Abstract
Objectives:
Quantify by immunohistochemistry (IHC) a partial epithelial-to-mesenchymal transition (p-EMT) population in oral cavity squamous cell carcinoma (OCSCC) and determine its predictive value for lymph node metastasis.
Methods:
Tissue microarrays (TMA) were created using 2 mm cores from 99 OCSCC patients (47 with low volume T2 disease, 52 with high volume T4 disease, and ∼50% in each group with nodal metastasis). IHC staining was performed for three validated p-EMT markers (PDPN, LAMB3, LAMC2) and one marker of well-differentiated epithelial cells (SPRR1B). Staining was quantified in a blinded manner by two reviewers. Tumors were classified as malignant basal subtype based on staining for the four markers. In this subset, the p-EMT score was computed as the average of p-EMT markers.
Results:
84 tumors were classified as malignant basal. There was 87% inter-rater consistency in marker quantification. There were associations of p-EMT scores with higher grade (2.15 vs. 1.92, p = 0.04), PNI (2.13 vs.1.83, p = 0.003), and node positivity (2.09 vs. 1.87, p = 0.02), including occult node positivity (56% vs. 19%, p = 0.005). P-EMT was independently associated with nodal metastasis in a multivariate analysis (OR 3.12, p = 0.039). Overall and disease free survival showed trends towards being diminished in the p-EMT high group.
Conclusions:
IHC quantification of p-EMT in OCSCC primary tumors is reliably associated with nodal metastasis, PNI, and high grade. With prospective validation, p-EMT biomarkers may aid in decision-making over whether to perform a neck dissection in the N0 neck and/or for adjuvant therapy planning.
Keywords: Head and neck squamous cell carcinoma, Oral cavity, Epithelial-mesenchymal transition, Lymph node, Metastasis, Immunohistochemistry
Introduction
The presence of lymph node metastasis has long been considered one of the most significant adverse prognostic features in oral cavity squamous cell carcinoma (OCSCC) [1,2]. Despite this, it remains challenging to definitively identify patients who would benefit from elective neck dissection (END) [1,2]. Traditionally, END has been recommended in patients with a 20 percent risk of occult nodal metastasis [3,4], suggesting not only that a negative neck dissection rate of 80 percent is acceptable, but also potentially missing occult disease in those with a low risk of nodal metastasis. Currently, primary tumor depth of invasion is the most reliable measure to estimate risk for occult nodal metastasis in OCSCC [5]. In addition, the need for adjuvant therapy in early stage disease is often controversial, with unclear treatment significance of adverse pathologic features such as tumor grade [6], perineural invasion (PNI) [7,8], and lymphovascular invasion (LVI) [9,10].
Epithelial-to-mesenchymal transition (EMT) is thought to be a potential driver of invasiveness and metastasis in a variety of epithelial cancers [11,12]. While most prior studies have focused on cell lines and animal models, our recent single cell expression analysis of oral cavity squamous cell carcinoma (OCSCC) identified the presence of a sub-population of mesenchymal cells expressing a partial EMT (p-EMT) program [13,14]. Immunohistochemical (IHC) staining for top markers from this program demonstrated a unique spatial localization to the invasive edge of tumor nests, in close apposition to the surrounding stroma [13]. Using single cell profiles to deconvolve bulk expression data from The Cancer Genome Atlas (TCGA), we also demonstrated associations of this program with a number of adverse pathologic features, including the presence of nodal metastasis and advanced nodal stage [13]. However, the ability to extrapolate such findings to clinical practice is limited by the high costs related to sequencing and inability to validate these findings in a well-annotated clinical cohort. Here, we sought to identify OCSCC tumors with p-EMT in an independent, institutional cohort using readily-accessible IHC markers and to determine whether there were associations between p-EMT and adverse clinical and pathologic features based on a more traditional IHC-based approach.
Patients and methods
Identification of Patients.
This study was deemed to be of minimal risk by the institutional review board. The institutional cancer registry was used to identify patients who underwent surgical management of previously untreated OCSCC between February 2000 and December 2014. The cohort was selected to attempt to be representative of smaller (low volume) and larger (high volume) tumors. In light of this, it was approximately evenly divided between T2 and T4 patients, with T2 patients thought to represent low volume tumors that would still have adequate tumor tissue for staining, and T4 tumors thought to represent larger, high volume tumors with more significant local invasion. In each group, approximately even numbers of node negative and node positive patients were chosen in order to facilitate a comparison between these two groups. Patient charts were reviewed, and relevant clinical and pathologic data were recorded.
Creation of Tissue Microarrays.
Hematoxylin and eosin (H&E) slides and formalin fixed, paraffin-embedded (FFPE) tissue blocks were obtained for all patients in the cohort. The H&E slides were reviewed and areas containing tumor were marked by a dedicated head and neck pathologist (W.C.F.). Tissue microarrays (TMA) were created using 2 mm cores. Five replicate microarrays were created using cores from at least three separate tumor blocks for each primary tumor. Creation of TMAs and sectioning were done by the Tissue Microarray and Imaging Core at the Dana-Farber/Harvard Cancer Center per standard protocols.All sections were 5 um thick.
Choice of Markers.
Four markers previously tested with IHC staining were chosen [13]. P-EMT quantification in our prior expression analysis was defined by an axis from epithelial to mesenchymal. We thus chose one epithelial marker (SPRR1B) and three of the top mesenchymal markers (LAMC2, LAMB3, and PDPN) [13]. All four markers were used to define the presence of this epithelial-mesenchymal axis, which we previously demonstrated to be relevant only in malignant-basal subtype tumors [13,14], and the three mesenchymal markers were subsequently used to quantify p-EMT in this subset of tumors.
Staining of Tissue Sections.
Double IHC staining tissue sections from FFPE HNSCC specimens was performed by the Massachusetts General Hospital Histopathology Core per standard protocols. Antigen retrieval was performed in a decloaker (Biocare Medical) using citrate buffer at pH 6.0. Sections were deparaffinized through xylenes and graded ethanol. Primary antibodies were visualized with HRP- or AP- linked secondary antibodies, followed by diaminobenzidine (DAB; Dako, Glostrup, Denmark) or AP-red (Dako) chromogens, respectively. Primary antibodies used included monoclonal mouse p63 (Biocare Medical, clone 4A4, cat#CM163A/B), polyclonal goat p63 (R&D Systems, cat#AF1916), polyclonal sheep PDPN (R&D Systems, cat#AF3670), polyclonal rabbit LAMB3 (Sigma-Aldrich, cat#HPA008069), monoclonal mouse LAMC2 (Novus Biologicals, clone CL2980, cat#NBP2–42388), and polyclonal rabbit SPRR1B (Sigma-Aldrich, cat#SAB1301567). Sections were stained for combinations of p63 with either PDPN, LAMB3, LAMC2, or SPRR1B. Sections were counterstained with hematoxylin (ThermoFisher Scientific). Stained sections were scanned into Aperio ImageScope (Leica Biosystems, Wetzlar, Germany).
Quantification of Marker Staining.
TMA cores were visualized at 10X magnification. Marker staining for each marker was quantified as 1+ (absent or minimal), 2+ (light or partial), or 3+ (dark or complete), focusing on marker staining at the invasive edge of tumor nests (Fig. 1). At least four cores for each marker from each primary tumor were quantified. Quantification was done in a blinded manner by the lead author (A.S.P.). Approximately 20% of all data points (including at least 1 core for each marker from each primary tumor) were independently quantified by a dedicated head and neck pathologicst (W.C.F.), and inter-rater agreement was assessed.
Fig. 1.
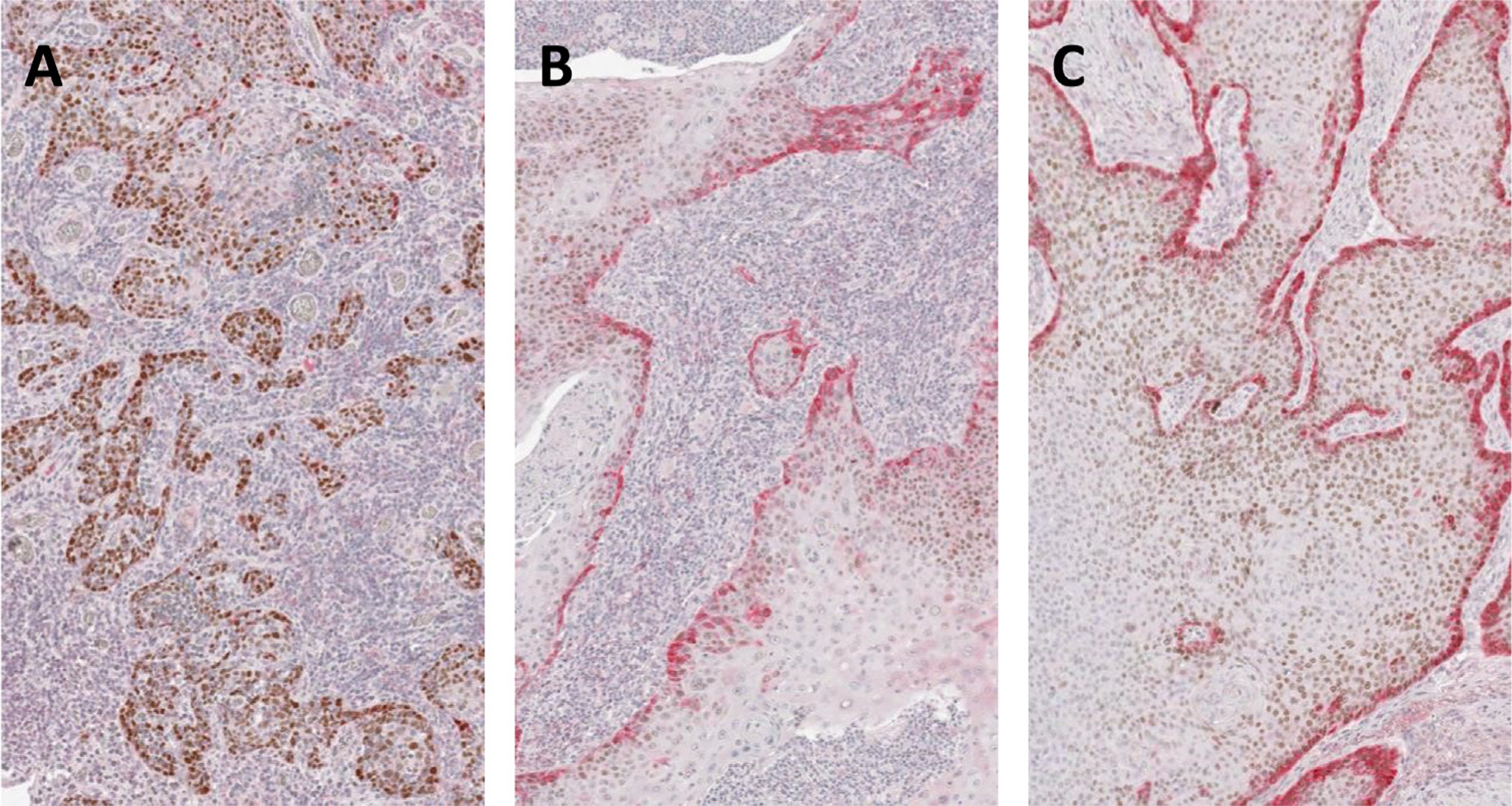
Quantification of p-EMT marker staining. Three cores from a single representative TMA with double IHC staining for p63 (brown) and a p-EMT marker (red). (A) Absent or minimal staining for red p-EMT marker (1+); (B) light or partial staining (2+); and (C) dark or complete staining (3+).
Survival Analysis.
Overall survival (OS) was defined as the time elapsed between the date of surgery and the date of last documented communication with the patient. For patients who did not suffer a recurrence, disease free survival (DFS) was equal to OS; for patients who recurred, DFS was calculated as the time elapsed between the date of surgery and the date of documented recurrence. For survival analyses, all patients were assumed to be censored unless documented as deceased (for analyses of OS) or having a recurrence (for analyses of DFS). JMP-Pro version 13 (SAS) was used to generate Kaplan-Meier survival curves.
Results
Patient Sample.
Ninety-nine patients were identified. Mean age at diagnosis was 63.3 years. 47 patients had T2 tumors, and 26 of these (55%) had nodal disease; 52 patients had T4 tumors, and 25 of these (48%) had nodal disease. 49 patients (49%) had PNI, 27 patients (27%) had LVI, 26 patients (26%) had high grade tumors (grade 3), and 22 patients (22%) had positive margins. Mean follow-up time was 43 months. There were 39 recurrences (39%). 74 patients (75%) received adjuvant radiation therapy, and 26 patients (26%) received adjuvant chemotherapy.
Quantification of Marker Staining.
Scores for each marker were calculated as the average of scores for each core. The mesenchymal score was calculated as the average of the scores for PDPN, LAMB3, and LAMC2 for a given sample. The epithelial score was the average SPRR1B score across cores. The mean mesenchymal score across all patients was 1.9 ± 0.5 and the mean epithelial score across all patients was 2.0 ± 0.6.
Inter-rater Correlation.
446/1900 cores (23.5%) were quantified independently by both A.S.P. and W.C.F. (Table 1). There was 86.8% consistency between raters (387/446 cores, chi sq = 578.6, p < 0.00001). Moreover, all inter-rater differences were within 1 point; i.e., there were no instances in which a given core was scored 1 + by one rater and 3 + by the other. Finally, there were no systematic differences between ratings by A.S.P. and W.C.F.
Table 1.
Inter-rater Agreement. Table of counts of 446 cores quantified by both A.S.P. and W.C.F. demonstrates strong inter-rater agreement (87%, chi sq = 587.6, p < 0.00001).
| A.S.P. | % Agree | |||||
|---|---|---|---|---|---|---|
| W.C.F. | - | 1 + | 2 + | 3 + | Total | - |
| 1 + | 133 | 11 | 0 | 144 | 92% | |
| 2 + | 17 | 143 | 17 | 177 | 81% | |
| 3 + | 0 | 14 | 111 | 125 | 89% | |
| Total | 150 | 168 | 128 | 446 | - | |
| % Agree | - | 89% | 85% | 87% | - | 87% |
Classification of Tumors as Malignant-Basal.
Our prior work demonstrated that p-EMT was of clinicopathologic significance only in malignant-basal subtype tumors [13,14] and that tumors not falling into this category were defined by low expression of both mesenchymal and epithelial markers [13]. Therefore, we classified all tumors falling below the mean for both mesenchymal and epithelial markers as non-basal; 15/99 tumors (15.2%) fell into this category and were eliminated from further analyses. These data suggest that identification of this cohort is possible solely based on IHC analyses and does not require bulk or single cell RNA sequencing.
Marker Distributions and Correlations.
84 patients were included in the final analysis. Mesenchymal scores for these patients were normally distributed, with a mean of 2.0 ± 0.5 and median of 2.0. Pearson correlations between markers were assessed. There was a strong and highly significant positive correlation between LAMB3 and LAMC2 (r = 0.65, p < 10−10) and weaker but significant positive correlations between LAMB3 and PDPN (r = 0.30, p < 0.006) and LAMC2 and PDPN (r = 0.28, p = 0.008). SPRR1B had a moderate but statistically significant negative correlation with each of the mesenchymal markers (LAMB3 r = −0.25, p = 0.02; LAMC2 r = −0.33, p = 0.002; PDPN r = −0.30, p = 0.006). It also had a highly significant negative correlation with the overall mesenchymal score (r = −0.38, p = 0.0004), confirming previously demonstrated inverse associations between these populations [13,14].
Association with Pathologic Features.
There was a significant association of higher mesenchymal scores with high grade (2.15 vs. 1.92, ANOVA F = 4.24, p = 0.04), PNI (2.13 vs. 1.83, F = 9.54, ANOVA p = 0.003), node positivity (2.09 vs. 1.87, ANOVA F = 5.39, p = 0.02), and T2 stage (2.09 vs. 1.89, ANOVA F = 4.01, p < 0.05), but not with LVI (2.11 vs. 1.92, ANOVA F = 2.59, p = 0.11) or positive margins (1.98 vs. 1.98, ANOVA F = 6 × 10−6, p > 0.99) (Fig. 2). Patients were then divided based on a mesenchymal score cutoff. In order to clearly delineate p-EMT tumors, tumors with mesenchymal score ≥2.2 were classified as mesenchymal high (28 patients, 33%), while those with mesenchymal score < 2.2 were classified as mesenchymal low (56 patients, 67%). There were significant associations between the mesenchymal high classification and higher grade, PNI, and node positivity, but not LVI or positive margins. In the mesenchymal high and low groups, 43% vs. 18% were high grade, respectively (chi sq = 6.04, p = 0.01); 63% vs. 38% had PNI, respectively (chi sq = 4.5, p = 0.03), 71% vs. 39% had positive nodes, respectively (chi sq = 7.71, p = 0.006); 37% vs. 20% had LVI, respectively (chi sq = 2.60, p = 0.11), and 29% vs. 16% had positive margins (chi sq = 1.81, p = 0.18) (Fig. 3).
Fig. 2.
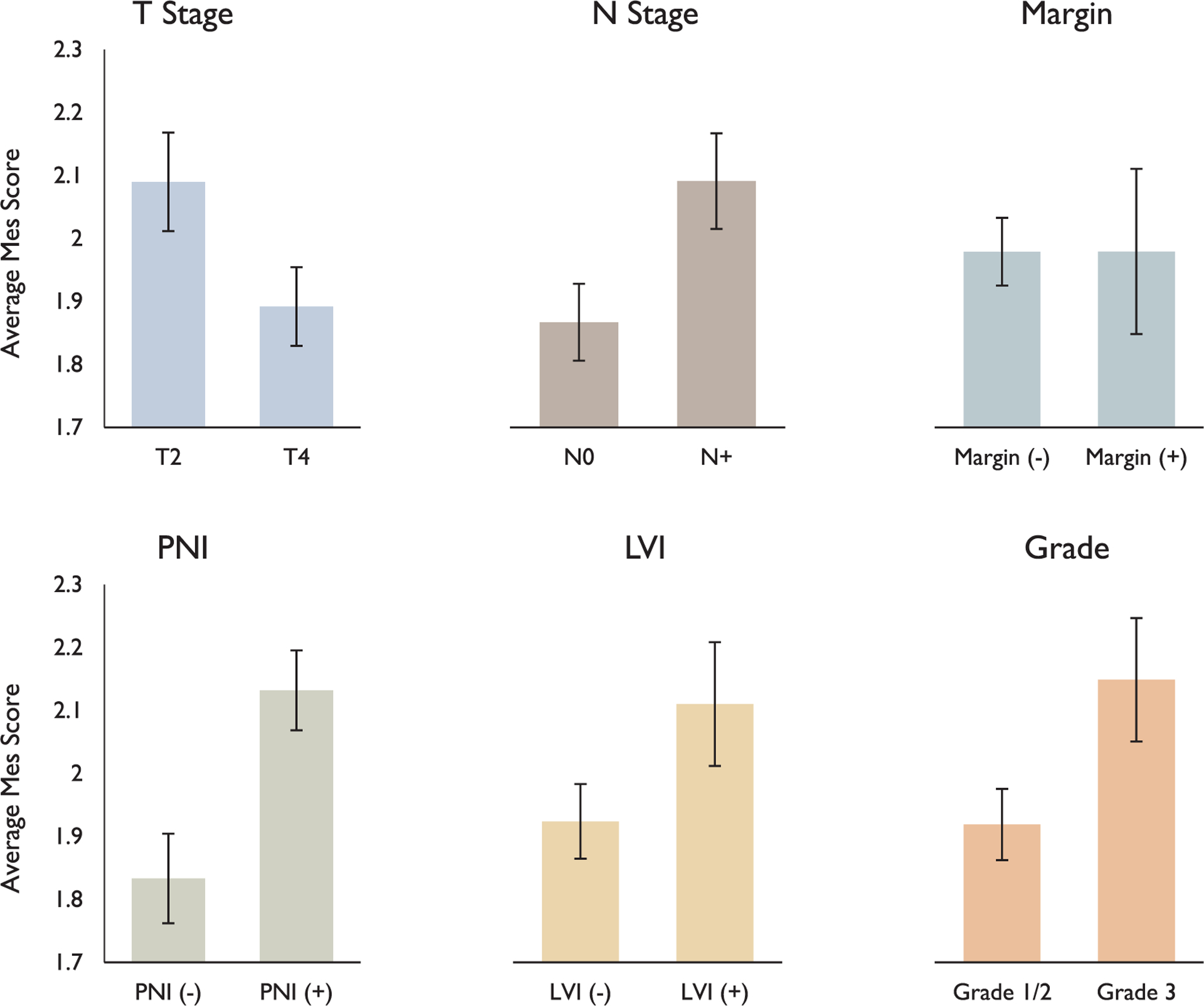
Association of mesenchymal scores with pathologic features. Bar plots demonstrate significant associations of p-EMT with T stage (p < 0.05), N stage (p = 0.02), PNI (p = 0.0003), and grade (p < 0.05), but not margin status (p = 0.99) or LVI (p = 0.11).
Fig. 3.
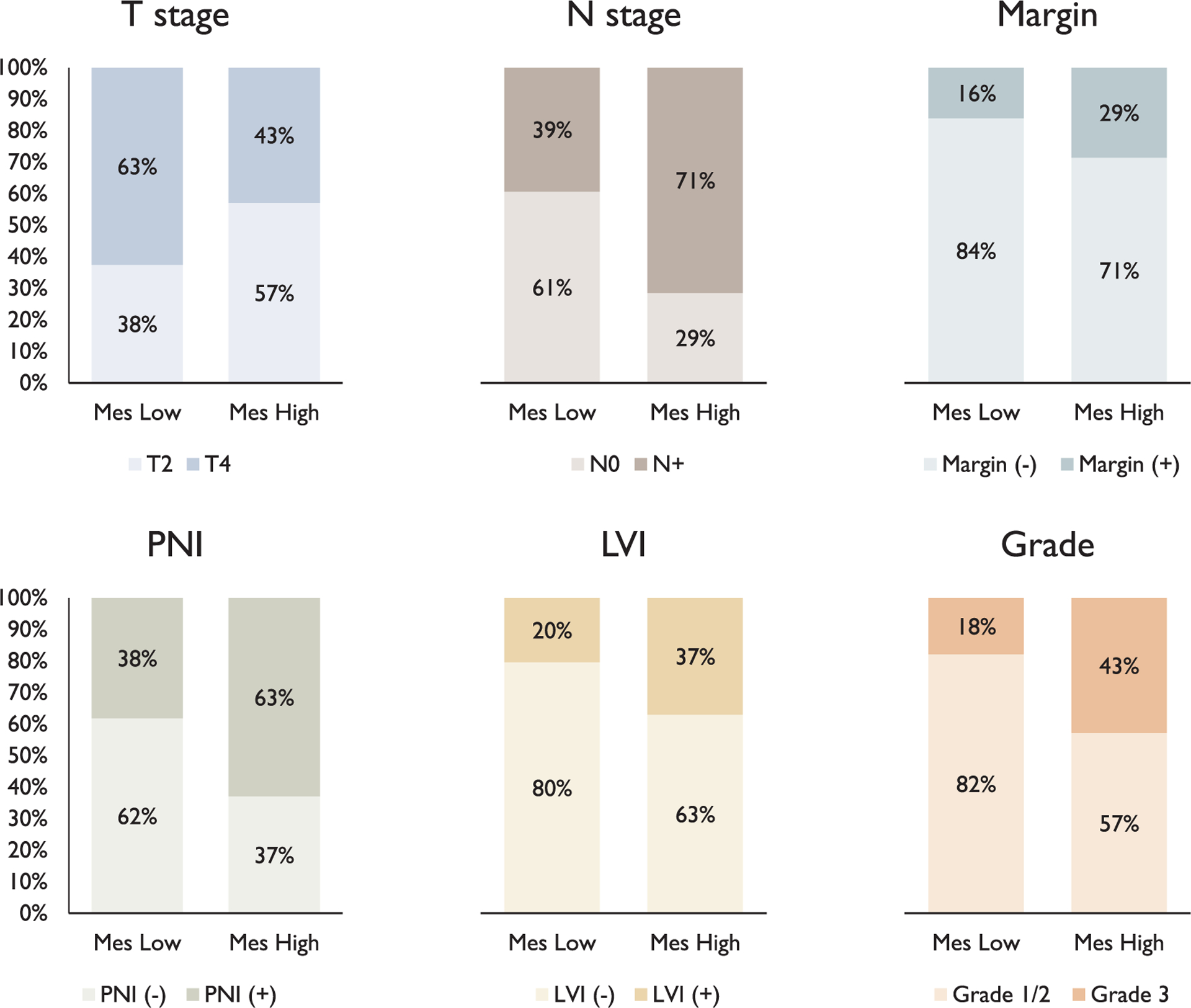
Association of mesenchymal classification with pathologic features. There were significant associations with N stage (chi sq = 7.71, p = 0.006), PNI (chi sq = 4.47, p = 0.03), and grade (chi sq = 6.04, p = 0.01), but not T stage (chi sq = 2.92, p = 0.09), margin status (chi sq = 1.81, p = 0.18), or LVI (chi sq = 2.6, p = 0.11). Error bars represent the standard error of the mean.
Predicting Nodal Disease.
Given the association of p-EMT with high grade and PNI and the known associations of the latter two factors with nodal metastasis, a multinomial logistic regression was performed to assess the independent effects of these three factors on predicting nodal disease (Table 2). T stage was left out of the analysis due to a cohort specifically selected to have approximately 50% node positive patients in each T stage, and DOI was left out of the analysis due to incomplete reporting of the data. Mesenchymal high (odds ratio (OR) = 3.12, p = 0.039) and PNI (OR = 2.90, p = 0.033) were found to be independently predictive of node positivity, while high grade (OR = 0.95, p = 0.93) was not.
Table 2.
Predicting Nodal Disease. Multinomial logistic regression was performed to assess the independent effects of p-EMT, high grade, and PNI on the presence of nodal disease. Odds ratios are shown. P-EMT and PNI were found to be independently predictive of node positivity. N+, node positive; N0, node negative; CI, confidence interval.
| Odds Ratio for N + /N0 [95% CI] | P-value | |
|---|---|---|
| Mesenchymal high | 3.12 [1.06–9.15] | 0.039 |
| PNI | 2.90 [1.09–7.69] | 0.033 |
| High grade | 0.95 [0.29–3.07] | 0.93 |
Predicting Occult Nodal Disease.
60/84 patients (71%) were clinically N0 at the time of presentation, and 18 of these patients (30%) had occult nodal disease on final pathology. Within this cN0 group of 60 patients, 10/18 mesenchymal high patients (56%) and 8/42 mesenchymal low patients (19%) had occult nodal disease (Table 3, chi sq = 8.0, p = 0.005). Depth of invasion (DOI) information was available for 24/60 patients (40%), as prior to the systematic reporting of DOI, tumor thickness was typically reported. In cN0 patients, average DOI was 10 mm for both pN0 and pN + patients. Assessed in another way, with a DOI cutoff of 4 mm in mind [5], of those with DOI < 4 mm 1/4 patients (25%) had occult nodal disease, while of those with DOI ≥ 4 mm, 5/20 patients (25%) had occult nodal disease.
Table 3.
Predicting Occult Nodal Disease. Of 60 malignant basal patients who were cN0, 30% were classified as mesenchymal high and 70% as mesenchymal low. Of mesenchymal low patients 81% were pN0 and of mesenchymal high patients 56% were pN+ (chi sq = 8.0, p = 0.005).
| Mesenchymal Low | Mesenchymal High | Total | |
|---|---|---|---|
| Node Negative | 34 (81%) | 8 (44%) | 42 |
| Node Positive | 8 (19%) | 10 (56%) | 18 |
| Total | 42 (100%) | 18 (100%) | 60 |
Survival Analysis.
OS at 2 and 5 years was 80% and 70%, respectively, for the mesenchymal low group, and 71% and 52%, respectively, for the mesenchymal high group (logrank test p = 0.19). DFS at 2 and 5 years was 69% and 50%, respectively, for the mesenchymal low group, and 50% and 35%, respectively, for the mesenchymal high group (log rank test p = 0.39) (Fig. 4). Although this was not statistically significant, there was a trend in the direction of poorer survival outcomes for the mesenchymal high group, with our results limited by sample size. In the mesenchymal low group 22/56 patients (39%) recurred, with an average time to recurrence of 16 months. In the mesenchymal high group 11/28 patients (39%) recurred, with an average time to recurrence of 17 months. There were 21 documented deaths (25%) during the follow up period.
Fig. 4.
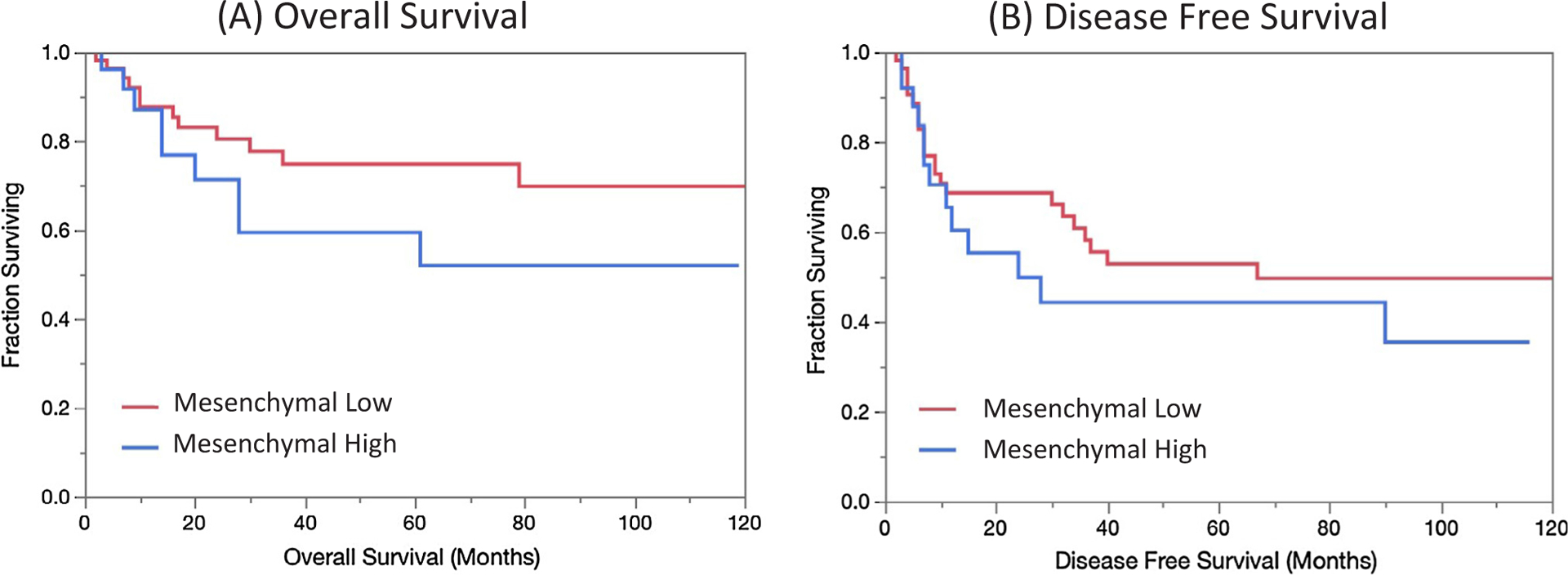
Survival analysis. Kaplan-Meier curves of overall (A, p = 0.19) and disease free (B, p = 0.39) survival stratified by mesenchymal classification demonstrate trends towards poorer survival outcomes in mesenchymal high patients.
Discussion
In this study, we utilized an independent, institutional cohort of patients to validate the clinical and pathologic associations of a previously described p-EMT population in malignant-basal subtype OCSCC [13,14]. In our cohort, p-EMT was associated with a number of adverse pathologic features, including grade, PNI, and, most notably, N-stage. We also demonstrate the feasibility of assessing p-EMT on 2 mm cores in a TMA, suggesting such an assessment on a primary tumor biopsy obtained via direct laryngoscopy or in-office may be feasible, as well. The utility of such an assay in the standard pathology workflow is supported by the use of IHC, which is already widely utilized, and the strong inter-rater agreement across samples.
P-EMT was strongly associated with the presence of nodal metastasis, with node positive patients having a higher p-EMT score (2.09 vs.
1.87, p = 0.02) and mesenchymal high patients having a higher rate of node positivity (71% vs. 39%, p = 0.006). Multinomial regression demonstrated that p-EMT was independently predictive of node positivity, even when controlling for PNI and high grade, other factors that are known to be associated with nodal disease (Table 2, OR = 3.12, p = 0.039). This relationship also included an association with occult nodal disease in clinically N0 (cN0) patients (Table 3, chi sq = 8.0, p = 0.005). DOI was not associated with occult nodal metastasis, though we were limited by the availability of DOI to 40% of our patients, as prior to systematic DOI reporting, tumor thickness, which included the exophytic component of the tumor, was often reported instead. Although further studies are needed, these preliminary results suggest p-EMT status in OCSCC may be helpful, in conjunction with other pathologic markers, to better determine which patients would benefit from elective neck dissection. We previously demonstrated in TCGA data that p-EMT score was associated with the proportion of justified neck dissection, as defined by cN0 neck dissections with pathologically confirmed occult disease on final pathology [13].
We also demonstrate associations of p-EMT with higher grade, supporting previously suggested associations in TCGA data [13], as well as the presence of PNI. Although these associations certainly do not suggest a causal relationship, they may suggest a biological explanation for the aggressive behavior of tumors with these characteristics. For example, the role of EMT in PNI has previously been postulated [15,16]. Interestingly, we did not appreciate an association of p-EMT with positive margins, suggesting the underlying biology of subtle microscopic disease that extends beyond a normal cuff of tissue may be independent of this program.
There are a few important limitations to the present study. Most notable is the retrospective nature of the data, with patients lost to follow up, incomplete reporting of pathologic features such as DOI and grade, and inability to clearly discern rationale for neck dissection and adjuvant therapy from the medical record. Moreover, as the cohort was specifically chosen to maximize the power to uncover an association between p-EMT and nodal metastasis, this was not a cohort of consecutive OCSCC patients. In addition, T1 and T3 patients were eliminated for the sake of maintaining uniform cohorts representing low volume and high volume disease. These limitations make it challenging to interpret the impact of p-EMT on survival outcomes. Certainly, an expanded analysis of a consecutive, preferably prospective, cohort of patients encompassing all T stages and with complete documentation of clinicopathologic features will be important in future efforts to determine the prognostic and predictive utility of p-EMT a marker of aggressive disease.
In addition, although quantification of p-EMT by IHC is an excellent method for validation and fits well into the existing anatomic pathology workflow, it provides only subjective semi-quantitative interpretation, though the excellent inter-rater agreement suggests this may not be a significant issue. In the present study, tumors were classified as malignant basal subtype based on the absence of staining for both meenchymal and epithelial markers; methods such as RNA-sequencing would be more definitive and could be performed as part of the development of a clinical assay but are out of the scope of the present study. In addition, a host of new technologies such as multi-spectral imaging may improve the ability to utilize a large number of markers with quantitative analysis all performed computationally. Application of this technology to p-EMT markers in OCSCC will be a natural next step in this line of investigation.
Conclusions
In a single institution cohort of patients, we demonstrate the association of p-EMT in primary tumors with adverse pathologic features, such as the presence of nodal disease, including occult nodal metastases in cN0 patients, high grade, and PNI. Our IHC staining in TMAs with 2 mm cores demonstrated good resolution between samples and strong inter-rater agreement, suggesting the possibility for clinical utility. With these findings, a prospective study assessing the impact of p-EMT on patient outcomes and whether it may serve to alter treatment algorithms may be beneficial.
Funding
Broadnext10 Catalytic Steps Translation Research Grant, #4300002, 2016, Broad Institute, Cambridge, MA (PI: Anuraag Parikh, MD and Sidharth Puram, MD PhD). Funds from the National Cancer Institute and NIH common fund (PI: Bradley Bernstein, MD PhD) were also used. The funding sources had no involvement in the design, conduct, and reporting of the research.
Footnotes
Disclosures
B.E.B. discloses financial interests in Fulcrum Therapeutics, 1CellBio, HiFiBio, Arsenal Biosciences, Cell Signaling Technologies and Nohla Therapeutics. The other authors have no financial disclosures or conflicts of interest.
Presented as podium presentation at: American Academy of Otolaryngology-Head and Neck Surgery Annual Meeting, 2018 Oct 7–10, Atlanta, GA.
References
- [1].Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg 1990;160(4):405–9. 10.1016/S0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- [2].De Silva RK, Siriwardena BSMS, Samaranayaka A, Abeyasinghe WAMU, Tilakaratne WM. A model to predict nodal metastasis in patients with oral squamous cell carcinoma. PLoS One 2018;13(8):1–16. 10.1371/journal.pone.0201755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weiss MH, Harrison LB, Isaacs RS. Use of a decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg 1994;120:699–702. [DOI] [PubMed] [Google Scholar]
- [4].Jalisi S Management of the clinically negative neck in early squamous cell carcinoma of the oral cavity. Otolaryngol Clin North Am 2005;38(1):37–46. [DOI] [PubMed] [Google Scholar]
- [5].D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 2015;373(6):521–9. [DOI] [PubMed] [Google Scholar]
- [6].Thomas B, Stedman M, Davies L. Grade as a prognostic factor in oral squamous cell carcinoma: a population-based analysis of the data. Laryngoscope 2014;124(3):688–94. 10.1002/lary.24357. [DOI] [PubMed] [Google Scholar]
- [7].Aivazian K, Ebrahimi A, Low THH, et al. Perineural invasion in oral squamous cell carcinoma: Quantitative subcategorisation of perineural invasion and prognostication. J Surg Oncol 2015;111(3):352–8. 10.1002/jso.23821. [DOI] [PubMed] [Google Scholar]
- [8].Nair D, Mair M, Singhvi H, et al. Perineural invasion: Independent prognostic factor in oral cancer that warrants adjuvant treatment. Head Neck 2018;40(8):1780–7. 10.1002/hed.25170. [DOI] [PubMed] [Google Scholar]
- [9].Adel M, Kao HK, Hsu CL, et al. Evaluation of lymphatic and vascular invasion in relation to clinicopathological factors and treatment outcome in oral cavity squamous cell carcinoma. Med (United States) 2015;94(43):1–7. 10.1097/MD.0000000000001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cassidy RJ, Switchenko JM, Jagdeesh N, et al. Association of lymphovascular space invasion with locoregional failure and survival in patients with node negative oral tongue cancers. JAMA Otolaryngol Head Neck Surg 2017;143(4):382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nieto MA, Huang RYYJ, Jackson RAA, Thiery JPP. Emt: 2016. Cell 2016;166(1):21–45. [DOI] [PubMed] [Google Scholar]
- [12].Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell 2017;168(4):670–91 http://www.ncbi.nlm.nih.gov/pubmed/28187288%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5308465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Puram SV, Tirosh I, Parikh AS, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 2017;171(7):1611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Puram SV, Parikh AS, Tirosh I. Single cell RNA-seq highlights a role for a partial EMT in head and neck cancer. Mol Cell Oncol 2018;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bakst RL, Wong RJ. Mechanisms of perineural invasion. J Neurol Surgery Part B Skull Base 2016;77(2):96–106. 10.1055/s-0036-1571835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang M, Li ZF, Wang HF, et al. MIF promotes perineural invasion through EMT in salivary adenoid cystic carcinoma. Mol Carcinog 2019;58(6):898–912. 10.1002/mc.22979. [DOI] [PubMed] [Google Scholar]


