Abstract
High prevalence of depression among people living with HIV (PLHIV) impedes antiretroviral therapy (ART) adherence and viral suppression. We estimate the effectiveness and cost-effectiveness of strategies to treat depression among PLHIV in Sub-Saharan Africa (SSA). We developed a microsimulation model of HIV disease and care in Uganda which captured individuals' depression status and the relationship between depression and HIV behaviors. We consider a strategy of screening for depression and providing antidepressant therapy with fluoxetine at ART initiation or re-initiation (if a patient has dropped out). We estimate that over 10 years this strategy would reduce prevalence of depression among PLHIV by 16.0% [95% uncertainty bounds 15.8%, 16.1%] from a baseline prevalence of 28%, increase adherence to ART by 1.0% [1.0%, 1.0%], and decrease rates of loss to followup by 3.7% [3.4%, 4.1%]. This would decrease first-line ART failure rates by 2.5% [2.3%, 2.8%] and increase viral suppression rates by 1.0% [1.0%, 1.0%]. This strategy costs $15/QALY compared to the status quo, and was highly cost-effective over a broad range of sensitivity analyses. We conclude that screening for and treating depression among PLHIV in SSA with fluoxetine would be effective in improving HIV treatment outcomes and would be highly cost-effective.
Keywords: HIV, Mental health, Depression, Cost-Effectiveness
INTRODUCTION
Despite considerable efforts devoted to improving antiretroviral therapy (ART) coverage and viral suppression in Sub-Saharan Africa (SSA) (Tanser, Bärnighausen, Grapsa, Zaidi, & Newell, 2013), HIV care falls short of UNAIDS 90-90-90 goals in most SSA countries (Joint United Nations Programme on HIV/AIDS, 2017). Two key targets for improving HIV care are reducing loss from care and increasing adherence to ART (Gupta, Dabla, Joshi, & Chakraborty, 2014; Joint United Nations Programme on HIV/AIDS, 2017; McCreesh et al., 2017). Developing effective and cost-effective approaches to attain these goals is central to many HIV control programs. Here, we assess the outcomes and cost-effectiveness of screening for, and treating, depression among people living with HIV (PLHIV) to improve population health.
Increasing evidence points to a high prevalence of depression among PLHIV. A meta-analysis found that 9-32% of PLHIV in SSA suffer from depression (Bernard, Dabis, & de Rekeneire, 2017). Compared to uninfected individuals, PLHIV have nearly twice the risk of depression (D. H. Akena, Musisi, & Kinyanda, 2010; Ciesla & Roberts, 2001; Spirig, 1998). Depression jeopardizes ART effectiveness and viral suppression by decreasing adherence, and reducing patient engagement (Bangsberg et al., 2000; Byakika-Tusiime et al., 2009; Cholera et al., 2017; Gonzalez, Batchelder, Psaros, & Safren, 2011; Horberg et al., 2008; Glenn J Wagner et al., 2014). Treatment of depression has been found to improve ART adherence and clinic attendance (Sin & DiMatteo, 2014; G. J. Wagner et al., 2017). Treatment approaches include pharmacological therapy and group and individual psychotherapy (Honagodu, Krishna, Sundarachar, & Lepping, 2013; Nakimuli-Mpungu et al., 2015).
We focus on a pharmacological intervention, fluoxetine, that is inexpensive and has good evidence of effectiveness in reducing depressive symptoms (Cipriani et al., 2016). Using data for Uganda, we assess the impact on HIV care cascade endpoints, clinical indicators, and cost-effectiveness of using fluoxetine to treat depression among PLHIV.
METHODS
We used a microsimulation model (Bendavid et al., 2008) that captures HIV transmission, HIV disease course, depression, and the benefits from treatment of both HIV and depression (Figure 1; Supplement). We consider a strategy of screening for depression and providing antidepressant therapy (ADT) with fluoxetine at ART initiation. Key data and sources are shown in Table 1 and Supplemental Tables S1 and S2.
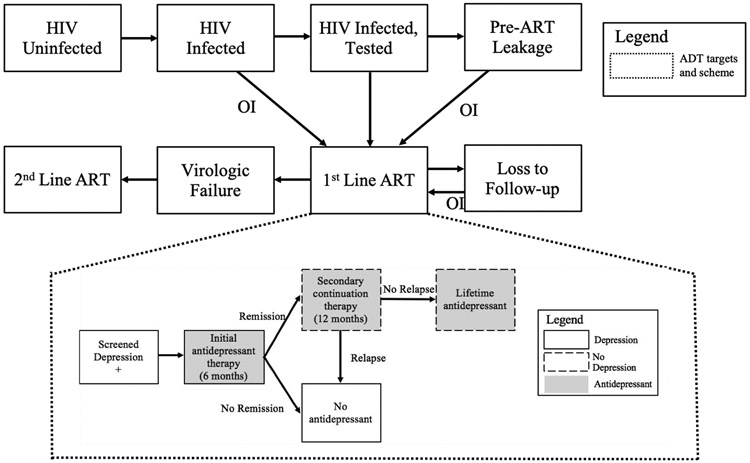
Figure 1.
Schematic representation of HIV natural history model and antidepressant treatment scheme
In each state and each time period, an individual can die, remain in the same state, or transition to another state following the direction of the arrows. Individuals enter the population during the various stages of HIV natural history (HIV uninfected, HIV infected and tested, etc.) and leave the population via death.
ADT: antidepressant treatment; ART: antiretroviral therapy; Pre-ART Leakage: patients are identified for ART but do not start ART; OI: opportunistic infection.
Table 1.
Data and Sources: Depression
| Parameter | Value | Range | Type
of Probability Distribution Used in PSA |
Sources |
|---|---|---|---|---|
| Depression prevalence (%) | ||||
| HIV negative | 17.0 | (12.0, 23.0) | Beta | (Ovuga, Boardman, & Wasserman, 2005) |
| HIV positive | 28.0 | (23.0, 33.0) | Beta | (Breuer, Myer, Struthers, & Joska, 2011) |
| Odds ratios for depression | ||||
| HIV positive, status known | 1.99 | (1.32, 3.00) | Lognormal | (Dickens Akena, Musisi, Joska, & Stein, 2012; Ciesla & Roberts, 2001) |
| CD4 <50 | 2.34 | (1.39, 3.93) | Lognormal | (Kaharuza et al., 2006) |
| Female | 1.85 | (1.24, 2.44) | Lognormal | (Kaharuza et al., 2006; Kinyanda, Hoskins, Nakku, Nawaz, & Patel, 2011) |
| Adherence prevalence (%) | 80.0 | (72.0, 88.0) | Beta | (Mills et al., 2006) |
| Odds ratios for HIV-infected individuals with depression | ||||
| Adherence | 0.32 | (0.11, 0.93) | Lognormal | (Byakika-Tusiime et al., 2009) |
| No leakage | 0.32 | (0.11, 0.93) | Lognormal | Assumed |
| No LTFU | 0.32 | (0.11, 0.93) | Lognormal | Assumed |
| Odds ratio for viral failure due to non-adherence | 9.90 | (3.20, 45.10) | Lognormal | (Obirikorang, Selleh, Abledu, & Fofie, 2013; Weidle et al., 2006) |
| PHQ-9 diagnostic test | ||||
| Sensitivity | 0.92 | (0.83, 1.00) | Beta | (D. Akena et al., 2013) |
| Specificity | 0.81 | (0.73, 0.89) | Beta | (D. Akena et al., 2013) |
| Treatment effects | ||||
| Probability of remission at month 6 of initial therapy | 0.70 | (0.32, 0.79) | Beta | (Ngo et al., 2014; Patel et al., 2003) |
| Probability of remission at month 12 of continuation therapy | 0.40 | (0.36, 0.50) | Beta | (Hollon, Stewart, & Strunk, 2006) |
ART: antiretroviral therapy; LTFU: loss to followup; PHQ-9: Patient Health Questionnaire-9; PSA: probabilistic sensitivity analysis.
Each individual is characterized by demographic features (age and gender), HIV-related features (HIV status, CD4 cell counts, viral load, and opportunistic infections), treatment features (treatment regimen and adherence), depression status (with or without depression), and depression treatment status. We track HIV care, including pre-ART loss from care (leakage), post-ART loss to follow-up (LTFU), and ART failure. Uninfected individuals can become infected through sexual contact with infected individuals.
The base case assumes individuals with depression suffer from depression until receiving ADT. Depression prevalence is stratified by gender, HIV infection status, and CD4 count. Individuals without depression may become depressed following either an HIV-positive test result or a decline in CD4 cell count below 50 cells/μL (Table 1). Depression lowers ART adherence rates, increases leakage, and increases LTFU (Tables 1, S2). ART adherence changes when depression status changes (Table 1).
We assume a depression screening tool with characteristics similar to the Patient Health Questionnaire-9 (PHQ-9) (D. Akena, Joska, Obuku, & Stein, 2013; Glenn J Wagner et al., 2014), a simple diagnostic questionnaire with estimated 91.6% sensitivity and 81.3% specificity for depression in Uganda (D. Akena et al., 2013; Kroenke, Spitzer, & Williams, 2001). We model ADT based on the cost and effectiveness of fluoxetine at a dosage of 40 mg/day. Our treatment algorithm (Figure 1, details in Supplement) is based on the clinical experience of long-term ADT that provide 6-month, 12-month, and long-term relapse and remission rates with 42% overall long-term remission (Table 1).
We used the model to assess costs and health outcomes measured in quality-adjusted life years (QALYs), discounted at 3%, and cost-effectiveness of providing ADT. We took a healthcare system perspective (Sanders, Neumann, Basu, & et al., 2016). Outcomes measured include prevalence of depression, annual rates of leakage and LTFU, percent of patients adherent to ART, percent of patients with virologic suppression, costs (2017 USD), life years, and QALYs. We measured the costs of fluoxetine, CD4 tests, viral load tests, ART (first-line and second-line), HIV tests, HIV-related inpatient and outpatient care, and non-HIV healthcare for each individual (Table S1). QALY weights were stratified based on HIV infection and depression status.
We performed one-way and two-way sensitivity analyses to examine critical assumptions; probabilistic sensitivity analysis varying all model parameters (distributions in Tables 1 and S1); and structural sensitivity analysis using a model that includes natural rates of developing and recovering from depression (details in Supplement).
We calibrated our model to data for Uganda (Figures S1-S4).
RESULTS
Base Case Analysis
The ADT intervention yields an additional 0.02 QALYs per person compared to the status quo (Table 2). The benefits of providing ADT accrue mainly from relief of depression leading to higher quality of life, improvements in retention and adherence, and reduction in new HIV infections. Compared to the status quo, providing ADT reduces depression prevalence among HIV-infected individuals by 16.0% [95% uncertainty bounds 15.8%, 16.1%] from a baseline prevalence of 28% (Table 3). All changes are percent change relative to the status quo.
Table 2.
Base Case Results: Costs, Life Years, Quality-Adjusted Life Years, and Incremental Cost-Effectiveness Ratio
| Strategy | LYs | Incremental LYs |
QALYs | Incremental QALYs |
Costs ($) | Incremental Costs ($) |
ICER ($/QALY gained) |
|---|---|---|---|---|---|---|---|
| Individual Level | |||||||
| Status Quo | 16.93 | 16.41 | -- | 2153.28 | -- | -- | |
| ADT Provision | 16.94 | 0.001 | 16.43 | 0.02 | 2153.52 | 0.25 | 15.43 |
| Population Level | |||||||
| Status Quo | 831.34 M | 805.67 M | -- | 105,687.20 M | -- | -- | |
| ADT Provision | 831.38 M | 0.04 M | 806.45 M | 0.79 M | 105,699.36 M | 12.13 M | 15.43 |
M: million; ADT: antidepressant therapy; LY: life year; QALY: quality-adjusted life year; ICER: incremental cost-effectiveness ratio, compared to the status quo
Table 3.
Base Case Results: Relative Percentage Changes (vs. Status Quo) in Care Cascade Parameters and 95% Uncertainty Bounds
| Strategy | Reduction
in Depression Prevalence |
Reduction
in Pre-ART Leakage |
Reduction in LTFU |
Increase in Adherence to ART |
Reduction in First Line ART Failure |
Increase
in Virologic Suppression |
|---|---|---|---|---|---|---|
| ADT Provision | 15.981 (15.841, 16.111) | −0.510 (−0.756, 0.349) | 3.722 (3.352, 4.092) | 1.014 (1.014, 1.015) | 2.526 (2.276, 2.775) | 1.005 (1.004, 1.006) |
ADT: antidepressant therapy; ART: antiretroviral therapy; LTFU: loss to followup.
Relief of depression improves several HIV care outcomes (Table 3). Rates of LTFU after ART initiation are reduced by 3.7% [3.4%, 4.1%]. ART adherence increases by a modest 1.0% [1.0%, 1.0%] and failure of first-line ART is reduced by 2.5% [2.3%, 2.8%]. Compared to the status quo, an additional 1.0% [1.0%, 1.0%] of the HIV-infected population is virologically suppressed.
The intervention costs $15/QALY gained compared to the status quo and thus would be considered very cost-effective by any criteria (Laxminarayan et al., 2006; Murray & Lopez, 2002; World Health Organization, 2009). Extrapolated to the population level, we estimate that the intervention would cost $12.13 million and gain 790,000 QALYs over the analytic time horizon (Table 3).
Sensitivity Analyses
At $2.52, the highest reported price (for Botswana) (World Health Organization, 2014), the ICER is $22/QALY. At the lowest reported effectiveness (32% in remission at 6 months), depression prevalence is reduced by 7.3% [7.2%, 7.4%] and the ICER is $42/QALY. If the long-term relapse rate is 50% (vs. 42% in the base case), the ICER is $18/QALY. If ADT uptake is only 50%, the ICER is $36/QALY.
The improvement in quality of life from ADT contributes significantly to the health benefits. If there is no quality-of-life improvement from treating individuals with depression, the ICER is $292/QALY. If we remove the quality-of-life benefits from treating depression and increase antidepressant cost to the upper bound of $2.52, the ICER is $419/QALY.
We performed one-way sensitivity analyses on the parameters characterizing the relationship between depression, ART adherence, and viral failure. In the base case, the OR for adherence among those depressed is 0.32, and the OR for viral failure is 9.9 with ART non-adherence. Changing the ORs to their bounds, 0.93 and 3.2, respectively, increases the ICER to $29/QALY.
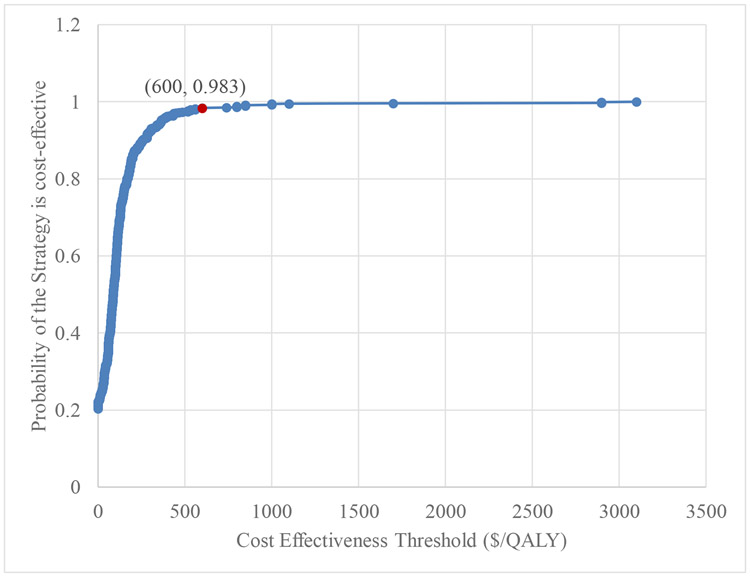
In probabilistic sensitivity analysis, the ADT intervention was very cost-effective for all model parameter realizations when using the Uganda GDP per capita ($631)(The World Bank, 2019) as the willingness-to-pay reference value (Figure 2).
Figure 2.
Cost-effectiveness acceptability curve
In structural sensitivity analysis, using a model that incorporates natural development of and remission from depression, the ICER was $28/QALY.
DISCUSSION
Our analysis indicates that screening PLHIV for depression and providing fluoxetine or a similar antidepressant is an effective and highly cost-effective strategy for improving HIV-related outcomes in Uganda, and likely throughout other regions of SSA with a generalized HIV epidemic. Beyond reducing depressive symptoms among PLHIV, ADT improves adherence and reduces virologic failure rates, thereby improving suppressions rates and reducing HIV transmission. Such a strategy is likely to be highly cost-effective, especially where the prices of generic antidepressant medications are low. At an estimated cost of $1.16 per month and an ICER of $15/QALY gained, provision of ADT to PLHIV with depression falls well below commonly accepted thresholds, and is more cost-effective than interventions such as home care treatment (Laxminarayan et al., 2006).
Treatment of depression in PLHIV in SSA has been limited to date. International funding agencies frequently focus on physical illnesses (such as HIV) rather than mental illness. Country ministries also provide few funds for mental health services. In 2010, Uganda devoted only 1% of public health expenditures to mental health care (Kigozi, Ssebunnya, Kizza, Cooper, & Ndyanabangi, 2010). Additionally, Uganda and other countries in SSA have very few trained mental health providers (Kigozi et al., 2010). Providing ADT can be accomplished as part of routine HIV care and thus would be practical to implement in settings with few trained mental health professionals.
Our analysis has several limitations. First, our model is instantiated with data from Uganda and therefore is most applicable to countries with a similar HIV context. Second, there is relatively little data on the natural course of depression in PLHIV and in lower-income countries (Stegenga, Kamphuis, King, Nazareth, & Geerlings, 2012). We made simplifying assumptions that match the existing evidence available from similar populations and interventions. While additional granularity about depression could be added, such detail is unlikely to impact our population-level outcomes. Third, we assumed that PLHIV on ART receive no depression treatment under the status quo. To the extent that PLHIV already receive depression treatment, our analysis may overstate the intervention benefits. Fourth, we did not consider side effects of fluoxetine such as sexual dysfunction (Ferguson et al., 2005). Our analysis may thus overestimate the health benefits of ADT. However, given the low fluoxetine cost and the significant improvement in QALYs among treated individuals, our conclusions regarding cost-effectiveness are unlikely to change. Fifth, our analysis assumes that ADT will improve ART adherence. Recent evidence suggests that adding another pill to ART treatment could reduce adherence (Hosseinipour et al., 2016). To the extent that this occurs, our analysis may overestimate the health benefits of ADT. Lastly, we only examined the cost-effectiveness of a pharmacological intervention for depression treatment. A promising area for depression treatment is group or interpersonal therapy, which may impact the appeal of pharmacological ADT (Chibanda et al., 2015; Hosseinipour et al., 2016).
We conclude that ADT for treatment of depression among PLHIV in Uganda and similar countries in SSA is likely both highly effective and cost-effective when judged by common criteria (Goldie et al., 2006; Murray & Lopez, 2002; World Health Organization, 2001). Use of available HIV funding to help alleviate depression in PLHIV would be strongly leveraged by the direct and indirect benefits from resulting improvements in the HIV care cascade, will generate both individual and population benefits, and compares favorably to other investments in HIV and other diseases.
Supplementary Material
ACKNOWLEDGMENTS
Financial support for this study was provided by Grant Number 1-R01-DA15612 from the National Institute on Drug Abuse and Grant Number R01-AI127250 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Financial Disclosures
None reported
Previous Presentations
2016 Institute for Operations Research and the Management Sciences (INFORMS) Annual Meeting in Nashville, Tennessee
REFERENCES
- Akena D, Joska J, Obuku EA, & Stein DJ (2013). Sensitivity and specificity of clinician administered screening instruments in detecting depression among HIV-positive individuals in Uganda. AIDS Care, 25(10), 1245–1252. doi: 10.1080/09540121.2013.764385 [DOI] [PubMed] [Google Scholar]
- Akena D, Musisi S, Joska J, & Stein DJ (2012). The association between AIDS related stigma and major depressive disorder among HIV-positive individuals in Uganda. PLoS One, 7(11), e48671. doi: 10.1371/journal.pone.0048671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akena DH, Musisi S, & Kinyanda E (2010). A comparison of the clinical features of depression in HIV-positive and HIV-negative patients in Uganda. Afr J Psychiatry (Johannesbg), 13(1), 43–51 [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, … Moss a. (2000). Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS, 14(4), 357–366 [DOI] [PubMed] [Google Scholar]
- Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, & Owens DK (2008). Cost-effectiveness of HIV monitoring strategies in resource-limited settings: a southern African analysis. Arch Intern Med, 168(17), 1910–1918. doi: 10.1001/archinternmed.2008.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Dabis F, & de Rekeneire N (2017). Prevalence and factors associated with depression in people living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One, 12(8), e0181960. doi: 10.1371/journal.pone.0181960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer E, Myer L, Struthers H, & Joska JA (2011). HIV/AIDS and mental health research in sub-Saharan Africa: a systematic review. Afr J AIDS Res, 10(2), 101–122. doi: 10.2989/16085906.2011.593373 [DOI] [PubMed] [Google Scholar]
- Byakika-Tusiime J, Crane J, Oyugi JH, Ragland K, Kawuma A, Musoke P, & Bangsberg DR (2009). Longitudinal antiretroviral adherence in HIV+ Ugandan parents and their children initiating HAART in the MTCT-Plus Family Treatment Model: role of depression in declining adherence over time. AIDS Behav, 13(1), 82–91. doi: 10.1007/s10461-009-9546-x [DOI] [PubMed] [Google Scholar]
- Chibanda D, Bowers T, Verhey R, Rusakaniko S, Abas M, Weiss HA, & Araya R (2015). The Friendship Bench programme: a cluster randomised controlled trial of a brief psychological intervention for common mental disorders delivered by lay health workers in Zimbabwe. Int J Ment Health Syst, 9, 21. doi: 10.1186/s13033-015-0013-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholera R, Pence BW, Gaynes BN, Bassett J, Qangule N, Pettifor A, … Miller WC (2017). Depression and engagement in care among newly diagnosed HIV-infected adults in Johannesburg, South Africa. AIDS Behav, 21(6), 1632–1640. doi: 10.1007/s10461-016-1442-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, & Roberts JE (2001). Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry, 158(5), 725–730. doi: 10.1176/appi.ajp.158.5.725 [DOI] [PubMed] [Google Scholar]
- Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, … Xie P (2016). Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet, 388(10047), 881–890. doi: 10.1016/S0140-6736(16)30385-3 [DOI] [PubMed] [Google Scholar]
- Ferguson NM, Donnelly CA, Hooper J, Ghani AC, Fraser C, Bartley LM, … Anderson RM (2005). Adherence to antiretroviral therapy and its impact on clinical outcomes in HIV-infected patients. J R Soc Interface, 2, 349–363. doi: 10.1098/rsif.2005.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, Walensky RP, … Freedberg KA (2006). Cost-effectiveness of HIV treatment in resource-poor settings — the case of Côte d'Ivoire. N Engl J Med, 355(11), 1141–1153. doi: 10.1056/NEJMsa060247 [DOI] [PubMed] [Google Scholar]
- Gonzalez JS, Batchelder AW, Psaros C, & Safren SA (2011). Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr, 58(2), 181–187. doi: 10.1097/QAI.0b013e31822d490a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Dabla V, Joshi BC, & Chakraborty S (2014). Challenges in retention of patients in continuum of HIV care in Delhi — experience of a decade & way ahead. World J AIDS, 4(4), 387–395 [Google Scholar]
- Hollon SD, Stewart MO, & Strunk D (2006). Enduring effects for cognitive behavior therapy in the treatment of depression and anxiety. Annu Rev Psychol, 57, 285–315. doi: 10.1146/annurev.psych.57.102904.190044 [DOI] [PubMed] [Google Scholar]
- Honagodu AR, Krishna M, Sundarachar R, & Lepping P (2013). Group psychotherapies for depression in persons with HIV: a systematic review. Indian J Psychiatry, 55(4), 323–330. doi: 10.4103/0019-5545.120541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, … Kovach D. a. (2008). Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr, 47(3), 384–390. doi: 10.1097/QAI.0b013e318160d53e [DOI] [PubMed] [Google Scholar]
- Hosseinipour MC, Bisson GP, Miyahara S, Sun X, Moses A, Riviere C, … Adult, A. C. T. G. A. S. T. (2016). Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet, 387(10024), 1198–1209. doi: 10.1016/S0140-6736(16)00546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS. (2017). Ending AIDS. Progress toward the 90-90-90 targets. Retrieved from http://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf
- Kaharuza FM, Bunnell R, Moss S, Purcell DW, Bikaako-Kajura W, Wamai N, … Mermin J (2006). Depression and CD4 cell count among persons with HIV infection in Uganda. AIDS Behav, 10(1), 105–111. doi: 10.1007/s10461-006-9142-2 [DOI] [PubMed] [Google Scholar]
- Kigozi F, Ssebunnya J, Kizza D, Cooper S, & Ndyanabangi S (2010). An overview of Uganda's mental health care system: results from an assessment using the World Health Organization's assessment instrument for mental health systems (WHO-AIMS). Int J Ment Health, 4(1), 1. doi: 10.1186/1752-4458-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyanda E, Hoskins S, Nakku J, Nawaz S, & Patel V (2011). Prevalence and risk factors of major depressive disorder in HIV/AIDS as seen in semi-urban Entebbe district, Uganda. BMC Psychiatry, 11(1), 1. doi: 10.1186/1471-244X-11-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JBW (2001). The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med, 16(9), 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R, Mills AJ, Breman JG, Measham AR, Alleyne G, Claeson M, … Jamison DT (2006). Advancement of global health: key messages from the Disease Control Priorities Project. Lancet, 367(9517), 1193–1208. doi: 10.1016/S0140-6736(06)68440-7 [DOI] [PubMed] [Google Scholar]
- McCreesh N, Andrianakis I, Nsubuga RN, Strong M, Vernon I, McKinley TJ, … White RG (2017). Universal test, treat, and keep: improving ART retention is key in cost-effective HIV control in Uganda. BMC Infect Dis, 17(1), 322. doi: 10.1186/s12879-017-2420-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, … Bangsberg DR (2006). Adherence to antiretroviral therapy in Sub-Saharan Africa and North America. JAMA, 296(6), 679–690. doi: 10.1001/jama.296.6.679 [DOI] [PubMed] [Google Scholar]
- Murray CJ, & Lopez A (2002). World Health Report 2002: Reducing risks, promoting healthy life. Geneva: http://www.who.int/whr/2002/en/whr02_en.pdf. [Google Scholar]
- Nakimuli-Mpungu E, Wamala K, Okello J, Alderman S, Odokonyero R, Mojtabai R, … Musisi S (2015). Group support psychotherapy for depression treatment in people with HIV/AIDS in northern Uganda: a single-centre randomised controlled trial. Lancet HIV, 2(5), e190–199. doi: 10.1016/S2352-3018(15)00041-7 [DOI] [PubMed] [Google Scholar]
- Ngo VK, Wagner GJ, Nakasujja N, Dickens A, Aunon F, & Musisi S (2014). Effectiveness of antidepressants and predictors of treatment response for depressed HIV patients in Uganda. Int J STD AIDS, 26(14), 998–1006. doi: 10.1177/0956462414564606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obirikorang C, Selleh PK, Abledu JK, & Fofie CO (2013). Predictors of adherence to antiretroviral therapy among HIV/AIDS patients in the upper west region of Ghana. ISRN AIDS, 2013. doi: 10.1155/2013/873939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovuga E, Boardman J, & Wasserman D (2005). The prevalence of depression in two districts of Uganda. Soc Psychiatry Psychiatr Epidemiol, 40(6), 439–445. doi: 10.1007/s00127-005-0915-0 [DOI] [PubMed] [Google Scholar]
- Patel V, Chisholm D, Rabe-Hesketh S, Dias-Saxena F, Andrew G, & Mann A (2003). Efficacy and cost-effectiveness of drug and psychological treatments for common mental disorders in general health care in Goa, India: a randomised, controlled trial. Lancet, 361(9351), 33–39. doi: 10.1016/S0140-6736(03)12119-8 [DOI] [PubMed] [Google Scholar]
- Sanders GD, Neumann PJ, Basu A, & et al. (2016). Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA, 316(10), 1093–1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- Sin NL, & DiMatteo MR (2014). Depression treatment enhances adherence to antiretroviral therapy: a meta-analysis. Ann Behav Med, 47(3), 259–269. doi: 10.1007/s12160-013-9559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirig R (1998). Support groups for people living with HIV/AIDS: a review of literature. J Assoc Nurses AIDS Care, 9(4), 43–55. doi: 10.1016/S1055-3290(98)80044-7 [DOI] [PubMed] [Google Scholar]
- Stegenga BT, Kamphuis MH, King M, Nazareth I, & Geerlings MI (2012). The natural course and outcome of major depressive disorder in primary care: the PREDICT-NL study. Soc Psychiatry Psychiatr Epidemiol, 47(1), 87–95. doi: 10.1007/s00127-010-0317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanser F, Bärnighausen T, Grapsa E, Zaidi J, & Newell M-L (2013). High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science, 339, 966–971. doi: 10.1126/science.1228160.High [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Bank. (2019). GDP per capita (current US$) - Sub-Saharan Africa. Retrieved from https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=ZG
- Wagner GJ, Ghosh-Dastidar B, Robinson E, Ngo VK, Glick P, Mukasa B, … Akena D (2017). Effects of depression alleviation on ART adherence and HIV clinic attendance in Uganda, and the mediating roles of self-efficacy and motivation. AIDS Behav, 21(6), 1655–1664. doi: 10.1007/s10461-016-1500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ, Ngo V, Glick P, Obuku EA, Musisi S, & Akena D (2014). INtegration of DEPression Treatment into HIV Care in Uganda (INDEPTH-Uganda): study protocol for a randomized controlled trial. Trials, 15(1), 1. doi: 10.1186/1745-6215-15-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidle PJ, Wamai N, Solberg P, Liechty C, Sendagala S, Were W, … Bunnell R (2006). Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet, 368(9547), 1587–1594. doi: 10.1016/S0140-6736(06)69118-6 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2001). Macroeconomics and health: investing in health for economic development. Retrieved from http://www1.worldbank.org/publicsector/pe/PEAMMarch2005/CMHReport.pdf
- World Health Organization. (2009). Threshold values for intervention cost-effectiveness by region. Retrieved from http://www.who.int/choice/costs/CER_levels/en/index.html
- World Health Organization. (2014). International drug price indicator guide. Retrieved from http://apps.who.int/medicinedocs/en/m/abstract/Js21982en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.