Abstract
Objective
The aim the study was to calculate remission, recovery and relapse rates in first episode patients with schizophrenia (FES) vs. patients at a later phase (non-FES).
Methods
Thirty-two FES and 101 non-FES patients took part in the study. The assessment included testing at baseline and at 1 year with the Positive and Negative Syndrome Scale (PANSS), Calgary Depression scale, State-Trait Anxiety Inventory (STAI), Udvalg for Kliniske Undersøgelser (UKU) scale, Simpson Angus, and General Assessment of Functioning (GAF) subscale. The statistical analysis included chi-square test and analysis of covariance.
Results
At baseline 15.62% FES vs. 10.89% non-FES patients were in remission; none of FES vs. 2.97% non-FES patients were in recovery. At endpoint, the respective figures were 12.50% vs. 25.00% and 3.12% vs. 3.96%. None of the differences in rates was significant between the two groups except from the percentage of patients being under medication (higher in the non-FES group). Baseline PANSS negative subscale (PANSS-N) was the only predictor of the outcome at endpoint.
Conclusion
The current study reported very low rates of remission and recovery of patients with schizophrenia without any differences between FES and non-FES patients. One possibility is that the increased antipsychotic treatment compensates for the worsening of the illness with time. An accumulating beneficial effect of antipsychotic treatment suggested that early lack of remission is not prognostic of a poor outcome.
Keywords: Antipsychotics, Early intervention, First episode schizophrenia, Long-term, Maintenance, Outcome, Schizophrenia
INTRODUCTION
Early intervention and particularly with specialized programs have been developed at many centers around the world for patients at their first episode of psychosis (FEP) aiming to optimize the overall outcome [1]. Although classically, schizophrenia is defined on the basis of its progressive and deteriorating nature leading to a poor outcome in comparison to other types of psychotic disorders, more recent findings suggest a more benign course especially during the early stages with remission of symptoms after the first episode to occur in almost 80% of patients [2]. If these reports reflect the actual condition of early-stage patients which could enjoy high rates of sustained remission and functional recovery for prolonged periods of time, especially in the frame of early intervention [3,4] the next question is whether prolonged treatment with antipsychotics is meaningful, after taking into consideration the risk this type of therapy is related with [5]. There is a wide agreement on the efficacy of antipsychotics during the acute phase and this is based on an abundance of hard data accompanied by a series of reliable meta-analyses [6-10]. The efficacy of maintenance treatment up to 1 year after the acute episode was confirmed by a meta-analysis of 65 trials [11,12]. The implementation of specific early intervention programs is also a question, due to their high cost.
Although naturalistic FEP outcome studies of increasing sophistication and duration have been published the longer-term outcomes for these patients in terms of remission and recovery rates remain uncertain. The most recent analysis suggests that within one year the risk of relapse is approximately 65% in patients who had discontinued and 27% in those who had continued medication [11]. A systematic review and meta-analysis concluded that only 13.5% of patients with schizophrenia eventually met the criteria for recovery, although the follow-up period was not given, and this review included people with both first-episode and multi-episode disorder [13]. Another systematic review in FEP identified ‘good’ outcomes for 42% of patients with psychosis and 31% of those with schizophrenia [14] whereas a later review of remission in FEP identified an average remission rate of 40% (range, 17−78%) [15] These reviews are limited by the wide variety of outcome definitions used [14], in keeping with a paucity of identified studies using standardized definitions of remission or recovery, the small number of included studies, 6 and the absence of a FEP review including a meta-analysis. Finally a meta-analysis reported that 58% of patients with FEP met criteria for remission and 38% met criteria for recovery over mean follow-up periods of 5.5 years and 7.2 years, respectively. Thirty percent of those with first-episode schizophrenia met the criteria for recovery [16].
The primary aim of the current study was to compare the one-year remission rate of patients in their first episode patients with schizophrenia (FES) with patients at a later phase, after their first episode, after controlling for a possible effect of age and sex.
Secondary aims were:
a. The calculation of the rate of patients in recovery in both groups
b. The calculation of the rate of patients who remained medication naïve at endpoint and their remission and recovery rates
c. The calculation of rates of patients under antipsychotics or not at both time points in both groups and their remission and recovery rates
d. Calculation of relapse rates of patients initially in remission
e. To test whether prediction of 12-month outcome by the baseline clinical picture is possible in FES patients
METHODS
Study Sample
One hundred and thirty-three stabilized patients with schizophrenia were included in the study. The diagnosis was put in two steps, at the initial step during baseline they should satisfly the criteria of schizophrenia according to Diagnostic and Statistical Manual of Mental Disorders 4th edition, text revision (DSM-IV-TR), except from the duration which should had been at least 4 weeks so that they would qualify for a diagnosis of schizophrenia according to International Classification of Diseases, 10th revision (ICD-10) or schizophrenicoform according to DSM-IV-TR. The final diagnosis of DSM schizophrenia was confirmed for all patients at endpoint.
They were 77 males and 56 females with a mean age of 33.55 ± 11.22 years (range, 18−65 years). Fifty-four of them (aged, 30.37 ± 11.46 years; 29 males [37.7%] and 25 females [44.6%] were not under antipsychotic medication at baseline.). Thirty-two of them (24.06%) were FES.
The FES group included 15 males (46.87%; aged, 23.33 ± 3.51 years old) and 17 females (53.12%; aged, 23.94 ± 6.18 years old). The non-FES group included 62 males (61.38%; aged, 34.69 ± 9.67 years old) and 39 females (38.61%; aged, 39.84 ± 11.89 years old).
The mean age at onset was 23.34 ± 5.08 years and 22.98 ± 6.52 years for the FES and the non-FES groups, respectively and the duration of illness was 0.31 ± 0.47 years and 13.07 ± 8.50 years, respectively.
All clinicodemographic characteristics by group are shown in Tables 1, 2.
The data were gathered in the frame of the doctorate thesis of one of the authors (PP) and the study sample is the same used in a previous publications of our group [17-21] where more details on the characteristics of the sample are given.
All were recruited from local hospitals but most of them from the local military hospital. They constituted a convenient sample but because of the screening of hospital admissions for a limited period of time, they are very close to the general population of FES psychosis which presents to mental health care facilities (this was especially because of the participation of the military hospital department). The diagnosis was made by two of the authors (KNF and PP) after a detailed clinical interview and with the additional use of the MINI to register specific criteria. Diagnosis was reached after consensus. In spite of being hospitalized, some of them refused and never received antipsychotics.
The study received ethical approval from the Ethics Committee of the School of Medicine, Aristotle University of Thessaloniki in Greece. Written informed consent was obtained from all participants.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008
Assessment Tools
The clinical symptoms of schizophrenia were assessed with the Positive and Negative Syndrome Scale (PANSS) [22], depression with the use of the Calgary Depression scale (CDS) [23,24], and anxiety with the State-Trait Anxiety Inventory form Y, State and Trait subscales (STAI-Y) [25]. For the assessment of extrapyramidal signs the Simpson- Angus scale [26] was used, the Udvalg for Kliniske Undersøgelser (UKU) scale [27] was used for the assessment of adverse events, and the General Assessment of Functioning (GAF) scale for the assessment of general functioning and impairment [28].
The criteria by Andreasen et al. [29] were used in order to define remission both at baseline as well as at 12-month. These criteria utilize PANSS individual item scores. As a general approach a score of mild or less (PANSS item scores of ≤ 3; simultaneously on all items as representative of an impairment level consistent with symptomatic remission of illness. However, in order to be more precise, these authors proposed parallel, cross-scale remission criteria items. These are the criteria used in the current study and they demand that specifically the PANSS items P1, P2, P3, N1, N4, N6, G5, and G9 each one to score ≤ 3.
Patients were considered as ‘recovered’ if GAF scores were > 70 (no more than slight impairment). This was an arbitrary choice by the authors on the basis of their expertise and knowledge of the literature.
12-Months Follow-up
At baseline patients were assessed with the tools mentioned above. Fortunately, all of them were able to be tracked and re-assessed 12 months later with the same protocol. This was achieved mainly because they were recruits or military staff and kept contact with the psychiatric department of the military hospital.
During the 12-month time duration some of them were treated by members of the study group while others were treated by other therapists and some of them were not under clinical care at all. All were treated according to the clinical judgment of the therapist without any kind of intervention relative to the needs of the current study, which is purely observational.
At both time points all patients were assessed by the same members of the research group (KNF and PP).
Statistical Analysis
It included:
All statistical analyses were conducted using IBM SPSS 25.0 (IBM SPSS Statistics for Windows ver. 25.0, 2011; IBM Corp., Armonk, NY, USA).
The calculation of descripted statistics so as to chart the course of all patients from baseline to endpoint (12-month).
Chi-square was used to test for differences in categorical variables (e.g., remission and recovery status, etc.) between the study groups.
Analysis of Covariance was performed to investigate for the possibility to predict remission by using baseline characteristics. The grouping variables, were the following: antipsychotic status (yes/no) at baseline by episode group (FES/non-FES) by sex and by remission rate at 12 months. Independent variables included baseline PANSS subscales, GAF, CDS, STAT-state (STAI-S) and STAT-trait subscales (STAI-T), UKU and Simpson Angus subscales. Age, duration of illness and dosage of antipsychotics in haloperidol equivalents at baseline were used as covariates. The Scheffe test was used as the post-hoctest.
Statistical significance was p < 0.05.
RESULTS
General Description of the Study Sample
Of one of 133 patients (0.75%) had no education at all, 25 (18.80%) had less than 6 years, 23 (17.29%) with 6−9 years, 51 (38.35%) with 9−12 years of education, while 31 (23.31%) had a university degree and 2 (1.50%) had post-graduate education.
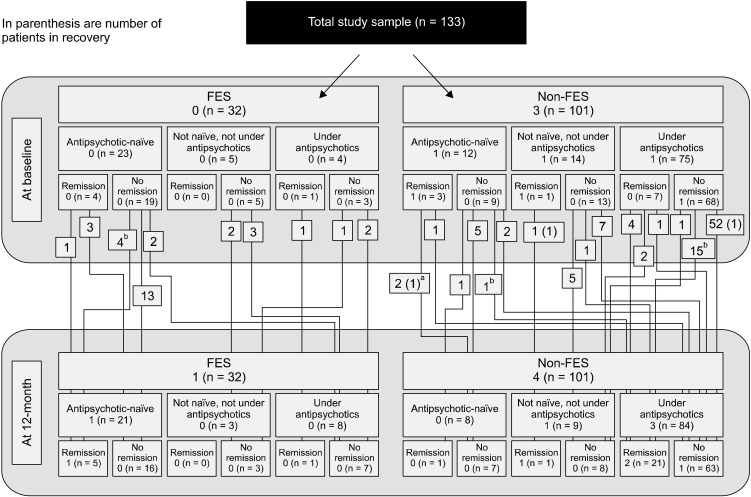
The results concerning the composition of the study sample at baseline and at endpoint in terms of medication status and remission and recovery are shown in detail in Figure 1, together with the trajectories of specific patient groups. The means and standard deviations of measurements are shown in Table 1 and in more detail in Table 2 concerning the groups defined by medication status and remission.
Fig. 1.
Graphic presentation of the trajectories of specific patient groups from baseline to 12-month.
FES, first episode patients with schizophrenia.
aNot in recovery at 12-month. bOne of them recovered at 12-month.
Table 1.
Means of measurements in the two basic patients groups at baseline and at endpoint
| Variable | Subheading | FES (n = 32) | Non-FESa (n = 101) | All groups (n = 133) |
|---|---|---|---|---|
| Age at baseline (yr) | 23.66 ± 5.04 | 36.68 ± 10.83 | 33.55 ± 11.22 | |
| Illness duration (yr) | 0.31 ± 0.47 | 13.70 ± 8.51 | 10.48 ± 9.38 | |
| Haloperidol equivalent | Baseline | 0.74 ± 2.28 | 5.83 ± 6.14 | 4.61 ± 5.88 |
| Endpoint | 1.22 ± 2.39 | 6.34 ± 6.18 | 5.11 ± 5.93 | |
| PANSS-P | Baseline | 15.50 ± 6.31 | 16.45 ± 5.66 | 16.22 ± 5.81 |
| Endpoint | 13.06 ± 4.29 | 14.56 ± 4.67 | 14.20 ± 4.61 | |
| PANSS-Nb | Baseline | 17.44 ± 8.53 | 18.40 ± 7.00 | 18.17 ± 7.37 |
| Endpoint | 19.59 ± 7.51 | 20.15 ± 6.47 | 20.02 ± 6.71 | |
| PANSS-G | Baseline | 25.94 ± 6.38 | 26.29 ± 7.25 | 26.20 ± 7.03 |
| Endpoint | 25.81 ± 6.22 | 26.27 ± 7.09 | 26.16 ± 6.87 | |
| PANSS-EP | Baseline | 7.56 ± 3.31 | 7.35 ± 3.22 | 7.40 ± 3.23 |
| Endpoint | 7.38 ± 2.85 | 7.29 ± 3.07 | 7.31 ± 3.01 | |
| Calgary Depression scale | Baseline | 0.44 ± 1.24 | 1.88 ± 3.74 | 1.53 ± 3.37 |
| Endpoint | 0.38 ± 1.21 | 0.71 ± 1.39 | 0.63 ± 1.35 | |
| STAI-S | Baseline | 50.03 ± 8.36 | 49.82 ± 12.29 | 49.87 ± 11.44 |
| Endpoint | 49.72 ± 7.99 | 47.84 ± 11.36 | 48.29 ± 10.65 | |
| STAI-T | Baseline | 49.97 ± 10.50 | 50.12 ± 11.78 | 50.08 ± 11.44 |
| Endpoint | 49.94 ± 9.84 | 50.27 ± 10.80 | 50.19 ± 10.55 | |
| GAF | Baseline | 51.41 ± 11.67 | 49.37 ± 12.22 | 49.86 ± 12.08 |
| Endpoint | 52.13 ± 11.93 | 52.41 ± 11.15 | 52.34 ± 11.29 | |
| UKU | Baseline | 5.28 ± 4.15 | 7.63 ± 5.55 | 7.07 ± 5.33 |
| Endpoint | 5.44 ± 3.87 | 6.93 ± 4.25 | 6.57 ± 4.19 | |
| Simpson Angus scale | Baseline | 1.03 ± 2.65 | 0.58 ± 1.24 | 0.69 ± 1.68 |
| Endpoint | 0.91 ± 3.20 | 0.51 ± 1.43 | 0.61 ± 2.00 |
Values are presented as mean ± standard deviation.
FES, first episode patients with schizophrenia; PANSS, Positive and Negative Symptoms Scale; P, positive subscale; N, negative subscale; G, general psychopathology subscale; EP, excitement component subscale; STAI, State-Trait Anxiety Inventory; S, state; T, trait subscales; GAF, General Assessment of Functioning; UKU, Udvalg for Kliniske Undersøgelser scale.
Patients at a latter episode. b PANSS-N score predicts remission after 12 months.
Table 2.
Detailed data with means of measurements in the various patients groups at baseline
| Variable | Subheading | FES | Non-FESa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TN | NRec | AP | TN | NRec | AP | ||||||||
| R (n = 4) | nR (n = 19) | R (n = 0) | nR (n = 5) | R (n = 1) | nR (n = 3) | R (n = 3) | nR (n = 9) | R (n = 1) | nR (n = 13) | R (n = 7) | nR (n = 68) | ||
| Age at baseline (yr) | 22.75 ± 2.87 | 23.21 ± 2.94 | 23.00 ± 2.92 | 46.00 | 21.33 ± 4.93 | 53.00 ± 7.00 | 36.00 ± 14.59 | 39.00 | 36.23 ± 9.69 | 36.71 ± 9.53 | 36.10 ± 10.52 | ||
| Illness duration (yr) | 0.50 ± 0.58 | 0.32 ± 0.48 | 0.00 ± 0.00 | 0.00 | 0.67 ± 0.58 | 28.33 ± 5.77 | 12.89 ± 11.29 | 11.00 | 14.46 ± 5.78 | 13.29 ± 9.96 | 13.10 ± 8.17 | ||
| Haloperidol equivalent | Baseline | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 7.89 | 5.27 ± 4.10 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 | 0.00 ± 0.00 | 5.90 ± 6.33 | 8.06 ± 5.88 | |
| Endpoint | 0.00 ± 0.00 | 0.37 ± 1.11 | 3.09 ± 3.52 | 7.89 | 2.87 ± 2.80 | 1.00 ± 1.74 | 1.99 ± 3.50 | 0.00 | 4.34 ± 4.39 | 4.42 ± 7.03 | 7.83 ± 6.31 | ||
| PANSS-P | Baseline | 9.75 ± 0.96 | 15.16 ± 6.80 | 20.60 ± 4.10 | 10.00 | 18.67 ± 2.08 | 10.33 ± 3.06 | 20.44 ± 8.37 | 8.00 | 19.23 ± 5.82 | 10.00 ± 1.73 | 16.44 ± 4.70 | |
| Endpoint | 9.75 ± 0.96 | 12.11 ± 4.18 | 16.60 ± 2.07 | 8.00 | 18.67 ± 2.08 | 10.33 ± 3.06 | 17.56 ± 7.95 | 6.00 | 16.54 ± 5.11 | 9.86 ± 1.68 | 14.56 ± 3.74 | ||
| PANSS-Nb | Baseline | 12.00 ± 7.44 | 16.47 ± 8.35 | 23.80 ± 10.62 | 16.00 | 20.67 ± 2.31 | 8.00 ± 0.00 | 17.89 ± 6.41 | 13.00 | 22.08 ± 6.98 | 14.14 ± 5.01 | 18.74 ± 6.88 | |
| Endpoint | 20.75 ± 2.22 | 18.00 ± 7.84 | 23.80 ± 10.62 | 18.00 | 22.33 ± 0.58 | 19.00 ± 1.73 | 20.00 ± 5.72 | 23.00 | 22.31 ± 7.05 | 18.86 ± 4.45 | 19.90 ± 6.82 | ||
| PANSS-G | Baseline | 18.75 ± 2.22 | 25.00 ± 5.39 | 33.20 ± 6.26 | 23.00 | 30.33 ± 2.52 | 22.33 ± 3.21 | 27.11 ± 7.88 | 22.00 | 31.38 ± 8.65 | 22.00 ± 4.51 | 25.88 ± 6.89 | |
| Endpoint | 19.00 ± 2.45 | 25.00 ± 5.14 | 33.20 ± 6.26 | 20.00 | 28.67 ± 4.73 | 22.67 ± 2.89 | 27.00 ± 7.86 | 21.00 | 31.31 ± 8.62 | 22.57 ± 4.58 | 25.81 ± 6.69 | ||
| PANSS-EP | Baseline | 5.00 ± 0.00 | 7.74 ± 3.65 | 10.00 ± 2.55 | 6.00 | 6.33 ± 2.31 | 6.33 ± 1.15 | 8.00 ± 5.81 | 5.00 | 8.23 ± 2.39 | 5.57 ± 1.51 | 7.35 ± 3.09 | |
| Endpoint | 5.25 ± 0.50 | 7.26 ± 2.94 | 10.00 ± 2.55 | 5.00 | 7.00 ± 2.65 | 6.33 ± 1.15 | 7.89 ± 5.49 | 5.00 | 8.23 ± 2.39 | 5.71 ± 1.50 | 7.26 ± 2.94 | ||
| Calgary Depression scale | Baseline | 0.00 ± 0.00 | 0.47 ± 1.50 | 0.20 ± 0.45 | 2.00 | 0.67 ± 1.15 | 1.33 ± 2.31 | 1.11 ± 2.26 | 0.00 | 2.38 ± 5.66 | 2.29 ± 2.93 | 1.90 ± 3.66 | |
| Endpoint | 0.00 ± 0.00 | 0.58 ± 1.54 | 0.00 ± 0.00 | 1.00 | 0.00 ± 0.00 | 0.33 ± 0.58 | 0.67 ± 1.12 | 0.00 | 0.69 ± 1.25 | 1.00 ± 0.82 | 0.72 ± 1.53 | ||
| STAI-S | Baseline | 52.50 ± 3.51 | 51.32 ± 7.27 | 45.80 ± 12.54 | 45.00 | 47.33 ± 13.32 | 43.00 ± 13.08 | 51.78 ± 9.92 | 39.00 | 45.85 ± 11.29 | 45.00 ± 10.86 | 51.28 ± 12.78 | |
| Endpoint | 50.50 ± 1.73 | 50.63 ± 8.00 | 48.20 ± 10.47 | 49.00 | 46.33 ± 12.42 | 43.00 ± 13.08 | 49.78 ± 12.47 | 37.00 | 45.31 ± 10.87 | 45.57 ± 10.16 | 48.65 ± 11.55 | ||
| STAI-T | Baseline | 56.00 ± 4.24 | 51.05 ± 8.53 | 46.00 ± 16.17 | 30.00 | 48.33 ± 13.32 | 45.67 ± 8.39 | 53.33 ± 14.18 | 40.00 | 46.38 ± 13.62 | 57.00 ± 6.68 | 50.04 ± 11.50 | |
| Endpoint | 55.50 ± 4.04 | 51.00 ± 8.19 | 46.20 ± 14.89 | 27.00 | 48.67 ± 11.02 | 46.67 ± 6.66 | 53.44 ± 13.13 | 41.00 | 46.38 ± 12.51 | 55.86 ± 5.79 | 50.31 ± 10.58 | ||
| GAF | Baseline | 62.25 ± 9.67 | 52.42 ± 9.79 | 47.00 ± 11.16 | 63.00 | 34.00 ± 6.56 | 67.00 ± 15.87 | 50.11 ± 13.75 | 71.00 | 46.54 ± 10.02 | 63.00 ± 9.00 | 47.31 ± 11.01 | |
| Endpoint | 53.25 ± 5.74 | 55.68 ± 10.79 | 47.00 ± 11.16 | 67.00 | 33.00 ± 7.94 | 61.67 ± 7.64 | 50.67 ± 13.11 | 69.00 | 49.23 ± 10.73 | 57.71 ± 5.91 | 52.01 ± 11.18 | ||
| UKU | Baseline | 4.50 ± 4.43 | 5.16 ± 4.10 | 2.80 ± 2.59 | 10.00 | 9.67 ± 4.04 | 7.00 ± 4.36 | 6.00 ± 5.39 | 3.00 | 9.85 ± 6.34 | 5.00 ± 4.97 | 7.79 ± 5.49 | |
| Endpoint | 5.25 ± 4.11 | 5.00 ± 3.87 | 3.80 ± 2.17 | 12.00 | 9.67 ± 4.04 | 7.00 ± 4.36 | 5.78 ± 4.52 | 3.00 | 9.54 ± 5.22 | 5.43 ± 5.83 | 6.79 ± 3.74 | ||
| Simpson Angus scale | Baseline | 0.00 ± 0.00 | 0.11 ± 0.32 | 0.00 ± 0.00 | 6.00 | 7.00 ± 8.89 | 0.00 ± 0.00 | 1.11 ± 3.33 | 0.00 | 1.08 ± 1.44 | 0.29 ± 0.76 | 0.38 ± 1.08 | |
| Endpoint | 0.25 ± 0.50 | 0.42 ± 0.69 | 0.20 ± 0.45 | 4.00 | 6.33 ± 7.09 | 0.00 ± 0.00 | 1.22 ± 2.59 | 0.00 | 1.15 ± 1.68 | 0.57 ± 1.13 | 0.43 ± 0.83 | ||
Values are presented as mean ± standard deviation.
FES, first episode patients with schizophrenia; TN, treatment naïve; NRec, not naïve, not receiving antipsychotics; AP, under antipsychotics; R, remission; nR, non-remission; PANSS, Positive and Negative Symptoms Scale; P, positive subscale; N, negative subscale; G, general psychopathology subscale; EP, excitement component subscale; STAI, State-Trait Anxiety Inventory; S, state; T, trait subscales; GAF, General Assessment of Functioning; UKU, Udvalg for Kliniske Undersøgelser scale.
Patients at a latter episode. b PANSS-N score predicts remission after 12 months.
Medication Status
The percentage of antipsychotic-naïve patients falls from 23 (71.87%) at baseline and 21 (65.62%) after one year in FES patients to 12 (11.88%) and 8 (7.92%), respectively in non-FES patients. The difference is significant both at baseline (chi-square = 45.11, degree of freedom [df] = 1; p < 0.0001) as well as after one year (chi-square = 13.89, df = 1; p = 0.0002).
Remission and Recovery Rates
At baseline 5 (15.62%) FES vs. 11 (10.89%) in non-FES patients were in remission (chi-square = 0.51, df = 1, p = 0.473) and none of FES vs. 3 (2.97%) in non-FES patients were in recovery (Yates corrected chi-square = 0.09, df = 1, p = 0.766).
At endpoint, remission was 6/32 (18.75%) in FES and 23/101 (22.77%) in non-FES patients (chi-square = 0.23, df = 1, p = 0.631), while one (3.12%) FES and 4 (3.96%) non-FES patients were in recovery (Yates chi-square = 0.10, df = 1, p = 0.751).
At endpoint remission in patients under antipsychotics is 12.50% in FES patients vs. 25.00% in non-FES patients (Yates chi-square = 0.13, df = 1, p = 0.720). Remission rate is almost double in non-FES and this is exclusively because of prolonged antipsychotic treatment.
At endpoint, an additional one patient in FES vs. 12 non-FES patients manifested remission after 12 months (Yates chi-square = 1.00, df = 1, p = 0.317). Both groups manifested one additional case of recovery after one year (chi-square = 0.00, df = 1, p = 0.985).
Of those in remission at baseline, 60% of the FES group relapsed (3/5) and 50% of the non-FES group (5/10). However several patients not in remission initially, eventually gained that status.
There seems to be no difference between FES and non-FES in terms of recovery, however while in FES this might occur spontaneously, in non-FES it is exclusively the product of prolonged antipsychotic treatment. If one hypothesizes that non-FES patients originally when at their first episode, had a profile similar to that of FES patients of the current study, then they should had started with a number of 72 (71.28%) antipsychotic naïve patients from which eventually only one (1.39%) would achieve some kind of sustained remission and none would achieve recovery. With prolonged treatment up to 25% would be expected to remit and 3.57% to recover.
At endpoint 21/32 (65.62%) for FES patients were still medication naïve and 5/21 (23.81%) were in remission with 1/21 (4.76%) in recovery. For non-FES patients the respected figures were 8/101 (7.92%), 1/8 (12.5%) and 0/8 (0%) (Yates corrected chi-square = 0.11, df = 1, p = 0.739 for remission).
Drop out from medication was present in 1/8 (12.5%) in the FES groups vs. 3/75 (4%) in the non-FES group (Yates corrected chi-square = 0.04, df = 1, p = 0.842). Of the 72 patients who were on medication at both time points, 19 (26.39%) were in remission at endpoint and from the 5 in remission at baseline who stayed under treatment, one (20%) relapsed.
Prediction of Treatment Outcome
The ANCOVA results returned no effect for medication status at baseline (p = 0.257), sex (p = 0.289) or FES group (p = 0.277), but did returned a significant effect for remission at 12 months (p = 0.004). Concerning covariates, a significant effect was returned for baseline treatment in terms of haloperidol equivalents (p = 0.035) but not for age (p = 0.141) or duration of illness (p = 0.486).
Scheffe post-hoctests revealed a significant difference concerning only PANSS negative subscale (PANSS-N) (p < 0.0001). Patients which remitted at endpoint had lower PANSS-N scores (13.5 ± 4.56 vs. 19.47 ± 7.43).
DISCUSSION
Since only the better doing stay naïve and the response rate is stable, it means that good outcome without treatment is highly unlikely
Answers to the Aims of the Study
Primary aim
At baseline 15.62% of FES vs. 10.89% of non FES patients were in remission. At endpoint, 18.75% of FES vs. 22.77% of non-FES patients were in remission. Differences were not statistically significant.
Secondary aims
The respected recovery rates were 0% vs. 2.97% at baseline and 3.12% vs. 3.96% at endpoint.
The percentage of antipsychotic-naïve patients was 71.87% at baseline and 65.62% after one year in FES patients vs. 11.88% 7.92%, respectively in non-FES patients. The difference was significant both at baseline as well as after one year.
Of naïve patients at baseline 23.81% were in remission and 4.76% in recovery for the FES group while for non-FES patients the respected figures were 7.92%, 12.5%, and 0%. The difference was not significant.
Of patients who were on medication at both time points 26.39% were in remission at endpoint and from those in remission at baseline who stayed under treatment, 20% relapsed.
Of those in remission at baseline, 60% of the FES group and 50% of the non-FES group relapsed.
Lower PANSS-N score at baseline was the only predictor of remission at endpoint,
Additional observations
• Patients previously not in remission or recovery achieved that status at endpoint were more than those who relapsed; therefore the general picture was that the group of patients improved by passing the time.
Relevance of the results of the current study to the existing literature, and implications for future research and clinical practice
There are some but not abundant data in the literature concerning the outcome of FES. However most papers deal with FEP and pool together schizophrenia with other psychotic illnesses including affective psychoses. Even the studies that claim to deal with schizophrenia alone, most often have their study samples contaminated with schizoaffective illness [15]. However they often tend to extrapolate their results specifically to schizophrenia [16]. On the contrary, our study sample included only patients with established diagnosis of schizophrenia. However this might include an opposite bias, that is only the worse end of patients with schizophrenia are included and not those with a favorable initial course which because of this, fail to receive the correct diagnosis early.
The first major finding was that the remission rates did not differ between FES and non-FES patients and this is in accord with a meta-analysis [15]. However these rates of remission/recovery were very low in comparison to the literature concerning FES patients. This was probably because of the very different design of the current study and the fact that most study samples include also schizoaffective patients, not pure schizophrenia [15]. On the other hand, there are studies with similar results [30-32]. It should be noted however that in our sample, not even those patients continuously under antipsychotics, did achieve the reported in the literature response rates which are as high as more than 80% for FES [33]. Our results agree with the report that seventy per cent of treatment resistant patients, and 23% of total patients are treatment resistant from illness onset [34].
Our results are at the lower end of the range reported by a meta-analysis specifically on schizophrenia and also in accord with the conclusion of that meta-analysis that there is no difference in remission rates between first and latter episodes of schizophrenia [15]. A more recent meta- analyses reported much higher rates (58% remission and 38% recovery) but it included not only FES patients but also first episodes of any psychotic disorder, including affective disorders [16]. Meta-analyses reported a recovery rate of 13.5−38% in FEP and 11−33% in multi-episode schizophrenia [13-16,35,36] but as already commented, the papers utilized suffer from heterogeneity of samples. Interestingly the recovery rates for studies from North America are somewhat higher than those reported from other regions of the world [16].
From a different point of view, concerning the relapse rate, our results are in accord with two previous reports which suggest that a significant percentage of patient relapse each year. However our relapse rate was much higher (25% in our sample vs. approx. 10% in these studies literature) [37,38]. Even relapse rates as high as 40% in patients under continuous treatment have been reported [39]. Other studies report a variety of results [39-44] including a number of other studies which reported no relapses at all in FEP patients receiving maintenance medication [41,45-48]. On the other hand, our results concerning relapses are in accord with previous studies [49] as well as with a meta-analysis which reported a 27% relapse rate after one year in patients under treatment [11]. Additionally, the bigger picture is that this loss is overcompensated by a larger number of non-remitters at baseline who achieve remitting status at endpoint.
In terms of prediction of outcome by utilizing baseline characteristics, our results that only PANSS-N constitutes a predictive factor is in accord with some previous studies [50-52]. However often the results by the same authors are conflicting [53] and the majority of the reports in the literature propose a more complex but rather not clinically useful prediction model [32,54-56]. It is important to note that depressive symptoms, traditionally considered to be a positive predictive feature, was not found to play any significant role, in contrast to previous studies [31,57].
Our results on one hand suggest that schizophrenia might not be a progressively deteriorating disorder but on the other hand also suggest that it is highly malignant and refractory to available treatments. Patients with a poor course and outcome manifest it early already during the first episode. This conclusion is not new; several authors have argued in this line of thought. This suggests that patients with worse outcomes are apparent in the earlier stages of illness, rather than that the course of illness is progressive for the majority of patients, but until today it is not widely accepted. Furthermore the argument that a significant minority of patients will do well without any medication for prolonged periods of time is not in accord with our results.
Additionally, our data suggest a continuous beneficial effect with remission cases accumulating as an effect of the interaction of time by antipsychotic treatment. No improvement exists in patients which discontinued treatment, while on the contrary, patients not on medication at baseline manifest a tendency to start medication. This is in direct contrast with the findings of some early studies who reported that medication free patients at endpoint had a better outcome.
Conclusion
The current study reported very low rates of remission (approximately 20%) and recovery (approximately 3%) of patients with schizophrenia with a relapse rate of 20% for those under continuous antipsychotic treatment and 60% for those not under treatment after one year follow-up. There were no differences between FES and non-FES patients except from antipsychotic utilization which was significantly higher in the non-FES group. One possibility which can not be ruled out is that this increased antipsychotic treatment with passing the episodes compensates for the worsening of the illness. There was an accumulating beneficial effect of antipsychotic treatment over a 12-month period and therefore, early lack of remission is not prognostic of a poor outcome. Only negative symptoms were a prognostic marker for the one-year outcome. Overall, both remission and recovery are difficult for patients with schizophrenia to achieve and characterize the minority of patients.
Strengths and Limitations of the Present Study
The advantages of the current study is that it is based on a sufficiently big study sample of patients solidly diagnosed with schizophrenia and not on an admixture of various types of psychoses with different prognosis and expected outcomes. It was made possible to locate all of them after one year and it is the only study who identified pathways of patient exchange between study groups.
The limitations of the current study includes the fact that all patients have been previously hospitalized which means they represent maybe a more severe form of schizophrenic illness.
For recruitment, the patient should had recently manifested a psychotic episode fulfilling the criteria for schizophrenia except from the time criterion; duration of the episode should had been of at least 4 weeks duration. Thus, at baseline patients were already fulfilling criteria for schizophrenia according to ICD-10 and schizophrenicoform according to DSM-IV-TR. In this way, the group of patients with schizophrenia which start with a more mild form of symptomatology and do not come to fulfill the criteria but only after several years [58] were excluded. These patients might be as many as 10% of the total population of patients with schizophrenia [58] and they definitely manifest better course and remission and recovery rates during their first episode.
Their exposure to antipsychotic treatment during the 12-month interval was heterogeneous and has not been recorded. The compliance of patients was not confirmed by following an objective method during the follow-up period.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Konstantinos N. Fountoulakis and Ioannis Nimatoudis conceived and designed the study. Konstantinos N. Fountoulakis and Panagiotis Panagiotidis participated in involving the subjects. Panagiotis Panagiotidis and Konstantinos N. Fountoulakis participated in clinical assessment and data acquisition and management. Konstantinos N. Fountoulakis and Antonis T. Theofilidis did the data analysis and wrote the first draft of the paper. All authors participated in interpreting the data and developing further stages of the paper.
REFERENCES
- Zipursky RB, Menezes NM, Streiner DL. Risk of symptom recurrence with medication discontinuation in first-episode psychosis: a systematic review. Schizophr Res. 2014:408–414. doi: 10.1016/j.schres.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Lieberman JA. Prediction of outcome in first-episode schizophrenia. J Clin Psychiatry. 1993;54(Suppl):13–17. [PubMed] [Google Scholar]
- Craig TK, Garety P, Power P, Rahaman N, Colbert S, Fornells-Ambrojo M, et al. The Lambeth Early Onset (LEO) Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. BMJ. 2004;329:1067. doi: 10.1136/bmj.38246.594873.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry P, Johanessen JO, Lewis S, Birchwood M, Malla A, Nordentoft M, et al. Early intervention in psychosis: keeping faith with evidence-based health care. Psychol Med. 2010;40:399–404. doi: 10.1017/S0033291709991346. [DOI] [PubMed] [Google Scholar]
- Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161:414–425. doi: 10.1176/appi.ajp.161.3.414. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kissling W, Davis JM. Second-generation antipsychotics for schizophrenia: can we resolve the conflict? Psychol Med. 2009;39:1591–1602. doi: 10.1017/S0033291709005455. [DOI] [PubMed] [Google Scholar]
- Leucht S, Pitschel-Walz G, Abraham D, Kissling W. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res. 1999;35:51–68. doi: 10.1016/S0920-9964(98)00105-4. [DOI] [PubMed] [Google Scholar]
- Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta- analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–447. doi: 10.1038/sj.mp.4002136. [DOI] [PubMed] [Google Scholar]
- Haddad PM, Correll CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 2018;8:303–318. doi: 10.1177/2045125318781475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–2071. doi: 10.1016/S0140-6736(12)60239-6. [DOI] [PubMed] [Google Scholar]
- Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2012;(5):CD008016. doi: 10.1002/14651858.CD008016.pub2. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen E, Juola P, Hirvonen N, McGrath JJ, Saha S, Isohanni M, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39:1296–1306. doi: 10.1093/schbul/sbs130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes NM, Arenovich T, Zipursky RB. A systematic review of longitudinal outcome studies of first-episode psychosis. Psychol Med. 2006;36:1349–1362. doi: 10.1017/S0033291706007951. [DOI] [PubMed] [Google Scholar]
- AlAqeel B, Margolese HC. Remission in schizophrenia: critical and systematic review. Harv Rev Psychiatry. 2012;20:281–297. doi: 10.3109/10673229.2012.747804. [DOI] [PubMed] [Google Scholar]
- Lally J, Ajnakina O, Stubbs B, Cullinane M, Murphy KC, Gaughran F, et al. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. Br J Psychiatry. 2017;211:350–358. doi: 10.1192/bjp.bp.117.201475. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Panagiotidis P, Gonda X, Kimiskidis V, Nimatoudis I. Neurological soft signs significantly differentiate schizophrenia patients from healthy controls. Acta Neuropsychiatr. 2018;30:97–105. doi: 10.1017/neu.2017.29. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Panagiotidis P, Kimiskidis V, Nimatoudis I, Gonda X. Neurological soft signs in familial and sporadic schizophrenia. Psychiatry Res. 2019;272:222–229. doi: 10.1016/j.psychres.2018.12.105. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Panagiotidis P, Kimiskidis V, Nimatoudis I, Gonda X. Prevalence and correlates of neurological soft signs in healthy controls without family history of any mental disorder: a neurodevelopmental variation rather than a specific risk factor? Int J Dev Neurosci. 2018;68:59–65. doi: 10.1016/j.ijdevneu.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Gonda X, Siamouli M, Panagiotidis P, Moutou K, Nimatoudis I, et al. Paternal and maternal age as risk factors for schizophrenia: a case-control study. Int J Psychiatry Clin Pract. 2018;22:170–176. doi: 10.1080/13651501.2017.1391292. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Panagiotidis P, Nimatoudis I. The effect of baseline antipsychotic status on the 12-month outcome in initially stabilized patients with schizophrenia. Hum Psychopharmacol. 2019;34:e2712. doi: 10.1002/hup.2712. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-R. [DOI] [PubMed] [Google Scholar]
- Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6:201–208. doi: 10.1016/0920-9964(92)90003-N. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Preliminary manual for the state-trait personality inventory (STPI) Universwity of South Florida; Tampa: 1979. [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Text revision. 4th ed. American Psychiatric Press; Washington: 2000. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Andreasen NC, Carpenter WT Jr, Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- de Haan L, van Nimwegen L, van Amelsvoort T, Dingemans P, Linszen D. Improvement of subjective well-being and enduring symptomatic remission, a 5-year follow-up of first episode schizophrenia. Pharmacopsychiatry. 2008;41:125–128. doi: 10.1055/s-2008-1076729. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Weiner E, Ball MP, McMahon RP, Carpenter WT, Buchanan RW. Remission in schizophrenia: the relationship to baseline symptoms and changes in symptom domains during a one-year study. J Psychopharmacol. 2009;23:436–441. doi: 10.1177/0269881108093883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Rabinowitz J, Medori R Early Psychosis Global Working Group. Remission in early psychosis: Rates, predictors, and clinical and functional outcome correlates. Schizophr Res. 2007;89:129–139. doi: 10.1016/j.schres.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Lieberman J, Jody D, Geisler S, Alvir J, Loebel A, Szymanski S, et al. Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry. 1993;50:369–376. doi: 10.1001/archpsyc.1993.01820170047006. [DOI] [PubMed] [Google Scholar]
- Lally J, Ajnakina O, Di Forti M, Trotta A, Demjaha A, Kolliakou A, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016;46:3231–3240. doi: 10.1017/S0033291716002014. [DOI] [PubMed] [Google Scholar]
- Hegarty JD, Baldessarini RJ, Tohen M, Waternaux C. One hundred years of schizophrenia: a meta-analysis of the outcome literature. Schizophr Res. 1993;9:134. doi: 10.1016/0920-9964(93)90221-4. [DOI] [PubMed] [Google Scholar]
- Warner R. Recovery from schizophrenia and the recovery model. Curr Opin Psychiatry. 2009;22:374–380. doi: 10.1097/YCO.0b013e32832c920b. [DOI] [PubMed] [Google Scholar]
- Ciudad A, Alvarez E, Bobes J, San L, Polavieja P, Gilaberte I. Remission in schizophrenia: results from a 1-year follow-up observational study. Schizophr Res. 2009;108:214–222. doi: 10.1016/j.schres.2008.12.004. [DOI] [PubMed] [Google Scholar]
- De Hert M, van Winkel R, Wampers M, Kane J, van Os J, Peuskens J. Remission criteria for schizophrenia: evaluation in a large naturalistic cohort. Schizophr Res. 2007;92:68–73. doi: 10.1016/j.schres.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Chen EY, Hui CL, Lam MM, Chiu CP, Law CW, Chung DW, et al. Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ. 2010;341:c4024. doi: 10.1136/bmj.c4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra G, Burger H, Grobbee DE, Kahn RS. Antipsychotic prophylaxis is needed after remission from a first psychotic episode in schizophrenia patients: results from an aborted randomised trial. Int J Psychiatry Clin Pract. 2011;15:128–134. doi: 10.3109/13651501.2010.534801. [DOI] [PubMed] [Google Scholar]
- Gaebel W, Riesbeck M, Wölwer W, Klimke A, Eickhoff M, von Wilmsdorff M, et al. Relapse prevention in first-episode schizophrenia--maintenance vs intermittent drug treatment with prodrome-based early intervention: results of a randomized controlled trial within the German Research Network on Schizophrenia. J Clin Psychiatry. 2011;72:205–218. doi: 10.4088/JCP.09m05459yel. [DOI] [PubMed] [Google Scholar]
- McCreadie RG, Wiles D, Grant S, Crockett GT, Mahmood Z, Livingston MG, et al. The Scottish first episode schizophrenia study. VII. Two-year follow-up. Scottish Schizophrenia Research Group. Acta Psychiatr Scand. 1989;80:597–602. doi: 10.1111/j.1600-0447.1989.tb03032.x. [DOI] [PubMed] [Google Scholar]
- Gardos G. Are antipsychotic drugs interchangeable? J Nerv Ment Dis. 1974;159:343–348. doi: 10.1097/00005053-197411000-00004. [DOI] [PubMed] [Google Scholar]
- Crow TJ, MacMillan JF, Johnson AL, Johnstone EC. A randomised controlled trial of prophylactic neuroleptic treatment. Br J Psychiatry. 1986;148:120–127. doi: 10.1192/bjp.148.2.120. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Suzuki T, Uchida H, Watanabe K, Mimura M. Antipsychotic treatment for schizophrenia in the maintenance phase: a systematic review of the guidelines and algorithms. Schizophr Res. 2012;134:219–225. doi: 10.1016/j.schres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Kane JM, Rifkin A, Quitkin F, Nayak D, Ramos-Lorenzi J. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry. 1982;39:70–73. doi: 10.1001/archpsyc.1982.04290010048009. [DOI] [PubMed] [Google Scholar]
- Rabiner CJ, Wegner JT, Kane JM. Outcome study of first-episode psychosis. I: relapse rates after 1 year. Am J Psychiatry. 1986;143:1155–1158. doi: 10.1176/ajp.143.9.1155. [DOI] [PubMed] [Google Scholar]
- Gitlin M, Nuechterlein K, Subotnik KL, Ventura J, Mintz J, Fogelson DL, et al. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry. 2001;158:1835–1842. doi: 10.1176/appi.ajp.158.11.1835. [DOI] [PubMed] [Google Scholar]
- Uçok A, Polat A, Cakir S, Genç A. One year outcome in first episode schizophrenia. Predictors of relapse. Eur Arch Psychiatry Clin Neurosci. 2006;256:37–43. doi: 10.1007/s00406-005-0598-2. [DOI] [PubMed] [Google Scholar]
- Cesková E, Radovan P, Tomás K, Hana K. One-year follow-up of patients with first-episode schizophrenia (comparison between remitters and non-remitters) Neuropsychiatr Dis Treat. 2007;3:153–160. doi: 10.2147/nedt.2007.3.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L, Thorup A, Øqhlenschlaeger J, Christensen TØ, Jeppesen P, Krarup G, et al. Predictors of remission and recovery in a first-episode schizophrenia spectrum disorder sample: 2-year follow-up of the OPUS trial. Can J Psychiatry. 2008;53:660–670. doi: 10.1177/070674370805301005. [DOI] [PubMed] [Google Scholar]
- Üçok A, Serbest S, Kandemir PE. Remission after first-episode schizophrenia: results of a long-term follow-up. Psychiatry Res. 2011;189:33–37. doi: 10.1016/j.psychres.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Ceskova E, Prikryl R, Kasparek T. Outcome in males with first-episode schizophrenia: 7-year follow-up. World J Biol Psychiatry. 2011;12:66–72. doi: 10.3109/15622975.2010.518625. [DOI] [PubMed] [Google Scholar]
- Emsley R, Oosthuizen PP, Kidd M, Koen L, Niehaus DJ, Turner HJ. Remission in first-episode psychosis: predictor variables and symptom improvement patterns. J Clin Psychiatry. 2006;67:1707–1712. doi: 10.4088/JCP.v67n1106. [DOI] [PubMed] [Google Scholar]
- Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163:743–745. doi: 10.1176/ajp.2006.163.4.743. [DOI] [PubMed] [Google Scholar]
- Addington J, Addington D. Symptom remission in first episode patients. Schizophr Res. 2008;106:281–285. doi: 10.1016/j.schres.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Geddes J, Mercer G, Frith CD, MacMillan F, Owens DG, Johnstone EC. Prediction of outcome following a first episode of schizophrenia. A follow-up study of Northwick Park first episode study subjects. Br J Psychiatry. 1994;165:664–668. doi: 10.1192/bjp.165.5.664. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168:1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]