Abstract
Purpose of Review
The optimal management of external ventricular drains (EVD) in the setting of acute brain injury remains controversial. Therefore, we sought to determine whether there are optimal management approaches based on the current evidence.
Recent Findings
We identified 2 recent retrospective studies on the management of EVDs after subarachnoid hemorrhage (SAH) which showed conflicting results. A multicenter survey revealed discordance between existing evidence from randomized trials and actual practice. A prospective study in a post-traumatic brain injury (TBI) population demonstrated the benefit of EVDs but did not determine the optimal management of the EVD itself. The recent CLEAR trials have suggested that specific positioning of the EVD in the setting of intracerebral hemorrhage with intraventricular hemorrhage may be a promising approach to improve blood clearance.
Summary
Evidence on the optimal management of EVDs remains limited. Additional multicenter prospective studies are critically needed to guide approaches to the management of the EVD.
Keywords: External ventricular drain, Wean, Ventriculoperitoneal shunt, Length of stay, Vasospasm
Introduction
Placement of an external ventricular drain (EVD) is frequently an emergent, lifesaving procedure in the acute management of hydrocephalus and intracranial hypertension in the critically ill neurological patient [1, 2, 3••]. After the EVD is in place, important additional management goals are to optimize brain tissue physiology, minimize complications, and determine if and when the drain can be removed. However, the best way to use the EVD to monitor intracranial pressure (ICP) or drain CSF is unclear. It also remains unknown if the manner in which the EVD is discontinued matters for clinically important outcomes such as ventriculoperitoneal shunt (VPS) placement, length of stay, and hospital complications.
The available evidence, in the form of single-center randomized trials, suggests that an intermittent CSF drainage approach and rapid weaning of the EVD may be associated with fewer complications and shorter length of stay in patients following subarachnoid hemorrhage (SAH). However, recent surveys of neurointensive care units across the USA and internationally demonstrate high practice variance, and the majority of institutions take the opposite approach of continuous CSF drainage and a gradual EVD wean [4, 5•]. This reflects a paucity of evidence either to lead to a practice change or to justify the current predominant practice. There is even less evidence to guide the management of the EVD in the setting of traumatic brain injury (TBI), intracerebral hemorrhage with intraventricular hemorrhage (ICH/IVH), or other disease processes. Furthermore, interpreting existing evidence is hindered by the variability in how ICPs are measured and reported in the literature.
The purpose of this review is to describe the rationale behind different EVD management approaches in the setting of disease processes encountered in critically ill brain injured patients, discuss controversies and the recent literature, and propose future research focused on improving patient outcomes and decreasing hospital complications and length of stay. Since the use of the EVD and corresponding evidence base differs based on clinical context, we have structured the following discussion primarily around disease states. We will also discuss ICP measurement issues common to all diseases.
Aneurysmal Subarachnoid Hemorrhage
Most existing evidence for management of the EVD is in the patient with aneurysmal SAH and acute hydrocephalus. The Neurocritical Care Society recently published an evidence-based consensus statement which stated that “EVD weaning should be accomplished as quickly as is clinically feasible so as to minimize the total duration of EVD monitoring and [ventriculostomy-related infection] risk.” [3••] However, no strong recommendations for how to specifically manage the EVD could be made based on the existing evidence. Here, we will review two older single-center randomized trials and provide an update with two newer, retrospective studies which have been published since a recent systematic review on EVD management (Table 1) [12•].
Table 1.
Studies of rapid vs. gradual weaning and intermittent vs. continuous CSF drainage of EVDs after SAH
| Reference | Design | Number of centers | Number of subjects | Comparison | Outcomes | Findings |
|---|---|---|---|---|---|---|
| Klopfenstein 2004 [6] | RCT | 1 | 81 | Rapid vs. gradual | VPS, LOS | Rapid had shorter ICU and hospital LOS |
| Amato 2011 [7] | Prospective observational | 1 | 37 | Intermittent vs. continuous | VSP, EVD complications | No difference |
| Kim 2011 [8] | Prospective observational | 1 | 37 | Intermittent vs. continuous | VSP, EVD complications | No difference |
| Olson 2013 [9] | RCT | 1 | 60 | Intermittent vs. continuous | VSP, EVD complications | Intermittent had fewer EVD complications |
| Jabbarli 2018 [10•] | Retrospective | 2 | 965 | Rapid vs. gradual | VPS, LOS, complications | Gradual had fewer VPS and longer LOS |
| Rao 2019 [11••] | Retrospective | 1 | 152 | Intermittent/rapid vs. continuous/gradual | VPS, VSP, LOS, EV complications | Intermittent/rapid had fewer VPS and shorter LOS |
RCT, randomized clinical trial; VSP, symptomatic vasospasm; VPS, ventriculoperitoneal shunt placement; LOS, length of stay
The most widely cited randomized trial on EVD management addresses the optimal manner of EVD discontinuation or weaning [6]. This study compared rapid versus gradual weaning protocols. When the clinical determination was made that the EVD was no longer needed, patients randomized to a rapid EVD wean underwent an immediate closing of the drain. If there were no clinical or radiographic signs of hydrocephalus over 24 h, then the EVD was discontinued. For the gradual wean group, the EVD was raised from 10 cm H2O to 25 cm H2O in increments of 5 cm H2O per day, then closed for 24 h. The investigators found that the rapid EVD weaning protocol decreased ICU and hospital length of stay as compared to the gradual weaning protocol. There was also a decrease in the number of EVD device-days, as would be expected. A caveat of the study is that the positive findings were prespecified secondary outcomes. The authors did not find a difference in the primary outcome of ventriculoperitoneal shunt (VPS) placement rate. This was likely due to the fact that the study was designed to tolerate only a single wean attempt failure prior to placing a VPS, which is reflected in an unusually high rate of VPS placement (approximately 63% in both groups). Additional limitations were that the study was not designed to see differences in rates of vasospasm, 31% of eligible patients did not complete the study protocol, functional outcomes were not reported, and statistical analyses were not adjusted for confounders.
Another controversy in EVD management is whether to keep the EVD continuously open to drainage or to only open when needed, referred to as intermittent or on-demand drainage. The rationale behind intermittent drainage is that continuous drainage and a low ICP may not allow for the pressure gradient necessary to re-establish the natural pathways of CSF egress. Continuously open EVDs have been associated with more EVD-related complications [7, 8]. There is conflicting evidence around other outcomes, with older retrospective data suggesting that continuous drainage is associated with increased rates of delayed cerebral ischemia (DCI) and VPS placement [13] and subsequent studies suggesting that more robust CSF drainage is associated with a decreased risk for vasospasm, delayed ischemic neurological deficits, and poor outcome [14–16]. To partially address the controversy, a single-center randomized trial was performed to determine rates of vasospasm using a continuous versus intermittent EVD management strategy [9]. The authors found no difference in their predefined primary outcome of vasospasm, though there was a non-significant increase in angiographic vasospasm in the intermittent group (35% vs. 21%). Both a major finding and caveat is that the study was stopped early by the Data Safety and Monitoring Board because an interim analysis revealed a large and significant increase in EVD complications in the continuous drainage group. The 53% versus 23% difference in complications was largely driven by more EVD clogging in the continuous group. There was also a higher, albeit not statistically significant, rate of ventriculitis in the continuous group, although the rates of ventriculitis were considerably higher than rates reported in other studies [17].
Despite the limitations of the 2 single-center randomized trials discussed above, they are the only available randomized evidence to guide management and suggest that rapid weaning and intermittent drainage strategies are at least safe and could potentially lead to reduced EVD duration, shorter ICU and hospital length of stay, and fewer EVD complications. Since neither of these studies can be considered practice changing, current practice variability is likely to continue until more definitive data is available. Furthermore, neither study was effectively powered to detect DCI or symptomatic vasospasm, and neither addressed the question of long-term functional outcomes associated with the studied management approach. These limitations represent important opportunities for future progress.
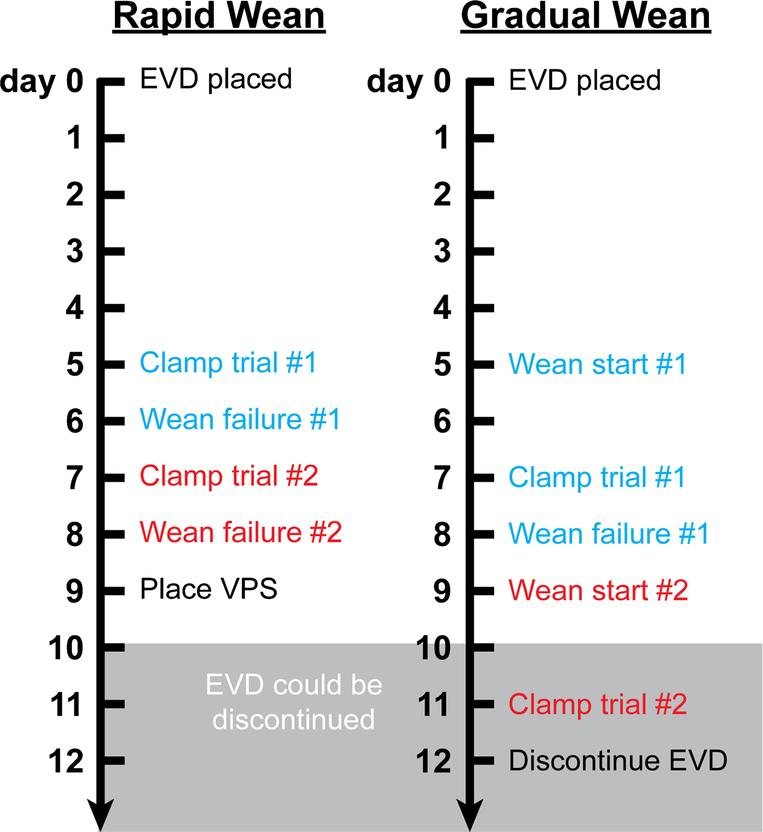
It is worth noting 2 recent retrospective studies that have looked at EVD management approaches after SAH. One compared rapid versus gradual weaning in two German hospitals within the same health care system [10•]. Standard of care at one of the hospitals was a rapid wean and at the other hospital was a gradual wean. The hospital with a rapid wean approach had higher VPS rates but fewer delayed VPSs and was associated with shorter hospital length of stay, consistent with the aforementioned rapid versus gradual wean randomized trial. A major limitation is that there was a set number of two wean failures before a VPS was placed. It is possible that the requirement that a VPS be placed after a predetermined number of failures—rather than the influence of a rapid or gradual wean—could have accounted for the greater number of VPS’s seen in the rapid group (Fig. 1). Indeed, a retrospective study that focused on the effect of clamp trials on VPS placement in aneurysmal SAH patients found that more clamp trials were associated with a lower VPS rate [18•]. Therefore, we recommend not limiting the number of wean attempts in clinical practice and in future studies.
Fig. 1.
Number of predefined wean attempts alone can determine rate of ventriculoperitoneal shunt (VPS) placement between rapid and gradual wean groups. The figure defines a hypothetical scenario where a rapid or gradual wean is started on day 5 following EVD placement. Only 2 wean failures are tolerated prior to placing a VPS. The rapid wean consists of immediate clamping of the EVD. The gradual wean consists of stepwise raising of the EVD that culminates in a clamp trial. A 24-h period is used to determine success or failure of wean. The scenario assumes in both cases that the EVD could be successfully discontinued on or after day 10, unbeknownst to the clinician.
Another recent retrospective study on EVD management after SAH is one that took a before-and-after study design approach during a change in EVD guidelines [11••]. The authors compared an epoch where CSF was by default continuously drained and EVDs were gradually weaned (continuous/gradual) to a shift in practice where CSF was intermittently drained and the EVD was rapidly weaned (intermittent/rapid). Importantly, there was no set limit to the number of EVD wean attempts prior to placing a VPS. The authors found that the intermittent/rapid group had a lower rate of VPS placement, including delayed VPSs. Furthermore, the study found shorter ICU and hospital length of stay and fewer EVD days in the intermittent/rapid group, consistent with the rapid versus gradual wean randomized trial. There were fewer EVD complications, consistent with the intermittent versus gradual drainage randomized trial. The study, however, was not powered to detect a difference in rates of symptomatic vasospasm, and rates of DCI and long-term functional outcomes were not assessed. Future studies should test the generalizability of these results and determine the effect of EVD management on long-term functional outcomes.
Traumatic Brain Injury
The role of an EVD in the setting of TBI is controversial [19–21], but it is often employed. Following TBI, an EVD may serve both to monitor ICP and to divert CSF for decreasing elevated ICP. In the modern era, a fiber optic intraparenchymal monitor can be used if all that is needed is measurement of ICP, and most clinicians in the industrialized world adhere to the Brain Trauma Foundation guidelines that advocate use of some form of ICP monitor [22•]. Current controversies around ICP monitoring itselfare discussed elsewhere [20, 21]. Here, we will focus on the issue of EVDs in the management of TBI for therapeutic drainage of CSF.
A recent randomized trial compared the use of an EVD to use of an intraparenchymal fiber optic ICP monitor alone [23]. The EVD group—which employed an intermittent CSF drainage approach—had fewer episodes of refractory ICP (52% intraparenchymal monitor versus 21% EVD, p > 0.001). There was better survival in the EVD group at both 1 and 6 months post-injury (90% vs. 77% p = 0.04 and 89% vs. 68% p = 0.006). Furthermore, the Glasgow Outcome Scale was better in the EVD group at 6 months post-injury (66% vs. 48% p = 0.009 for favorable outcome). The study demonstrates that the therapeutic benefit of draining CSF in the setting of elevated ICP outweighed additional procedures and complications associated with EVDs. A major limitation of the study was the inability to blind clinicians to the group. Regardless, the study provides a reasonable level of evidence that EVDs can be useful as a therapeutic measure [24, 25].
Notably, the above study used an intermittent CSF drainage approach in the EVD group, advocated by the American College of Surgeons Trauma Quality Improvement Program [26]. Intermittent drainage—whereby the drain is kept closed and only opened for the amount of time needed to control ICP—allows for continuous ICP monitoring since it is not typically possible to simultaneously drain CSF and measure ICP. However, there is no high-quality evidence supporting the use of an intermittent CSF drainage approach. Furthermore, the Brain Trauma Foundation guidelines provide a conflicting recommendation that “continuous drainage of CSF may be considered to lower ICP burden more effectively than intermittent use” [22•], based on a retrospective study which observed a greater ICP burden in patients managed with intermittent drainage [27]. Although there are limited data to support either approach, among the 7 institutions represented by the authors of this manuscript, all employ a continuous CSF drainage approach.
The rationale behind the continuous approach is that constant drainage could reduce the need for hyperosmolar therapy, which could then be used in a rescue situation. However, there is the disadvantage of not being able to monitor ICP while the EVD is open, with the exception of cases where there is a concomitant fiber optic intraparenchymal probe or dual EVDs. While the development of EVDs that can simultaneously drain and record ICP could potentially resolve this issue [28•, 29•], the utility and efficacy of these devices are under debate [30, 31] and these novel EVDs have not yet seen widespread adoption. Another consideration with the continuously open EVD approach is that in the setting of refractory intracranial hypertension, the ventricles often collapse, leaving limited additional CSF available for drainage.
In conclusion, continuous and intermittent drainage are both reasonable approaches in the setting of TBI. The single randomized study addressing the use of an EVD as compared to a fiber optic intraparenchymal monitor took an intermittent drainage approach in the EVD group and obtained superior outcomes. Otherwise, there are no compelling data to make a recommendation in favor of intermittent versus continuous drainage or rapid versus gradual weaning of an EVD in the setting of TBI.
Intracerebral hemorrhage with intraventricular extension
The management of the EVD in the setting of ICH/IVH is different from SAH and TBI, owing to the difference in non-communicating and communicating hydrocephalus. The evidence to guide practice is very limited. There have been no high-quality studies since the publication of a review article appearing in this journal specifically focused on EVD management after ICH/IVH [32]. Therefore, we will focus this section on promising new approaches that are in development.
The most well-known series of studies involving the management of EVDs following ICH/IVH have been carried out by the CLEAR investigators [33]. Their goal has been to determine the effect of intrathecal alteplase through an EVD to lyse the blood clot, decrease mechanical factors that alter CSF dynamics, and clear downstream effects ofthe blood itself that may worsen patient outcome. Early studies established the safety of the approach [34]. Further analysis of these early studies revealed that blood clearance was primarily enhanced in the midline ventricles [35]. This led the investigators to test unique EVD placement strategies to improve blood clearance from the lateral ventricles as well.
Phase III of CLEAR-IVH (CLEAR-III) investigated the effect of clearing IVH in the third and fourth ventricles with alteplase on functional outcomes [36••]. This was a negative study as the primary outcome of good functional outcome (mRS ≤ 3) at 6 months was not different between the alteplase and the control saline groups. However, additional analysis revealed that good functional outcome was associated with greater IVH clearance, and that IVH clearance was associated with bilateral EVDs or an EVD placed ipsilateral to the greatest clot burden. Although the level of evidence here is low, it raises the possibility that more efficient IVH clearance via unique EVD placement strategies could result in better outcomes.
Limitations of the CLEAR III trial include the lack of evidence-based guidelines in the management of the IVH itself, which could have affected participant selection and treatment. There were also no uniform guidelines on which patients should receive an EVD, or multiple EVDs. Furthermore, only 33% of patients in the alteplase group and 10% in the saline group achieved the 80% IVH removal end point. Therefore, studies designed specifically to determine whether EVD placement strategies can influence IVH clearance and functional outcome represent promising areas of future research.
ICP Measurement and Reporting
Determining best EVD management practices depends on the accuracy and precision of ICP measurement. However, recent observational studies, practice surveys, and reviews highlight inconsistencies in how ICPs through the EVD are measured, recorded, and reported [4, 37•, 38•, 39•, 40–44]. A discussion of ICP thresholds for intervention is beyond the scope of this review. Here, we will highlight critical principles to keep in mind regarding a few technical aspects of EVD management. If ICP is to be a useful measurement it is important that the reported values use a standardized unit of measure, have standardized reference points, and adhere to uniform reporting practices. Furthermore, it is important for clinicians to recognize that ICP is a dynamic process and that any one reported number cannot fully capture the true ICP.
There are two dominant units of measure: millimeters of mercury (mm Hg), and centimeters of water (cm H2O). Millimeters of water (mm H2O) has also rarely been reported in the literature. In a recent practice survey, 50% of clinicians measured ICP in mm Hg, 29% in cm H2O, and 21% used both mm Hg and cm H2O in the same patient [4]. In an observational study, roughly 31% measured ICP in mm Hg, 23 % in cm H2O, and 46% did not specify the unit of measure. The fact that the survey study and observational practice study results differ substantially highlights that differences can occur with differences in research methodology. Another study summarized 357 manuscripts wherein ICP was a reported variable [45•]. This study found that 96% of those reporting ICP use mm Hg. It is unclear why there is such a discrepancy between units reported in research as compared to clinically. However, it seems reasonable to move towards a standard of reporting ICP in mm Hg. Whereas many commercially available EVD drainage management systems display both cm H2O and mm Hg, most (if not all) commercially available bedside monitors output pressure recordings as mm Hg and not as cm H2O. Furthermore, modern electronic medical record (EMR) systems auto-populate discrete values directly from the bedside monitor [39], and it is likely that most EMR values for ICP are documented in mm Hg. At a minimum, it is clear that clinicians and investigators need to be aware that units are important to be aware of, and to apply the appropriate conversions when communicating the values to others.
The reference point for ICP measurements is important. In a typical EVD system, fluid-filled tubing is connected to an external strain-gauge transducer that converts mechanical pulsations into an electronic signal. The accuracy of the ICP measurement requires that the system has an uninterrupted column of fluid and is properly leveled (referenced) to an anatomical landmark; however, there is no universally agreed upon standard reference point [4, 43, 45•]. The most common landmark is the tragus ofthe ear (48.6%) as an approximation of the center of the skull. The second most commonly-reported reference point is immediately adjacent to the external auditory meatus (35.4%) [4]. However, using either of these locations on the left, for example, in a patient who is lying on his or her right side would result in the transducer being leveled (referenced) higher than the center of the patient’s skull. One group found that fewer than 50% of citations reported ICP site and only 23 % reported patient position [45•]. Therefore, it is important that future studies involving ICP measurements include these methodological details.
Because ICP values vary over time, it is important to clearly communicate the meaning of reported values. For example, over a given time span, which ICP should be reported? A random time point? The mean ICP? A range? One study used simultaneous video and continuous ICP measurements and analyzed 15-min epochs after EVD clamping [39•]. They found that there is a very low probability that any single observation made during the 15-min period is reflective of the entire period. This concept is reinforced by others who have examined ICP dynamics and noted that at rest ICP values may fluctuate up to 4 mm Hg within a single respiratory cycle [46]. Furthermore, nursing care procedures common to critically ill patients have been associated with >30 mm Hg changes in ICP over <5 min [46, 47].
In the setting of an open EVD, there are minimal data to suggest how long the EVD should be leveled and closed (set to monitor) prior to recording an ICP value [48]. (For accurate measurement of ICP, the EVD must be closed.) Current practice was likely set by an influential study from 1991 which recommended use of the end-hour ICP—defined as a single value documented at the end of a 60-min period—as an estimate of ICP [49]. Following this recommendation, clinical practice started leaning towards a single measure reported at the end of an epoch of time. As mentioned by the authors of the original study, this method is only an estimate of “true” ICP and fails to capture key features of clinical interest such as ICP waveform, maximum and minimum values, and transient elevations. Therefore, it is important to keep in mind that although it remains a common practice, the end-hour ICP should be interpreted as only a snapshot of the ICP during the previous several minutes, not for the duration of the epoch.
The dynamic nature of ICP actually represents an important opportunity to capture additional data about acutely brain injured patients. For example, investigators have been developing methods of ICP waveform analysis to predict the success of an EVD wean trial. One such study tested the hypothesis that when in homeostasis, pulses with similar mean ICP will have a similar waveform morphology [50•]. Conversely, when perturbed, ICP pulses will be morphologically different, even with the same mean ICP. Therefore, by evaluating the ICP pulse morphological difference, EVD wean failure could be predicted. This study needs to be validated in a prospective cohort, but represents the kind of future research that could be accomplished when ICP dynamics are taken into account.
Conclusions and Recommendations
Management of the EVD in the setting of SAH, TBI, and ICH/IVH likely influences patient outcomes and length of stay. The optimal approach to EVD management, however, remains unclear. At a minimum, rapid or gradual EVD weans and intermittent or continuous CSF drainage strategies appear safe. Therefore, determination of best EVD management approaches for specific disease states should be goals for future multicenter prospective studies.
Acknowledgments
The authors would like to thank Anna Cervantes-Arslanian, MD, for helpful comments.
Funding Information This work was supported by the National Institutes of Health (R25NS065743, KL2TR002542, and K08NS112601); the American Heart Association and American Stroke Association (18POST34030369); the Andrew David Heitman Foundation; the Aneurysm and AVM Foundation; and the Brain Aneurysm Foundation’s Timothy P. Susco and Andrew David Heitman Foundation Chairs of Research.
Footnotes
Conflict of Interest David Y. Chung, Sayona John, Wazim Mohamed, Monisha A. Kumar, and Guy A. Rordorf each declare no potential conflicts of interest. DaiWai M. Olson is the Editor in Chief, Journal of Neuroscience Nursing. Bradford B. Thompson reports Investigator Meeting travel-related reimbursements from BARD.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Critical Care
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Gigante P, Hwang BY, Appelboom G, Kellner CP, Kellner MA, Connolly ES. External ventricular drainage following aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 2010;24(6):625–32. [DOI] [PubMed] [Google Scholar]
- 2.Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711–37. 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 3.Fried HI, Nathan BR, Rowe AS, Zabramski JM, Andaluz N, Bhimraj A, et al. The insertion and management of external ventricular drains: an evidence-based consensus statement: a statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care. 2016;24(1):61–81•• A consensus statement from the Neurocritical Care Society which systematically reviewed the evidence on the insertion and management of EVDs and concluded that “EVD weaning should be accomplished as quickly as is clinically feasible so as to minimize the total duration of EVD monitoring and [ventriculostomy-related infection] risk.”.
- 4.Olson DM, Batjer HH, Abdulkadir K, Hall CE. Measuring and monitoring ICP in Neurocritical Care: results from a national practice survey. Neurocrit Care. 2014;20(1):15–20. [DOI] [PubMed] [Google Scholar]
- 5.Chung DY, Leslie-Mazwi TM, Patel AB, Rordorf GA. Management of external ventricular drains after subarachnoid hemorrhage: a multi-institutional survey. Neurocrit Care. 2016. 10.1007/s12028-016-0352-9• This study surveyed neurocritical care units in the United States. The authors found high practice variance and that the majority of institutions take a continuous CSF drainage and a gradual EVD wean approach.
- 6.Klopfenstein JD, Kim LJ, Feiz-Erfan I, Hott JS, Goslar P, Zabramski JM, et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. J Neurosurg. 2004;100(2):225–9. [DOI] [PubMed] [Google Scholar]
- 7.Amato A, Britz GW, James ML, Graffagnino C, Zomorodi AR, Zomorodi ME, et al. An observational pilot study of CSF diversion in subarachnoid haemorrhage. Nurs CritCare. 2011;16(5):252–60. [DOI] [PubMed] [Google Scholar]
- 8.Kim GS, Amato A, James ML, Britz GW, Zomorodi A, Graffagnino C, et al. Continuous and intermittent CSF diversion after subarachnoid hemorrhage: a pilot study. Neurocrit Care. 2011;14(1):68–72. [DOI] [PubMed] [Google Scholar]
- 9.Olson DM, Zomorodi M, Britz GW, Zomorodi AR, Amato A, Graffagnino C. Continuous cerebral spinal fluid drainage associated with complications in patients admitted with subarachnoid hemorrhage. J Neurosurg. 2013;119(4):974–80. [DOI] [PubMed] [Google Scholar]
- 10.Jabbarli R, Pierscianek D, ROlz R, Reinhard M, Darkwah Oppong M, Scheiwe C, et al. Gradual external ventricular drainage weaning reduces the risk of shunt dependency after aneurysmal subarachnoid hemorrhage: a pooled analysis. Oper Neurosurg (Hagerstown). 2018;15(5):498–504. 10.1093/ons/opy009• A 2 center retrospective study comparing rapid and gradual EVD weans after SAH. The authors found that the center that took a gradual EVD approach had a lower rate of ventriculoperitoneal shunt placement but a longer hospital length of stay.
- 11.Rao SS, Chung DY, Wolcott Z, Sheriff F, Khawaja AM, Lee H et al. Intermittent CSF drainage and rapid EVD weaning approach after subarachnoid hemorrhage: association with fewer VP shunts and shorter length of stay. J Neurosurg. 2019;1–6. doi: 10.3171/2019.1.JNS182702.••A before-and-after retrospective single center study which found that a change from a continuous/gradual EVD approach to an intermittent/rapid EVD approach resulted in fewer ventriculoperitoneal shunt placements, fewer EVD complications, and shorter length of stay.
- 12.Chung DY, Mayer SA, Rordorf GA. External ventricular drains after subarachnoid hemorrhage: is less more? Neurocrit Care. 2018;28(2):157–61. 10.1007/s12028-017-0443-2•A review focused on management and discontinuation of the EVD in the setting of SAH. The authors advocated for an early clamp trial, intermittent, and rapid weaning EVD strategy.
- 13.Kasuya H, Shimizu T, Kagawa M. The effect of continuous drainage of cerebrospinal fluid in patients with subarachnoid hemorrhage: a retrospective analysis of 108 patients. Neurosurgery. 1991; 28(1):56–9. [DOI] [PubMed] [Google Scholar]
- 14.Klimo P Jr, Kestle JR, MacDonald JD, Schmidt RH. Marked reduction of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 2004;100(2): 215–24. 10.3171/jns.2004.100.2.0215. [DOI] [PubMed] [Google Scholar]
- 15.Kramer AH, Fletcher JJ. Locally-administered intrathecal thrombolytics following aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurocrit Care. 2011;14(3):489–99. 10.1007/s12028-010-9429-z. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto S, Tsutsumi K, Yoshikawa G, Shinozaki MH, Yako K, Nagata K, et al. Effectiveness of the head-shaking method combined with cisternal irrigation with urokinase in preventing cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 2004;100(2):236–43. 10.3171/jns.2004.100.2.0236. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Desai NS, Ricci J, Stieg PE, Rosengart AJ, Hartl R, et al. Factors contributing to ventriculostomy infection. World Neurosurg. 2012;77(1):135–40. 10.1016/j.wneu.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Ascanio LC, Gupta R, Adeeb N, Moore JM, Griessenauer CJ, Mayeku J, et al. Relationship between external ventricular drain clamp trials and ventriculoperitoneal shunt insertion following nontraumatic subarachnoid hemorrhage: a single-center study. J Neurosurg. 2018;130(3):956–62. 10.3171/2017.10.JNS171644•A retrospective study which found that more clamp trials were associated with a lower rate of ventriculoperitoneal shunt placement.
- 19.Bales JW, Bonow RH, Buckley RT, Barber J, Temkin N, Chesnut RM. Primary external ventricular drainage catheter versus intraparenchymal ICP monitoring: outcome analysis. Neurocrit Care. 2019;31(1):11–21. 10.1007/s12028-019-00712-9. [DOI] [PubMed] [Google Scholar]
- 20.Chesnut RM. Intracranial pressure monitoring: headstone or a new head start. The BEST TRIP trial in perspective. Intensive Care Med. 2013;39(4):771–4. 10.1007/s00134-013-2852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471–81. 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. 10.1227/NEU.0000000000001432• These are widely cited guidelines for the management of severe TBI, including consensus-based recommendations for management of an EVD.
- 23.Liu H, Wang W, Cheng F, Yuan Q, Yang J, Hu J, et al. External ventricular drains versus intraparenchymal intracranial pressure monitors in traumatic brain injury: a prospective observational study. World Neurosurg. 2015;83(5):794–800. 10.1016/j.wneu.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 24.Servadei F, Picetti E. Intracranial pressure monitoring and outcome in traumatic brain injury: the probe does matter? World Neurosurg. 2015;83(5):732–3. 10.1016/j.wneu.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Valadka AB. Are external ventricular drains better than parenchymal intracranial pressure monitors in trauma patients? World Neurosurg. 2015;84(2):211–3. 10.1016/j.wneu.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 26.ACS TQIP best practices in the management of traumatic brain injury. 2015. https://www.facs.org/-/media/files/quality-programs/trauma/tqip/tbi_guidelines.ashx?la=en Accessed January 2015.
- 27.Nwachuku EL, Puccio AM, Fetzick A, Scruggs B, Chang Y-F, Shutter LA, et al. Intermittent versus continuous cerebrospinal fluid drainage management in adult severe traumatic brain injury: assessment of intracranial pressure burden. Neurocrit Care. 2014;20(1): 49–53. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Zimmermann LL, Ho N, Vespa P, Liao X, Hu X. Evaluation of a new catheter for simultaneous intracranial pressure monitoring and cerebral spinal fluid drainage: a pilot study. Neurocrit Care. 2018. 10.1007/s12028-018-0648-z• The authors validate dynamic ICP measurements taken from a new, commercially-available EVD which can drain CSF and measure ICP simultaneously.
- 29.Liu X, Zimmermann LL, Ho N, Vespa P, Liao X, Hu X. Cerebral vascular changes during acute intracranial pressure drop. Neurocrit Care. 2018. 10.1007/s12028-018-0651-4• The authors use a newly-validated EVD which can drain CSF and measure ICP simultaneously to dynamically measure ICP drops during CSF drainage.
- 30.Liu X, Zimmermann L, Vespa P, Hu X. Response to Letter to the Editor: Evaluation of a new catheter for simultaneous intracranial pressure monitoring and cerebral spinal fluid drainage: a pilot study. Neurocrit Care. 2019;31(1):227–8. 10.1007/s12028-019-00756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson DM, Atem F, Busch DR. Evaluation of a new catheter for simultaneous intracranial pressure monitoring and cerebral spinal fluid drainage: a pilot study. Neurocrit Care. 2019;31 (1):225–6. https://doi.org/10d007/s12028-019-00722-7. [DOI] [PubMed] [Google Scholar]
- 32.Dey M, Jaffe J, Stadnik A, Awad IA. External ventricular drainage for intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2012;12(1):24–33. 10.1007/s11910-011-0231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinson HE, Hanley DF, Ziai WC. Management of intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2010;10(2):73–82. 10.1007/s11910-010-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naff N, Williams MA, Keyl PM, Tuhrim S, Bullock MR, Mayer SA, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42(11):3009–16. 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb AJ, Ullman NL, Mann S, Muschelli J, Awad IA, Hanley DF. Resolution of intraventricular hemorrhage varies by ventricular region and dose of intraventricular thrombolytic: the Clot Lysis: Evaluating Accelerated Resolution of IVH (CLEAR IVH) program. Stroke. 2012;43(6):1666–8. 10.1161/STROKEAHA.112.650523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603–11. 10.1016/S0140-6736(16)32410-2•• A randomized trial which investigated the effect of clearing IVH in the third and fourth ventricles with alteplase through an EVD on functional outcomes. The primary outcome of good functional outcome was no different between alteplase and control groups, but there was a suggestion that EVD location could influence resolution of intraventricular blood.
- 37.Olson DM, Ortega Perez S, Ramsay J, Venkatasubba Rao CP, Suarez JI, McNett M, et al. Differentiate the source and site of intracranial pressure measurements using more precise nomenclature. Neurocrit Care. 2019;30(2):239–43. 10.1007/s12028-018-0613-x• A review focused on appropriate terminology and reporting of ICP measurements.
- 38.Zhang X, Medow JE, Iskandar BJ, Wang F, Shokoueinejad M, Koueik J, et al. Invasive and noninvasive means of measuring intracranial pressure: a review. Physiol Meas. 2017;38(8):R143–R82. 10.1088/1361-6579/aa7256• A review on invasive and non-invasive approaches to ICP measurement.
- 39.Rogers M, Stutzman SE, Atem FD, Sengupta S, Welch B, Olson DM. Intracranial pressure values are highly variable after cerebral spinal fluid drainage. J Neurosci Nurs. 2017;49(2):85–9. 10.1097/JNN.0000000000000257• A prospective study which found a very low probability that any single ICP observation made during a 15-minute period is reflective of the entire period.
- 40.O’Phelan K, Shepard S, DeJesus-Alvelo I. Controversies in intracranial pressure monitoring In: Koenig M, editor. Cerebral herniation syndromes and intracranial hypertension. New Brunswick, New Jersey: Rutgers University Press; 2016. [Google Scholar]
- 41.Zacchetti L, Magnoni S, Di Corte F, Zanier ER, Stocchetti N. Accuracy of intracranial pressure monitoring: systematic review and meta-analysis. Crit Care. 2015;19:420 10.1186/s13054-015-1137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson DM, Rogers MS, Stutzman SE. Electronic medical record validation: exploring the reliability of intracranial pressure data abstracted from the electronic medical record-pilot. J Nurs Meas. 2015;23(3):532–40. 10.1891/1061-3749.233.532. [DOI] [PubMed] [Google Scholar]
- 43.Olson DM, Lewis LS, Bader MK, Bautista C, Malloy R, Riemen KE, et al. Significant practice pattern variations associated with intracranial pressure monitoring. J Neurosci Nurs. 2013;45(4): 186–93. [DOI] [PubMed] [Google Scholar]
- 44.Wijayatilake DS, Talati C, Panchatsharam S. The monitoring and management of severe traumatic brain injury in the United Kingdom: is there a consensus?: A National Survey. J Neurosurg Anesthesiol. 2015;27(3):241–5. [DOI] [PubMed] [Google Scholar]
- 45.Samudra NP, Park SM, Gray SE, Sebai MA, Olson DM. Inconsistency in reporting variables related to intracranial pressure measurement in scientific literature. J Nurs Meas. 2018;26(3):415–24. 10.1891/1061-3749.26.3.415• A review of clinical trials that reported ICP as variables and which found that fewer than 50% of citations reported ICP site and only 23% reported patient position.
- 46.Hickey JV, Olson DM, Turner DA. Intracranial pressure waveform analysis during rest and suctioning. Biol Res Nurs. 2009;11(2): 174–86. 10.1177/1099800409332902. [DOI] [PubMed] [Google Scholar]
- 47.Yao LL, Hu XY. Factors affecting cerebrospinal fluid opening pressure in patients with spontaneous intracranial hypotension. J Zhejiang Univ Sci B. 2017;18(7):577–85. 10.1631/jzus.B1600343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hockel K, Schuhmann MU. ICP monitoring by open extraventricular drainage: common practice but not suitable for advanced neuromonitoring and prone to false negativity. Acta Neurochir Suppl. 2018;126:281–6. 10.1007/978-3-319-65798-1_55. [DOI] [PubMed] [Google Scholar]
- 49.Marmarou A, Anderson RL, Ward JD, Choi SC, Young HF, Eisenberg HM, et al. NINDS Traumatic Coma Data Bank: intracranial pressure monitoring methodology. J Neurosurg. 1991;75(Supplement):S21–S7. [Google Scholar]
- 50.Arroyo-Palacios J, Rudz M, Fidler R, Smith W, Ko N, Park S, et al. Characterization of shape differences among ICP pulses predicts outcome of external ventricular drainage weaning trial. Neurocrit Care. 2016;25(3):424–33. 10.1007/s12028-016-0268-4• A retrospective study of SAH patients which found that a morphological clustering analysis of ICP pulse could predict the outcome of an EVD weaning trial.