Abstract
Introduction
The mastic tree (Pistacia lentiscus), belonging to the Anacardiaceae family, has anti-inflammatory and antioxidant properties. This study aims to assay the anti-inflammatory effects of mastic in rats with colitis.
Methods
Forty-eight male Sprague-Dawley rats were randomly divided into six groups of control, colitis without treatment, colitis with mastic (400 mg/kg/daily) administered orally or intra-rectally, colitis with prednisolone (5 mg/kg of body weight), and colitis with sesame oil for seven successive days. Treatment effects were evaluated by determining cytokines (TNFα, IL6) and myeloperoxidase (MPO) activity, macroscopic scores, and histopathological parameters. The results of each group are compared with colitis without treatment group.
Results
After administering sesame oil, the MPO level was reduced significantly compared to colitis without the treatment group (P=0.025). The mastic oil (400 mg/kg orally) administration was effective in reducing colitis severity through the reduction in the total colitis index (p=0.046) after 7 days. The Intra-rectal administration of mastic decreased TNF-α significantly, similar to prednisolone and control groups compared to the colitis without treatment group (p=0.024). The IL-6 did not change in the mastic and sesame oil groups.
Conclusion
According to our results, mastic and sesame oil have anti-inflammatory properties, suggesting that they could be used as natural sources to lessen the ulcerative colitis inflammation.
Keywords: mastic, colitis, inflammation, rats, sesame
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disorder, which involves the colon, and in 95% of all cases, the rectum as well.1,2 The two main treatment methods are pharmacotherapy and surgery (for those with severe inflammation). Corticosteroid and aminosalicylates are the routine treatments for mild to moderate forms of the disease.1,2 This disorder does not have a complete cure yet and traditional medicine with anti-inflammatory effects might be the sole treatment to have favorable effects in controlling the disease. Furthermore, long-term consumption of anti-inflammatory medicines is likely to give rise to side effects; thus, alternative herbal or natural medicines are desirable. Growing abundantly in the Mediterranean region, Pistacia lentiscus (var. chia) tree of the Anacardiaceae family produces mastic which is rich in triterpenoids.3 The word “mastic” is originally Greek, meaning to chew or gnash. Mastic trees could grow up to 4 meters in height and produces red or yellow resin gum. The therapeutic properties of mastic are as follows: memory enhancement, removing cough suppression, and anticancer effects.4,5 More than 120 chemical compounds like natural polymer, acidic and neutral triterpenes have been identified in the mastic. Mastic has anti-oxidant and anti-inflammatory effects due to its Oleanolic acidic properties and, in the form of powder mixed with inulin, experimental colitis mastic was successfully used to reduce levels of inflammatory markers.3 In Gioxari et al study on rats with Crohn’s disease, mastic powder was used and the results showed a reduction in the interleukin and inflammatory cytokines within 3 days.5 Naouar et al found preventive effects of mastic on bowel inflammation in rats with colitis.6 Another study revealed therapeutic uses of mastic regarding experimental colitis, reporting histological amelioration of colitis.3
This natural resin from Pistacia lentiscus tree has been traditionally used as a dietary flavoring agent in traditional Persian medicine as well as traditional Greek medicine. It is collected by making incisions in the branches and trunks of the mastic trees and used for cleaning teeth and eliminating bad breath. It contains triterpenes of the oleanane, euphane and lupine type, alpha-tocopherol and polyphenols. The main triterpenes in samples collected traditionally were isomasticadienonic acid, masticadienonic acid and 28-norolean-17-en-3-one.7 In a study, the Pistacia lentiscus oil inhibited tumor growth, through a mechanism of increased apoptosis, reduced neovascularization, and inhibition of chemokine expression.8 Mastic oil has anti-bacterial, anti-fungal, and anti-inflammatory effects.6
The present study aims to evaluate the effects of mastic and sesame oil (as a solvent of mastic powder) on experimental colitis in rats. Mastic oil was applied either orally or via intra-colonic injection. Acetic acid induced colitis in rats as a model of Crohn’s disorder or ulcerative colitis.
Materials and Methods
The ethics committee of Tabriz University of Medical Sciences in Iran approved the protocol of study (IR.TBZ.rec.1398.328).
Chemicals
Prednisolone (Darupakhsh Company, Iran), dissolved in normal saline, as well as Diethyl ether, formalin, and acetic acid (Merck, Darmstadt, Germany) were used. Sesame oil (Moosavi Co., Kazeroon, Iran) was used as the control in our study. TNF-α, IL-6 and MPO kits (Bioassay Technology Laboratory, China) were used to measure cytokines.
Mastic Oil Preparation
Mastic resin from Pistacia lentiscus was collected from Shiraz, Fars, Iran, identified by the Pharmacognosy Department of Shiraz University of Medical Sciences (SUMS). Mastic resin powder 40 g was dissolved in 200cc sesame oil under Bain-marie. Then, the solution was mixed until it became homogeneous.9 Mastic oil dose (400 mg/kg) was selected based on traditional experiments.7 P. lentiscus powder was dissolved in sesame oil and then administered orally or intra-rectally to rats. Presumably, low solubility of mastic in the water during its transit through the bowel may have decreased its bioavailability and we solved it in an oral oil like sesame oil and hence its bioactivity at the mucosal interface of the more distal bowel regions. The major components of the mastic were α-pipene, β-pipene, β-myrcine, linalool, trans-caryophyllene and camphene and its nutritional value showed zero calories and 0% fat, 0% cholesterol, 0% sodium, 0% carbohydrate, 0% protein and 3% calcium, 0% iron, 0% vitamin A and Vitamin C.9
Animals
In total, 48 male Sprague-Dowley 6–8 weeks-old rats, weighing 200–250 g were purchased from the animal lab of SUMS. They were kept in groups of 8 in temperature and humidity-controlled rooms (20–23°C, 50–60%) with a 12-h light/dark cycle and free access to standard food and tap water. The animals were kept and handled according to the local guidelines of care and work with laboratory animals at SUMS.
Induction of Experimental Colitis
The animals were kept in stainless steel cages while fasting but having free access to water for 24 h before colitis induction by administering 3% acetic acid intra-rectally.10 The rats were lightly anesthetized by diethyl ether. An 8 cm tube was inserted into rat’s colon via the anus. Then, acetic acid (2 mL, 3% v/v in normal saline) was injected into the colon gradually. Next, for 30 sec, they were held in a head-down position to reduce leakage from the anus.
Experimental Design
The following six groups were designed into which the 48 rats were randomly allocated:
Control group: normal saline (2 mL/kg, intra-colonic) without colitis induction; Colitis group: normal saline (2 mL/kg, intra-colonic) administered 24 h after the induction of colitis; Prednisolone group: prednisolone (5 mg/kg)11 administered orally 24 h after the induction of colitis as reference drug; Test group 1, 2 (n=8): mastic (400 mg/kg/day, administered intra-colonic or orally) 24 h after the induction of colitis, Test group 3 sesame oil (2 mL/kg/day) administered 24 h after the induction of colitis. Treatments administration was done by a gavage needle for anesthetized rats. Medication was administered for six consecutive days following the induction of colitis.
The animals were euthanized by CO2 on the seventh day of the experiment and the colons were removed and washed with normal saline.12 The 8 cm distal colons were assayed for macroscopic evaluations and then the colons were bisected for histopathology or biochemical assessments. The tissue segments for histopathology assessment were kept in 10% formalin and the segments for measuring MPO activity and cytokine levels were kept frozen (−85°C).
Determination of the Cytokines and MPO Levels in the Colon Tissue
The cytokines tumor necrosis factor-α, interleukin (IL)-6, and myeloperoxidase levels in the colon tissues were evaluated by enzyme-linked immunosorbent assay (ELISA). Segments of the colon were thawed in a laboratory environment, chopped to small pieces and homogenized in 0.01 M PBS (pH=7.2–7.6) containing 8.5 g NaCl, 0.2 g NaH2PO4 and 1.4 g Na2HPO4 to 1 Liter distilled water. One mL PBS per 1 g tissue was used to prepare the supernatant. The cytokines levels were measured according to kit instructions provided by the manufacturer (Bioassay Technology Laboratory, China).
Gross Morphologic Study
We used the criteria described by Morris et al 1989.13 “Inflammation” was defined as regions of hyperemia and bowel wall thickening (Table 1). After scoring, samples were excised from each colon. The tissue samples were fixed in 10% formalin and embedded in paraffin for histopathological study.
Table 1.
Criteria to Scale the Macroscopic Morphological Damage of the Colonic Mucosa
| Score Gross Morphology | |
|---|---|
| 0 | No macroscopic damage |
| 1 | Localized hyperemia, but no ulcers or erosions |
| 2 | Ulcers or erosions with no significant inflammation |
| 3 | Ulcers or erosions with inflammation at one site |
| 4 | Two or more sites of ulceration and/or inflammation |
| 5 | Two or more major sites of inflammation and ulceration or one major site of inflammation and ulceration extending >1 cm along the length of the colon |
Histopathological Assessment of Colon Injury
The colon pieces in paraffin were cut into slices of 5 μm thickness. Then, paraffin was removed by xylene solution and the slices were dyed, using the “H & E” method (hematoxylin and eosin staining protocol). Finally, the dyed slices were scored as described previously4 with slight modifications. The total colitis index was measured by summing up the scores of inflammation severity (0–3), inflammation extent (0–3), crypt damage (0–4).12
Statistical Analysis
The normality of distribution of their data was assayed by Kolmogorov–Smirnov test. Data of parametric distribution are expressed as mean ± SD and analysed by one-way ANOVA. And for nonparametric distribution, median accompanied by minimum and maximum value was presented. The Mann–Whitney test was applied to compare quantitative data and Chi-square test was used for qualitative data. All statistical analyses were performed using SPSS software (version 22). The level of significance presented by p-value.
Results
Effect of Mastic Oil on Macroscopic Parameters and Histopathological Features
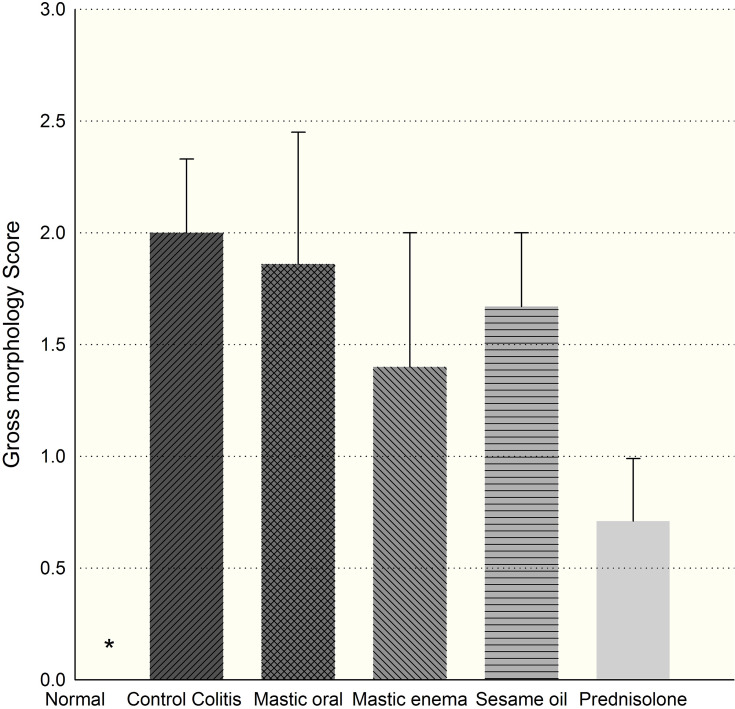
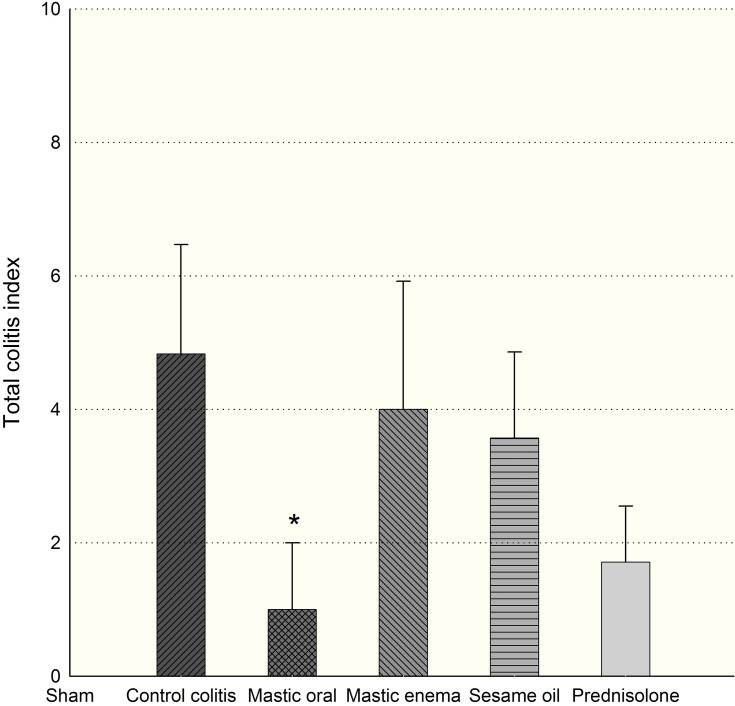
Results from the macroscopic study showed that the colons in the colitis group displayed severe inflammation while colons of the control group showed no damage (Figure 1). Treatment with mastic oil (400 mg/kg) did not affect the gross score, but significantly reduced the total colitis index of the colon compared to the colitis group (Figures 2 and 3). The total colitis index of mastic oil (400 mg/kg) was almost the same as that of prednisolone (5 mg/kg) (Figure 3).
Figure 1.
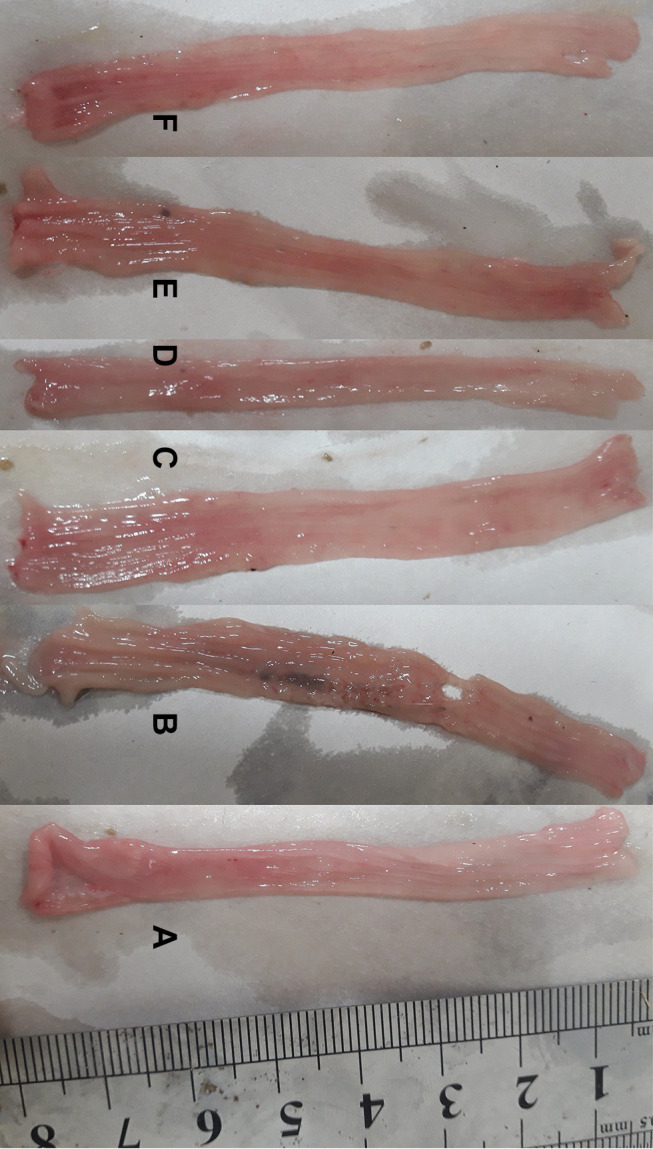
Macroscopic presentation of rat colons in treatment groups. Control (A) which shows intact colon, Colitis without treatment (B) which shows the highest level of tissue injuries like edema, erythema, ulcer, necrosis and thickening of tissue. Mastic oil: orally (C) and enema (D), prednisolone (E) and sesame oil (F) treated colons represents healing of ulcers and obvious improvement in tissue injuries.
Figure 2.
Gross morphology score in groups. *Normal tissues were significantly different compared with colitis (p=0.016) and prednisolone decreased gross score but statistically non-significant (p=0.057).
Figure 3.
Effect of mastic oil (400 mg/kg) and prednisolone (5 mg/kg) shows on total colitis index. Data are analyzed as mean ± SD. *P<0.05 in comparison with colitis group.
There was no histological damage in the control group; hence, colonic mucosa can be said to have retained an intact epithelium. But, the colitis group showed edema and severe inflammation (Figure 4). Mastic oil effects on histopathological features are shown in Figure 5 (Table 2).
Figure 4.
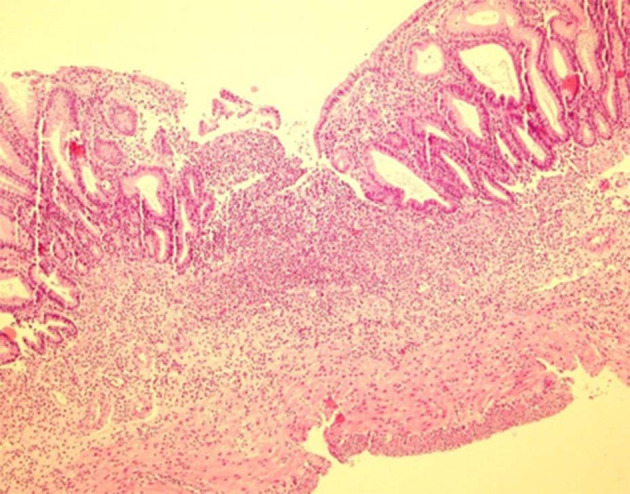
Microscopic section shows severe transmural acute inflammation with crypt and epithelium lost in colitis group. (Hematoxylin and Eosin, 100×).
Figure 5.
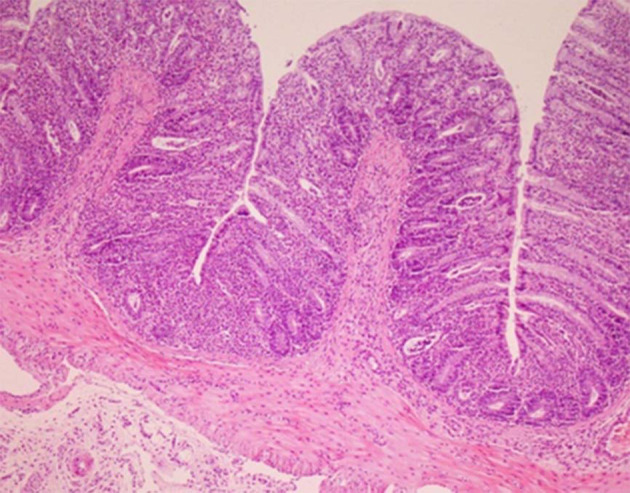
Microscopic section shows mild transmural acute inflammation with basal crypt damage and complete regeneration after mastic oil orally consumption. (Hematoxylin and Eosin, 100×).
Table 2.
Histopathological Characteristics Score in Different Groups, Mean Score (SD)
| Groups (n=8) | Inflammation | Extent | Regeneration | Crypt Damage |
|---|---|---|---|---|
| Colitis control | 1.50±0.50 | 1.67±0.56 | 1.33±0.49 | 1.67±0.61 |
| Mastic oil | 0.33±0.33 | 0.33±0.33 | 0.33±0.33 | 0.33±0.33 |
| Sesame oil | 1.28±0.42 | 1.14±0.4 | 0.86±0.55 | 1.14±0.55 |
| Prednisolone | 0.71±0.36 | 0.43±0.20 | 0.14±0.14 | 0.57±0.30 |
| Mastic oil enema | 1.00±0.45 | 1.40±0.68 | 1.00±0.63 | 1.60±0.81 |
One-way ANOVA tests show the total colitis index, including inflammation severity, inflammation extent, and crypt damage scores, to be significantly reduced in the mastic oil group (400 mg/kg administered orally) in comparison with colitis group (p=0.046), while there were no significant score changes with sesame oil alone (Figure 3).
Effect of Mastic Oil on Myeloperoxidase (MPO) Activity
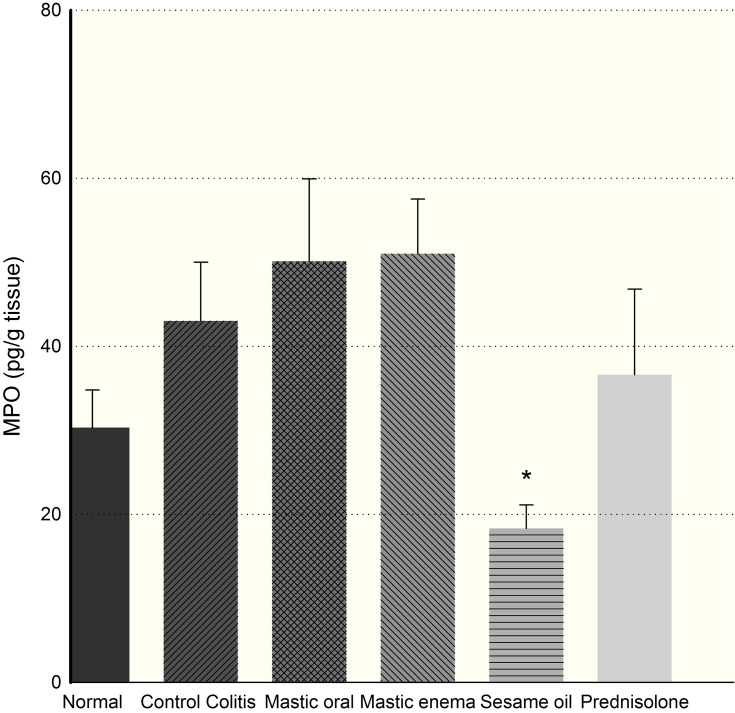
Myeloperoxidase activity levels in colonic tissues of the sesame oil group (P=0.025) were reduced significantly in comparison with the colitis group after 7 days (Figure 6). The mastic oil group did not show any significant change in MPO levels (p>0.05). The sesame oil group demonstrates a significant difference with regard to mastic oil in MPO levels (p<0.001). On the other hand, no significant differences were observed in MPO levels after using the prednisolone, which could be dose-dependent.11
Figure 6.
Effect of sesame oil and prednisolone (5 mg/kg) on MPO activity of rat colon. Data are analyzed as mean ± SD. *P<0.05 in comparison with colitis group.
Effect of Mastic Oil on the Colon Tissue Cytokines (IL-6 and TNF-α)
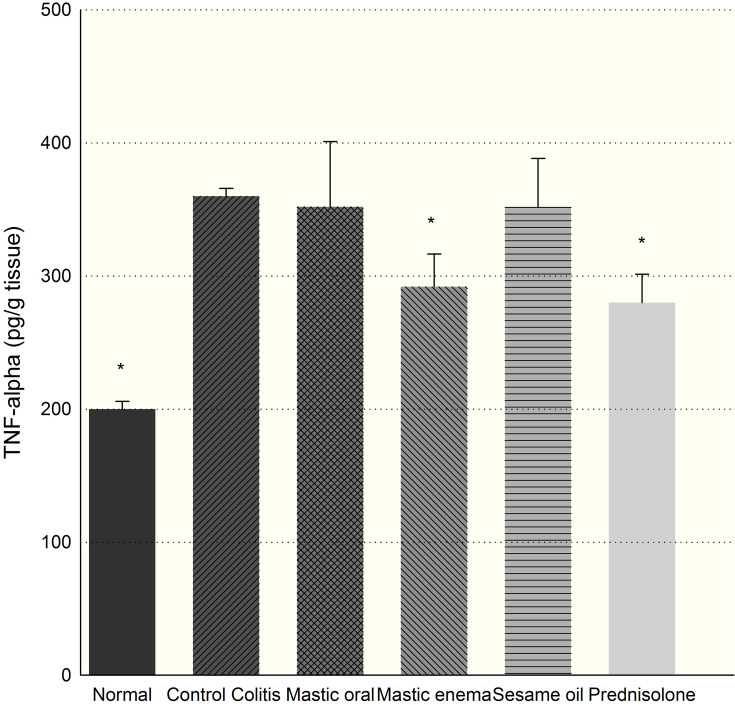
Induction of colitis increased TNF-α tissue levels significantly (p<0.01) in comparison with the control group (Figure 7). Mastic oil administered intra-rectally decreased TNF-α level significantly after 7 days compared to other groups (p=0.024). Mastic oil did not produce any significant changes in IL-6 levels (p>0.05). Furthermore, sesame oil did not produce any significant changes in IL-6 or TNF-α levels (p>0.05).
Figure 7.
Effect of mastic oil (400 mg/kg) and prednisolone (5 mg/kg) on TNF-α. Data are analyzed as mean ± SD. *P<0.05, in comparison with colitis group.
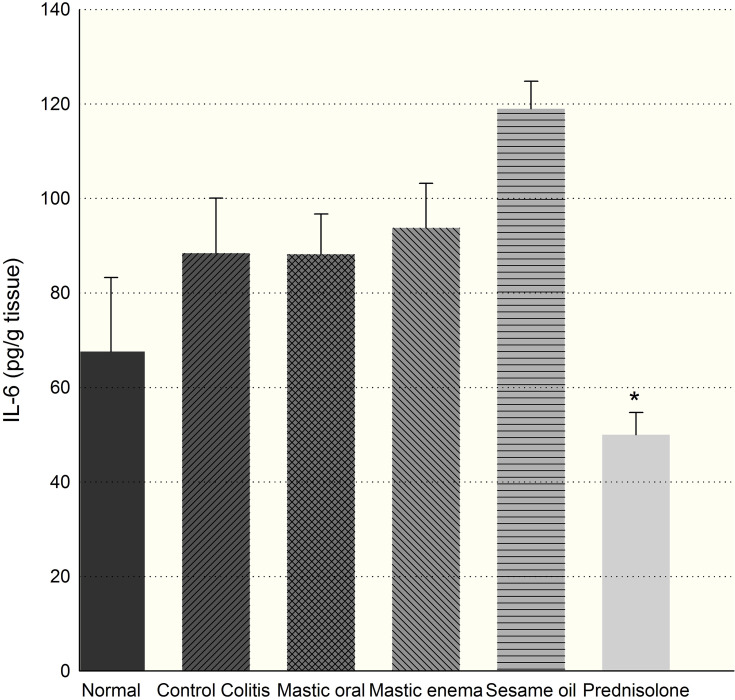
Compared to other groups, prednisolone significantly decreased IL-6 or TNF-α levels after 7 days, which is even lower than normal tissue (p<0.05). Mastic oil in enema usage and sesame oil increased IL-6 level in tissue (Figure 8).
Figure 8.
Effect of mastic oil (400 mg/kg) and prednisolone (5 mg/kg) on IL6. Data are analyzed as mean ± SD. *P<0.05 in comparison with colitis group.
Discussion
Although the causes of ulcerative colitis are still unknown, recent investigations have confirmed genetic agents, individual and environmental immunological factors and stress as the most important factors in this disorder.2 Inflammation mediators and oxidative stress play an important role in the pathogenesis of this disease. In addition, the infiltration of macrophage and neutrophil to intestinal mucosa are other important specifications of this disease.2 Inflammation control and the reduction of drug side effects are the main goals in the treatment of UC and several herbal remedies could be good candidates to improve parameters associated with colitic damage.
Five main substance classes comprise mastic are terpenes, flavonoids, phenol carboxylic acids, volatile oil, and coumarines.14 Flavonoids and phenol carboxylic acid contain radical scavenging properties and interest in Pistacia lentiscus has been primarily due to its antioxidant effects.
The current study demonstrated that the mastic significantly reduced the total colitis index of the colon compared to the colitis group in the acetic acid–induced model of ulcerative colitis in rats. In the present study, the partial reduction in acute inflammation by sesame oil, as indicated by the decrease of MPO activity, could strengthen the potential role of sesame oil as a potent anti-oxidant and anti-inflammatory agent. Other inflammatory cytokines like TNF-α and IL-6 in colon tissue clarified the anti-inflammatory effects of sesame oil and mastic. Consistent with earlier studies, Murthy et al reported intra-rectal administration of mastic oil for its rapid onset of action, which lowered the risk inherent in systemic administration in the treatment of UC.15 Hosseini et al showed that the honey reduced the ulcerative colitis inflammation when administered rectally.16 In this research, we evaluated the effect of mastic oil administered orally and rectally on ulcerative colitis. This finding has not been reported previously, the difference between mastic oil and sesame oil showed the anti-inflammatory effects of the two oils to be significantly different. The dose of mastic oil (400 mg/kg) did not result in the reduction of MPO level, but sesame oil without P. lentiscus reduced MPO level. These results show that adding P. lentiscus changed the concentration and nature of sesame oil. On the other hand, mastic oil preparation in sesame oil could change some parameters, such as the MPO activity of sesame oil. Our results are in agreement with previous studies, in which Tanideh et al showed that Pistacia Atlantica oil can improve colitis in rats with its anti-inflammatory activity.17 Another study showed that mastic powder (100 mg/kg/day) administered after 3 days could decrease inflammatory cytokines, such as IL-10, amelioration of colitis histologically and significant differences in colonic indices.5
The TNF-α and IL-6 from inflammatory cytokines are involved in inflammatory reaction and UC treatment.18,19 The present data show that TNF-α levels were down-regulated in the gut homogenate of mastic oil-treated rats similar to the prednisolone group. Nevertheless, prednisolone decreased IL-6 levels more significantly than other groups. Moreover, the beneficial effects of sesame oil might be partly due to MPO-activity level. Nevertheless, other immunological mechanisms might be involved, which has to be clarified in future studies.
Limitations
The limitation of the study was limited samples in each group because the animal studies should be done under ethical considerations.
Conclusion
Mastic oil in enema form reduces TNF-α significantly, similar to prednisolone and the control group. In our experiment, MPO activity was reduced by sesame oil, but IL-6 did not change significantly in mastic and sesame oil groups. The total colitis index in the mastic oil group had decreased significantly via oral administration and it is suggested that mastic and sesame oils could be used as natural remedies to lessen the ulcerative colitis inflammation.
Funding Statement
This study was financially supported by Vice-Chancellor of Research, Tabriz University of Medical Sciences, Tabriz, Iran (no. 62504).
Highlights
Mastic and sesame oil as two traditional medicines showed anti-inflammatory properties and it is suggested that these oils could be used as natural sources to alleviate the inflammation of ulcerative colitis.
Ethical Statement
The ethics committee of Tabriz University of Medical Sciences in Iran approved the protocol of study (IR.TBZ.rec.1398.328).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Thoreson R, Cullen JJ. Pathophysiology of inflammatory bowel disease: an overview. Surg Clin North Am. 2007;87:575–585. doi: 10.1016/j.suc.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S39. doi: 10.1097/01.mib.0000195385.19268.68 [DOI] [PubMed] [Google Scholar]
- 3.Papalois A, Gioxari A, Kaliora AC, et al. Chios mastic fractions in experimental colitis: implication of the nuclear factor kappaB pathway in cultured HT29 cells. J Med Food. 2012;15:974–983. doi: 10.1089/jmf.2012.0018 [DOI] [PubMed] [Google Scholar]
- 4.Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gioxari A, Kaliora AC, Papalois A, Agrogiannis G, Triantafillidis JK, Andrikopoulos NK. Pistacia lentiscus resin regulates intestinal damage and inflammation in trinitrobenzene sulfonic acid-induced colitis. J Med Food. 2011;14:1403–1411. doi: 10.1089/jmf.2010.0240 [DOI] [PubMed] [Google Scholar]
- 6.Naouar MS, Mekki LZ, Charfi L, Boubaker J, Filali A. Preventive and curative effect of Pistacia lentiscus oil in experimental colitis. Biomed Pharmacother. 2016;83:577–583. doi: 10.1016/j.biopha.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 7.Assimopoulou AN, Papageorgiou VP. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part I. Pistacia lentiscus var. Chia. Biomed Chromatogr. 2005;19:285–311. doi: 10.1002/bmc.454 [DOI] [PubMed] [Google Scholar]
- 8.Magkouta S, Stathopoulos GT, Psallidas I, et al. Protective effects of mastic oil from Pistacia lentiscus variation chia against experimental growth of lewis lung carcinoma. Nutr Cancer. 2009;61:640–648. doi: 10.1080/01635580902825647 [DOI] [PubMed] [Google Scholar]
- 9.Dimas KS, Pantazis P, Ramanujam R. Chios mastic gum: a plant-produced resin exhibiting numerous diverse pharmaceutical and biomedical properties. In Vivo. 2012;26:777–786. [PubMed] [Google Scholar]
- 10.MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978;17:135–150. doi: 10.1159/000198104 [DOI] [PubMed] [Google Scholar]
- 11.Yano H, Hirayama F, Kamada M, Arima H, Uekama K. Colon-specific delivery of prednisolone-appended alpha-cyclodextrin conjugate: alleviation of systemic side effect after oral administration. J Control Release. 2002;79:103–112. doi: 10.1016/s0168-3659(01)00532-6 [DOI] [PubMed] [Google Scholar]
- 12.Minaiyan M, Hajhashemi V, Rabbani M, Fattahian E, Mahzouni P. Evaluation of anti-colitic effect of fluvoxamine against acetic acid-induced colitis in normal and reserpinized depressed rats. Eur J Pharmacol. 2015;746:293–300. doi: 10.1016/j.ejphar.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 13.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. doi: 10.1016/S0016-5085(89)80079-4 [DOI] [PubMed] [Google Scholar]
- 14.Mashtoub S, Hoang BV, Vu M. Clinical and structural effects of traditional chinese medicine and the herbal preparation, iberogast, in a rat model of ulcerative colitis. J Evid Based Complementary Altern Med. 2014;19(1):10–19. doi: 10.1177/2156587213503660 [DOI] [PubMed] [Google Scholar]
- 15.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;(38):1722–1734. doi: 10.1007/bf01303184 [DOI] [PubMed] [Google Scholar]
- 16.Hosseini SV, Abodrab EAM, Khazraei H, Zamani M, Mokhtari M. Effect of egg white and honey enema on ulcerative colitis in an animal model. Comp Clin Path. 2019. doi: 10.1007/s00580-019-03023-9 [DOI] [Google Scholar]
- 17.Tanideh N, Masoumi S, Hosseinzadeh M, et al. Healing effect of pistacia atlantica fruit oil extract in acetic acid-induced colitis in rats. Iran J Med Sci. 2014;39:522–528. [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15:1548–1553. doi: 10.3748/wjg.15.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abtahi Froushani SM, Mashouri S. The beneficial effects of hypiran in ameliorating rat model of ulcerative colitis. Zahedan J Res Med Sci. 2018;20:e58919. doi: 10.5812/zjrms.58919 [DOI] [Google Scholar]