had a minor effect on H3K18 and H3K27 acetylation. Treatment with the SIRT1 activator, SRT1720, reduced H3K9ac activity.
FIGURE 5.
Acetylation of H4K16, H3K9, H3K18, or H3K27 in emerin-deficient myogenic progenitors upon treatment with L002, Nu9056, or SRT1720. Wild-type or emerin-deficient myogenic progenitors were treated with DMSO, L002, Nu9056, or SRT1720, and whole cell lysates were obtained after 36 hours. A, Western blotting was done with the indicated antibodies to monitor histone acetylation. B, Quantitation of histone acetylation normalized to γ-tubulin and plotted as fold-change in emerin-deficient cells, as compared with wild-type cells. Results are expressed as mean ± standard deviation for each condition. DMSO, dimethylsulfoxide; H3K9, histone 3 lysine 9; H3K18, histone 3 lysine 18; H3K27, histone 3 lysine 27; H4K16, histone 4 lysine 16; N.S., not significant; *P ≤ .05 using paired two-tailed t tests
4 |. DISCUSSION
Upon serum withdrawal, transcriptional reprogramming of myogenic progenitors is initiated and these cells exit the cell cycle. Transcriptional reprogramming activates the myogenic differentiation program and represses the proliferative program, thereby committing myoblasts to differentiation.37 This reprogramming is compromised in emerin-deficient progenitors.38 The functional interaction between emerin and HDAC3 coordinates the spatiotemporal nuclear envelope localization of genomic regions containing important transcription factors that regulate the temporal expression of differentiation genes.23,26,27,39,40 The failure of emerin-deficient progenitors to coordinate the temporal reorganization of their genome during differentiation is predicted to cause their defective transcriptional reprogramming.
The results presented herein support the role of emerin in controlling histone acetylation dynamics by regulating HDAC3 activity. Using HATi specifically targeting acetylation of residues deacetylated by HDAC3 (eg, H4K5ac), we found HAT inhibition rescued impaired differentiation in emerin deficiency. This recapitulated the rescue seen by treatment of emerin-deficient progenitors with an HDAC3 activator. Thus, H4K5 acetylation dynamics are predicted to be important for ensuring proper transcriptional reprogramming upon differentiation induction (Figure 6). Similar to HDAC3 activation, HDAC3 inhibition primarily affected later differentiation transitions,23 suggesting emerin regulation of HDAC3 activity controls the temporal expression of these later genes. This may result from failure to completely reprogram the transcriptome upon differentiation induction or by specifically regulating the latter steps of the gene expression program. Nu9056 exhibits high specificity, as the IC50 of Nu9056 for
FIGURE 6.
Effects of altered H4K5 acetylation dynamics on myogenic differentiation. A, Wild-type myogenic differentiation. B, Lack of emerin results in impaired differentiation with loss of Myf5 localization and increased H4K5 and H3 acetylation states. C, D, Treatment with HAT inhibitors (C) and HDAC3 (D) activators restore the H4K5 and H4K5ac equilibrium and rescue myotube formation with no effect on cell cycle exit. C, HAT inhibitor L002 partially rescues myoblast commitment by targeting unknown histone modifications to alter chromatin architecture at the nuclear envelope. D, Treatment with HDAC3 activators induces Myf5 nuclear envelope localization. Red arrows indicate impaired differentiation programming, solid dark green arrows indicate normal differentiation programming, and dashed light green arrow signifies partially impaired differentiation. HAT, histone acetyltransferase; H4K5, histone 4 lysine 5; H4K5ac, histone 4 acetylated on lysine 5; HAT, histone acetyltransferase; HDAC3, histone deacetylase 3
Tip60/KAT5 is 20- to 40-fold lower than for the histone acetyltransferase p300 or the histone acetyltransferase pCAF/GCN5. L002 was identified as a p300-specific inhibitor.30 p300 acetylates H3K18 and H3K27; it has also been reported to acetylate H4K5, H4K8, H4K12, and H4K16.41,42 L002 was reported to decrease H4 acetylation,30 but this study was the first to show L002 inhibits acetylation of H4K5. Although H3K9ac, H3K18ac, and H3K27ac were increased in emerin-deficient myogenic progenitors, treatment with Nu9056 had no significant effect on acetylation of H3K9, H3K18, or H3K27; L002 treatment had a small effect on H3K18 and H3K27 acetylation; and SRT1720 only affected H3K9 acetylation. Collectively, the distinct specificities of these compounds at the concentrations used in this study demonstrates that changes in acetylation of H3K9, H3K18, H3K27, and H4K16 cannot be responsible for the rescued myotube formation. Rather, these results suggest rescue by L002 and Nu9056 occurs primarily through rescuing H4K5 acetylation dynamics. This is consistent with our previous studies using HDAC3 inhibitors and activators.23,26 It is possible L002 may act to rescue impaired myogenic commitment by p300-mediated acetylation of H3K18 or H3K27. Alternatively, L002 may act through an unknown mechanism or act on other HATs to rescue myoblast commitment, as L002 appears to be more promiscuous at lower concentrations. Targeting these dynamic epigenetic changes represents a new potential strategy for ameliorating the skeletal muscle wasting seen in EDMD1.
It is important to elucidate how emerin regulates the dynamic epigenetic changes occurring during myogenic differentiation to control the transcriptional programs needed for passage through specific transition points. HAT inhibition and HDAC3 activation successfully rescued the latter steps of emerin-deficient myogenic differentiation (this study),23,26 suggesting emerin regulation of H4K5ac dynamics during transcriptional reprogramming likely impairs the expression of genes acting at later stages of differentiation (eg, myotube formation). Consistent with these results, HDAC3 inhibition by RGFP966 blocked MyHC expression and myotube fusion in both differentiating wild-type and emerin-deficient myogenic progenitors.23 We propose H4K5 acetylation levels are tightly regulated and that increases or decreases in H4K5 acetylation levels impair the transition from committed, differentiating myoblasts to myotubes by altering transcription reprogramming upon differentiation induction. Collectively, our results support pharmacological targeting of H4K5 acetylation as a potential therapeutic strategy for rescuing muscle regeneration in EDMD.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the members of the Holaska Laboratory for the many helpful discussions regarding these studies and preparation of this manuscript.
Funding information
National Institutes of Health, Grant/Award Number: R15AR069935; National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Number: 2R15AR069935
Abbreviations
- BSA
bovine serum albumin
- DAPI
4’,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle medium
- DMSO
dimethylsulfoxide
- ECL
enhanced chemiluminescence
- EDL
extensor digitorum longus
- EDMD
Emery-Dreifuss muscular dystrophy
- EdU
5-ethynyl-20-deoxyuridine
- FBS
fetal bovine serum
- H3K18
histone 3 lysine 18
- H3K18ac
histone 3 acetylated on lysine 18
- H3K27
histone 3 lysine 27
- H3K27ac
histone 3 acetylated on lysine 27
- H3K9
histone 3 lysine 9
- H3K9ac
histone 3 acetylated on lysine 9
- H4
histone 4
- H4K16
histone 4 lysine 16
- H4K16ac
histone 4 acetylated on lysine 16
- H4K5
histone 4 lysine 5
- H4K5ac
histone 4 acetylated on lysine 5
- HAT
histone acetyltransferase
- HATi
histone acetyltransferase inhibitor
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitor
- HRP
horseradish peroxidase
- IC
half-maximal 50 inhibitory concentration
- MyHC
myosin heavy chain
- NAD
nicotinamide adenine dinucleotide
- NCoR
nuclear corepressor
- PBS
phosphate-buffered saline
- PBST
phosphate-buffered saline with Tween
- SIRT1
sirtuin 1
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Holaska JM. Diseases of the nucleoskeleton. Compr Physiol 2016;6: 1655–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tatli M, Medalia O. Insight into the functional organization of nuclear lamins in health and disease. Curr Opin Cell Biol. 2018;54:72–79. [DOI] [PubMed] [Google Scholar]
- 3.Gruenbaum Y, Foisner R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem. 2015;84:131–164. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez Y, Saito A, Sazer S. Fission yeast Lem2 and Man1 perform fundamental functions of the animal cell nuclear lamina. Nucleus 2012;3:60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korfali N, Wilkie GS, Swanson SK, et al. The leukocyte nuclear envelope proteome varies with cell activation and contains novel transmembrane proteins that affect genome architecture. Mol Cell Proteomics. 2010;9:2571–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schirmer EC, Florens L, Guan T, Yates JR 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive pro-teomics. Science. 2003;301:1380–1382. [DOI] [PubMed] [Google Scholar]
- 7.Worman HJ, Schirmer EC. Nuclear membrane diversity: underlying tissue-specific pathologies in disease? Curr Opin Cell Biol. 2015;34: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Las Heras JI, Meinke P, Batrakou DG, et al. Tissue specificity in the nuclear envelope supports its functional complexity. Nucleus 2013;4: 460–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkie GS, Korfali N, Swanson SK, et al. Several novel nuclear envelope transmembrane proteins identified in skeletal muscle have cytoskeletal associations. Mol Cell Proteomics. 2011;10:M110 003129:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik P, Korfali N, Srsen V, et al. Cell-specific and lamin-dependent targeting of novel transmembrane proteins in the nuclear envelope. Cell Mol Life Sci. 2010;67:1353–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korfali N, Wilkie GS, Swanson SK, et al. The nuclear envelope proteome differs notably between tissues. Nucleus 2012;3:552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton LJ, Soshnev AA, Geyer PK. Networking in the nucleus: a spotlight on LEM-domain proteins. Curr Opin Cell Biol. 2015;34:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bione S, Maestrini E, Rivella S, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8:323–327. [DOI] [PubMed] [Google Scholar]
- 14.Mendez-Lopez I, Worman HJ. Inner nuclear membrane proteins: impact on human disease. Chromosoma. 2012;121:153–167. [DOI] [PubMed] [Google Scholar]
- 15.Vlcek S, Foisner R. Lamins and lamin-associated proteins in aging and disease. Curr Opin Cell Biol. 2007;19:298–304. [DOI] [PubMed] [Google Scholar]
- 16.Worman HJ. Nuclear lamins and laminopathies. J Pathol. 2012;226: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonne G, Leturcq F, Ben Yaou R. Emery-Dreifuss muscular dystrophy. In: Pagon RA, Adam MP, Ardinger HH, et al. , editors. GeneReviews. [Internet]. Seattle (WA): University of Washington; 1993–2020. [PubMed] [Google Scholar]
- 18.Melcon G, Kozlov S, Cutler DA, et al. Loss of emerin at the nuclear envelope disrupts the Rb1/E2F and MyoD pathways during muscle regeneration. Hum Mol Genet. 2006;15:637–651. [DOI] [PubMed] [Google Scholar]
- 19.Ozawa R, Hayashi YK, Ogawa M, et al. Emerin-lacking mice show minimal motor and cardiac dysfunctions with nuclear-associated vacuoles. Am J Pathol. 2006;168:907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakay M, Wang Z, Melcon G, et al. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain. 2006;129:996–1013. [DOI] [PubMed] [Google Scholar]
- 21.Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20:486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber MD, Guan T, Gerace L. Overlapping functions of nuclear envelope proteins NET25 (Lem2) and emerin in regulation of extracellular signal-regulated kinase signaling in myoblast differentiation. Mol Cell Biol. 2009;29:5718–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins CM, Ellis JA, Holaska JM. MAPK signaling pathways and HDAC3 activity are disrupted during differentiation of emerin-null myogenic progenitor cells. Dis Model Mech. 2017;10:385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedeic Z, Cetera M, Cohen TV, Holaska JM. Emerin inhibits Lmo7 binding to the Pax3 and MyoD promoters and expression of myoblast proliferation genes. J Cell Sci. 2011;124:1691–1702. [DOI] [PubMed] [Google Scholar]
- 25.Koch AJ, Holaska JM. Loss of emerin alters myogenic signaling and miRNA expression in mouse myogenic progenitors. PLoS One 2012; 7:e37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demmerle J, Koch AJ, Holaska JM. Emerin and histone deacetylase 3 (HDAC3) cooperatively regulate expression and nuclear positions of MyoD, Myf5, and Pax7 genes during myogenesis. Chromosome Res. 2013;21:765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demmerle J, Koch AJ, Holaska JM. The nuclear envelope protein emerin binds directly to histone deacetylase 3 (HDAC3) and activates HDAC3 activity. J Biol Chem. 2012;287:22080–22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anamika K,Krebs AR,Thompson J,Poch O,Devys D,Tora L.Lessons from genome-wide studies: an integrated definition of the coactivator function of histone acetyl transferases. Epigenetics Chromatin. 2010;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffey K, Blackburn TJ, Cook S, et al. Characterisation of a Tip60 specific inhibitor, NU9056, in prostate cancer. PLoS One. 2012;7: e45539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Pinello CE, Luo J, et al. Small-molecule inhibitors of acetyltransferase p300 identified by high-throughput screening are potent anticancer agents. Mol Cancer Ther. 2013;12:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao J, Sack MN. Protein deacetylation by sirtuins: delineating a posttranslational regulatory program responsive to nutrient and redox stressors. Cell Mol Life Sci. 2010;67:3073–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalkiadaki A, Guarente L. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer. 2015;15:608–624. [DOI] [PubMed] [Google Scholar]
- 33.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feige JN, Lagouge M, Canto C, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. [DOI] [PubMed] [Google Scholar]
- 35.Jat PS, Noble MD, Ataliotis P, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan JE, Beauchamp JR, Pagel CN, et al. Myogenic cell lines derived from transgenic mice carrying a thermolabile T antigen: a model system for the derivation of tissue-specific and mutation-specific cell lines. Dev Biol. 1994;162:486–498. [DOI] [PubMed] [Google Scholar]
- 37.Mashinchian O, Pisconti A, Le Moal E, Bentzinger CF. The muscle stem cell niche in health and disease. Curr Top Dev Biol. 2018;126: 23–65. [DOI] [PubMed] [Google Scholar]
- 38.Iyer A, Koch AJ, Holaska JM. Expression profiling of differentiating emerin-null myogenic progenitor identifies molecular pathways implicated in their impaired differentiation. cells. 2017;6(4):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holaska JM, Wilson KL. An emerin "proteome": purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry. 2007; 46:8897–8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poleshko A, Shah PP, Gupta M, et al. Genome-nuclear lamina interactions regulate cardiac stem cell lineage restriction. Cell. 2017;171: 573–587, e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry RA, Kuo YM, Bhattacharjee V, Yen TJ, Andrews AJ. Changing the selectivity of p300 by acetyl-CoA modulation of histone acetylation. ACS Chem Biol. 2015;10:146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henry RA, Kuo YM, Andrews AJ. Differences in specificity and selectivity between CBP and p300 acetylation of histone H3 and H3/H4. Biochemistry. 2013;52:5746–5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.