Abstract
Objectives:
The purpose of this study is to quantify breast radiologists’ performance at predicting occult invasive disease when DCIS presents as calcifications on mammography and to identify imaging and histopathological features that are associated with radiologists’ performance.
Materials and Methods:
Mammographically detected calcifications that were initially diagnosed as DCIS on core biopsy and underwent definitive surgical excision between 2010 and 2015 were identified. Thirty cases of suspicious calcifications upstaged to invasive ductal carcinoma and 120 cases of DCIS confirmed at the time of definitive surgery were randomly selected. Nuclear grade, estrogen and progesterone receptor status, patient age, calcification long axis length, and breast density were collected. Ten breast radiologists who were blinded to all clinical and pathology data independently reviewed all cases and estimated the likelihood that the DCIS would be upstaged to invasive disease at surgical excision. Subgroup analysis was performed based on nuclear grade, long axis length, breast density and after exclusion of microinvasive disease.
Results:
Reader performance to predict upstaging ranged from an AUC of 0.541 to 0.684 with a mean AUC of 0.620 (95% CI: 0.489–0.751). Performances improved for lesions smaller than 2 cm (AUC: 0.676 vs 0.500; p = 0.002). The exclusion of micro-invasive cases also improved performance (AUC: 0.651 vs 0.620; p = 0.005). There was no difference in performance based on breast density (p=0.850) or nuclear grade (p=0.270)
Conclusion:
Radiologists were able to predict invasive disease better than chance, particularly for smaller DCIS lesions (<2 cm) and after the exclusion of microinvasive disease.
Keywords: ductal carcinoma in situ, breast cancer, upstaging, mammography, calcifications
Introduction
Ductal carcinoma in situ (DCIS) is a preinvasive form of breast carcinoma (1, 2). DCIS typically presents as asymptomatic calcifications on mammography and is primarily diagnosed by core needle biopsy (3). The current standard of care is to remove DCIS with definitive surgery (3), but the final surgical pathology may reveal occult invasive disease. The reported rate of upstaging of DCIS to invasive disease after surgical excision is 17–26% (4, 5). The clinical implications of indentifying invasive carcinoma at the time of definitive surgery include the need for additional surgery with sentinel node biopsy and possible chemotherapy (6, 7). In addition, new management strategies for DCIS, such as active surveillance, depend on differentiation of DCIS and invasive disease as surgical excision is not performed (8–12). Preoperative methods that correctly differentiate DCIS from invasive disease are thus needed to provide more personalized care.
Currently the primary means of risk-stratifying women for the presence of occult invasive disease relies on histopathological features from core needle biopsy and some limited radiology features. Histological features such as nuclear grade have been shown to be prognostically relevant (13), with high nuclear grade being associated with higher upstaging of disease (4, 14–22). Although hormone receptors such as estrogen receptor (ER) and progesterone receptor (PR) status have been associated with an increased risk of disease recurrence, their utility to predict upstaging has shown mixed results (4, 23). Radiologists’ assessement of imaging features, such as lesion size and Breast Imaging Reporting and Data System (BI-RADS) classification have been evaluated, but are currently underutilized (24). The combined use of imaging, clinical, and histopathologic features has not been thoroughly tested.
The purpose of this study is to quantify breast radiologists’ performance at predicting occult invasive disease when DCIS presents as calcifications on mammography and to identify imaging and histopathological features that are associated with radiologists’ performance.
Methods
Case Selection
Following Institutional Review Board approval, we queried our institutional database to identify all cases of asymptomatic mammographically detected calcifications that were initially diagnosed as DCIS via a stereotactic core needle biopsy and that subsequently underwent definitive surgical excision between 2010 and 2015. This query did not include women with a history of breast cancer or those with imaging reports describing an associated mass, asymmetry or architectural distortion as these patients have a higher likelihood of invasive disease. This yielded 329 cases of pure DCIS and 68 cases of DCIS upstaged to IDC at surgical excision for an overall upstaging rate of 17.1%. From this master pool, cases were randomly shuffled and the first 120 cases of pure DCIS as well as the first 30 cases of upstaged IDC were selected to maintain a rate of upstaging (20%) typical in clinical practice (4, 5).
Data Collection
From the pre-biopsy diagnostic mammogram report, the BI-RADS breast density was recorded and categorized as low (predominately fatty or scattered fibroglandular) or high (heterogeneously dense or extremely dense) (25). For 13 cases, the breast density was not available as the clinical reports were not available. In these 13 cases, a radiologist who was blinded to the pathology outcome recorded the breast density. From the core needle biopsy pathology report, the nuclear grade, ER, and PR status were recorded. HER2 status is not typically tested for DCIS at our institution. Surgical reports were reviewed to determine the final pathology: DCIS (pure) or invasive cancer (upstaged). The long-axis length of the calcifications was calculated by a computer vision algorithm following annotation by a radiologist.
Radiologist Review
Cases were reviewed in a random order on a Mammography Quality Standards Act certified 5 megapixel Picture Archiving and Communication System monitor (Figure 1). Reader experience ranged from breast imaging fellow-in-training (n = 3) to attending (n = 7) with 3 to 25 years of post-breast imaging fellowship experience. The radiologists were told that all cases were DCIS at core needle biopsy but were blinded to all clinical and pathology information as well as prior images and radiology reports. The diagnostic mammograms that preceded the biopsy, including the magnification views, were available for review. It is standard of practice for all cases of calcifications to have one or more magnification views in conjunction with full field mammogram views available for review. No prior comparison studies were utilized. Readers reported a percentage likelihood of upstaging to invasive disease from 0–100% using a slider scale. Readers also recorded the morphology and distribution of the calcifications according to the BI-RADS Atlas, and reported the presence of a secondary mass, asymmetry, or architectural distortion at the site of the calcifications (25). Of note, no mass, asymmetry, or architectural distortion was reported on the original clinical interpretation, but readers were given the opportunity to report whether they felt one was present in addition to the biopsied calcifications during this review. The option to report secondary features was provided because there is interobserver variability in these features and they are associated with higher upstaging rates.
Figure 1:

(a) 57-year-old woman with pure DCIS. (b) 43-year-old woman with DCIS upstaged to invasive disease at surgical excision.
Statistical Analysis
Fleiss’ coefficient (κ) was used to evaluate the agreement between readers for the BI-RADS morphology and distribution. We utilized area under the receiver operating characteristic curve (AUC) using the pROC_1.0–7 package in R to determine the readers’ ability to predict occult invasive disease upon surgical removal (26). The pROC package utilized DeLong’s method to determine confidence intervals (27). In our analysis, the cancer estimation ratio was considered a prediction probability, thus no training or cross-validation was included. Subgroup analysis was performed based on long-axis length (≤2 cm or > 2 cm), breast density (low or high), and nuclear grade (low/intermediate or high). The 2 cm threshold was used as this differentiates a T1 from a T2 cancer according to the TNM staging system (28). The nuclear grade split into low/intermediate or high was based on the selection criteria used for the active surveillance trials in progress (8–12). Secondary analysis was performed after the exclusion of upstaged cases with microinvasion (defined as no invasive focus >1 mm) as the only form of invasive disease.
Results
The distribution of clinical and pathology variables is shown in Table 1. The average patient age was 58.8 +/− 11.3 years. DCIS was most commonly high nuclear grade (60%), estrogen receptor positive (82%), and progesterone receptor positive (77%). The mean long axis length of DCIS was 16.2 +/− 5.9 mm. There were no significant differences between pure and upstage groups in age, long axis length, breast density, and estrogen receptor/progesterone receptor status. Microinvasion was present on final pathology in 6 cases (4%).
Table 1:
Distribution of clinical, imaging, and histopathological information overall and for cases of pure DCIS and DCIS upstaged to invasive disease at surgical excision.
| Total (n=150) | Pure (n=120) | Upstage (n=30) | p-value | |
|---|---|---|---|---|
| Age (years) | 58.8 +/− 11.3 | 59.3 +/− 11.4 | 56.9 +/− 10.6 | 0.243 |
| Long axis length | 0.100 | |||
| Average (mm) | 16.2 +/− 5.9 | 15.9 +/− 6.1 | 17.2 +/− 4.6 | |
| ≥2 cm | 112 (75%) | 92 (77%) | 20 (67%) | |
| >2 cm | 38 (25%) | 28 (23%) | 10 (33%) | |
| Density | 0.364 | |||
| Low | 61 (41%) | 51 (43%) | 10 (33%) | |
| High | 89 (59%) | 69 (58%) | 20 (66%) | |
| Nuclear Grade | 0.198 | |||
| Low & Intermediate | 60 (40%) | 51 (43%) | 9 (30%) | |
| High | 90 (60%) | 69 (57%) | 21 (70%) | |
| ER Positive | 123 (82%) | 101 (84%) | 22 (73%) | 0.170 |
| PR Positive | 116 (77%) | 96 (80%) | 20 (67%) | 0.240 |
| Micro-invasive | 6 (4%) | 0 (0%) | 6 (20%) | N/A |
Note – Number refer to counts and column percentage in parentheses or mean +/− standard deviation
Calcifications were most frequently described as fine pleomorphic (53%) and grouped (65%) as shown in Table 2. There was fair agreement for the use of BI-RADS morphology (κ=0.271) and distribution (κ=0.371) descriptors. In addition to the biopsied calcifications, readers reported a secondary mass in 4% of cases, asymmetry in 9% of cases, and architectural distortion in 4% of cases. Of note, the initial clinical reports did not describe masses, asymmetries, or architectural distortion as these were exclusion criteria. Readers reported higher upstaging estimates if they felt there was an associated mass (41% vs 27%), asymmetry (40% vs 27%), or architectural distortion (51% vs 27%). The actual upstaging rates were not significantly higher if a mass (28% vs 20%, p=0.113) or asymmetry (26% vs 19%, p=0.076) was reported, but significantly higher if architectural distortion (37% vs 19%, p<0.001) was reported as shown in Table 2.
Table 2.
BI-RADS descriptor counts with mean reader upstaging estimates and actual upstaging percentages.
| Morphology | Count | Upstaging estimate | Actual upstaging |
|---|---|---|---|
| Amorphous | 291 (20%) | 17% | 43 (15%) |
| Coarse Heterogenous | 96 (6%) | 29% | 18 (19%) |
| Fine Pleomorphic | 794 (53%) | 29% | 168 (21%) |
| Fine Linear or fine-linear branching | 301 (20%) | 34% | 70 (23%) |
| Typical Benign* | 10 (1%) | 22% | 1 (10%) |
| Distribution | |||
| Diffuse | 1 (0%) | 5% | 0 (0%) |
| Regional | 122 (8%) | 36% | 31 (25%) |
| Grouped | 955 (65%) | 25% | 185 (19%) |
| Linear | 145 (10%) | 27% | 26 (18%) |
| Segmental | 255 (17%) | 38% | 54 (21%) |
| Mass | |||
| Yes | 65 (4%) | 41% | 18 (28%) |
| No | 1435 (96%) | 27% | 282 (20%) |
| Asymmetry | |||
| Yes | 140 (9%) | 40% | 36 (26%) |
| No | 1360 (91%) | 27% | 264 (19%) |
| Architectural distortion | |||
| Yes | 67 (4%) | 51% | 25 (37%) |
| No | 1433 (96%) | 27% | 275 (19%) |
Note – The count column refers to the number of times a descriptor was used by any reader for any case.
Typically benign BI-RADS morphologies include skin, vascular, coarse, large rod-like, round, rim, dystrophic, milk of calcium, and suture.
Reader performance to predict upstaging of DCIS to invasive disease ranged from an AUC of 0.541 to 0.684 with a mean AUC of 0.620 (95% CI: 0.489–0.751) as shown in Figure 2 and Table 3. Readers performed significantly better (p=0.002) when evaluating lesions 2 cm or smaller (AUC=0.676, 95% CI: 0.517–0.835) compared to lesions larger than 2 cm (AUC=0.500, 95% CI: 0.228–0.772). There was no difference in performance (p=0.104) between low breast density (AUC=0.473, 95% CI: 0.272–0.672) and high breast density cases (AUC=0.681, 95% CI: 0.517–0.846). The performance of readers for low/intermediate nuclear grade DCIS (AUC=0.617, 95% CI: 0.385–0.848) was not significantly different (p=0.27) than high nuclear grade DCIS (AUC=0.614, 95% CI: 0.449–0.778). When cases with microinvasion only were excluded from the upstaged group, the performance of readers significantly improved (AUC=0.651, 95% CI: 0.508–0.795, p = 0.005). There was a non-significant difference (p=0.164) in performance between breast imaging fellows (AUC=0.630, 95% CI: 0.438–0.718) and faculty (AUC=0.630, 95% CI: 0.587–0.673).
Figure 2:
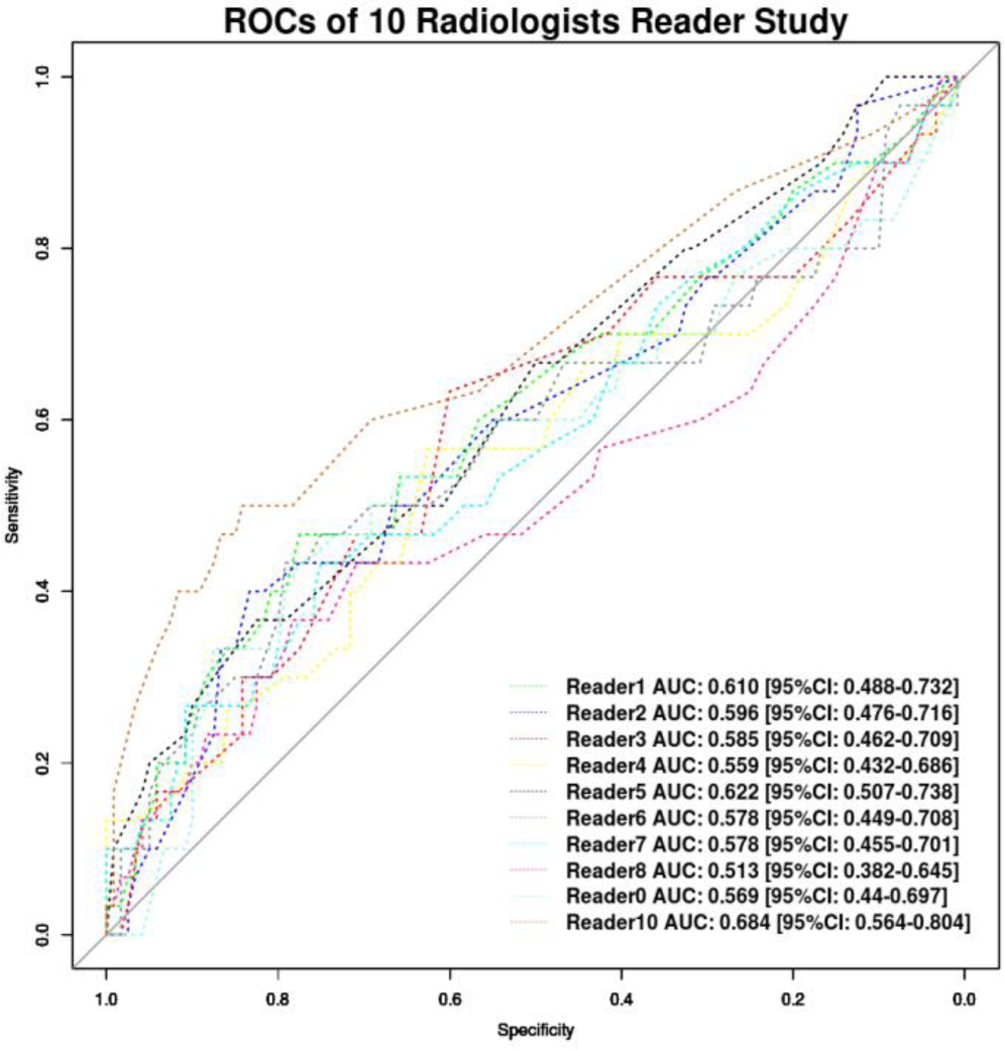
The receiver operating characteristic (ROC) curve for 10 readers is displayed for all 150 cases (n=120 Pure DCIS; n= 30 Invasive). No sub-group analysis is included in this figure.
Table 3:
Reader performance to predict upstaging of DCIS to invasive disease with subgroup analysis.
| N | AUC | |
|---|---|---|
| Overall | 150 | 0.620 [95% CI: 0.489–0.751] |
| Lesion Size | ||
| <2 cm | 38 | 0.676 [95% CI: 0.517–0.835] |
| ≥2 cm | 112 | 0.500 [95% CI: 0.228–0.772] |
| Breast Density | ||
| Low | 61 | 0.473 [95% CI: 0.272–0.672] |
| High | 89 | 0.681 [95% CI: 0.517–0.846] |
| Nuclear Grade | ||
| Low & Intermediate | 60 | 0.617 [95% CI: 0.385–0.848] |
| High | 90 | 0.614 [95% CI: 0.449–0.778] |
| Microinvasion | ||
| All | Upstaged = 30 Pure = 120 |
0.620 [95% CI: 0.489–0.751] |
| Excluded | Upstaged = 24 Pure = 126 |
0.651 [95% CI: 0.508–0.795] |
Discussion
Our study analyzed the performance of breast radiologists to predict upstaging of DCIS diagnosed at core needle biopsy to invasive disease at surgical excision. Reader performance overall was better than chance (AUC>0.5) and was improved for cases less than 2 cm in long axis length and after excluding cases of microinvasion, but not improved based on the nuclear grade or breast density.
Individual reader performance was variable ranging from an AUC at the low end of 0.541 up to a maximum AUC of 0.684 (Figure 2). Although all readers were either fellowship trained or current fellows in breast imaging, this is not the same task as differentiating cancer from not cancer that breast imagers are typically asked to perform. Nonetheless, we hypothesized that the skills utilized in clinical practice may translate to this task and the performance of at least some readers suggests that this is possible. However, the range of performances indicates that readers may be incorporating different factors into their prediction estimates. The use of traditional BI-RADS morphology and distribution descriptors suffered from fair interobserver variability, which was likely exacerbated by over half of cases described with a fine pleomorphic morphology and two thirds of cases described with a grouped distribution descriptor. This demonstrates the limited predictive capacity of BI-RADS features. It is possible that readers are incorporating non-BI-RADS morphology and distribution features into their estimates and further work is needed to identify and categorize such features, if they exist.
There were two features that were predictive of improved reader performance: smaller size and the exclusion of microinvasive disease. The improved performance of readers when evaluating lesions 2 cm or less was an unexpected finding since larger lesions provide more calcifications for evaluation. However, larger areas of calcifications may have more heterogeneity in appearance. We theorize that readers may be assessing the calcifications based on their overall appearance which is likely guided by the most common presentation. Since invasive disease can occur in just one small area it may be most appropriate to perform upstaging estimates based on the most suspicious appearance of any one area, no matter how small or unrepresentative of the entirety. Furthermore, biopsy planning might similarly need to be guided to target the most suspicious subgroups of calcifications. Additionally, we found that the exclusion of microinvasive disease improved reader performance. Microinvasion is defined as less than 1 mm of invasive disease and is included with T1 disease for staging purposes (28). However, given the extremely small component of invasion, it is likely that any mammographic features associated with invasion may either not manifest or be too small to appreciate by readers (29). This presents a challenge for the prediction of upstaging and one which may represent a fixed limitation of this type of investigation.
Although all cases selected for this study were originally interpreted as pure calcifications, readers were asked to record any secondary features they thought might be present and in discussion with readers they were fairly liberal in their reporting of these secondary features. When readers reported these secondary features, they notably increased their upstaging estimates, although this increase was only statistically significant for architectural distortion. DCIS presenting as a mass has been previously shown to be predictive of upstaging, but this is a slightly different scenario whereby the primary presentation is calcifications and only a secondary mass was appreciated (4). These findings suggest that close scrutiny for an underlying secondary process may be warranted to guide biopsy planning. However, as our study demonstrated, there is not universal agreement on the presence or absence of these secondary processes. Furthermore, the improved ability of tomosynthesis to evaluate for an underlying secondary process may also be helpful and warrants further evaluation.
The performance of the readers in this study (mean AUC = 0.62) are comparable to those of other investigators who have used alternative methods to predict DCIS upstaging to invasive disease. Computer vision and deep learning algorithms have been applied to mammographic calcifications and shown slightly better AUCs of 0.68 and 0.70 respectively (30, 31). Several investigators have applied clinical and pathological factors to predict upstaging, such as the nomogram developed by Jakub et al. which demonstrated a c-statistic of 0.71 (32). The value of other standard breast imaging modalities such as MRI or even more exploratory modalities such as gamma imaging have been explored in a limited fashion (33, 34). The baseline reader performance described in this study is alone likely not sufficient for immediate clinical use, but it provides a meaningful baseline metric for future studies. Predicting upstaging of DCIS to invasive disease will likely always be a challenging task and the best performance may ultimately be achieved using a combination of imaging, clinical, and histopathological factors. Furthermore, although AUC is a useful overall performance metric for analysis, depending on the specific clinical scenario, it may be beneficial to instead focus on maximizing sensitivity at the cost of specificity (or vice versa). Detailed analyses of these performance tradeoffs and how they might influence clinical treatment decision making are promising areas of future research.
Limitations
Our study has several limitations. Predicting occult invasive disease is a novel task for radiologists and training readers may improve performance. However, it is unclear what features radiologists should be identifying to improve their performance and so further work is needed in order to develop improved training strategies. Additionally, readers knew they were participating in a reader study so their performance might be different than in routine clinical practice, as evident in the use of the secondary descriptors. Radiologists also typically have access to additional clinical, pathological, and radiologic information including one or more prior studies which may influence their prediction performance. There is a very small risk of recall bias in this study as the cases were all imaged 4–9 years prior to the reader study. Finally, subgroup analysis, while informative, is limited by the size of the subgroup populations. For example, 20% of invasive cases demonstrated microinvasion only, thus, reducing the limited upstage cases to 24. Of note, this study was designed to assess reader performance to predict upstaging rather than to determine which clinical, pathologic, or demographic features are predictive of upstaging.
Conclusion
Radiologists were able to predict upstaging of DCIS to invasive disease better than chance, which improved for smaller cases of calcifications and after the exclusion of DCIS with microinvasion. Although radiologists are not currently asked to perform this task, if active surveillance as a management strategy becomes more widespread then radiologists will need to develop the skills to better identify occult invasive disease. However, at baseline the performance of radiologists is comparable to that of recently published manuscripts using quantitative image analysis. Further work is needed to determine if radiologists’ performance can be sufficiently improved through additional training and whether specific imaging features can be identified to improve this performance.
Abbreviations:
- AUC
area under the receiver operating characteristic curve
- DCIS
ductal carcinoma in situ
- ER
estrogen receptor
- PR
progesterone receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2017–2018. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf.
- 2.Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. J Natl Cancer Inst Monogr 2010; 2010(41):134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell KB, Kuerer H. Ductal Carcinoma In Situ: Treatment Update and Current Trends. Curr Oncol Rep 2015; 17(11):48. [DOI] [PubMed] [Google Scholar]
- 4.Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011; 260(1):119–28. [DOI] [PubMed] [Google Scholar]
- 5.Grimm LJ, Ryser MD, Partridge AH, et al. Surgical Upstaging Rates for Vacuum Assisted Biopsy Proven DCIS: Implications for Active Surveillance Trials. Ann Surg Oncol 2017; 24(12):3534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nounou MI, ElAmrawy F, Ahmed N, Abdelraouf K, Goda S, Syed-Sha-Qhattal H. Breast Cancer: Conventional Diagnosis and Treatment Modalities and Recent Patents and Technologies. Breast Cancer (Auckl) 2015; 9(Suppl 2):17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhankhar R, Vyas SP, Jain AK, Arora S, Rath G, Goyal AK. Advances in novel drug delivery strategies for breast cancer therapy. Artif Cells Blood Substit Immobil Biotechnol 2010; 38(5):230–49. [DOI] [PubMed] [Google Scholar]
- 8.Gierisch JM, Myers ER, Schmit KM, et al. Prioritization of research addressing management strategies for ductal carcinoma in situ. Ann Intern Med 2014; 160(7):484–91. [DOI] [PubMed] [Google Scholar]
- 9.Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22–24, 2009. J Natl Cancer Inst 2010; 102(3):161–9. [DOI] [PubMed] [Google Scholar]
- 10.Elshof LE, Tryfonidis K, Slaets L, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ - The LORD study. Eur J Cancer. 2015; 51(12):1497–510. [DOI] [PubMed] [Google Scholar]
- 11.Francis A, Fallowfield L, Rea D. The LORIS Trial: Addressing overtreatment of ductal carcinoma in situ. Clin Oncol (R Coll Radiol) 2015; 27(1):6–8. [DOI] [PubMed] [Google Scholar]
- 12.Hwang ES, Hyslop T, Lynch T, et al. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open. 2019; 9(3):e026797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffer S, Bleiweiss IJ. Histologic classification of ductal carcinoma in situ. Microsc Res Tech 2002; 59(2):92–101. [DOI] [PubMed] [Google Scholar]
- 14.Bagnall MJ, Evans AJ, Wilson AR, et al. Predicting invasion in mammographically detected microcalcification. Clin Radiol 2001; 56(10):828–32. [DOI] [PubMed] [Google Scholar]
- 15.Dillon MF, McDermott EW, Quinn CM, O’Doherty A, O’Higgins N, Hill AD. Predictors of invasive disease in breast cancer when core biopsy demonstrates DCIS only. J Surg Oncol 2006; 93(7):559–63. [DOI] [PubMed] [Google Scholar]
- 16.Kurniawan ED, Rose A, Mou A, et al. Risk factors for invasive breast cancer when core needle biopsy shows ductal carcinoma in situ. Arch Surg 2010; 145(11):1098–104. [DOI] [PubMed] [Google Scholar]
- 17.Lee CH, Carter D, Philpotts LE, et al. Ductal carcinoma in situ diagnosed with stereotactic core needle biopsy: can invasion be predicted? Radiology. 2000; 217(2):466–70. [DOI] [PubMed] [Google Scholar]
- 18.Lee CW, Wu HK, Lai HW, et al. Preoperative clinicopathologic factors and breast magnetic resonance imaging features can predict ductal carcinoma in situ with invasive components. Eur J Radiol 2016; 85(4):780–9. [DOI] [PubMed] [Google Scholar]
- 19.O’Flynn EA, Morel JC, Gonzalez J, et al. Prediction of the presence of invasive disease from the measurement of extent of malignant microcalcification on mammography and ductal carcinoma in situ grade at core biopsy. Clin Radiol 2009; 64(2):178–83. [DOI] [PubMed] [Google Scholar]
- 20.Park HS, Park S, Cho J, Park JM, Kim SI, Park BW. Risk predictors of underestimation and the need for sentinel node biopsy in patients diagnosed with ductal carcinoma in situ by preoperative needle biopsy. J Surg Oncol 2013; 107(4):388–92. [DOI] [PubMed] [Google Scholar]
- 21.Renshaw AA. Predicting invasion in the excision specimen from breast core needle biopsy specimens with only ductal carcinoma in situ. Arch Pathol Lab Med 2002; 126(1):39–41. [DOI] [PubMed] [Google Scholar]
- 22.Sim YT, Litherland J, Lindsay E, et al. Upgrade of ductal carcinoma in situ on core biopsies to invasive disease at final surgery: a retrospective review across the Scottish Breast Screening Programme. Clin Radiol 2015; 70(5):502–6. [DOI] [PubMed] [Google Scholar]
- 23.Harowicz MR, Saha A, Grimm LJ, et al. Can algorithmically assessed MRI features predict which patients with a preoperative diagnosis of ductal carcinoma in situ are upstaged to invasive breast cancer? J Magn Reson Imaging. 2017; 46(5):1332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 2010; 102(3):170–8. [DOI] [PubMed] [Google Scholar]
- 25.Sickles E, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS® Mammography ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology; 2013. [Google Scholar]
- 26.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011; 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44(3):837–45. [PubMed] [Google Scholar]
- 28.Breast Cancer Staging, 7th Edition. Available at: https://cancerstaging.org/references-tools/quickreferences/Documents/BreastMedium.pdf. Accessed June 27, 2019.
- 29.Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017; 67(4):290–303. [DOI] [PubMed] [Google Scholar]
- 30.Shi B, Grimm LJ, Mazurowski MA, et al. Can Occult Invasive Disease in Ductal Carcinoma In Situ Be Predicted Using Computer-extracted Mammographic Features? Acad Radiol 2017; 24(9):1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi B, Grimm LJ, Mazurowski MA, et al. Prediction of Occult Invasive Disease in Ductal Carcinoma in Situ Using Deep Learning Features. J Am Coll Radiol 2018; 15(3 Pt B):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakub JW, Murphy BL, Gonzalez AB, et al. A Validated Nomogram to Predict Upstaging of Ductal Carcinoma in Situ to Invasive Disease. Ann Surg Oncol 2017; 24(10):2915–24. [DOI] [PubMed] [Google Scholar]
- 33.Doyle AJ, Prakash S, Wang K, Cranshaw I, Taylor E, Oldfield R. DCIS of the breast: The value of preoperative MRI. J Med Imaging Radiat Oncol 2016; 60(2):194–8. [DOI] [PubMed] [Google Scholar]
- 34.Yoo J, Kim BS, Yoon HJ. Predictive significance of breast-specific gamma imaging for upstaging core-needle biopsy-detected ductal carcinoma in situ to invasive cancer. Ann Nucl Med 2018; 32(5):328–36. [DOI] [PubMed] [Google Scholar]


