Abstract
Purpose:
This review highlights the development of long-acting injectable cabotegravir (CAB LA) for HIV pre-exposure prophylaxis (PrEP), with a focus on Phase 2 studies and later development.
Recent findings:
Early studies of CAB LA for HIV prevention offered promising PK data and paved the way for Phase 2 studies which have now been completed. Based on Phase 2 data, dosing of CAB LA at 8-week intervals consistently delivers target trough concentrations in both males and females. Recent studies have shown no required dose adjustments for hepatic or renal disease and minimal drug-drug interactions. Additionally, injectable PrEP is desired by potential PrEP candidates. Still, gaps in knowledge remain with respect to implementation and delivery, the clinical significance of the pharmacologic tail, and dosing in key populations. Phase 3 trials are underway that are anticipated to inform some of these questions and provide efficacy and safety data to support regulatory submissions for CAB LA as a potential PrEP agent.
Summary:
Recent studies have defined an appropriate CAB LA dosing interval and offered insight into its safety profile. Phase 3 studies will provide much-anticipated efficacy data. If efficacious, CAB LA may provide a desirable PrEP option for those who face challenges to daily pill adherence. A more complete understanding of how to best integrate LA PrEP into service delivery models will be critical for success.
Keywords: injectable PrEP, long-acting cabotegravir, PrEP 2.0, Next generation PrEP
Introduction
Large clinical trials have demonstrated the efficacy of tenofovir disoproxyl fumarate/emtricitabine (TDF/FTC) as pre-exposure prophylaxis (PrEP) for the prevention of HIV infection.1,2 Results of the DISCOVER trial are anticipated to lead to upcoming regulatory approvals for daily oral tenofovir alafenamide/emtricitabine (TAF/FTC) for PrEP in men who have sex with men (MSM) and transgender women (TGW), since the efficacy of TAF/FTC for HIV prevention was non-inferior to TDF/FTC in these populations.3 In PrEP trials of TDF/FTC, individuals with consistent adherence to TDF/FTC achieved substantial protection from HIV infection. However, it quickly became apparent that the effectiveness of TDF/FTC is closely associated with adherence, as individuals who became infected were significantly less likely to have detectable drug concentrations compared to those who were not infected.4 Barriers to adherence using daily tablets may be related to forgetfulness, competing priorities, safety concerns, stigma, or partners’ negative sentiments toward PrEP.5 To overcome these barriers, development of alternative forms of PrEP are likely to be required. Parallels can be drawn to the reproductive health literature, in which contraceptive use increases as more delivery options become available;6 it is possible, and even likely, that expanding PrEP options will lead to enhanced uptake and coverage.
The “PrEP 2.0” pipeline includes a variety of prevention agents and delivery mechanisms, including many with analogy to female contraceptive products. Investigations of injectable agents, rings, implants, patches, inserts, enemas, douches and more are currently in progress.7 Injectable PrEP is most advanced in clinical development, with progress facilitated by the availability of anti-retroviral medications that allow for formulation as long-acting suspentions for delivery via intramuscular injection. Furthermore, injectable technologies have repeatedly been viewed favorably among key popultions.8,9
Rilpivirine, a non-nucleoside reverse trancriptase inhibitor (NNRTI), has been shown to have efficacy in HIV treatment when delivered as injectable anti-retroviral maintenance therapy in combination with cabotegravir in persons living with HIV (PLWH) who have not previously experienced virologic failure.10 However, despite being safe, well-tolerated, and acceptable for PrEP in key populations, long-acting rilpivirine for PrEP has not moved forward into phase 3 studies due to storage and transportation limitations (cold chain required) and a low barrier to resistance.11 Long-acting injectable cabotegravir (CAB LA) has been studied in Phase 1 and 2a/b studies and is currently being investigated in Phase 3 clinical trials of long-acting PrEP, HIV Prevention Trials Network (HPTN) Study 083 (NCT02720094) in HIV-uninfected men who have sex with men and transgender women, and HPTN 084 (NCT03164564) in HIV-uninfected women in Sub-Saharan Africa. This review will provide an overview of current literature on CAB LA for the prevention of HIV infection, highlighting recent key studies and developments in the field.
The Case for CAB
Cabotegravir (formerly GSK1265744) is an integrase strand transfer inhibitor (INSTI) with potent antiviral activity.12 A chemical congener of dolutegravir, it has a high barrier to resistance and retains activity in the setting of common INSTI mutations.13,14 Cabotegravir is amenable to formulation as a long-acting injectable suspension. The injectable suspension is effectively composed of pure active drug in crystalline form, meaning near complete drug loading per unit volume (200 mg/ml) and a small injection load.15
Early Phase Studies
Phase I studies have provided the initial characterization of the pharmacokinetics (PK) of CAB LA in humans.16,17 In an open-label, 9-cohort parallel study, intramuscular (IM) doses of 100 to 800 mg as well as subcutaneous doses of 100 to 400 mg were administered to healthy participants.16 CAB LA was generally safe and well-tolerated, although most participants reported mild injection site reactions (ISRs). The 800 mg IM dose achieved mean concentrations above the protein adjusted-IC90 (PA-IC90) for approximately 16 weeks, leading to the hypothesis that this was an appropriate dose for quarterly administration in the prevention context. Adequate exposure was also observed when the 400 mg dose was administered as two simultaneous 200 mg injections. In a second study, repeated 800 mg IM doses administered at 12-week intervals maintained cabotegravir concentrations above targets for the full dosing interval.17
Macaque models of rectal, vaginal, penile, and parenteral exposures have further supported the development of CAB LA as a promising PrEP agent.18-21 In a low-dose rectal challenge model, plasma CAB concentrations above 3x PA-IC90 provided 100% protective efficacy, concentrations 1x-3x PA-IC90 provided 97% protective efficacy, and concentrations <1x PA-IC90 provided 45% protective efficacy compared to control untreated macaques (CM).19 In a vaginal challenge model, plasma levels >4x PA-IC90 were 87% protective compared to CM, while plasma levels <4x PA-IC90 were not protective compared to CM.22 Finally, in a study of intravenous SIV challenge, CAB LA was found to be highly protective; 21 of 24 CAB LA-treated macaques remained aviremic after IV challenge (88% protected). These studies paved the way for the further clinical development of CAB LA for PrEP in humans.
Drug-Drug Interactions
Cabotegravir is primarily metabolized by UGT1A1 with some contribution from UGT1A9.23 CAB inhibits OAT1 and OAT3 in vitro, although the clinical impact is considered a concern only for substrates of OAT1 and OAT3 with narrow therapeutic windows (e.g. methotrexate).24 Potent enzyme inducers (e.g. rifampin, carbamazepine) have been shown to decrease CAB levels by almost 60% and should be avoided.25 Rifabutin has a more modest effect on plasma CAB concentrations—approximately 20% reduction—and so there is less concern about co-administration.26 A small study of oral CAB with a regimen of a levonorgestrel and ethinyl estradiol in healthy women found no significant effect on hormonal PK, and also no effect on CAB PK.27
Dosing in hepatic and renal dysfunction
Moderate hepatic impairment has minimal impact on CAB PK, and no dose adjustment is necessary in individuals with mild to moderate hepatic impairment.28 Additionally, no dose adjustment is necessary in individuals with severe renal impairment.29 CAB exposure is also not impacted by ingestion in the fed versus fasting state.30
Phase 2 Human Studies:
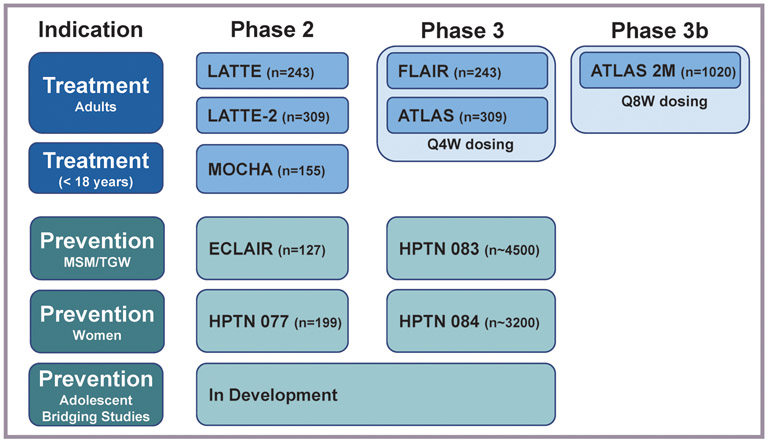
Two significant Phase 2a studies have been recently conducted in the global CAB LA development program for prevention (Figure 1).
Figure 1. Past, Ongoing, and Future CAB LA Development.
MSM, men who have sex with men; TGW, transgender women; Q4W, every 4 weeks; Q8W, every 8 weeks
ECLAIR
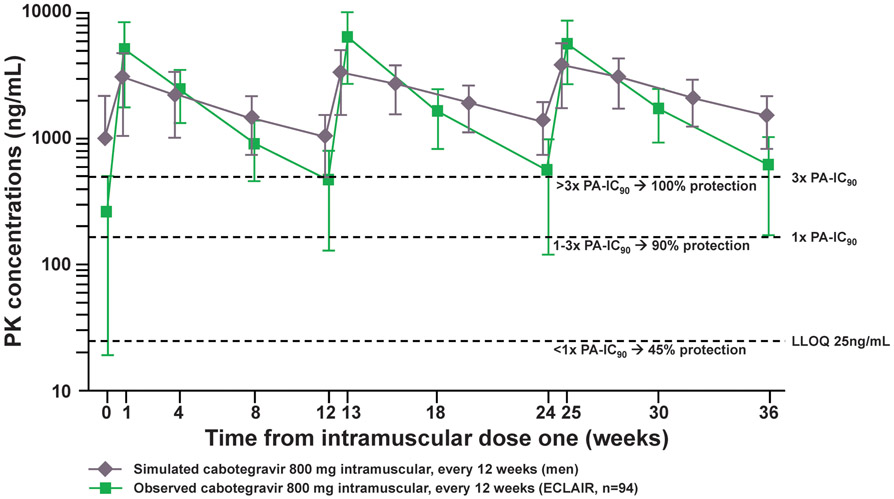
The ECLAIR study (NCT02076178), enrolled healthy HIV-uninfected men aged 18-65 years at low risk of HIV infection at 10 sites in the US.31 In total, 127 participants were randomized 5:1 to cabotegravir (n=106) or placebo (n=21). Participants first received a 4 week oral lead-in (CAB 30 mg or placebo) to ensure safety and tolerability. After a one week washout period, participants received a series of 3 injections every 12 weeks: 800 mg CAB LA as 2 x 2mL injections in the active drug arm and 0.9% saline placebo injections of the same volume in the control arm. Unexpectedly, 15-31% of participants had CT below 1x PA-IC90 while 32-55% of participants had CT between 1x and 4x PA-IC90. These observations raised concern that 800 mg IM every 12 weeks might not consistently provide levels anticipated to be highly protective based on data from macaque models (Figure 2).16,17
Figure 2. Simulated and Observed CAB Concentrations of Men Enrolled in ECLAIR.
PK, pharmacokinentic; ng, nanogram; mL, milliter; PA-IC90, protein binding–adjusted 90% inhibitory concentration; LLOQ, lower limit of quantification; mg, milligram
During the injection phase of ECLAIR, 80% of participants in the CAB LA group experienced Grade 2 or higher adverse events (AEs), as compared to 48% in the placebo group. Grade 2 or higher injection site pain was significantly higher in the CAB group (59% vs 5%), as was the mean duration of injection site pain (5.4 vs 2 days). Injection intolerability led four (4%) participants in the CAB group to withdraw consent and discontinue the study drug; other AEs led to withdrawal in additional participants (7%). There were no significant differences in laboratory abnormalities, electrocardiogram, and vital sign assessments between the CAB and placebo groups.
HPTN 077
HPTN 077 (NCT02178800) was designed to supplement data from ECLAIR with pharmacokinetic data for CAB LA in females and males in regions particularly impacted by the HIV epidemic.32 HPTN 077 enrolled healthy men and women aged 18-65 years at low risk of HIV infection at 8 sites in Brazil, Malawi, South Africa, and the US. Two sequential cohorts enrolled 199 particpants who were randomized 3:1 to CAB or placebo (0.9% saline) injections. As with ECLAIR, all participants first received 30 mg oral CAB or placebo for 4 weeks, and after serial safety assessments, continued to injections. The first cohort received 800 mg CAB LA IM (two 400 mg [2 ml] injections, identical to ECLAIR) or a matching volume saline placebo every 12 weeks. The second cohort was added after ECLAIR results became known; participants received 600 mg CAB LA IM (one 600 mg [3ml] injection) or saline placebo every eight weeks after an initial two doses spaced four weeks apart, for a total of 5 injections.
In CAB recipients in the first cohort (800 mg IM every 12 weeks), CT fell below the steady state target of 4x PA-IC90 for 72%, 35%, and 32% of male participants and for 24%, 5%, and 0% of female participants after injections 1, 2, and 3, respectively. In CAB recipients in the 8-week dosing cohort, CT was above the target of 4x PA-IC90 for at minimum 79% of both male and female participants after all injections.
In HPTN 077, rates of injectable product discontinuation were not different between the CAB and placebo arms. One study product discontinuation in the CAB arm was for a seizure event in a participant with pre-existing history of seizure disorder. Rates of any grade 2 or higher AE were not different between the CAB and placebo arms (91% vs 88%). The only grade 2 or higher clinical AE that occurred more often in the CAB arm was ISR (38% vs 2%, p<0.001). For the CAB arm, ISR events declined with repeated injections. One grade 3 ISR occurred in one participant receiving CAB in each of 12-week and 8-week dosing cohorts, although neither participant discontinued study product as a result.
Gaps in Knowledge
ECLAIR and HPTN 077 demonstrated that CAB LA at a dose of 800 mg every 12 weeks did not consistently meet target trough concentrations. Based on data from HPTN 077, an eight week dosing interval with a 600 mg dose delivers consistent trough concentrations at or above macaque model pharmacokinetic targets in both males and females. However, only Phase 3 studies and clinical experience will define the pharmacokinetic correlates of protection.
In both ECLAIR and HPTN 077, participants with lower BMI generally had higher peak CAB concentrations as well as higher AUC concentrations. The mechanism, significance, and additional correlates of pharmacokinetic variability will hopefully become clearer with Phase 3 investigation. In addition, data on the pharmokinetics of CAB LA in transgender individuals are needed: HPTN 077 enrolled 6 transmasculine and 1 transfeminine participants; larger cohorts will help to define any potential interactions between CAB and cross-sex hormone therapy. HPTN 083, an ongoing Phase 3 trial comparing CAB LA to daily oral TDF-FTC in MSM and TGW is pre-specified to enroll at minimum 10% of its planned 4500 participants from transgender communities. CAB has not been studied among individuals on dialysis or those with severe hepatic impairment, and further studies will be needed to understand dosing in these populations.
Data from pregnant and breastfeeding women are also needed. HPTN 077 excluded women who were pregnant or breastfeeding, and all women of childbearing potential were required to use contraception as an enrollment criterion. The ongoing Phase 3 safety and efficacy study, HPTN 084, comparing CAB LA to daily oral TDF-FTC in cisgender women in sub-Saharan Africa has a substudy of both mothers and infants who will undergo intensive PK and safety monitoring. It is reassuring that recent data among HIV-infected mothers demonsate only a slightly higher prevalence of neural-tube defects in the setting of periconception dolutegravir use, relative to other types of ART, given the structural and class similarities of CAB and dolutegravir.33
The Pharmacologic Tail: Asset or Liability?
An important focus in CAB LA studies has been on the pharmacologic tail. In ECLAIR, participants were followed for safety and pharmacokinetic assessments until 52 weeks following the final injection.31 Unexpectedly, 17% of participants still had detectable concentrations of CAB (assay lower limit of quantification [LLOQ] = 25 ng/mL) at 52 weeks following the final injection. In HPTN 077, 13% of males and 42% of females had detectable CAB concentrations 76 weeks following the last injection.34 The median time to reach the LLOQ was 43 weeks for males and 66 weeks for females. CAB’s prolonged half-life is attractive in considering its potential power and dosing forgiveness as a prophylactic agent; it also could represent a period of vulnerability to seroconversion with potentially CAB or INSTI resistant virus. HPTN 083 and 084 should provide further information about declining CAB levels and the potential for resistance development, although it will more likely not be until clinical implementation and scale up that the clinical significance of the tail will be clarified. In the current PrEP landscape, HIV drug resistance is most likely to occur when TDF/FTC is started during undiagnosed acute HIV infection;35 a recent macaque modeling study of acute infection demonstrated the development of CAB mutations in this setting as well.36 Reassuringly, in earlier macaque studies of rectal and vaginal SHIV and SIV challenges, no major integrase resistance mutations were selected when infections occurred during the pharmacokinetic tail.18,19
CAB and Weight Change
In a number of studies, use of integrase inhibitors as part of an anti-retroviral treatment regimen has been associated with weight gain and increasing waist circumference.37-40 A post-hoc analysis of HPTN 077 in 134 CAB recipients and 43 placebo recipients demonstrated that CAB LA in HIV-uninfected patients was not associated with weight changes.41 Median weight gain among 146 participants with paired weights (week 0 and week 41) was 1.1 kg for CAB recipients and 1.0 kg for placebo recipients. A gain of 5% total body weight or greater was seen in 22% of CAB recipients and 18% of placebo participants. The sample size in this study was modest, and this finding will need to be confirmed in ongoing Phase 3 studies that are in progress. These weight change analyses in HIV-uninfected populations absent confounding by both the pro-inflammatory milieu of HIV infection and additional ARV agents will be critical to the understanding of the role of INSTIs in weight gain.
Acceptability of Long-Acting PrEP
Numerous studies have assessed the hypothetical acceptability of long-acting injectable PrEP, or assessed acceptability in low-risk individuals. In ECLAIR, side effects were common yet almost all participants reported being interested in continuing injections, and most reported overall satisfaction with CAB LA.42,43 Similarly, in HPTN 077, there was a strong preference for a long-acting injectable PrEP at baseline and follow-up, and product attributes were moderately to highly acceptable for most participants, particularly those outside of the US.44 In populations at elevated risk of HIV infection, long-acting injectable regimens have also been hypothetically viewed favorably, although fear of needles and concerns about the duration of therapeutic efficacy may be barriers in some individuals.8,9,45-48 Again, retention rates, and quantitative and qualitative perspectives in direct head-to-head comparisons with daily oral TDF/FTC in ongoing Phase 3 studies, will be very informative for implementation and scale-up should safety and efficacy be demonstrated.
Beyond Phase 3 Studies
The Oral lead-in
Current studies of CAB for HIV prevention leverage the strategy or an oral lead-in (approximately 4 weeks in duration) to assess safety and tolerability prior to administration of the long-acting injectable formulation. In the HIV treatment space, a larger safety database has supported investigation of the strategy of going directly to the long-acting injectable formulation without oral precursor in virologically suppressed patients. It is anticipated that should HIV prevention efficacy and safety in HIV-uninfected individuals be demonstrated, additional studies will be planned to support the safety of a “direct to inject” approach.
Considering Alternative Injection “Sites”
Currently, the only anatomical site approved for CAB LA injection is the buttock region (gluteus medius or maximus). If CAB LA ultimately receives regulatory approvals for prevention, consideration may be given to self-administration and the possibility of alternative muscle injection sites (e.g. rectus femoris). However, additional evaluation will be needed before the use of other IM sites can be considered. With the current volume of injection required, deltoid or other small muscle sites will not be feasible. Of note, persons with buttock implants or fillers have been excluded from current clinical trials out of concern for altered pharmacokinetics.
Should regulatory approvals be granted, the best site for service delivery will also need to be determined. In prior and current trials, study product has been administered in a clinic based setting. Whether the location of service delivery can be broadened will require further investigation, but consideration may be given to community-based services such as pharmacies or mobile health vans. Alternatively, “de-medicalizing” PrEP with self-administration, in-home services, or delivery by community based organizations or in other non-medical community settings could allow for greater convenience, and thus potentially greater uptake, on the part of patients. In the era of an urgent push to “End the HIV Epidemic” and expand PrEP coverage,49 novel mechanisms of delivery that address consumer needs and preferences will need to be strongly integrated into service delivery models, and there is an urgent need for this research now, in anticipation of regulatory approvals in the not-too-distant future.
Conclusions
Phase 2 studies exploring CAB LA for PrEP, ECLAIR and HPTN 077, have been encouraging, providing PK data and defining the appropriate dose and interval for Phase 3 studies. Other recent work has clarified the length and correlates of the pharmacokinetic tail, although its clinical significance remains unclear. Phase 2 substudies have demonstrated that participants and potential patients are enthusiastic about an option for LA injectable PrEP. Phase 3 study results for 083 and 084 are anticipated in 2021. If CAB LA is shown to be safe and effective, it is possible that this injectable option for PrEP may lead to enhanced PrEP uptake among populations who most critically need it. In places and populations where stigma is rampant, a discreet product could be especially impactful. Ultimately, optimizing the implementation strategy for injection administration to suit consumer needs will be critical.
Key Points.
Cabotegravir has characteristics that make it a promising long-acting injectable prevention option including potent anti-viral activity, few drug interactions, and a favorable side effect profile
When studied for HIV prevention, dosing of long-acting cabotegravir at 8-week intervals consistently delivers target trough concentrations in both males and females, and this dosing frequency is now being studied in large Phase 3 Clinical Trials
In populations at elevated risk of HIV infection, long-acting injectable regimens have been viewed favorably in hypothetical scenarios
If CAB LA is shown to be safe and effective, it is possible that this injectable option for PrEP may lead to enhanced PrEP uptake among populations who most critically need it
Acknowledgments
Financial support and sponsorship
This work was also supported by the UCLA Center for HIV Identification, Prevention, and Treatment Services (CHIPTS) NIMH grant P30MH58107; the UCLA Center for AIDS Research (CFAR) grant 5P30AI028697; the UCLA Clinical Translational Science Institute (CTSI) Grant UL1TR001881; and NIH/NIAID grant K23AI137121
Footnotes
Conflicts of interest
RJL is an Advisory Board member for Gilead Sciences and Merck and receives consultation fees from Roche. MEC receives consulting fees from FHI360.
Contributor Information
Meredith E. Clement, Louisiana State University Health Sciences Center, New Orleans, 1542 Tulane Avenue, New Orleans, LA 70112.
Ryan Kofron, UCLA Center for Clinical AIDS Research and Education, 1399 S. Roxbury Drive, Suite 100, Los Angeles, CA 90035.
Raphael J. Landovitz, UCLA Center for Clinical AIDS Research and Education, 1399 S. Roxbury Drive, Suite 100, Los Angeles, CA 90035.
References
- 1.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hare CB, Coll J, Ruane P, et al. The Phase 3 DISCOVER Study: Daily F/TAF or F/TDF for HIV Pre-exposure Prophylaxis Abstract #0104. CROI, Seattle, WA, 2019. [Google Scholar]
- 4.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corneli A, Perry B, McKenna K, et al. Participants’ Explanations for Nonadherence in the FEM-PrEP Clinical Trial. J Acquir Immune Defic Syndr. 2016;71(4):452–461. [DOI] [PubMed] [Google Scholar]
- 6.Ross J, Stover J. Use of modern contraception increases when more methods become available: analysis of evidence from 1982-2009. Glob Health Sci Pract. 2013;1(2):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mugwanya KK, Baeten JM. Next-Generation Pre-Exposure Prophylaxis: Choices for Effective HIV Prevention. J Infect Dis. June 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RR, Crane JS, López J, et al. Pre-exposure prophylaxis for HIV prevention preferences among young adult African American men who have sex with men. PLoS ONE. 2018;13(12):e0209484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery ET, Atujuna M, Krogstad E, et al. The Invisible Product: Preferences for Sustained-Release, Long-Acting Pre-exposure Prophylaxis to HIV Among South African Youth. J Acquir Immune Defic Syndr. 2019;80(5):542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A. Long-Acting Cabotegravir + Rilpivirine as Maintenance Therapy: ATLAS Week 48 Results Abstract #0139. CROI, Seattle, WA, 2019. [Google Scholar]
- 11.Penrose KJ, Parikh UM, Hamanishi KA, et al. Selection of Rilpivirine-Resistant HIV-1 in a Seroconverter From the SSAT 040 Trial Who Received the 300-mg Dose of Long-Acting Rilpivirine (TMC278LA). J Infect Dis. 2016;213(6):1013–1017. [DOI] [PubMed] [Google Scholar]
- 12.Spreen W, Min S, Ford SL, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013;14(5):192–203. [DOI] [PubMed] [Google Scholar]
- 13.Karmon SL, Mohri H, Spreen W, Markowitz M. GSK1265744 demonstrates robust in vitro activity against various clades of HIV-1. J Acquir Immune Defic Syndr. 2015;68(3):e39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshinaga T, Kobayashi M, Seki T, et al. Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. Antimicrob Agents Chemother. 2015;59(1):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spreen WR, Margolis DA, Pottage JC. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013;8(6):565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67(5):481–486. [DOI] [PubMed] [Google Scholar]
- 17.Spreen W, Williams P, Margolis D, et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr. 2014;67(5):487–492. [DOI] [PubMed] [Google Scholar]
- 18.Andrews CD, Yueh YL, Spreen WR, et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med. 2015;7(270):270ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews CD, Spreen WR, Mohri H, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science. 2014;343(6175):1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31(4):461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobard C, Makarova N, Nishiura K, et al. Long-Acting Cabotegravir Protects Macaques Against Repeated Penile SHIV Exposures Abstract #83. CROI. Boston, MA, 2018. [DOI] [PubMed] [Google Scholar]
- 22.Spreen W, Lowry A, Pal R, et al. Correlation of In Vivo Cabotegravir Concentration and Prevention of SIV in Macaques Abstract #996LB. CROI. Seattle, WA, 2015. [Google Scholar]
- 23.Bowers GD, Culp A, Reese MJ, et al. Disposition and metabolism of cabotegravir: a comparison of biotransformation and excretion between different species and routes of administration in humans. Xenobiotica. 2016;46(2):147–162. [DOI] [PubMed] [Google Scholar]
- 24.Reese MJ, Bowers GD, Humphreys JE, et al. Drug interaction profile of the HIV integrase inhibitor cabotegravir: assessment from in vitro studies and a clinical investigation with midazolam. Xenobiotica. 2016;46(5):445–456. [DOI] [PubMed] [Google Scholar]
- 25.Ford SL, Sutton K, Lou Y, et al. Effect of Rifampin on the Single-Dose Pharmacokinetics of Oral Cabotegravir in Healthy Subjects. Antimicrob Agents Chemother. 2017;61(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford SL, Lou Y, Lewis N, et al. Effect of rifabutin on the pharmacokinetics of oral cabotegravir in healthy subjects. Antivir Ther (Lond). 21 2019. [DOI] [PubMed] [Google Scholar]
- 27.Trezza C, Ford SL, Gould E, et al. Lack of effect of oral cabotegravir on the pharmacokinetics of a levonorgestrel/ethinyl oestradiol-containing oral contraceptive in healthy adult women. Br J Clin Pharmacol. 2017;83(7):1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Shaik JSB, Ford SL, Lou Y, et al. A Phase 1 Study to Evaluate the Pharmacokinetics and Safety of Cabotegravir in Patients With Hepatic Impairment and Healthy Matched Controls. Clin Pharmacol Drug Dev. 2019;8(5):664–673.This study evaluated pharmacokinetic (PK) parameters of cabotegravir (CAB) in 8 individuals with moderate hepatic impairment compared to 8 individuals who served as healthy controls. Those with hepatic impairment had a modest decrease in total CAB plasma exposure that was not considered clinically relevant, and the authors concluded that CAB may be administered without dose adjustment in patients with mild to moderate hepatic impairment.
- *29.Parasrampuria R, Ford SL, Lou Y, et al. A Phase I Study to Evaluate the Pharmacokinetics and Safety of Cabotegravir in Adults With Severe Renal Impairment and Healthy Matched Control Participants. Clin Pharmacol Drug Dev. 2019;8(5):674–681.This PK study evaluated CAB levels in 8 individuals with severe renal impairment (not requiring renal replacement therapy [RRT]) compared to 8 individuals who served as healthy controls. Severe renal impairment did not impact plasma CAB exposure. The authors concluded that CAB may be administered without dose adjustment in those with renal impairment not receiving RRT.
- *30.Patel P, Ford SL, Lou Y, et al. Effect of a High-Fat Meal on the Pharmacokinetics of the HIV Integrase Inhibitor Cabotegravir. Clin Pharmacol Drug Dev. 2019;8(4):443–448.In this study, oral CAB was administered to healthy participants after fasting or following a high-fat meal. CAB administration in the fed state increased the AUC and maximal drug concentration by 14% relative to the fasting state, although this difference was not considered clinically significant.
- *31.Markowitz M, Frank I, Grant RM, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. The Lancet HIV. 2017;4(8):e331–e340.This double-blind, placebo controlled Phase 2a trial assessed the safety, tolerability, and PK for CAB LA in healthy men. Participants were randomized 5:1 to receive placebo or intramuscular CAB LA injections at quarterly intervals, following a 4-week oral lead-in period. Grade 2 or higher AEs were more frequent in the CAB LA group, mostly driven by injection site reactions. Plasma concentrations of CAB were below targets following all three injections.
- **32.Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15(11):e1002690.The second Phase 2a study of CAB LA was a double-blind, placebo controlled trial conducted in healthy men and women who were randomized 3:1 to CAB or placebo. This study included two cohorts of CAB participants, one receiving doses every 12 weeks and the other receiving doses every 8 weeks. Grade 2 or higher injection site reactions occurred more frequently in participants receiving CAB. The 8-week dosing regimen led to maintenance of CAB concentrations above pre-specified targets, although this was not the case in the 12-week regimen.
- *33.Zash R, Holmes L, Diseko M, et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N Engl J Med. 2019. July 22.In a large surveillance study of birth outcomes in Botswana, 0.30% of mothers taking dolutegravir at conception had deliveries complicated by neural tube defects, relative to 0.10% of deliveries when the mother was taking any non-dolutegravir ART at conception.
- *34.Landovitz R, Li S, Eron J, et al. Tail-phase Safety, Tolerability and Phamacokinetics of Long-acting Injectable Cabotegravir in HIV-uninfected Individuals: HPTN 077 Final Results Abstract # OA15.06LB. HIV R4P. Madrid, Spain, 2018.Here data were presented on the pharmacologic tail of CAB LA. Participants in HPTN were followed for 76 weeks after the last injection, at which time a significant proportion of participants (13% of males and 42% of females) still had detectable CAB concentrations.
- 35.Gibas KM, van den Berg P, Powell VE, Krakower DS. Drug Resistance During HIV Pre-Exposure Prophylaxis. Drugs. 2019;79(6):609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radzio-Basu J, Council O, Cong M-E, et al. Drug resistance emergence in macaques administered cabotegravir long-acting for pre-exposure prophylaxis during acute SHIV infection. Nat Commun. 2019;10(1):2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gatell JM, Assoumou L, Moyle G, et al. Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS. 2017;31(18):2503–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111–118. [DOI] [PubMed] [Google Scholar]
- **39.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med. July 2019. doi: 10.1056/NEJMoa1902824In this Phase 3, randomized, open-label trial conducted in South Africa, HIV treatment with dolutegravir and emtricitabine (FTC) with either tenofovir alefenomide or tenofovir disoproxyl fumarate (TDF) was non-inferior to a standard-care regimen with efavirenz with TDF and FTC at 48 weeks. Those receiving dolutegravir gained significantly more weight than those receiving the standard-care regimen.
- **40.NAMSAL ANRS 12313 Study Group. Dolutegravir-Based or Low-Dose Efavirenz-Based Regimen for the Treatment of HIV-1. N Engl J Med. July 2019.The Phase 3, randomized, open-label study of HIV-infected patients in Cameroon demonstrated that a dolutegravir-based HIV treatment regimen was non-inferior to an efavirenz-based treatment regimen at 48 weeks. More weight gain was observed in those receiving dolutegravir than in those receiving efavirenz.
- *41.Landovitz RJ, Zangeneh SZ, Chau G, et al. Cabotegravir is Not Associated with Weight Gain in HIV-uninfected Individuals in HPTN 077. Clin Infect Dis. May 2019.This study was a post-hoc analysis of HPTN 077 to study changes in weight. In 146 participants with paired weights, median weight gain was 1.1 kg for CAB recipients and 1.0 kg for placebo recipients. The authors concluded that CAB LA in HIV-uninfected patients was not associated with weight changes, although the study was limited by small sample size.
- 42.Kerrigan D, Mantsios A, Grant R, et al. Expanding the Menu of HIV Prevention Options: A Qualitative Study of Experiences with Long-Acting Injectable Cabotegravir as PrEP in the Context of a Phase II Trial in the United States. AIDS Behav. 2018;22(11):3540–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray MI, Markowitz M, Frank I, et al. Satisfaction and acceptability of cabotegravir long-acting injectable suspension for prevention of HIV: Patient perspectives from the ECLAIR trial. HIV Clin Trials. 2018;19(4):129–138. [DOI] [PubMed] [Google Scholar]
- 44.Tolley E, Zangeneh SZ, Chau G, et al. Future; Demand for an Injectable PrEP: Acceptability of Long-Acting Injectable Cabotegravir (CAB LA) in HIV-uninfected Individuals: HPTN 077 Abstract #OA05.01. HIV R4P. Madrid, Spain, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Straten A, Agot K, Ahmed K, et al. The Tablets, Ring, Injections as Options (TRIO) study: what young African women chose and used for future HIV and pregnancy prevention. J Int AIDS Soc. 2018;21(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyers K, Wu Y, Qian H, et al. Interest in Long-Acting Injectable PrEP in a Cohort of Men Who have Sex with Men in China. AIDS Behav. 2018;22(4):1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyers K, Rodriguez K, Brill AL, et al. Lessons for Patient Education Around Long-Acting Injectable PrEP: Findings from a Mixed-Method Study of Phase II Trial Participants. AIDS Behav. 2018;22(4):1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.John SA, Whitfield THF, Rendina HJ, Parsons JT, Grov C. Will Gay and Bisexual Men Taking Oral Pre-exposure Prophylaxis (PrEP) Switch to Long-Acting Injectable PrEP Should It Become Available? AIDS Behav. 2018;22(4):1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisinger RW, Fauci AS. Ending the HIV/AIDS Pandemic1. Emerging Infect Dis. 2018;24(3):413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]