Abstract
Aim
The aim of this paper is to provide a succinct literature review of the different clinical applications for AMT usage in an ophthalmic setting, ranging from commonly used applications to less mainstream approaches. The hope is that this review enables the reader to have a better understanding of the biological properties of amnion as well as the indications and scenarios in which AMT can be used, whilst presenting relevant evidence from within the literature which may be of interest. We also provide an update on the methods of preservation of amniotic membrane and the application methodologies.
Methods
Literature search. A PubMed search was performed using the search terms “amniotic membrane transplant”, “amnion AND cornea”, amnion AND ophthalmology”, “amnion AND ocular surface” and “Amnion AND eye”. A full review of the literature using the PubMed database was conducted up until 01/05/20. The articles used were written in English, with all articles accessed in full. Both review articles and original articles were used for this review. All full publications related to ophthalmology were considered.
Keywords: amnion, amniotic membrane transplant, amniotic membrane graft, amniotic membrane
Introduction
The placenta is usually discarded as a waste material following birth. However, amnion, the innermost layer of the placental sac can be harvested as a transplant material. AMT is useful clinically due to its unique structure, biocompatible composition and subsequent biological functions.1,2 Amnion was first introduced into clinical medicine as a substrate for skin transplantation3 and has since been adopted into a wide range of surgical applications including adhesion reduction,4 restoration of hearing,5 and replacing the vaginal6 and urethral7 mucous membranes.
The role of an AMT in an ophthalmic setting is usually to support damaged tissue, protect and shield defects from further degeneration or breakdown from external factors and to promote re-cellularisation.1 This is possible due to a myriad of biological properties, including a lack of immunogenicity, thus reducing the risk of inciting an immune response.8 AMT also preserves and supports stem cells9 whilst inhibiting neoplastic,10,11 inflammatory,12,14 angiogenic and fibroblastic cells.1,12 When applied in combination these biological properties support and facilitate wound healing.8,15 Amnion has also been shown to improve pain management16,17 in conjunction with delivering anti-microbial benefits.18,20
The Historical Background of Amniotic Membrane Transplantation
In 1910, AMT was first utilised surgically as a skin graft substitute material.3 The first ophthalmic usage was in the 1940s as a conjunctival substitute after removal of fibrotic tissue,21 with good outcomes reported when compared with the widely used alternative of rabbit peritoneum. Its usage then fell out of favour, potentially as in this era only fresh amniotic membrane was available which was difficult to obtain, and carried a risk of bloodborne virus infection. By the early 1990s, alternative applications began to gain popularity amongst ophthalmologists for a second time. After different preservation methods were developed and refined, better storage and distribution techniques increased tissue accessibility.
The Wide-Ranging Properties of Amniotic Membrane
General Structure
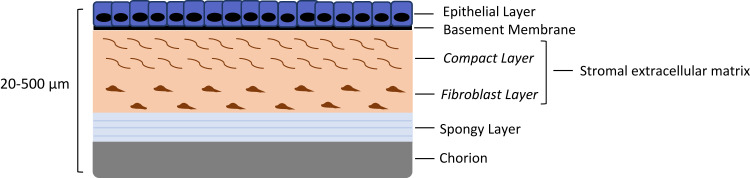
Amnion is composed of five layers, usually between 20–500µm thick in total22 (see Figure 1). It consists of an epithelial monolayer supported by a basement membrane, and an extracellular matrix (ECM) stromal layer, consisting of an acellular compact layer and sparsely populated fibroblast layer. The innermost layer, called the spongy layer, acts as the interface between the fibroblastic layer of the amnion and the reticular layer of the chorion.23,26 By the second month of gestation, the mesenchymal cells separate from the epithelium by a layer of tissue containing loosely packed collagen fibrils and occasional fibroblastic cells. It is predominantly the collagen component of the mesenchymal layer that provides additional tensile strength.8 Structural proteins such as laminin, fibronectin and collagens in the amnion ECM and basement membrane provide a scaffold with which cells can interact to promote epithelial regeneration.27,29 At full term, a single layer of amniotic cells exists, firmly adherent to a mesenchymal layer usually six to eight cells in thickness.30,31 Unusually it is avascular with no direct blood supply.8,32,33 Although the exact role that AM performs in the homeostasis of amniotic fluid currently remains uncertain, it has an exceptional metabolical activity during pregnancy.34 As it does not have a blood supply of its own, it derives its nutrition and oxygen supply from the surrounding chorionic fluid, amniotic fluid, and fetal surface blood vessels. Energy is derived primarily through anaerobic glycolysis pathways.8
Figure 1.
A diagram of the general structure of an amniotic membrane.
Anti-Angiogenic, Anti-Inflammatory, Anti-Scarring and Anti-Fibrotic Factors
The tissue’s anti-inflammatory properties have been well documented within the literature.17,35,36 Although the exact mechanism of action is not yet understood, evidence points towards a range of properties that summate to this, such as the expression of anti-inflammatory and regulatory mediators coupled with the added ability to remove infiltrating inflammatory cells.
The amnion ECM complex contains protease inhibitors of heavy chain 1 of inter-α-trypsin and hyaluronan/pentraxin 3 (HC-HA/PTX3), which is thought to contribute to the anti-inflammatory, anti-scarring and anti-angiogenic therapeutic action.37,39 In addition, inhibition of the ever recognised TGF-ß signal transduction within fibroblasts by mediators from the stromal layer provides additional anti-scarring properties by up-regulating matrix metalloproteinases,12 with reduced numbers of fibroblasts shown in the cornea, limbus and conjunctiva after the application of AMT stromal matrix.12 IL-10 is also present, which is known to reduce IL-6 and TNF alpha levels. This blocks the process of fibrosis formation by removing the activation of the profibrogenic cytokines. Authors have also shown the glycoprotein Lubricin to be present in AM, which is a naturally occurring boundary lubricant with anti-inflammatory, anti-adhesive, and anti-friction properties and this is likely to play a role in the wide-ranging functions of AMT.40
Fibrosis development is driven by the formation of myofibroblasts, which form following the activation of keratocytes, as a response to the disruption of the ocular surface. The anti-fibrotic features of amniotic membrane act in a complementary fashion to the anti-inflammatory nature of the tissue.
Anti-Microbial Action
The antibacterial effects of both amnion and chorion are active against a range of bacteria, including Hemolytic streptococcus group A, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa.41,42 Several factors have been demonstrated to be present in amniotic fluid including bactricidin, beta-lysin, lysozyme, transferrin, and immunoglobulins. Progesterone hormones present in the amniotic fluid are also said to be bacteriostatic against some gram-positive organisms.41,43
Amnion secretes elafin (peptidase inhibitor 3) and secretory leukocyte proteinase inhibitor, both of which have antimicrobial action and are components of the innate immune system.44 Amnion is also reported to have anti-viral properties due to the analogue of a cystine proteinase inhibitor, cystatin E.44
Promotion of Epithelisation
Amnion’s ECM and epithelial layers are interspersed with a complement of trophic components, in particular, epidermal growth factor, keratocyte growth factor and neurotrophic substances that support the wound healing properties of the matrix mentioned above. This complex interplay of healing components uniquely regulates and promotes regenerative healing.45,46 The structure and especially the collagen composition of AM’s basement membrane closely resemble that of the conjunctiva and cornea. This allows it to act as a substrate on which epithelial cells can easily replicate. AM is said to have multiple effects on the regenerating corneal epithelium, including facilitation of migrating epithelial cells47,48 and reinforcing basal epithelial cell adhesion.49 It also plays a role in the promotion cell differentiation50,51 and blockade of apoptosis.52,53 These features mean it can be useful in cases of non-healing or persistent epithelial defects, as will be discussed later on in this review.
Amniotic Membrane Procurement and Manufacture
Procurement of Amniotic Membrane
Amnion is procured from consenting mothers who are undergoing elective caesarean sections. All donors answer a lifestyle questionnaire, which is used to minimise the hazard of any transmissible diseases by removing potentially “high risk” donors. For all consenting donors, blood samples are extensively tested for any viral infections or disease markers to ensure the risk of transmission from transplantation is minimised.
The physical processing of amniotic membrane is conducted under sterile conditions and the tissue is washed using antibacterial and antifungal agents. Following this the chorion is usually removed prior to processing,54,55 but this does vary between amnion manufacturers. Amnion is then preserved to allow long-term storage of the tissue; this can be conducted in multiple ways as discussed below.
Methods of Amniotic Membrane Preservation
It must be noted that all preservation methods cause some degree of compromise to integrity of the tissue.
Cryopreservation
Cryopreservation is the most common technique of preservation. Cryopreservation involves adding a storage medium to the tissue, such as Dulbecco’s Modified Eagle Medium containing cryoprotectants such as glycerol, and freezing to −80°C. When cooled to these temperatures, degradation through unregulated enzymatic reactions is said to be limited. Some reports within the literature suggest that freezing may lead to ice crystal formation which potentially damages cellular integrity, and the thawing process prior to application may result in a loss of soluble proteins that are important to the wound healing process55,56 such as the aforementioned angiogenic factors.56
Freeze-Drying/Lyophilisation
Freeze-drying involves cooling the amnion to −80°C and then a process of sublimation follows in order to remove the water from the tissue. Gamma irradiation is used in order to sterilise the tissue. This process can inflict similar ice crystal damage to the tissue as cryopreservation, but unlike cryopreserved products, these do not have cold chain storage logistical issues and do not require thawing prior to use.57 Lyophilisation is a method that consists of removing water by a sublimation process. This results in the inhibition of chemical reactions that lead to adverse alteration of tissues.57 Lyophilised tissue can then be stored at room temperature for long periods facilitating transportation, thus resolving the two main disadvantages of cryopreservation. Authors comparing cryopreserved and lyophilized samples have shown the presence of type IV collagen throughout the basement membranes, in both cryopreserved and lyophilized samples.57 Growth factors and total protein content were not significantly different in either preservation method, other than certain fibroblast growth factors were higher in cryopreserved samples.
Dehydration and Low-Temperature Vacuum Evaporation
Air or heat can be utilised to remove the moisture and dehydrate the tissue. Dehydrated amniotic membrane does not have the issues related to lyophilised or cryopreserved amnion, as it does not involve a step where the tissue is frozen. Gamma irradiation is used to sterilise the tissue.
This evaporation process uses a sugar protectant, such as trehalose,58 before undergoing a low-temperature vacuum process to remove any water content from the tissue in a controlled process. Sugar protectants such as trehalose replace intracellular water during dehydration or freezing to form a glassy matrix, therefore preventing the major disruption of internal cell organelles, keeping the tissue viable.56,59 Similar to dehydrated amnion, this process does not include any freezing steps throughout. Some authors suggest that this method provides a superior substrate compared to conventional cryopreserved AM,56 although there are some conflicting reports.54 In addition, this product is stable allowing it to be transported globally for use in clinical and military sectors.56
Application Methods
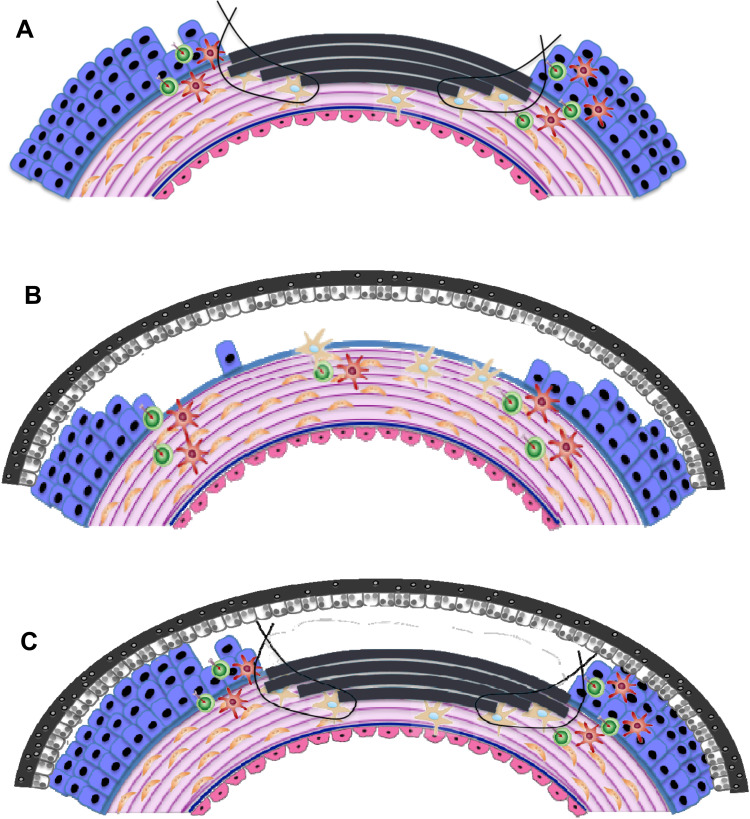
The application method for transplantation will depend on the aetiology of the disease. The depth and size of the wound and the area of the ocular or mucosal surface that has been affected, are all contributing factors. Publications available from within the literature have excellent images detailing the different forms of transplant methods.60 In addition, please see Figure 2.
Figure 2.
Schematic depicting potential applications and orientation of AMT (depicted in grey) on the ocular surface. (A) Inlay (graft) amnion transplantation. Epithelial-side-up: amnion replaces lost stromal tissue, up to the basement membrane. (B) Onlay (patch) transplantation where amnion is placed epithelial-side-down over the wound periphery as a temporary biological dressing. (C) Combinatorial/Sandwich AMT.
Inlay Transplantation (Graft)
Inlay transplantation involves the amnion being grafted into the damaged ocular surface after the defective tissue has been removed from the site. Amnion acts a replacement for the lost tissue. A graft is permanently incorporated and remodelled into the host corneal matrix over time. Dependent on the depth of the wound, single or multilayer grafts may be used. Amnion’s ability to integrate into the corneal stroma is possible due to the formation of hemidesmosomes and desmosomes.61 These keratinocytes provide both stability and anchorage, which allow for amnion to help improve the structural quality of the tissue.62,63 Amnion is placed epithelial side up, as the membrane is able to act as a substrate for epithelial regeneration. Usually, a rim of epithelium is removed from the periphery of the stromal defect to ensure that no overlapping epithelium remains, reducing the chances of proliferation underneath the graft. This helps the epithelium to grow over the inlay graft, acting as a basement membrane.60 At times, residual membrane may remain visible as wavy white lines or superficial “scar” tissue has been incorporated into the cornea.
Onlay Transplantation (Patch)
Onlay or “patch” amnion transplantation is the addition of the tissue epithelial side down over the periphery of a superficial wound.8,55 The transplant acts as a temporary biological dressing, which is able to protect the wound by providing a physical barrier against: environmental damage; the formation of symblepharon and ankyloblepharon; and any further physical insult. Proteins are secreted from the amniotic membrane onto the ocular surface. These proteins limit fibrosis and scarring, allowing for an environment for effective wound healing. Onlay transplantations will remain on the surface of the eye until removed or the product self-degrades. The transplant is not intended to become integrated into the cornea.
Combinatorial (Sandwich) Transplantation
Combinatorial transplantation utilises both the inlay and onlay methodologies of transplantation. The graft provides structural integrity, whilst the patch allows for the protection of the graft.1,8 Both of the transplants are able to deliver anti-inflammatory and pro-epithelisation factors.61 The epithelium is expected to grow between the two layers8 if the orientation of the graft (epithelial side up) and patch (epithelial side down) is used in this manner.
Ocular Surface Reconstruction: Graft
Corneal Ulceration and Persistent Epithelial Defects
AMT can be incorporated into the treatment of corneal ulcers by any of the methods of application mentioned above: i) Inlay, ii) Onlay, iii) Combinatorial Technique. The chosen method of application will depend on the depth of the ulceration, desired effect from AMT and surgeon preference.
Schuerch et al64 ‘s retrospective analysis studying non-epithelialising corneal ulcers refractory to standard medical therapy included 149 eyes. It is important to note that a variety of ulcer aetiologies were included, with the majority being herpetic infections or patients that had undergone previous penetrating keratoplasty. In brief, an amniotic membrane was placed with the epithelium up within the corneal epithelial defect and fixed using nylon 10–0 interrupted sutures. A second amniotic membrane with the epithelium down was placed overlapping the first layer and fixed with a continuous nylon 10–0 suture to the conjunctiva. The mean duration from the diagnosis of the corneal ulcer to AMT was 42 (range 6–46 days).64
Ulcers due to bullous keratopathy, bacterial ulcers, herpetic ulcers and neurotrophic ulcers had the highest overall closure rates (79%, 80%, 85%, and 93%, respectively) and achieved epithelial closure mainly within the first 3 months after AMT. Of note, those ulcers related to rheumatic disease only epithelialised in 52.5% of cases.64
A meta-analysis by Lui et al65 studied three different methodologies for the application of amniotic membrane in ulcer treatment (as mentioned above). The meta-analysis pooled 18 eligible studies and found the highest rate of epithelial healing was amongst the “sandwich application” group, although this technique resulted in the smallest visual improvement outcomes, making technique selection difficult.65
Tabatabaei et al35 presented the largest of the trials, studying 99 eyes, all of which were diagnosed with bacterial keratitis. After 2–5 days of topical antibiotic treatment, AMT was performed in the treatment group and standard care was continued in both groups. The tissue transplantation was deemed safe and showed better outcomes in regard to visual acuity at 6 months (p<0.001). Other studies show favourable outcomes in terms of pain management and epithelial healing when early AMT intervention (at 48 hours) is combined with topical steroid application (at 72 hours).66,68
Further evidence of AMT efficacy is detailed by the findings of Fuchsluger et al69 and Khokhar et al,70 the latter specifically examining the use of AMT in corneal ulcers relating to neurotrophic keratopathy in a randomised control trial. AMT was found to be as effective as conventional tarsorrhaphy or bandage contact lens placement for refractory neurotrophic corneal ulcers.
Similarly, other authors have shown that usage for symptomatic, painful bullous keratopathy can improve pain scores and epithelialisation,71 although it has been suggested that given the ease and reduced cost of anterior stromal puncture techniques, these may be preferred in certain scenarios to AMT.72 Similar findings are seen when used in conjunction with the surgical removal of band keratopathy.73 It should be noted that these studies all have relatively small patient numbers and a variety of methodologies utilised amongst them, making direct comparison of outcomes difficult.
It is also worth noting that AMT can be used in eyes that have undergone penetrating keratoplasty to treat PEDs. Seventy percent of eyes showed successful epithelial closure within 4 weeks of AMT and, interestingly, the success rate appeared to be inversely proportional to the number of previous transplants.74
Limbal Stem Cell Deficiency (LSCD)
Progenitor stem cells for the conjunctiva and cornea have been shown to reside in the conjunctival fornices and limbal area, respectively, they then migrate onto the ocular surface and differentiate into daughter cells that continuously regenerate into the conjunctival and corneal surface epithelia. Corneal epithelial cells have been shown to migrate rapidly when limbal explants are placed on AM denuded of amniotic epithelial cells but with an intact basement membrane. Interestingly, they migrate relatively slowly when the amniotic epithelium is left intact, and at their slowest when they are grown on the stromal surface. Culturing limbal explants on an intact AM with devitalized epithelium has been shown to be conducive to the expansion of an epithelial phenotype that closely resembles limbal stem cells.75
Amnion can be used in conjunction with limbal stem cell transplantation (LSCT) for the treatment of LSCD. Two differing in-vivo expansion surgical procedures have been developed to tackle the issue regarding the conjunctival-cornea epithelial cell admixture that forms following LSCT procedures. Currently, sector sequential conjunctival epitheliectomy (SSCE) is used to mechanically remove any conjunctival epithelial cells which are migrating onto the cornea.76 However, this procedure requires multiple clinic appointments following surgery and is unpleasant for patients. Both of the in-vivo stem cell expansion techniques aim to prevent conjunctival epithelial admixture contamination and therefore increase the success of LSCT.
Simple Limbal Epithelial Transplantation (SLET)
In 2012, Sangwan et al77 reported a novel surgical technique for the treatment of limbal stem cell deficiency. Simple limbal epithelial transplantation technique involves harvesting limbal stem cells from a donor area of 2x2 mm from the superior limbus using sub-conjunctival dissection. In the recipient eye, a 360° peritomy is performed, and any vascular pannus removed. A human amnion graft was placed over the bare ocular surface and tucked under the conjunctiva, then secured in place with fibrin glue. The donor tissue was cut into small pieces and these explants placed epithelial side up on top of the amniotic membrane in a circular manner around the cornea. This technique has been further explored by a number of studies, such as Shanbhag et al78 and Borroni et al.79 Both these studies show SLET surgery to be an effective and safe surgical technique. One retrospective multicentre study using 68 eyes showed the success of SLET in 57 cases (83.8%) with a survival probability of greater than 80% at one-year post operatively.80
Amnion-Assisted Conjunctival Epithelial Redirection (ACER)
ACER utilises an amnion patch to redirect the conjunctival cells and allows for the corneal epithelial cells to regenerate with potentially less dilution from any invading conjunctival epithelium. Dua et al81 reported the surgical procedure using a 360° peritomy, with donor conjunctivo-kerato-limbal grafts sutured at the six and twelve positions with an inlay amnion graft sutured into the wound bed. Following this, a large AMT is tucked under the edge of the recessed conjunctiva between the ends of the limbal explants. This outer AMT patch is held in place through the use of fibrin glue and is large enough to cover the cornea and adjacent sclera.
The aim is to ensure the conjunctival epithelial cells are redirected to only grow over the amnion, meaning the conjunctival cells are theoretically prevented from mixing with the limbal explant-derived epithelial cells; conjunctival cells expand over the outer amnion, whilst the regeneration of the corneal epithelial surface is nurtured beneath, unhindered. Authors have shown that patients undergoing ACER require less outpatient appointments and can hopefully avoid the painful SSCE procedures.81
Cultivated Stem Cell Transplantation
In situations where there is significant bilateral limbal damage without residual LSCs, or if there is not enough healthy limbal tissue in an unaffected eye to harvest sufficient LSCs, one must consider the transplantation of ex vivo cultured and expanded cells. The usual source of cells is usually human limbal82 (CLET) or oral epithelium83 (COMET). The cells can be taken either from the patient (autologous), a living relative or cadaver (allogenic). The reported advantage is that less than 1mm2 of donor tissue is required for transplantation.84,85
AM is used as described as in other conditions to act as a basement membrane scaffold for cultured cells and as a cell carrier substrate in CLET or COMET procedures. Although both de-epithelialized and intact AM has been used, de-epithelialized AM is said to be superior to intact AM because as it preserves the properties of LSCs whilst promoting the migration of LSCs.86
Indeed, there is some evidence that using intact AM allows some LSCs to undergo epithelial–mesenchymal transition and invade the limbal stroma.87,88 AM has been shown to preserve limbal epithelial cells in an undifferentiated state, maintaining its naturally occurring slow cycling.88,91 Evidence suggests that AM provides a unique stromal microenvironment beneficial for LSC survival and expansion, whilst acting as an IL-1 antagonist to prevent cell apoptosis.92 Many authors show CLET to be successful, with grafts remaining stable one year post operatively with low rejection rates.93,100
It is important to note however that many variables exist within the different reported studies, with success rates depending on confounding factors such as age, donor source, and cell quality.85,88,98,101 Fibrin glue techniques have been shown to be just as effective, although AM has wider accessibility in many countries.88
COMET procedures have been shown to have good outcomes two years post operatively.102,108 There currently exists no comparative trial comparing CLET and COMET procedures so it is difficult to directly compare the clinical outcomes from different studies, although some reports show substrate-free oral mucosal cell sheet transplants to have higher success rates.88,106,107 Some authors would dispute this as although a stable phenotype is achieved post operatively with this method, CK12 the corneal epithelial marker and the PAX6 eye specific transcription factor, is not expressed in transplanted oral mucosal cells,109 indicating that they do not undergo a true cellular transdifferentiation.88
Glaucoma
AMT may be used in conjunction with bleb revision for persistent leaks following trabeculectomy surgery or aqueous shunt revision. The amnion can be grafted onto the epithelial surface over the bare sclera to replace lost or damaged conjunctiva.
In 2012, Bochmann et al110 published a meta-analysis examining various interventions involving AMT for late-onset bleb leaks. The meta-analysis searched for a range of techniques but only a single study by Budenz et al111 met the required criteria. In this review, amnion was not shown as an effective alternative to conjunctival advancement for reducing bleb leaks. Other reports from the literature agree with this finding.112 Authors also report success from using AMT to repair bleb leaks113,115 across a range of post operative time points, incorporating both early and late post operative bleb leaks.
Published meta-analysis data116 suggest that that amnion usage intraoperatively was effective at reducing IOP when compared with trabeculectomy alone. Amnion application during trabeculectomy is the most well-documented indication in glaucoma patients, and Sheha et al117 report that amnion combined with mitomycin-c increased the surgical success rate of secondary trabeculectomies in refractory surgical cases (93.7%). The authors describe the technique in their publication, using amnion sutured stromal side down under the trabeculectomy flap. AMT augmented trabeculectomies have been shown to increase success in primary trabeculectomy surgery.117 There are reports to suggest it can be used as a patch graft during drainage device insertion, providing good tectonic support and allowing direct visualisation of the tube,118 despite not improving clinical success.119,120 AMT has also been used to repair conjunctival defects causing bleb leaks and hypotony following GDDs and MIGS device insertion.121,122
Neoplasia
Amnion is used to act as a substrate for conjunctival migration and reconstruction following excision of both malignant and benign tumours. Qin et al123 studied 24 eyes with intraepithelial epithelioma in a randomised trial, assessing whether a concave-convex (sandwich technique) AMT had better post operative outcomes when compared with standard AMT. Epithelialisation rates were similar in each group, although the tumour recurrence rate at one year was significantly lower in the concave-convex AMT treated group (p<0.05).
Similarly, Agraval et al124 report improved local surgical outcomes following conjunctival melanoma excision combined with AMT improved conjunctival healing, resulting in minimal symblepharon, granuloma or scar formation. Other authors have also commented that the transparency of amniotic membrane allows for monitoring of tumour recurrence in deeper tissues, and may also provide a superior cosmesis when compared with thicker (for example, buccal) mucous membrane grafts.125
Although the anti-neoplastic mechanism of amnion is not well understood,10 it is attributed to the secretion of anti-angiogenic, pro-apoptotic and immune-modulatory factors.11,126,129 Amnion contains interleukins (IL-2, IL-3 and IL-4) and can express cytotoxic cytokines, these are known to enhance the cytotoxicity of natural killer cells, which are able to attack mitotic cancer cells.11
Oculoplastics
As mentioned briefly above, AMT has been suggested as a potential alternative to mucosal membrane transplantation in lid revision, orbital linings or symblepharolysis. Reports exist describing forniceal reconstruction utilising AM,130,132 with other authors stating that AM can be a viable alternative to established mucous membrane grafting, causing less patient morbidity, faster recovery times and better prosthesis fitting in anophthalmic-contracted sockets.132,134 It can also be particularly useful if a patient has an insufficient amount of mucosal membrane available, producing effective results when combined with antimetabolites such as MMC.135
Cicatricial eyelid abnormalities can be difficult to treat as tissue loss needs to be overcome. Good outcomes have been reported using a gray line lid split procedure with vertical anterior lamella repositioning, in patients with moderate to severe cicatricial entropion.136 AMT was used to cover the bare tarsus up to the lid margin and secured with 7–0 Vicryl sutures. Good cosmetic outcomes were reported, with rapid epithelialisation of the previously bare tarsus. No lashes were abrading the globe in 88% of cases 12 months post operatively. In addition, it has been used following conjunctival reconstruction in advanced mucous membrane pemphigoid. After scar tissue was carefully excised, AMT was placed over the cornea, bulbar and tarsal conjunctiva, and secured with 8–0 Vicryl sutures to the conjunctival edges and the deep fornices with double-armed 6–0 silk sutures.137 Immunosuppressive systemic therapy and topical steroids were administered to all patients for at least 6 months following surgery. Post operatively the forniceal depth was significantly improved up to 28 weeks following surgery, although the effect was said to deteriorate over time.137 Comparable outcomes have been shown in Stevens–Johnson Syndrome patients, using a similar surgical technique.138
There are reports of authors using AMT in conjunction with an Hughes procedure to restore tissue loss after excision of conjunctival melanoma involving the fornix. In addition, despite being a rare condition, AMT has been used effectively to manage cases of cryptophthalmos. One centre reports surgical success in terms of both acceptable functional and cosmetic outcomes in 20 of 24 patients receiving repair procedures from a single centre over a 12-year period. The authors conclude that a one-stage reconstruction of both the eyelid and fornix with scleral and amniotic grafts is an effective strategy to correct abortive cryptophthalmos.139
Pterygium
The use of AMT in pterygium is one of the most well-documented indications. However, it is also one of the most controversial areas for its usage, with the current treatment standard for pterygium excision being a repair of the defect with a conjunctival autograft. A Cochrane systematic meta-analysis review140 compared the use of autograft with AMT following pterygium excision. Conjunctival autograft was associated with a lower risk of recurrence at six months’ following surgery than AMT. Participants with recurrent pterygia, in particular, were found to have a lower risk of recurrence when they received conjunctival autografts. The obvious advantage AMT has in this setting is no donor site defect is created. There are few studies comparing the two techniques with respect to visual acuity outcomes, and no studies report on vision-related quality of life or direct or indirect costs.140 Unfortunately, an insufficient number of studies have used adjunctive mitomycin C to be able to draw conclusions on the effects on pterygium recurrence following conjunctival autograft or AMT.
Prajna et al141 studied double pterygium excision (combined nasal and temporal) comparing autograft to AMT. Again, the autograft group was shown to have statistically significant lower recurrence rates than AMT, which has also been reported by other studies.142
Strabismus
Cryopreserved AMT has been utilised during strabismus surgery either to cover defects or to reduce muscle fibrosis and adhesion. Kassem et al143 reported a randomised controlled study of revisional strabismus patients, where one group received amnion wrapped around the rectus muscle. Although no adverse events were reported from the amnion group, the authors concluded that no clinical benefits were shown from this technique. The patients in the treatment group were followed up on a long-term basis (up to 85 months) in a further study,144 with the conclusion that the effect of amniotic membrane transplantation on the success of strabismus reoperations was moderate in terms of ocular alignment, but more pronounced in terms of ocular motility range, likely due to a reduction in fibrosis and subsequent muscle restriction.143,145
There are also reports of AMT usage in complex strabismus cases, with successful outcomes in patients that suffer diplopia following vitreoretinal surgery.146 It has also been used with adjunctive MMC in cases with congenital fibrosis of the extraocular muscles.147
Fresh human AMT has been used successfully in secondary strabismus cases, where authors report the placement of two AM sheets, one between the operated muscle and tenon’s capsule, with the stromal side facing tenon’s capsule. The other AMT is placed between the muscle and sclera with the stroma facing the sclera. Areas of bare sclera were covered using AMT with the stroma against the sclera. The authors concluded that AMT placement around the extraocular muscle improved ductions and reduced the residual deviation angle due to less post operative scar formation.148 This was attributed to the prevention of adhesions by the AMT as it formed a temporary biological barrier between the layers of perimuscular connective tissue. Despite these outcomes, the use of fresh AMT is discouraged due to the risk of communicable diseases, which is significantly reduced in other forms of AMT. Interestingly, dried AMT has resulted in poor outcomes in strabismus cases145,149,150 apart from one case report.151
The orientation of AMT varies throughout these studies and conclusions are hard to draw from the outcomes. One histopathological study using rabbits compared three groups; one with stroma orientated towards the muscle, another with the epithelium towards the muscle, and one with folded AM where the epithelium was in contact with muscle and sclera. The authors concluded that any AMT orientation was effective in preventing adhesions, with no advantage seen in either group.152 Although there is limited evidence within the literature to support a sutured or sutureless technique, one review article recommends the use of a sutureless technique as it induces less of an inflammatory reaction and results in a shorter operating time.145
Ocular Surface Wound Healing: Patch
Chemical/Thermal Injury
Amnion has a range of biological properties that make it attractive for use in the treatment of chemical burns, such as its epitheliotrophic; anti-inflammatory and anti-neovascular effects.153 As well as offering increased oxygen permeation and reducing mechanical epithelial trauma from eyelid friction,8 as previously discussed, it can reduce pain whilst increasing patient comfort.8,154
As mentioned above, AMT can be utilised in LSCD, which can occur as a sequelae of chemical and thermal injuries. However, AMT has a role to play in the treatment of the acute phase of these injuries. Tandon et al36 presented the first randomised controlled trial, where amnion was transplanted epithelial side down within the first seven days of injury for chemical injuries of Roper Hall grading II–IV.155 The study recruited 100 patients, split between moderate (grade II–III) and severe (grade IV) burns. AMT significantly increased the rate of epithelial healing in the moderate burns patients (p = 0.0004). However, no difference was seen in the secondary long-term outcome measures, such as final visual acuity, symblepharon formation, corneal clarity or neovascularisation. Patients with severe burns showed no improved rate of epithelial healing. In 2012, Clare et al156 published a meta-analysis, which analysed only the Tandon et al paper as this was the only available randomised control trial. It concluded that the evidence for amnion use in chemical burns is equivocal.
Sharma et al17 compared three treatment groups: standard care (SC) alone, SC with amniotic membrane transplantation and SC with umbilical cord serum. Both the umbilical cord serum group and amniotic membrane transplantation group promoted epithelisation faster than SC. However, at three months’ there was no difference between the three groups in terms of visual outcome, symblepharon formation, tear film status, and lid abnormalities. The AMT and serum group saw a reduction of pain for patients at day seven of treatment, although the serum group had the largest reduction in pain scores at all measured time-points.
Most recently, Eslani et al157 enrolled 60 eyes with grade IV Roper Hall chemical injuries. Patients were randomised to receive medical management or AMT in conjunction with medical management. No significant difference was found between the rates of corneal epithelisation between the two groups (p=0.610), with the authors concluding that medical therapy with AMT does not accelerate corneal epithelialisation or improve final visual acuity in patients with severe chemical injuries. Although the authors suggest that in milder cases AMT may be beneficial based on the outcomes of other studies, it may not actually be a necessary treatment. It is suggested that the LSC function may be so poor in these severe injuries that the anti-inflammatory properties of AMT are not able to overcome the extensive damage. Moreover, its anti-angiogenic effect may reduce the effect of the recovering LSCs.
The current data suggests that the success of amnion transplantation in chemical burns is dependent on the severity of the burn, especially when considering the rate of epithelisation. However, other outcome measures, such as reduction of pain and inflammation, can be improved with amnion usage, with most of the studies indicating the need to apply the AMT within the first week of any injury.
Dry Eye Disease (DED)
The DEWS II report158 proposes the use of AMT for use in severe dry eye, as a ‘step 4ʹ therapeutic intervention. Reports from within the literature suggest that sutureless AMT (PROKERA®) can result in a sustained symptomatic improvement for four months in dry eye subjects when worn for approximately five days on average,159 demonstrating reduced corneal and conjunctival staining and improved visual acuity. Other studies show similar findings.160,161 One retrospective review by Macdonald et al162 applied AMT for 5.4 (±2.8 days) on average with a sustained reduction in the DED marker (3.25±0.5 to 1.47±0.6) three months post operatively with improvement in both patient reported symptoms and clinical signs. Finally, other authors report how corneal nerve density can be significantly improved following AMT application,163 potentially accelerating the recovery of the ocular surface health in patients with DED.
Cicatrising Conjunctivitis – Steven’s Johnson Syndrome (SJS), Toxic Epidermal Necrolysis (TEN), Graft versus Host Disease and Pemphigoid
The only published randomised controlled trial examining AMT in SJS patients, comparing medical management and AMT with solely medical management, is by Sharma et al.154 In terms of outcomes, the AMT groups showed significantly improved visual outcomes, longer tear break up times and improved Schirmer test outcomes. Importantly, no case in the AMT group demonstrated corneal haze, limbal stem cell deficiency, symblepharon, ankyloblepharon, or lid-related complications. Other authors have shown amnion to be safe and effective in paediatric populations with similar conditions.164
Ma et al165 published a novel technique for amnion application in SJS and TEN patients, which involved the use of 10cm x 5cm amnion rectangle combined with a custom-made forniceal ring to provide coverage of the full mucosal surface and eyelids. Amnion is placed on the upper eyelid and secured with two nylon sutures, laid across the ocular surface, and the forniceal ring is placed onto the eye. The technique minimises the manipulation of ocular tissues and decreases surgical time.
Following Ma’s study, Shanbhag et al166 published data using cyanoacrylate glue instead of suturing the AMT into position, making it easier to perform at a patient’s bedside, providing clinicians with the potential to avoid the risk of visiting theatre with a medically unstable patient.
Recurrent Corneal Erosion (RCE)
RCE has been highlighted as a therapeutic indication in which amniotic membrane could be utilised. In a case series by Huang et al,167 11 eyes of 9 patients, each treated with epithelial debridement followed by sutureless AMT. During the follow-up period (average 12 months), only one eye suffered symptoms of recurrent erosions. A comprehensive review by Miller et al168 in 2019 listed amnion as an effective treatment for acute RCE attacks and suggested its use once conservative treatment of topical lubrication and punctal occlusion has failed, instead of inserting a bandage contact lens.
Vitreoretinal – Amnion Plugs
Following Pars Plana Vitrectomy: Macular Holes and Tears
Amnion plugs can be implanted in the subretinal space with the chorion layer facing the RPE in an attempt to close macular holes.169 Evidence suggests that human retinal pigmented epithelial cells seed over amnion within 24 hours of implantation. Moreover, these cells maintain epithelial features and can proliferate over epithelium-free amnion, resulting in a tight monolayer with well-defined intercellular and cell–substrate interactions, secreting several growth factors important for maintaining retinal homeostasis.170 Recently, Caporossi et al, 2019169 studied 16 eyes of 16 patients with recurrent macular holes in a prospective case series of highly myopic eyes. All patients underwent a 23-gauge par plana vitrectomy with AMT implantation, with either 20% SF6 or air tamponade. Mean post operative BCVA was 0.94±0.24 logMAR, which improved to 0.67±0.26 logMAR post operatively . All patients experienced complete closure anatomically, although one hole reopened, requiring a repeat amniotic membrane implant two weeks post operatively.
Similarly, Caporossi et al171 presented a case series of two eyes of two patients with retinal detachment and large macular tears. Pars plana vitrectomy was performed in conjunction with an AMT, and standard silicone oil tamponade. For both patients, BCVA improved from light perception to 1.3 logMAR. At three months, both patients’ optical coherence tomography showed neuroretinal in-growth over the amniotic membrane plug.171
The use of amnion in vitreoretinal surgery is a novel and innovative technique that requires greater understanding and research but certainly appears encouraging.
Potential Risks of Amniotic Membrane Transplantation
As amnion is an allogenic tissue from a single donor, there will be an implicit risk of acquiring infectious diseases from its usage. Adequate donor screening, testing, handling, processing and storage, should be employed in order to minimize this risk. Legislation should stipulate that HIV, hepatitis B, C, and HTLV tests are undertaken on the donor serum at the time of membrane procurement, with a repeated HIV test 6 months later to cover the latent infectious period of the virus. In order to safely achieve this, the membrane is quarantined for 6 months. Additionally, as with any human-derived product, the risk of prion infection must also be considered. Very few complications have been reported despite AMT’s use in a wide range of clinical applications. Hypopyon formation has been reported in one case following repeated applications to a neurotrophic ulcer.172 This was resolved with topical steroid treatment. Haemotomas may form post operatively, which may be need to be drained if they cause discomfort or dislodge the transplant. Granulomas can also form around sutured membranes, and dislocated membranes can be frustrating. The authors also report persistent subepithelial membranes that are thought to be from thicker membranes harvested near the umbilical cord. This can reduce the visual potential if they reside on the visual axis.8
Conclusion
We have shown the wide-ranging applications of AMT that exist within ophthalmic settings, alongside a discussion of the relevant literature. We hope this review gives the reader greater understanding of the clinical scenarios in which AMT may provide benefit. Given its widespread use, there are likely to be scenarios where usage will be beneficial, but similarly, there will be times when it is not required. Our understanding of AMT is not yet complete, but it is improving. It is clear that AMT is beneficial in some conditions, and, as discussed above, offers a good option in some challenging situations. With time, our understanding will improve, along with clarifying the clinical scenarios for which it is required.
Abbreviations
AM, amniotic membrane; AMT, amniotic membrane transplant; LSC, limbal stem cell; LSCD, limbal stem cell deficiency; SLET, simple limbal epithelial transplantation; CLET, cultivated limbal epithelial transplantation; COMET, cultivated oral mucosal epithelial transplantation; ACER, amnion-assisted conjunctival epithelial redirection; SSCE, sector sequential conjunctival epitheliectomy.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Meller D, Pauklin M, Thomasen H, Westekemper H, Steuhl KP. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011;108(14):243–248. doi: 10.3238/arztebl.2011.0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng SC, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf. 2004;2(3):177–187. [DOI] [PubMed] [Google Scholar]
- 3.Davis JS II. Skin grafting at the johns hopkins hospital. Ann Surg. 1909;50(3):542–549. doi: 10.1097/00000658-190909000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karnitschnigg H. [Amniotic membrane in surgical therapy of adhesion ileus and adhesion complications]. Wien Med Wochenschr. 1950;100(29–30):502–503. [PubMed] [Google Scholar]
- 5.Schrimpf WJ. Repair of tympanic membrane perforations with human amniotic membrane; report of fifty-three cases. Ann Otol Rhinol Laryngol. 1954;63(1):101–115. doi: 10.1177/000348945406300109 [DOI] [PubMed] [Google Scholar]
- 6.Ashworth MF, Morton KE, Dewhurst J, Lilford RJ, Bates RG. Vaginoplasty using amnion. Obstet Gynecol. 1986;67(3):443–446. [PubMed] [Google Scholar]
- 7.Brandt FT, Albuquerque CD, Lorenzato FR. Female urethral reconstruction with amnion grafts. Int J Surg Investig. 2000;1(5):409–414. [PubMed] [Google Scholar]
- 8.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51–77. doi: 10.1016/j.survophthal.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 9.Tejwani S, Kolari RS, Sangwan VS, Rao GN. Role of amniotic membrane graft for ocular chemical and thermal injuries. Cornea. 2007;26(1):21–26. [DOI] [PubMed] [Google Scholar]
- 10.Hossain L, Siddika A, Adnan MH, Diba F, Hasan Z, Asaduzzaman SM. Human Amniotic Membrane and Its Anti-cancer Mechanism: a Good Hope for Cancer Therapy. SN Compr Clin Med. 2019;1(7):487–495. doi: 10.1007/s42399-019-00090-5 [DOI] [Google Scholar]
- 11.Niknejad H, Yazdanpanah G. Anticancer effects of human amniotic membrane and its epithelial cells. Med Hypotheses. 2014;82(4):488–489. doi: 10.1016/j.mehy.2014.01.034 [DOI] [PubMed] [Google Scholar]
- 12.Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179(3):325–335. doi: [DOI] [PubMed] [Google Scholar]
- 13.Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348–352. doi: 10.1097/00003226-200005000-00018 [DOI] [PubMed] [Google Scholar]
- 14.Ogawa Y, He H, Mukai S, et al. Heavy Chain-hyaluronan/pentraxin 3 from amniotic membrane suppresses inflammation and scarring in murine lacrimal gland and conjunctiva of chronic graft-versus-host disease. Sci Rep. 2017;7(1):42195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meller D, Pires RT, Mack RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107(5):980–989. [DOI] [PubMed] [Google Scholar]
- 16.Tamhane A, Vajpayee RB, Biswas NR, et al. Evaluation of amniotic membrane transplantation as an adjunct to medical therapy as compared with medical therapy alone in acute ocular burns. Ophthalmology. 2005;112(11):1963–1969. doi: 10.1016/j.ophtha.2005.05.022 [DOI] [PubMed] [Google Scholar]
- 17.Sharma N, Singh D, Maharana PK, et al. Comparison of Amniotic Membrane Transplantation and Umbilical Cord Serum in Acute Ocular Chemical Burns: a Randomized Controlled Trial. Am J Ophthalmol. 2016;168:157–163. doi: 10.1016/j.ajo.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 18.Jensen OL, Gluud BS. Bacterial growth in the conjunctival sac and the local defense of the outer eye. Acta Ophthalmol Suppl. 1985;173:80–82. doi: 10.1111/j.1755-3768.1985.tb06849.x [DOI] [PubMed] [Google Scholar]
- 19.Sotozono C, Inagaki K, Fujita A, et al. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections in the cornea. Cornea. 2002;21(7 Suppl):S94–S101. doi: 10.1097/01.ico.0000263127.84015.3f [DOI] [PubMed] [Google Scholar]
- 20.Goodman DF, Gottsch JD. Methicillin-resistant Staphylococcus epidermidis keratitis treated with vancomycin. Arch Ophthalmol. 1988;106(11):1570–1571. doi: 10.1001/archopht.1988.01060140738046 [DOI] [PubMed] [Google Scholar]
- 21.de ROTTH A. PLASTIC REPAIR OF CONJUNCTIVAL DEFECTS WITH FETAL MEMBRANES. Arch Ophthalmol. 1940;23(3):522–525. doi: 10.1001/archopht.1940.00860130586006 [DOI] [Google Scholar]
- 22.Rao TV, Chandrasekharam V. Use of Dry Human and Bovine Amnion as a Biological Dressing. Arch Surg. 1981;116(7):891–896. doi: 10.1001/archsurg.1981.01380190029007 [DOI] [PubMed] [Google Scholar]
- 23.McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: Identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14(1):237–241. doi: 10.1093/humrep/14.1.237 [DOI] [PubMed] [Google Scholar]
- 24.McParland P, Bell SC. THE FETAL MEMBRANES AND MECHANISMS UNDERLYING THEIR LABOUR-ASSOCIATED AND PRE-LABOUR RUPTURE DURING PREGNANCY. Fetal Matern Med Rev. 2004;15(1):73–108. doi: 10.1017/S0965539504001238 [DOI] [Google Scholar]
- 25.Karteris E, Grammatopoulos D, Dai Y, et al. The human placenta and fetal membranes express the corticotropin-releasing hormone receptor 1alpha (CRH-1alpha) and the CRH-C variant receptor. J Clin Endocrinol Metab. 1998;83(4):1376–1379. doi: 10.1210/jcem.83.4.4705 [DOI] [PubMed] [Google Scholar]
- 26.Wu ZY, Hui GZ. Materials for neuro-transplantation and the amnion. Chin Med J (Engl). 2006;119(16):1323–1326. doi: 10.1097/00029330-200608020-00001 [DOI] [PubMed] [Google Scholar]
- 27.Tseng SCG, Espana EM, Kawakita T, et al. How Does Amniotic Membrane Work? Ocul Surf. 2004;2(3):177–187. doi: 10.1016/S1542-0124(12)70059-9 [DOI] [PubMed] [Google Scholar]
- 28.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type iv collagen and laminin among the amniotic membrane, cornea, and Conjunctiva. Cornea. 1999;18(1):73–79. doi: 10.1097/00003226-199901000-00013 [DOI] [PubMed] [Google Scholar]
- 29.Hopkinson A, Shanmuganathan VA, Gray T, et al. Optimization of Amniotic Membrane (AM) Denuding for Tissue Engineering. Tissue Eng Part C Methods. 2008;14(4):371–381. doi: 10.1089/ten.tec.2008.0315 [DOI] [PubMed] [Google Scholar]
- 30.Bourne GL. The microscopic anatomy of the human amnion and chorion. Am J Obstet Gynecol. 1960;79(6):1070–1073. doi: 10.1016/0002-9378(60)90512-3 [DOI] [PubMed] [Google Scholar]
- 31.Danforth D, Hull RW. The microscopic anatomy of the fetal membranes with particular reference to the detailed structure of the amnion. Am J Obstet Gynecol. 1958;75(3):536–547. doi: 10.1016/0002-9378(58)90610-0 [DOI] [PubMed] [Google Scholar]
- 32.Trelford JD, Trelford-Sauder M. The amnion in surgery, past and present. Am J Obstet Gynecol. 1979;134(7):833–845. doi: 10.1016/0002-9378(79)90957-8 [DOI] [PubMed] [Google Scholar]
- 33.Matthews RN, Faulk WP, Bennett JP. A review of the role of amniotic membranes in surgical practice. Obstet Gynecol Annu. 1982;11:31–58. [PubMed] [Google Scholar]
- 34.van Herendael BJ, Oberti C, Brosens I. Microanatomy of the human amniotic membranes. A light microscopic, transmission, and scanning electron microscopic study. Am J Obstet Gynecol. 1978;131(8):872–880. doi: 10.1016/S0002-9378(16)33135-0 [DOI] [PubMed] [Google Scholar]
- 35.Tabatabaei SA, Soleimani M, Behrouz MJ, Torkashvand A, Anvari P, Yaseri M. A randomized clinical trial to evaluate the usefulness of amniotic membrane transplantation in bacterial keratitis healing. Ocul Surf. 2017;15(2):218–226. doi: 10.1016/j.jtos.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 36.Tandon R, Gupta N, Kalaivani M, Sharma N, Titiyal JS, Vajpayee RB. Amniotic membrane transplantation as an adjunct to medical therapy in acute ocular burns. Br J Ophthalmol. 2011;95(2):199–204. doi: 10.1136/bjo.2009.173716 [DOI] [PubMed] [Google Scholar]
- 37.He H, Kuriyan AE, Su CW, et al. Inhibition of proliferation and epithelial mesenchymal transition in retinal pigment epithelial cells by heavy chain-hyaluronan/pentraxin 3. Sci Rep. 2017;7(1):43736. doi: 10.1038/srep43736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa Y, He H, Mukai S, et al. Heavy chain-hyaluronan/pentraxin 3 from amniotic membrane suppresses inflammation and scarring in murine lacrimal gland and conjunctiva of chronic graft-versus-host disease. Sci Rep. 2017;7(1):42195. doi: 10.1038/srep42195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shay E, He H, Sakurai S, Tseng SC. Inhibition of angiogenesis by HC.HA, a complex of hyaluronan and the heavy chain of inter-alpha-inhibitor, purified from human amniotic membrane. Invest Ophthalmol Vis Sci. 2011;52(5):2669–2678. doi: 10.1167/iovs.10-5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Chen D, Sullivan DA, Xie H, Li Y, Liu Y. Expression of Lubricin in the Human Amniotic Membrane. Cornea. 2020;39(1):118–121. doi: 10.1097/ICO.0000000000002151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kjaergaard N, Hein M, Hyttel L, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94(2):224–229. doi: 10.1016/S0301-2115(00)00345-6 [DOI] [PubMed] [Google Scholar]
- 42.Kjaergaard N, Helmig RB, Schonheyder HC, Uldbjerg N, Hansen ES, Madsen H. Chorioamniotic membranes constitute a competent barrier to group b streptococcus in vitro. Eur J Obstet Gynecol Reprod Biol. 1999;83(2):165–169. doi: 10.1016/S0301-2115(99)00009-3 [DOI] [PubMed] [Google Scholar]
- 43.Gabric N, Mravicic I, Dekaris I, Karaman Z, Mitrovic S. Human amniotic membrane in the reconstruction of the ocular surface. Doc Ophthalmol. 1999;98(3):273–283. doi: 10.1023/A:1002423621010 [DOI] [PubMed] [Google Scholar]
- 44.Sangwan VS, Basu S. Antimicrobial properties of amniotic membrane. Br J Ophthalmol. 2011;95(1):1. doi: 10.1136/bjo.2010.184259 [DOI] [PubMed] [Google Scholar]
- 45.Rinastiti M, Santoso ALS, Sosroseno W. Histological evaluation of rabbit gingival wound healing transplanted with human amniotic membrane. Int J Oral Maxillofac Surg. 2006;35(3):247–251. doi: 10.1016/j.ijom.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 46.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/eCM.v015a07 [DOI] [PubMed] [Google Scholar]
- 47.Meller D, Pires RT, Tseng SC. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86(4):463–471. doi: 10.1136/bjo.86.4.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40(5):878–886. [PubMed] [Google Scholar]
- 49.Keene DR, Sakai LY, Lunstrum GP, Morris NP, Burgeson RE. Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol. 1987;104(3):611–621. doi: 10.1083/jcb.104.3.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115(5):1383–1395. doi: 10.1083/jcb.115.5.1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurpakus MA, Stock EL, Jones JC. The role of the basement membrane in differential expression of keratin proteins in epithelial cells. Dev Biol. 1992;150(2):243–255. doi: 10.1016/0012-1606(92)90239-D [DOI] [PubMed] [Google Scholar]
- 52.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267(5199):891–893. doi: 10.1126/science.7531366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci U S A. 1996;93(8):3509–3513. doi: 10.1073/pnas.93.8.3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooke M, Tan EK, Mandrycky C, He H, O’Connell J, Tseng SC. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care. 2014;23(10):465–74, 476. doi: 10.12968/jowc.2014.23.10.465 [DOI] [PubMed] [Google Scholar]
- 55.Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111–121. doi: 10.5500/wjt.v4.i2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen CL, Clare G, Stewart EA, et al. Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PLoS One. 2013;8(10):e78441. doi: 10.1371/journal.pone.0078441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Ares MT, Lopez-Valladares MJ, Tourino R, et al. Effects of lyophilization on human amniotic membrane. Acta Ophthalmol. 2009;87(4):396–403. doi: 10.1111/j.1755-3768.2008.01261.x [DOI] [PubMed] [Google Scholar]
- 58.Nakamura T, Yoshitani M, Rigby H, et al. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45(1):93–99. doi: 10.1167/iovs.03-0752 [DOI] [PubMed] [Google Scholar]
- 59.Wolkers WF, Tablin F, Crowe JH. From anhydrobiosis to freeze-drying of eukaryotic cells. Comp Biochem Physiol a Mol Integr Physiol. 2002;131(3):535–543. doi: 10.1016/S1095-6433(01)00505-0 [DOI] [PubMed] [Google Scholar]
- 60.Rock T, Bartz-Schmidt KU, Landenberger J, Bramkamp M, Rock D. Amniotic membrane transplantation in reconstructive and regenerative ophthalmology. Ann Transplant. 2018;23:160–165. doi: 10.12659/AOT.906856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting—a review. Cell Tissue Bank. 2017;18(2):193–204. [DOI] [PubMed] [Google Scholar]
- 62.Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting‚Äîa review. Cell Tissue Bank. 2017;18(2):193–204. doi: 10.1007/s10561-017-9618-5 [DOI] [PubMed] [Google Scholar]
- 63.Resch MD, Schlotzer-Schrehardt U, Hofmann-Rummelt C, et al. Adhesion structures of amniotic membranes integrated into human corneas. Invest Ophthalmol Vis Sci. 2006;47(5):1853–1861. doi: 10.1167/iovs.05-0983 [DOI] [PubMed] [Google Scholar]
- 64.Schuerch K, Baeriswyl A, Frueh BE, Tappeiner C. Efficacy of Amniotic Membrane Transplantation for the Treatment of Corneal Ulcers. Cornea. 2019;39:479. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, Li L, Li X. Effectiveness of Cryopreserved Amniotic Membrane Transplantation in Corneal Ulceration: a Meta-Analysis. Cornea. 2019;38(4):454–462. doi: 10.1097/ICO.0000000000001866 [DOI] [PubMed] [Google Scholar]
- 66.Gicquel JJ, Bejjani RA, Ellies P, Mercie M, Dighiero P. Amniotic membrane transplantation in severe bacterial keratitis. Cornea. 2007;26(1):27–33. doi: 10.1097/ICO.0b013e31802b28df [DOI] [PubMed] [Google Scholar]
- 67.Abdulhalim BE, Wagih MM, Gad AA, Boghdadi G, Nagy RR. Amniotic membrane graft to conjunctival flap in treatment of non-viral resistant infectious keratitis: a randomised clinical study. Br J Ophthalmol. 2015;99(1):59–63. doi: 10.1136/bjophthalmol-2014-305224 [DOI] [PubMed] [Google Scholar]
- 68.Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123(3):303–312. [DOI] [PubMed] [Google Scholar]
- 69.Fuchsluger T, Tuerkeli E, Westekemper H, Esser J, Steuhl KP, Meller D. Rate of epithelialisation and re-operations in corneal ulcers treated with amniotic membrane transplantation combined with botulinum toxin-induced ptosis. Graefes Arch Clin Exp Ophthalmol. 2007;245(7):955–964. doi: 10.1007/s00417-006-0493-1 [DOI] [PubMed] [Google Scholar]
- 70.Khokhar S, Natung T, Sony P, Sharma N, Agarwal N, Vajpayee RB. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005;24(6):654–660. doi: 10.1097/01.ico.0000153102.19776.80 [DOI] [PubMed] [Google Scholar]
- 71.Altiparmak UE, Oflu Y, Yildiz EH, et al. Prospective comparison of two suturing techniques of amniotic membrane transplantation for symptomatic bullous keratopathy. Am J Ophthalmol. 2009;147(3):442–446.e1. doi: 10.1016/j.ajo.2008.08.036 [DOI] [PubMed] [Google Scholar]
- 72.Paris Fdos S, Goncalves ED, Campos MS, Sato EH, Dua HS, Gomes JA. Amniotic membrane transplantation versus anterior stromal puncture in bullous keratopathy: a comparative study. Br J Ophthalmol. 2013;97(8):980–984. doi: 10.1136/bjophthalmol-2013-303081 [DOI] [PubMed] [Google Scholar]
- 73.Anderson DF, Prabhasawat P, Alfonso E, Tseng SC. Amniotic membrane transplantation after the primary surgical management of band keratopathy. Cornea. 2001;20(4):354–361. doi: 10.1097/00003226-200105000-00004 [DOI] [PubMed] [Google Scholar]
- 74.Seitz B, Das S, Sauer R, Mena D, Hofmann-Rummelt C. Amniotic membrane transplantation for persistent corneal epithelial defects in eyes after penetrating keratoplasty. Eye (Lond). 2009;23(4):840–848. doi: 10.1038/eye.2008.140 [DOI] [PubMed] [Google Scholar]
- 75.Maharajan VS, Shanmuganathan V, Currie A, Hopkinson A, Powell-Richards A, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction: indications and outcomes. Clin Exp Ophthalmol. 2007;35(2):140–147. doi: 10.1111/j.1442-9071.2006.01408.x [DOI] [PubMed] [Google Scholar]
- 76.Dua HS. Sequential Sectoral Conjunctival Epitheliectomy (SSCE) In: Holland EJ, Mannis MJ, editors. Ocular Surface Disease Medical and Surgical Management.:New York: Springer New York; 2002:168–174. [Google Scholar]
- 77.Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(7):931. doi: 10.1136/bjophthalmol-2011-301164 [DOI] [PubMed] [Google Scholar]
- 78.Shanbhag S, Patel C, Goyal R, Donthineni P, Singh V, Basu S. Simple limbal epithelial transplantation (SLET): review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J Ophthalmol. 2019;67(8):1265–1277. doi: 10.4103/ijo.IJO_117_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borroni D, Wowra B, Romano V, et al. Simple limbal epithelial transplantation: a review on current approach and future directions. Surv Ophthalmol. 2018;63(6):869–874. doi: 10.1016/j.survophthal.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 80.Vazirani J, Ali MH, Sharma N, et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: multicentre results. Br J Ophthalmol. 2016;100(10):1416–1420. doi: 10.1136/bjophthalmol-2015-307348 [DOI] [PubMed] [Google Scholar]
- 81.Dua HS, Miri A, Elalfy MS, Lencova A, Said DG. Amnion-assisted conjunctival epithelial redirection in limbal stem cell grafting. Br J Ophthalmol. 2017;101(7):913–919. doi: 10.1136/bjophthalmol-2015-307935 [DOI] [PubMed] [Google Scholar]
- 82.Tsai RJ, Li L, Chen J. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells(1). Am J Ophthalmol. 2000;130(4):543. doi: 10.1016/S0002-9394(00)00746-7 [DOI] [PubMed] [Google Scholar]
- 83.Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88(10):1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kethiri AR, Basu S, Shukla S, Sangwan VS, Singh V. Optimizing the role of limbal explant size and source in determining the outcomes of limbal transplantation: an in vitro study. PLoS One. 2017;12(9):e0185623. doi: 10.1371/journal.pone.0185623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–155. [DOI] [PubMed] [Google Scholar]
- 86.Shortt AJ, Secker GA, Lomas RJ, et al. The effect of amniotic membrane preparation method on its ability to serve as a substrate for the ex-vivo expansion of limbal epithelial cells. Biomaterials. 2009;30(6):1056–1065. [DOI] [PubMed] [Google Scholar]
- 87.Li W, Hayashida Y, He H, Kuo CL, Tseng SC. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48(2):605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Le Q, Deng SX. The application of human amniotic membrane in the surgical management of limbal stem cell deficiency. Ocul Surf. 2019;17(2):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch Ophthalmol. 2002;120(6):783–790. [DOI] [PubMed] [Google Scholar]
- 90.Harkin DG, Barnard Z, Gillies P, Ainscough SL, Apel AJ. Analysis of p63 and cytokeratin expression in a cultivated limbal autograft used in the treatment of limbal stem cell deficiency. Br J Ophthalmol. 2004;88(9):1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pathak M, Olstad OK, Drolsum L, et al. The effect of culture medium and carrier on explant culture of human limbal epithelium: a comparison of ultrastructure, keratin profile and gene expression. Exp Eye Res. 2016;153:122–132. [DOI] [PubMed] [Google Scholar]
- 92.Sun CC, Su Pang JH, Cheng CY, et al. Interleukin-1 receptor antagonist (IL-1RA) prevents apoptosis in ex vivo expansion of human limbal epithelial cells cultivated on human amniotic membrane. Stem Cells. 2006;24(9):2130–2139. [DOI] [PubMed] [Google Scholar]
- 93.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease(1). Am J Ophthalmol. 2000;130(4):543–544. [DOI] [PubMed] [Google Scholar]
- 94.Sangwan VS, Matalia HP, Vemuganti GK, et al. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006;54(1):29–34. [DOI] [PubMed] [Google Scholar]
- 95.Parihar JKS, Parihar AS, Jain VK, Kaushik J, Nath P. Allogenic cultivated limbal stem cell transplantation versus cadaveric keratolimbal allograft in ocular surface disorder: 1-year outcome. Int Ophthalmol. 2017;37(6):1323–1331. [DOI] [PubMed] [Google Scholar]
- 96.Shimazaki J, Higa K, Morito F, et al. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143(6):945–953. [DOI] [PubMed] [Google Scholar]
- 97.Zakaria N, Possemiers T, Dhubhghaill SN, et al. Results of a Phase I/II clinical trial: standardized, non-xenogenic, cultivated limbal stem cell transplantation. J Transl Med. 2014;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95(11):1525–1529. [DOI] [PubMed] [Google Scholar]
- 99.Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2012;153(4):643–650. [DOI] [PubMed] [Google Scholar]
- 100.Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(12):1504–1509. doi: 10.1136/bjophthalmol-2012-301869 [DOI] [PubMed] [Google Scholar]
- 101.Pauklin M, Fuchsluger TA, Westekemper H, Steuhl KP, Meller D. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57–70. [DOI] [PubMed] [Google Scholar]
- 102.Dobrowolski D, Orzechowska-Wylegala B, Wowra B, et al. Cultivated oral mucosa epithelium in ocular surface reconstruction in aniridia patients. Biomed Res Int. 2015;2015:281870. doi: 10.1155/2015/281870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakamura T, Takeda K, Inatomi T, Sotozono C, Kinoshita S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95(7):942–946. doi: 10.1136/bjo.2010.188714 [DOI] [PubMed] [Google Scholar]
- 104.Prabhasawat P, Ekpo P, Uiprasertkul M, et al. Long-term result of autologous cultivated oral mucosal epithelial transplantation for severe ocular surface disease. Cell Tissue Bank. 2016;17(3):491–503. doi: 10.1007/s10561-016-9575-4 [DOI] [PubMed] [Google Scholar]
- 105.Satake Y, Higa K, Tsubota K, Shimazaki J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118(8):1524–1530. doi: 10.1016/j.ophtha.2011.01.039 [DOI] [PubMed] [Google Scholar]
- 106.Kim YJ, Lee HJ, Ryu JS, et al. Prospective clinical trial of corneal reconstruction with biomaterial-free cultured oral mucosal epithelial cell sheets. Cornea. 2018;37(1):76–83. doi: 10.1097/ICO.0000000000001409 [DOI] [PubMed] [Google Scholar]
- 107.Hirayama M, Satake Y, Higa K, Yamaguchi T, Shimazaki J. Transplantation of cultivated oral mucosal epithelium prepared in fibrin-coated culture dishes. Invest Ophthalmol Vis Sci. 2012;53(3):1602–1609. doi: 10.1167/iovs.11-7847 [DOI] [PubMed] [Google Scholar]
- 108.Inatomi T, Nakamura T, Kojyo M, Koizumi N, Sotozono C, Kinoshita S. Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. Am J Ophthalmol. 2006;142(5):757–764. doi: 10.1016/j.ajo.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 109.Madhira SL, Vemuganti G, Bhaduri A, Gaddipati S, Sangwan VS, Ghanekar Y. Culture and characterization of oral mucosal epithelial cells on human amniotic membrane for ocular surface reconstruction. Mol Vis. 2008;14:189–196. [PMC free article] [PubMed] [Google Scholar]
- 110.Bochmann F, Azuara-Blanco A. Interventions for late trabeculectomy bleb leak. Cochrane Database Syst Rev. 2012;9:Cd006769. [DOI] [PubMed] [Google Scholar]
- 111.Budenz DL, Barton K, Tseng SC. Amniotic membrane transplantation for repair of leaking glaucoma filtering blebs. Am J Ophthalmol. 2000;130(5):580–588. doi: 10.1016/S0002-9394(00)00600-0 [DOI] [PubMed] [Google Scholar]
- 112.Kiuchi Y, Yanagi M, Nakamura T. Efficacy of amniotic membrane-assisted bleb revision for elevated intraocular pressure after filtering surgery. Clin Ophthalmol. 2010;4:839–843. doi: 10.2147/OPTH.S12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sethi P, Patel RN, Goldhardt R, Ayyala RS. Conjunctival advancement with subconjunctival amniotic membrane draping technique for leaking cystic blebs. J Glaucoma. 2016;25(2):188–192. doi: 10.1097/IJG.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 114.Kitagawa K, Yanagisawa S, Watanabe K, et al. A hyperdry amniotic membrane patch using a tissue adhesive for corneal perforations and bleb leaks. Am J Ophthalmol. 2009;148(3):383–389. doi: 10.1016/j.ajo.2009.03.030 [DOI] [PubMed] [Google Scholar]
- 115.Nagai-Kusuhara A, Nakamura M, Fujioka M, Negi A. Long-term results of amniotic membrane transplantation-assisted bleb revision for leaking blebs. Graefes Arch Clin Exp Ophthalmol. 2008;246(4):567–571. doi: 10.1007/s00417-007-0727-x [DOI] [PubMed] [Google Scholar]
- 116.Wang X, Khan R, Coleman A. Device-modified trabeculectomy for glaucoma. Cochrane Database Syst Rev. 2015;12:Cd010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sheha H, Kheirkhah A, Taha H. Amniotic membrane transplantation in trabeculectomy with mitomycin C for refractory glaucoma. J Glaucoma. 2008;17(4):303–307. doi: 10.1097/IJG.0b013e31815c3a47 [DOI] [PubMed] [Google Scholar]
- 118.Anand A, Sheha H, Teng CC, Liebmann JM, Ritch R, Tello C. Use of amniotic membrane graft in glaucoma shunt surgery. Ophthalmic Surg Lasers Imaging. 2011;42(3):184–189. doi: 10.3928/15428877-20110426-01 [DOI] [PubMed] [Google Scholar]
- 119.Yazdani S, Mahboobipour H, Pakravan M, Doozandeh A, Ghahari E. Adjunctive mitomycin c or amniotic membrane transplantation for ahmed glaucoma valve implantation: a randomized clinical trial. J Glaucoma. 2016;25(5):415–421. doi: 10.1097/IJG.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 120.Eliezer RN, Kasahara N, Caixeta-Umbelino C, Pinheiro RK, Mandia C Jr, Malta RF. Use of amniotic membrane in trabeculectomy for the treatment of glaucoma: a pilot study. Arq Bras Oftalmol. 2006;69(3):309–312. doi: 10.1590/S0004-27492006000300005 [DOI] [PubMed] [Google Scholar]
- 121.Fea A, Cannizzo PM, Consolandi G, Lavia CA, Pignata G, Grignolo FM. Managing drawbacks in unconventional successful glaucoma surgery: a case report of stent exposure. Case Rep Ophthalmol Med. 2015;2015:847439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arnould L, Theillac V, Moran S, Gatinel D, Grise-Dulac A. Recurrent Exposure of XEN Gel Stent Implant and Conjunctival Erosion. J Glaucoma. 2019;28(3):e37–e40. doi: 10.1097/IJG.0000000000001146 [DOI] [PubMed] [Google Scholar]
- 123.Qin H. Excision combined with concavity-convex amniotic membrane transplantation in the treatment of intraepithelial epithelioma. Int J Ophthalmol. 2017;17:404–408. [Google Scholar]
- 124.Agraval U, Rundle P, Rennie IG, Salvi S. Fresh frozen amniotic membrane for conjunctival reconstruction after excision of neoplastic and presumed neoplastic conjunctival lesions. Eye (Lond). 2017;31(6):884–889. doi: 10.1038/eye.2016.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paridaens D, Beekhuis H, van Den Bosch W, Remeyer L, Melles G. Amniotic membrane transplantation in the management of conjunctival malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol. 2001;85(6):658–661. doi: 10.1136/bjo.85.6.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Niknejad H, Yazdanpanah G, Ahmadiani A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell Tissue Res. 2016;363(3):599–608. doi: 10.1007/s00441-016-2364-3 [DOI] [PubMed] [Google Scholar]
- 127.Niknejad H, Khayat-Khoei M, Peirovi H, Abolghasemi H. Human amniotic epithelial cells induce apoptosis of cancer cells: a new anti-tumor therapeutic strategy. Cytotherapy. 2014;16(1):33–40. doi: 10.1016/j.jcyt.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 128.Niknejad H, Yazdanpanah G, Mirmasoumi M, Abolghasemi H, Peirovi H, Ahmadiani A. Inhibition of HSP90 could be possible mechanism for anti-cancer property of amniotic membrane. Med Hypotheses. 2013;81(5):862–865. doi: 10.1016/j.mehy.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 129.Kamiya K, Wang M, Uchida S, et al. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp Eye Res. 2005;80(5):671–679. doi: 10.1016/j.exer.2004.11.018 [DOI] [PubMed] [Google Scholar]
- 130.Oliphant H, Rajak SN. Dried amniotic membrane in fornix reconstruction. Clin Exp Ophthalmol. 2019;47(8):1090–1091. doi: 10.1111/ceo.13566 [DOI] [PubMed] [Google Scholar]
- 131.Kheirkhah A, Ghaffari R, Kaghazkanani R, Hashemi H, Behrouz MJ, Raju VK. A combined approach of amniotic membrane and oral mucosa transplantation for fornix reconstruction in severe symblepharon. Cornea. 2013;32(2):155–160. doi: 10.1097/ICO.0b013e318247983d [DOI] [PubMed] [Google Scholar]
- 132.Thatte S, Jain J. Fornix reconstruction with amniotic membrane transplantation: a cosmetic remedy for blind patients. J Ophthalmic Vis Res. 2016;11(2):193–197. doi: 10.4103/2008-322X.183916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumar S, Sugandhi P, Arora R, Pandey PK. Amniotic membrane transplantation versus mucous membrane grafting in anophthalmic contracted socket. Orbit. 2006;25(3):195–203. doi: 10.1080/01676830600575527 [DOI] [PubMed] [Google Scholar]
- 134.Bajaj MS, Pushker N, Singh KK, Chandra M, Ghose S. Evaluation of amniotic membrane grafting in the reconstruction of contracted socket. Ophthalmic Plast Reconstr Surg. 2006;22(2):116–120. doi: 10.1097/01.iop.0000200887.26015.d4 [DOI] [PubMed] [Google Scholar]
- 135.Tseng SC, Di Pascuale MA, Liu DT, Gao YY, Baradaran-Rafii A. Intraoperative mitomycin C and amniotic membrane transplantation for fornix reconstruction in severe cicatricial ocular surface diseases. Ophthalmology. 2005;112(5):896–903. doi: 10.1016/j.ophtha.2004.11.041 [DOI] [PubMed] [Google Scholar]
- 136.Ti SE, Tow SL, Chee SP. Amniotic membrane transplantation in entropion surgery. Ophthalmology. 2001;108(7):1209–1217. doi: 10.1016/S0161-6420(01)00599-1 [DOI] [PubMed] [Google Scholar]
- 137.Barabino S, Rolando M, Bentivoglio G, et al. Role of amniotic membrane transplantation for conjunctival reconstruction in ocular-cicatricial pemphigoid. Ophthalmology. 2003;110(3):474–480. doi: 10.1016/S0161-6420(02)01892-4 [DOI] [PubMed] [Google Scholar]
- 138.Honavar SG, Bansal AK, Sangwan VS, Rao GN. Amniotic membrane transplantation for ocular surface reconstruction in Stevens-Johnson syndrome. Ophthalmology. 2000;107(5):975–979. doi: 10.1016/S0161-6420(00)00026-9 [DOI] [PubMed] [Google Scholar]
- 139.Ding J, Hou Z, Li Y, Lu N, Li D. Eyelid and fornix reconstruction in abortive cryptophthalmos: a single-center experience over 12 years. Eye (Lond). 2017;31(11):1576–1581. doi: 10.1038/eye.2017.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clearfield E, Muthappan V, Wang X, Kuo IC, Johnston V. Conjunctival autograft for pterygium. Cochrane Database Syst Rev. 2016;2:Cd011349. doi: 10.1002/14651858.CD004158.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Prajna NV, Devi L, Seeniraj SK, Keenan JD. Conjunctival autograft versus amniotic membrane transplantation after double pterygium excision: a randomized trial. Cornea. 2016;35(6):823–826. doi: 10.1097/ICO.0000000000000812 [DOI] [PubMed] [Google Scholar]
- 142.Akbari M, Soltani-Moghadam R, Elmi R, Kazemnejad E. Comparison of free conjunctival autograft versus amniotic membrane transplantation for pterygium surgery. J Curr Ophthalmol. 2017;29(4):282–286. doi: 10.1016/j.joco.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kassem RR, Kamal AM, El-Mofty RMA, Elhilali HM. A controlled study of the role of cryopreserved amniotic membrane transplant during strabismus reoperations. J AAPOS. 2017;21(2):97–102 e1. doi: 10.1016/j.jaapos.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 144.Kassem RR, Kamal AM, El-Mofty RMA, Elhilali HM. Long-term follow-up of cryopreserved amniotic membrane transplant during strabismus reoperations: up 85 months’ follow-up. Eur J Ophthalmol. 2018;28(4):365–371. doi: 10.1177/1120672118757432 [DOI] [PubMed] [Google Scholar]
- 145.Kassem RR, El-Mofty RMA. Amniotic Membrane Transplantation in Strabismus Surgery. Curr Eye Res. 2019;44(5):451–464. doi: 10.1080/02713683.2018.1562555 [DOI] [PubMed] [Google Scholar]
- 146.Yamada M, Shinoda K, Hatakeyama A, Nishina S, Mashima Y. Fat adherence syndrome after retinal surgery treated with amniotic membrane transplantation. Am J Ophthalmol. 2001;132(2):280–282. doi: 10.1016/S0002-9394(01)00930-8 [DOI] [PubMed] [Google Scholar]
- 147.Kersey JP, Vivian AJ. Mitomycin and amniotic membrane: a new method of reducing adhesions and fibrosis in strabismus surgery. Strabismus. 2008;16(3):116–118. doi: 10.1080/09273970802405493 [DOI] [PubMed] [Google Scholar]
- 148.Tugcu B, Helvacioglu F, Yuzbasioglu E, Gurez C, Yigit U. Amniotic membrane in the management of strabismus reoperations. Jpn J Ophthalmol. 2013;57(2):239–244. doi: 10.1007/s10384-012-0217-5 [DOI] [PubMed] [Google Scholar]
- 149.Kassem RR, Abdel-Hamid MA, Khodeir MM. Effect of lyophilized amniotic membrane on the development of adhesions and fibrosis after extraocular muscle surgery in rabbits. Curr Eye Res. 2011;36(11):1020–1027. doi: 10.3109/02713683.2011.601842 [DOI] [PubMed] [Google Scholar]
- 150.Chun BY, Kim HK, Shin JP. Dried human amniotic membrane does not alleviate inflammation and fibrosis in experimental strabismus surgery. J Ophthalmol. 2013;2013:369126. doi: 10.1155/2013/369126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mehendale RA, Dagi LR. Amniotic membrane implantation to reduce extraocular muscle adhesions to a titanium implant. J AAPOS. 2011;15(4):404–406. doi: 10.1016/j.jaapos.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 152.Kassem RR, El-Mofty RMA, Khodeir MM, Hamza WM. A comparative study of different amniotic membrane orientations during extraocular muscle surgery in rabbits. Curr Eye Res. 2018;43(3):325–332. doi: 10.1080/02713683.2017.1401645 [DOI] [PubMed] [Google Scholar]
- 153.Meller D, Pires RTF, Mack RJS, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107(5):980–989. doi: 10.1016/S0161-6420(00)00024-5 [DOI] [PubMed] [Google Scholar]
- 154.Sharma N, Thenarasun SA, Kaur M, et al. Adjuvant Role of amniotic membrane transplantation in acute ocular stevens-johnson syndrome: a randomized control trial. Ophthalmology. 2016;123(3):484–491. doi: 10.1016/j.ophtha.2015.10.027 [DOI] [PubMed] [Google Scholar]
- 155.Roper-Hall MJ. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631–653. [PubMed] [Google Scholar]
- 156.Clare G, Suleman H, Bunce C, Dua H. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst Rev. 2012;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eslani M, Baradaran-Rafii A, Cheung AY, et al. Amniotic membrane transplantation in acute severe ocular chemical injury: a randomized clinical trial. Am J Ophthalmol. 2019;199:209–215. doi: 10.1016/j.ajo.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 158.Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15(4):802–812. doi: 10.1016/j.jtos.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 159.Cheng AM, Zhao D, Chen R, et al. Accelerated restoration of ocular surface health in dry eye disease by self-retained cryopreserved amniotic membrane. Ocul Surf. 2016;14(1):56–63. doi: 10.1016/j.jtos.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suri K, Kosker M, Raber IM, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: short-term results. Eye Contact Lens. 2013;39(5):341–347. doi: 10.1097/ICL.0b013e3182a2f8fa [DOI] [PubMed] [Google Scholar]
- 161.Kolomeyer AM, Do BK, Tu Y, Chu DS. Placement of ProKera in the management of ocular manifestations of acute Stevens-Johnson syndrome in an outpatient. Eye Contact Lens. 2013;39(3):e7–e11. doi: 10.1097/ICL.0b013e318255124f [DOI] [PubMed] [Google Scholar]
- 162.McDonald MB, Sheha H, Tighe S, et al. Treatment outcomes in the DRy Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol. 2018;12:677–681. doi: 10.2147/OPTH.S162203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.John T, Tighe S, Sheha H, et al. Corneal Nerve Regeneration after Self-Retained Cryopreserved Amniotic Membrane in Dry Eye Disease. J Ophthalmol. 2017;2017:6404918. doi: 10.1155/2017/6404918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wildes MT, Cherof AM, Cerda AM, Lynch AM, Wagner BD, Gregory DG. Outcomes of amniotic membrane transplantation for treatment of Stevens-Johnson syndrome in the pediatric population: a retrospective review. J Am Assoc Pediatr Ophthalmol Strabismus. 2016;20(4):e24–e25. doi: 10.1016/j.jaapos.2016.07.093 [DOI] [Google Scholar]
- 165.Ma KN, Thanos A, Chodosh J, Shah AS, Mantagos IS. A Novel technique for amniotic membrane transplantation in patients with acute stevens-johnson syndrome. Ocul Surf. 2016;14(1):31–36. doi: 10.1016/j.jtos.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 166.Shanbhag SS, Chodosh J, Saeed HN. Sutureless amniotic membrane transplantation with cyanoacrylate glue for acute Stevens-Johnson syndrome/toxic epidermal necrolysis. Ocul Surf. 2019;17(3):560–564. doi: 10.1016/j.jtos.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang Y, Tseng SH. SCG, Self-retained amniotic membrane for recurrent corneal erosion. J Clin Exp Ophthalmol. 2013;4:272. [Google Scholar]
- 168.Miller DD, Hasan SA, Simmons NL, Stewart MW. Recurrent corneal erosion: a comprehensive review. Clin Ophthalmol. 2019;13:325–335. doi: 10.2147/OPTH.S157430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Caporossi T, Pacini B, De Angelis L, Barca F, Peiretti E, Rizzo S. Human amniotic membrane to close recurrent, high myopic macular holes in pathologic myopia with axial length OF >/=30 mm. Retina. 2019;Publish Ahead of Print. doi: 10.1097/IAE.0000000000002699 [DOI] [PubMed] [Google Scholar]
- 170.Ohno-Matsui K, Ichinose S, Nakahama K, et al. The effects of amniotic membrane on retinal pigment epithelial cell differentiation. Mol Vis. 2005;11:1–10. [PubMed] [Google Scholar]
- 171.Caporossi T, Tartaro R, De Angelis L, Pacini B, Rizzo S. A human amniotic membrane plug to repair retinal detachment associated with large macular tear. Acta Ophthalmol. 2019;97(8):821–823. doi: 10.1111/aos.14109 [DOI] [PubMed] [Google Scholar]
- 172.Gabler B, Lohmann CP. Hypopyon after repeated transplantation of human amniotic membrane onto the corneal surface. Ophthalmology. 2000;107(7):1344–1346. doi: 10.1016/S0161-6420(00)00167-6 [DOI] [PubMed] [Google Scholar]