Highlights
-
•
Cycle threshold values were associated with the time to negative qPCR of SARS-CoV-2.
-
•
Olfactory and taste disorders occurred after development of fever and sore throat.
-
•
Fever and olfactory and taste disorders were correlated with higher viral burden.
-
•
Assessment of olfactory and taste disorders may be important to prevent transmission.
Keywords: COVID-19, SARS-CoV-2, Olfactory/taste disorders (OTDs), Real-time polymerase chain reaction (qPCR), Cycle threshold (Ct) values
Abstract
This study investigated, using cycle threshold (Ct) qPCR values, the association between symptoms and viral clearance in 57 patients with asymptomatic/mild SARS-CoV-2 infection. Patients with olfactory/taste disorders (OTDs) exhibited lower qPCR Ct values and longer time to negative qPCR than those without OTDs, suggesting an association between OTDs and high viral burden.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread globally. In Tokyo, Japan, the total number of confirmed cases has increased, particularly from March to April 2020. Real-time polymerase chain reaction (qPCR) using clinical specimens such as nasopharyngeal swabs or sputum is the standard of reference for diagnosis, and recent studies have shown an association between qPCR cycle threshold (Ct) values and disease severity (Liu et al., 2020, Yu et al., 2020). Specifically, Ct values from qPCR tests conducted on nasopharyngeal or sputum specimens of patients on admission were negatively associated with disease severity and progression to severe illness, and mild patients showed an early viral clearance using Ct values (Liu et al., 2020, Yu et al., 2020). However, little is known about the association between symptoms and viral clearance. This study investigated this association in mild/asymptomatic cases using qPCR Ct values of nasopharyngeal swab samples.
Method
Fifty-seven patients with asymptomatic/mild SARS-CoV-2 infection (without pneumonia or any organ failure) who were admitted to Keio University Hospital for isolation and/or treatment from March 28 to April 23, 2020 were included in this study. All patients had no symptoms of dyspnea and no findings of pneumonia on chest x-ray on admission. They were diagnosed with SARS-CoV-2 infection using qPCR conducted upon admission and did not receive any antiviral treatments. Pre-admission symptoms were collected at admission. After admission, patients were prospectively checked daily by physicians for symptoms such as fever, sore throat, nasal drip/congestion, cough/sputum, olfactory and taste disorders (OTDs), headache, fatigue, joint pain, and diarrhea. qPCR was performed on all nasopharyngeal swab samples using the SARS-CoV-2 CDC assay protocol and Ct values were reported for two genetic markers: the N1 and N2 viral nucleocapsid protein gene regions (Centers for Disease Control and Prevention, 2020). A value of ≤45 cycles indicated a positive result for SARS-CoV-2. qPCR testing was repeated every 3–7 days until the results were negative. This study retrospectively assessed the association between symptoms and Ct values or the period to negative qPCR.
Results
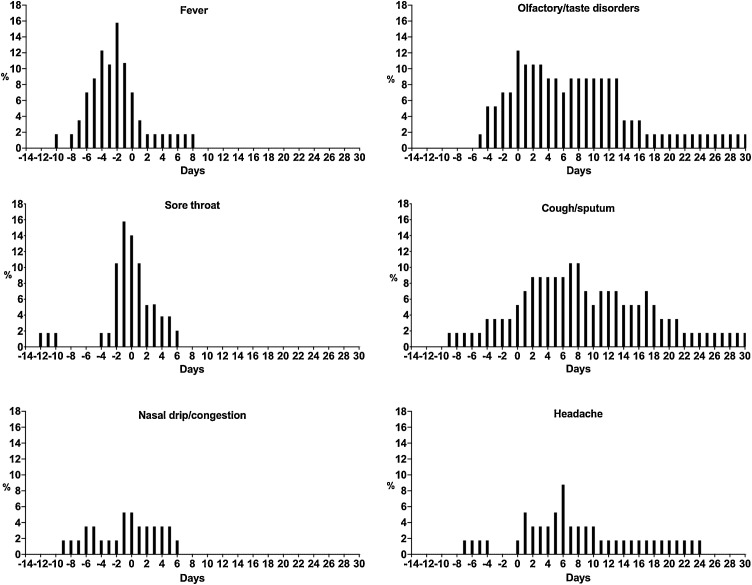
The clinical characteristics and symptoms of the patients are shown in Appendix A. The mean age was 35.4 years and 25 patients (43.9%) were males. Thirty-four patients (59.6%) had comorbidities (all under control). The most common disease was neoplasia (12.3%), followed by asthma (8.8%) and psychiatric disorders (7.0%). Twenty-eight patients (49.1%) were asymptomatic, with common symptoms on clinical courses being fever (15; 26.3%), sore throat (11; 19.3%), acute onset OTDs (10; 17.5%), headache (7; 12.3%), cough/sputum (8; 14.0%), and nasal drip/congestion (4; 7.0%). Figure 1 shows the prevalence of each symptom over time. Fever, sore throat and nasal drip congestion were relatively early and prolonged for about 1 week. The median duration of fever was 3 days (IQR 2.0–4.75 days, minimum: 1 day, maximum: 10 days). Cough and headache may be prolonged from early to late onset. The median duration of cough and sputum was 10 days (IQR 6.0–16.0 days, minimum: 2 days, maximum: 30 days).
Figure 1.
Prevalence of symptoms during the time course. Days 0 indicates the date of the first positive RT-PCR result.
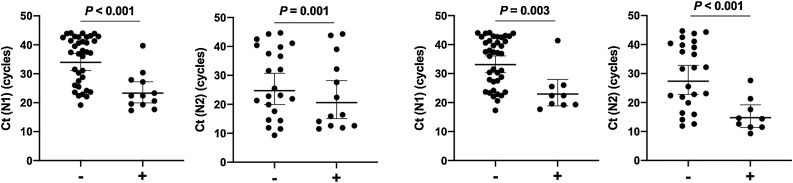
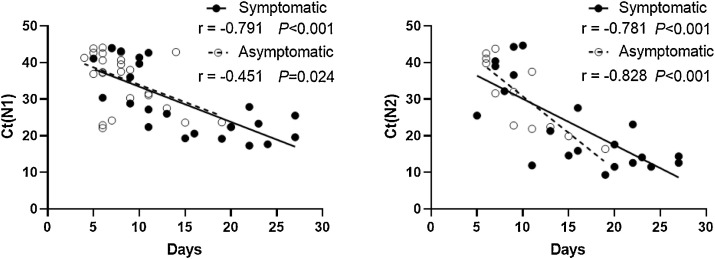
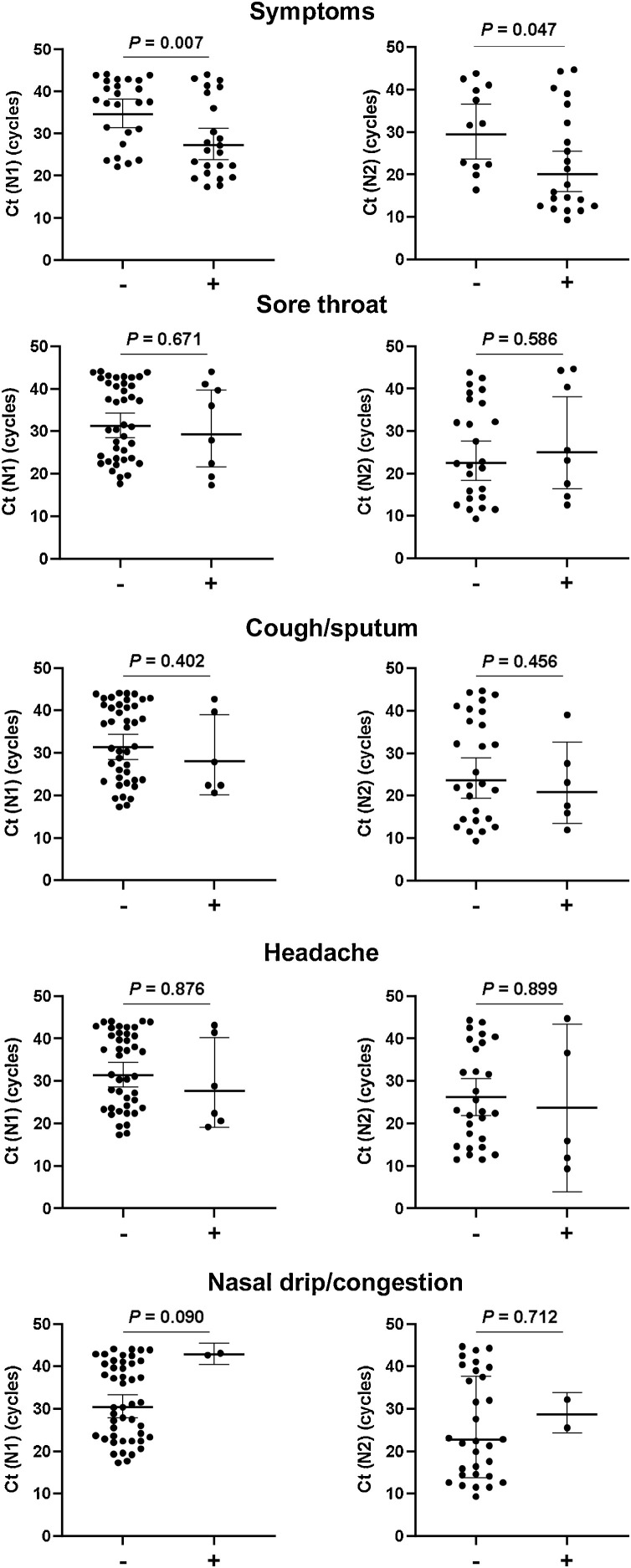
OTDs occurred in the later phase of disease compared with fever and sore throat. The median duration of OTDs was 9 days (IQR 4.5–16.3 days, minimum: 2 days, maximum: >30 days). Patients required a median of two rounds of qPCR testing (range: 2–9 times) to achieve a negative result. The median time to first negative qPCR was 8.5 days (range: 4–24 days). Ct values at admission were significantly associated with the time to negative qPCR (Appendix B). Furthermore, Ct values upon admission and the time to negative qPCR were significantly lower in patients with OTDs and fever (Figure 2) but not the other symptoms (Appendix C).
Figure 2.
Comparisons of Ct values of the N1/N2 gene in SARS-CoV-2-infected patients with/without fever or acute onset olfactory/taste disorders.
These results show that patients with asymptomatic or mild SARS-CoV-2 infection with a higher viral load required a longer time to exhibit negative qPCR results, suggesting that Ct values reflect viral load in principle (Arons et al., 2020). Moreover, the results demonstrated that fever and OTDs were significantly associated with a higher viral burden and longer time to negative qPCR. Compared with fever, data on OTDs are less available, unless intentionally assessed, even though OTDs are a common symptom in SARS-CoV-2 infection (Sedaghat et al., 2020, Beltrán-Corbellini et al., 2020, Giacomelli et al., 2020).
Recent studies have revealed that ACE2 (SARS-CoV-2 entry receptor) and TMPRSS2 (viral entry-associated protease) mRNAs are highly expressed in nasal epithelial cells, which suggests that the nasal cavity may be an important site for viral replication (Sungnak et al., 2020, Hou et al., 2020). Consistent with data from basic studies, patients with OTDs had a higher viral burden and required a longer time for viral clearance. The assessment of OTDs, including the avoidance of mechanical contact with the nasal cavity in OTD patients, may be important in preventing SARS-CoV-2 transmission.
This study had some limitations. First, it did not include severe patients because all study participants recovered well. Second, the diagnosis of OTDs was self-reported and no objective or physiological tests were performed. However, the symptoms that were prospectively collected reflected viral burden, even though the questionnaire was predetermined.
In conclusion, higher qPCR Ct values at diagnosis were associated with longer time to negative qPCR results, fever and OTDs. Physicians should carefully assess OTDs in patients with SARS-CoV-2 infection during disease, even in mild cases, as they may be associated with a high viral burden.
Conflict of interest
None.
Funding source
None.
Ethical approval
This study protocol was approved by the hospital’s Ethics Committee.
Acknowledgement
We would like to thank Editage (www.editage.com) for English language editing.
Appendix A. Characteristics of mild symptomatic and asymptomatic cases of SARS-CoV-2 infection
| Study population | |
| No. of patients | 57 |
| Age, mean ± SD | 35.4 ± 13.6 |
| Sex, male, no. (%) | 25 (43.9%) |
| Pregnant | 2 (3.5%) |
| Comorbidities | no. (%) |
| No comorbidity | 23 (30.4) |
| Malignant tumor/Neoplasm | 7 (12.3) |
| Asthma | 5 (8.8) |
| Psychiatric disorder | 4 (7.0) |
| Gynecologic disease | 3 (5.3) |
| Allergic rhinitis | 2 (3.5) |
| Atopic dermatitis | 2 (3.5) |
| Gastrointestinal disease | 2 (3.5) |
| Cardiovascular disease | 1 (1.8) |
| Traumatic subarachnoid hemorrhage | 1 (1.8) |
| Diabetes | 1 (1.8) |
| Dyslipidemia | 1 (1.8) |
| Herpes labialis | 1 (1.8) |
| Migraine | 1 (1.8) |
| Symptoms | no. (%) |
| Fever (>37.4 °C) | 15 (26.3) |
| Sore throat | 11 (19.3) |
| Acute onset olfactory/taste disorders | 10 (17.5) |
| Cough/sputum | 8 (14.0) |
| Headache | 7 (12.3) |
| Fatigue | 4 (7.0) |
| Nasal drip/congestion | 4 (7.0) |
| Joint pain | 1 (1.8) |
| Diarrhea | 1 (1.8) |
Appendix B. Association between Ct values of the N1/N2 gene and time to the first negative RT-PCR result
Appendix C. Comparisons of Ct values of N1/N2 gene in SARS-CoV-2-infected patients with/without symptoms
References
- Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Corbellini Á, Chico-García J.L., Martínez-Poles J., Rodríguez-Jorge F., Natera-Villalba E., Gómez-Corral J. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020 doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. 2019-novel coronavirus (2019-nCoV) real-time rRT-PCR panel primers and probes. Available from:https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html. [Accessed 9 May 2020] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa330. ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;S0092-8674(20):30675–30679. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat A.R., Gengler I., Speth M.M. Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820926464. [DOI] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Sun S., Shi Y., Wang H., Zhao R., Sheng J. SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit Care. 2020;24:170. doi: 10.1186/s13054-020-02893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]