Abstract
Purpose:
To investigate whether there are differences in diversity, taxonomic composition, and predicted functional pathways of the gut microbiome between Island Hispanic Puerto Ricans (HPR) and mainland non-Hispanic whites (NHW) measured before and at the end of chemo-radiation for Rectal Cancer.
Methods:
Fifty six stool samples of newly diagnosed rectal cancer patients (25 HPR and 31 NHW) were amplicon-sequenced during chemo-radiotherapy. 16S rRNA gene data was analyzed using QIIME2, phyloseq and LEfSe.
Results:
We observed similar within-sample alpha diversity for HPR and NHW participants during chemo-radiation. However, at the end of CRT, several taxa were present at significantly different abundances across both groups. Taxa enriched in the gut of HPR compared to NHW included Muribaculaceae, Prevotella 2, and 7, Gemella, Bacillales Family XI, Catenibacterium, Sutterella, Pasteurellales and Pasteurellaceae genera, whereas over-represented taxa in NHW participants were Turicibacter and Eubacteriaceae. Significant differences in predicted HPR microbiota functions included pathways for synthesis of L-methionine and degradation of phenylethylamine and phenylacetate.
Conclusion:
In this pilot study, taxonomic analyses and functional predictions of the gut microbiomes suggest greater inflammatory potential in gut microbial functions among HPR rectal cancer patients undergoing CRT compared to that of NHW participants.
Introduction
Island Hispanic Puerto Ricans (HPR) and mainland non-Hispanic whites (NHW) have different incidence, severity and health outcomes of cancer treatment contributing to health disparities.1 Although heath disparities may be attributed to multiple known factors (e.g., socio-economic position, complex socio-cultural and geographic factors, excess weight and physical inactivity),2–4 recent evidence underlining gut microbiota alterations in colorectal cancer (CRC) suggest potential ethnic differences in gut microbiomes that may contribute to disparities in patients undergoing chemo-radiation (CRT) for Rectal Cancer (RC).5–6 This pilot study is aligned with that area of interest. The objectives of this pilot study were to investigate whether there are differences in diversity, taxonomic composition, and predicted functional pathways of the gut microbiome between Island HPR and mainland NHW measured before and at the end of chemo-radiation for RC. Uncovering these potential differences may be a first step in identifying a source of health disparities in treatment outcomes for the optimization of clinical care, quality of life, and health outcomes of these patients.
Materials and methods
Study Population
Newly diagnosed RC patients of at least 18 years of age or older scheduled to receive CRT were recruited for this study. Exclusion criteria included history of intestinal chronic inflammatory diseases or history of previous abdominal surgery, diagnosed psychiatric and/or sleep disorders, comorbidities associated with sleep disorders (e.g. sleep apnea), use of insomnia medications, antibiotics, prebiotics, probiotics, steroids, and/or immune-suppressants agents within one month prior to sample collection at each assessment time-point. Data collection was conducted from September 2017 to April 2019. Ethics approval from both the Southeastern Academic Medical Center and the University of Puerto Rico Medical Science Campus were obtained prior to data collection. All participants included in the study provided written informed consent (IC).
16S rRNA Gene Sequencing
After obtaining IC, participants completed demographics and clinical information (i.e. age, weight, height). Participants collected approximately 5 g of stool using a sterile plastic container at two time-points: before, and at the end (after 24-28 treatments) of CRT. DNA was extracted from stool samples using the Power Soil DNA Isolation kit, (MoBio, Carlsbad, CA). V3-V4 regions of the 16S rRNA gene were amplified and sequenced on the MiSeq 2x300 bp platform (Illumina, San Diego, CA) following existing protocols.
Data Analysis
Demultiplexed reads were quality-checked and trimmed at a Q=25 cutoff using the Trim Galore! v0.4.4, (https://github.com/FelixKrueger/TrimGalore) wrapper package. Trimmed reads were imported into QIIME2-2019.17 and denoised with DADA2 8 without further trimming. For taxonomic assignment, a naïve Bayes classifier was trained on reference sequences from the SILVA v132 database 9 matching the sequencing primer pair. The resulting feature table was rarefied to 4,226 sequences per sample (smallest four-digit number), after eliminating one sample. Alpha and beta diversity metrics were calculated from the rarefied table using QIIME2 (alpha diversity) and the phyloseq R package (beta diversity).10 Metagenomic inference (Enzyme Commission [EC] and MetaCyc pathways) was performed on the rarefied table using QIIME2’s q2-picrust211 plugin. Linear discriminant analysis Effect Size (LEfSe)12 was performed on subsets (before and end-CRT) of the rarefied feature table using default parameters with per-sample normalization to a sum of 1 million.
Results
Participant characteristics
The sample consisted of 25 islander HPR and 31 mainlander NHW participants. The HPR participants were accrued from an ambulatory RT facility located in San Juan, Puerto Rico, while the NHW participants were accrued from two RT facilities located in the Tampa Bay, Florida area. Demographics and clinical characteristics are presented in Table 1. There were no significant differences between HPR and NHW participants in age, body mass index (BMI), years of education, and chemotherapy treatment (infusion of 5-FU vs. those on oral capecitabine) (Table 1).
Table 1.
Clinical characteristics of sample (n=56)
| Variables | All participants (n=56) | NHW (n=31) | HW (n=25) | |
|---|---|---|---|---|
| Gender | ||||
| M | 31 (55%) | 14 (45%) | 17 (68%) | |
| F | 25 (45%) | 17 (55%) | 8 (32%) | |
| Occupation | ||||
| Working | 54% | 50% | 58% | |
| Retired | 17% | 17% | 17% | |
| Handicapped | 8% | 8% | 8% | |
| Chemotherapy | ||||
| 5FU | 54% | 50% | 58% | |
| Xeloda | 42% | 42% | 42% | |
| Mean (SD) | Mean (SD) | Mean (SD) | p-value | |
| Age | 60.5 (13.1) | 59.1 (12.4) | 62.4 (14.1) | 0.34 |
| Education | 13.3 (3.0) | 13.8 (2.6) | 12.5 (3.6) | 0.56 |
| # treatment | 28.7 (3.7) | 29.0 (3.5) | 27.8 (4.1) | 0.43 |
| BMI | 27.1 (5.1) | 27.6 (4.6) | 26.0 (6.3) | 0.55 |
Composition of the Gut Microbiota
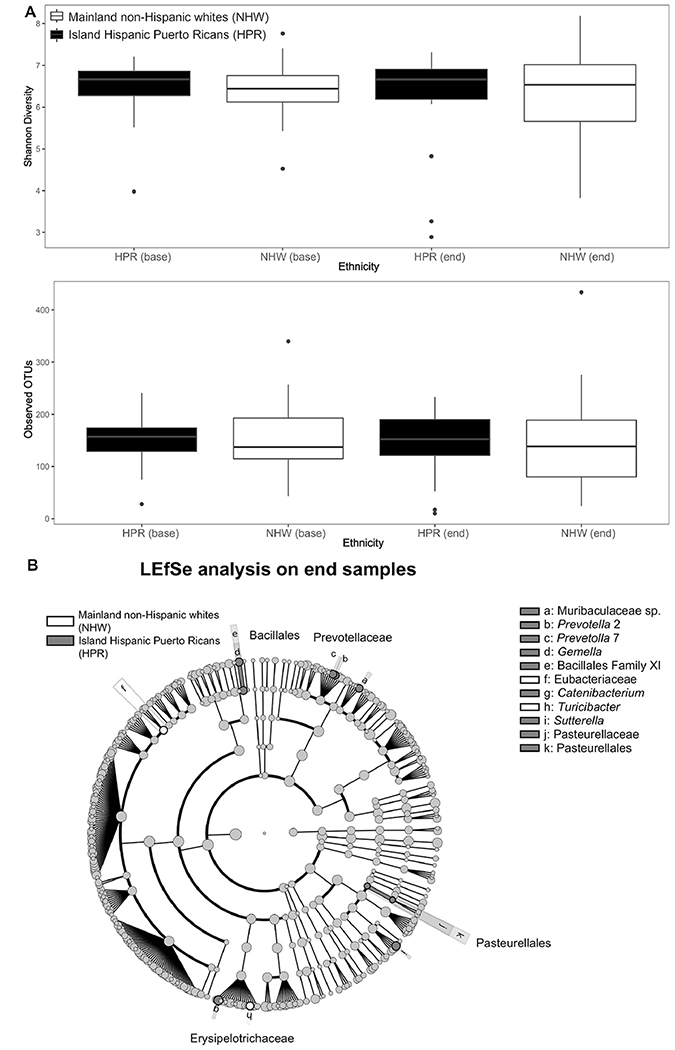
Shannon Diversity and number of observed OTUs indicated similar alpha diversity for HPR and NHW participants at each time of assessment (Figure 1a). Beta diversity analyses did not reveal consistent statistically significant groupings based on ethnicity and treatment time points (Figure S1). At the end of CRT, however, statistical comparisons using the LEfSe algorithm showed fine-scale differences in bacterial taxon abundances between both groups (Figure 1b). Bacterial genera enriched in HPR compared to NHW participants included Muribaculaceae, Prevotella 2, and 7, Gemella, Bacillales Family XI, Catenibacterium, Sutterella, Pasteurellales and Pasteurellaceae. At the end of CRT, two taxa classified to Turicibacter and Eubacteriaceae were over-represented in the NHW compared to the HPR group. Further, LEfSe comparison showed marked differences of functional gene pathways overrepresented in gut microbiota of HPR and NHW participants at the end of CRT.
Figure 1.
(A) Alpha diversity measures within HPR and NHW, split between CRT time points (before, and at the end of CRT for RC) and (B) LEfSe analyses of taxon abundances between HPR and NHW samples collected at end of CRT.
Discussion
Emerging evidence suggest that diversity of the gut microbiome in different ethnic groups 13–14 may contribute to disparities in treatment outcomes and/or influence the susceptibility to chronic disease in the intestinal tract (e.g., IBS).15 We observed no differences in alpha or beta diversity in our cohort of HPR and NHW, suggesting homogeneity across participants. Similar findings have been reported comparing healthy African American (n = 47) and NHW women (n = 33).5
At the end of CRT, we did, however, observe ethnic differences in bacterial communities down to the genera level. For example, certain strains of Catenibacterium enriched in HPR relative to NHW participants have been linked to the high-sugar, high-fat “Western” diet.15–16 Western diet-associated dysbiosis affects host gastrointestinal tract metabolism and immune homeostasis.15,17 Contrarily, other Catenibacterium species produce short chain fatty acids, such as butyric acids, from glucose fermentation.18–19 Butyric acid may serve beneficial roles in colonic anti-inflammation and metabolic health parameters.20 Another genus enriched in HPR relative to NHW participants, Sutterella, may be related to worse outcomes of cancer treatment such as chemoresistance among CRC patients.21 Similarly, other gram-negative genera enriched in the HPR compared to NHW group, such as Prevotella and Pasteurella, are associated with worse outcomes, including chemotherapy-induced oral mucositis, respiratory tract infection, or even sepsis among cancer patients.22–23 From the ethnic perspective, higher abundances of Prevotella were found in stool samples from a healthy Hispanic Cohort compared to Human Microbiome Project,24 although Prevotella can also be related to cancer. Further, Gemella and Prevotella are among intestinal microbes previously associated with CRC.25 The contribution of specific bacterial genera to negative outcomes in HPR participants is an area of future research.
Conversely, Turicibacter enriched in the NHW compared to HPR group is correlated with the anti-inflammatory compound butyric acid.20 This agrees with our previous report that gut Turicibacter abundances were associated with lower sleep disturbance and depression scores among RC participants during CRT.26 Eubacteriaceae, which is also enriched in NHW compared to HPR participants, participates in the production of medium-chain fatty acids.27 Medium-chain fatty acids exert beneficial effects on the intestinal health, including energy production, integrity support of the intestinal tissue, and immune modulation.28
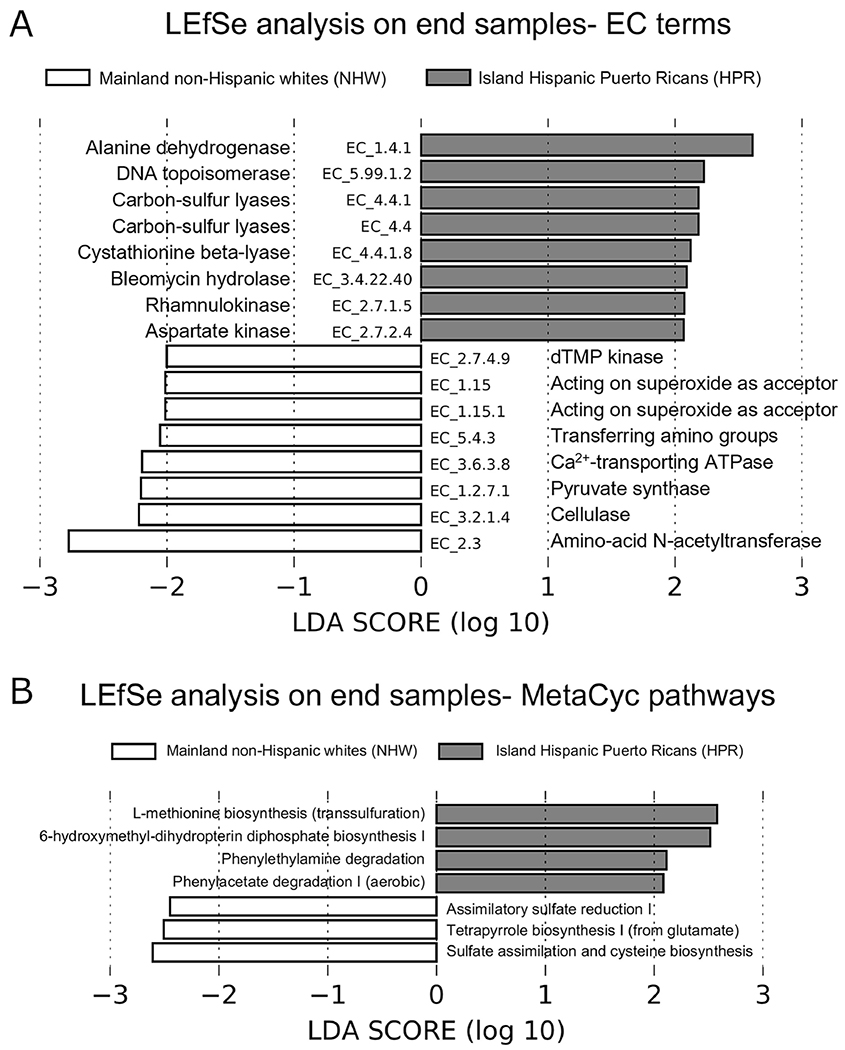
Exploratory functional analyses suggest that after CRT, the gut of HPR participants is enriched in L-methionine biosynthesis (via transsulfuration) and carbon-sulfur lyase pathways, implying the presence of hydrogen sulfide in the gut,29–30, although these metabolites were not directly measured. Hydrogen sulfide can be detrimental to the intestinal epithelia cells via inhibition of mitochondrial cytochrome C oxidase, activation of pro-inflammatory T helper 17 cell, and inhibition of butyrate catabolism in the colonocytes.31 Hydrogen sulfide can also protect gut bacteria from reactive oxygen species.31 At the end of CRT, HPR participants also showed over-representation of pathways that degrade phenylethylamine and phenylacetate, suggesting the presence/availability of these metabolites in the gut. Increased phenylethylamine levels have been positively associated with inflammatory conditions such as Crohn’s and inflammatory bowel disease,32 while elevated phenylacetate concentrations has been linked to CRC.33
The enrichment of cysteine and tetrapyrrole biosynthesis pathways suggest some recovery of gut microbiota functions in NHW versus HPR in this cohort. Cysteine is used for protein and glutathione synthesis, and glutathione and other cysteine derivatives are important for protection against oxidative stress toxicity,34 amelioration of intestinal inflammation,35 and survival in RC patients who received RT.36 The tetrapyrrole biosynthetic pathway encompasses the synthesis of porphyrins (such as heme, chlorophyll and coproporphyrin III) and corrinoids (such as cobalamin).37 While cobalamin is nutritionally beneficial and some porphyrins are anti-inflammatory,38 elevated endogenous porphyrins have been associated with CRC.39
Limitations
Limitations of this pilot study include the moderate sample size and the use of fecal samples, which may not fully represent the structure of the mucosal microbiota. However, tissue-based studies can be invasive, riskier, and more expensive compared to fecal samples which are more commonly used to study microbial communities.40 Another limitation of our 16S rRNA gene-based analyses is the inability to classify beyond the genus level, inaccuracy in resolving functional potential among taxa sharing high 16S rRNA gene identity, or inability to confirm functional activity. Clinical data analyses are also limited by the exclusion of variables (e.g. diet) that were assessed but not yet analyzed, and data that was not available to us (tumor characteristics [e.g., tumor target volume, rectal dose].
Conclusions
Our study suggests that, compared to NHW, HPR may have a greater abundance of CRC- and pro-inflammatory-associated bacterial taxa, which could potentially be related to poorer health outcomes of cancer treatment and contribute to health disparities. A large-scale, multi-center study is needed to validate these findings and confirm associations between gut microbiomes, metabolites, geography, diet, and/or lifestyle and comorbidities, including fatigue among RC survivors, of different ethnic groups.
Supplementary Material
Figure 2.
LEfSe analyses of over-represented (A) EC terms and (B) MetaCyc pathways between HPR and NHW samples collected at the end of CRT.
Acknowledgments
We thank the staff at Tampa General Hospital Cancer Center, Advent Heath Cancer Center, Tampa Bay, St. Joseph’s Hospital Cancer Institute, Tome and Ubiñas Radiotherapy Center, and Oncology Hospital (Dr. Isaac Gonzalez Martinez) for access to patients. We are grateful to volunteers of this study and the College of Nursing’s Biobehavioral Laboratory at University of South Florida for 16S rRNA gene sequencing support.
Funding
This work is supported through the National Institute of Nursing Research (NINR) program of the National Institutes of Health (Award Number F32NR016618 to VGM). The article does not represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Velda J. González-Mercado, Postdoctoral Scholar, College of Nursing, University of South Florida, Tampa, FL, United States.
Jean Lim, Postdoctoral Scholar, College of Nursing, University of South Florida, Tampa, FL, United States.
Lawrence Berk, Chief, Radiation Oncology, Medicine Professor, College of Medicine Radiology, University of South Florida, Tampa, FL, United States.
Mary Esele, Regional Clinical Coordinator, School of Nursing, South University, Tampa, FL, United States.
Carmen S. Rodriguez, Associate Professor, College of Nursing, University of South Florida, Tampa, FL, United States.
Gerardo Colón-Otero, Division of Hematology-Oncology, Mayo Clinic Cancer Center, Professor of Medicine, Mayo Clinic College of Medicine, Jacksonville, FL, United States.
References
- 1-.Gonzalez-Mercado VJ, Saligan LN, Ji M, et al. Differences in the Severity, Distress, Interference, and Frequency on Cancer-Related Symptoms Between Island Hispanic Puerto Ricans and Mainland Non-Hispanic Whites. Journal of immigrant and minority health. 2018; 20: 1029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2-.Goding Sauer A, Siegel RL, Jemal A, et al. Current Prevalence of Major Cancer Risk Factors and Screening Test Use in the United States: Disparities by Education and Race/Ethnicity. Cancer Epidemiol Biomarkers Prev. 2019; 28: 629–42. [DOI] [PubMed] [Google Scholar]
- 3-.Negron R, Vasquez A, Nieves M, et al. Body mass index affects the diagnosis and progression of prostate cancer in Hispanics. Ethn Dis. 2010; 20: S1-168-72. [PMC free article] [PubMed] [Google Scholar]
- 4-.Hughes DC, Tirado-Gomez M, Vallejo L, et al. Comparing determinants of physical activity in Puerto Rican, Mexican-American, and non-Hispanic white breast cancer survivors. SpringerPlus. 2015; 4: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5-.Carson TL, Wang F, Cui X, et al. Associations Between Race, Perceived Psychological Stress, and the Gut Microbiota in a Sample of Generally Healthy Black and White Women: A Pilot Study on the Role of Race and Perceived Psychological Stress. Psychosom Med. 2018; 80: 640–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6-.Hester CM, Jala VR, Langille MG, et al. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol. 2015; 21: 2759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7-.Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019; 37: 852–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8-.Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016; 13: 581–3. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9-.Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013; 41: D590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10-.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013; 8: e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013; 31: 814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12-.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011; 12: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13-.Brooks AW, Priya S, Blekhman R, et al. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018; 16: e2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14-.Liao M, Xie Y, Mao Y, et al. Comparative analyses of fecal microbiota in Chinese isolated Yao population, minority Zhuang and rural Han by 16sRNA sequencing. Sci Rep. 2018; 8: 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15-.Brown K, DeCoffe D, Molcan E, et al. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012; 4: 1095–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16-.Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009; 1: 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17-.Sekirov I, Russell SL, Antunes LC, et al. Gut microbiota in health and disease. Physiol Rev. 2010; 90: 859–904. [DOI] [PubMed] [Google Scholar]
- 18-.McIlroy SJ, Kirkegaard RH, McIlroy B, et al. MiDAS 2.0: an ecosystem-specific taxonomy and online database for the organisms of wastewater treatment systems expanded for anaerobic digester groups. Database (Oxford). 2017; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19-.Kageyama A, Benno Y. Catenibacterium mitsuokai gen. nov., sp. nov., a gram-positive anaerobic bacterium isolated from human faeces. Int J Syst Evol Microbiol. 2000; 50 Pt 4: 159–59. [DOI] [PubMed] [Google Scholar]
- 20-.Zhong Y, Nyman M, Fak F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res. 2015; 59: 2066–76. [DOI] [PubMed] [Google Scholar]
- 21-.Deng X, Li Z, Li G, et al. Comparison of Microbiota in Patients Treated by Surgery or Chemotherapy by 16S rRNA Sequencing Reveals Potential Biomarkers for Colorectal Cancer Therapy. Front Microbiol. 2018; 9: 1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22-.Hong BY, Sobue T, Choquette L, et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome. 2019; 7: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23-.Giordano A, Dincman T, Clyburn BE, et al. Clinical Features and Outcomes of Pasteurella multocida Infection. Medicine (Baltimore). 2015; 94: e1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24-.Ross MC, Muzny DM, McCormick JB, et al. 16S gut community of the Cameron County Hispanic Cohort. Microbiome. 2015; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25-.Kwong TNY, Wang X, Nakatsu G, et al. Association Between Bacteremia From Specific Microbes and Subsequent Diagnosis of Colorectal Cancer. Gastroenterology. 2018; 155: 383–90 e8. [DOI] [PubMed] [Google Scholar]
- 26-.González-Mercado VJ, Sakar A, Penedo FJ, et al. Gut microbiota perturbation is associated with acute sleep disturbance among rectal cancer patients. J Sleep Res. 2019;00:e12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27-.Scarborough MJ, Lawson CE, Hamilton JJ, et al. Metatranscriptomic and Thermodynamic Insights into Medium-Chain Fatty Acid Production Using an Anaerobic Microbiome. mSystems. 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28-.Chen J, Li Y, Tang Z, et al. Regulatory Functions of Fatty Acids with Different Chain Lengths on the Intestinal Health in Pigs and Relative Signaling Pathways. Curr Protein Pept Sci. 2019; 20: 674–82. [DOI] [PubMed] [Google Scholar]
- 29-.Chiku T, Padovani D, Zhu W, et al. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009; 284: 11601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30-.Ferla MP, Patrick WM. Bacterial methionine biosynthesis. Microbiology. 2014; 160: 1571–84. [DOI] [PubMed] [Google Scholar]
- 31-.Barton LL, Ritz NL, Fauque GD, et al. Sulfur Cycling and the Intestinal Microbiome. Dig Dis Sci. 2017; 62: 2241–57. [DOI] [PubMed] [Google Scholar]
- 32-.Santoru ML, Piras C, Murgia A, et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci Rep. 2017; 7: 9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33-.Vernocchi P, Del Chierico F, Putignani L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front Microbiol. 2016; 7: 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34-.Hicks JL, Mullholland CV. Cysteine biosynthesis in Neisseria species. Microbiology. 2018; 164: 1471–80. [DOI] [PubMed] [Google Scholar]
- 35-.Sina C, Lipinski S, Gavrilova O, et al. Extracellular cathepsin K exerts antimicrobial activity and is protective against chronic intestinal inflammation in mice. Gut. 2013; 62: 520–30. [DOI] [PubMed] [Google Scholar]
- 36-.Kotti A, Holmqvist A, Albertsson M, et al. SPARCL1 expression increases with preoperative radiation therapy and predicts better survival in rectal cancer patients. Int J Radiat Oncol Biol Phys. 2014; 88: 1196–202. [DOI] [PubMed] [Google Scholar]
- 37-.Zappa S, Li K, Bauer CE. The tetrapyrrole biosynthetic pathway and its regulation in Rhodobacter capsulatus. Advances in experimental medicine and biology. 2010; 675: 229–50. doi: 10.1007/9781-441915283_13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38-.Alonso-Castro AJ, Zapata-Morales JR, Hernandez-Munive A, et al. Synthesis, antinociceptive and anti-inflammatory effects of porphyrins. Bioorganic & medicinal chemistry. 2015; 23: 2529–37. 10.1016/j.bmc.2015.03.043 [DOI] [PubMed] [Google Scholar]
- 39-.Lualdi M, Battaglia L, Colombo A, et al. Colorectal cancer detection by means of optical fluoroscopy. A study on 494 subjects. Front Biosci (Elite Ed) 2010; 2:694–700. [DOI] [PubMed] [Google Scholar]
- 40-.Sze MA, Schloss PD (2018) Leveraging Existing 16S rRNA Gene Surveys To Identify Reproducible Biomarkers in Individuals with Colorectal Tumors. MBio, 9(3). doi: 10.1128/mBio.0063018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.