Abstract
The commonest etiologies of new-onset pancytopenia are congenital bone marrow failure syndromes, marrow space-occupying lesions, infections, and peripheral destruction. Nutritional deficiencies, including folate and vitamin B12, can occasionally cause pancytopenia. We report a 48-year-old gentleman who presented with a 1-week history of dizziness and upper gastrointestinal bleeding. Laboratory evaluation revealed pancytopenia, macrocytosis, toxic neutrophils, hemolysis, suppressed reticulocyte count, positive direct anti-globulin test (DAT), severely reduced B12 levels, and positive anti-intrinsic factor and anti-parietal cell antibodies. He was started on weekly intramuscular B12 supplementation and showed improvement in blood cell counts during follow-up. Recognition of B12 deficiency as a cause of pancytopenia and DAT-positive autoimmune hemolytic anemia can help to avoid unwanted investigations and aid in early diagnosis and treatment.
Keywords: Pernicious anemia, Pancytopenia, Megaloblastic anemia, B12 deficiency
Introduction
The commonest etiologies of new-onset pancytopenia include congenital bone marrow failure syndromes, aplastic anemia, paroxysmal nocturnal hemoglobinuria, myelofibrosis, drugs, such as chloramphenicol, nonsteroidal anti-inflammatory drugs, antithyroid drugs, corticosteroids, penicillamine, allopurinol, and gold, infections, autoimmune diseases and peripheral destruction [1]. Nutritional deficiencies are a rare cause of pancytopenia. Copper deficiency can cause pancytopenia, and many such patients are initially misdiagnosed with myelodysplastic syndrome and referred for allogeneic bone marrow transplantation [2]. Classic clinical symptoms of B12 deficiency include features of peripheral nervous system dysfunction, such as sensory loss, hyporeflexia, and paresthesia, autonomic nervous system dysfunction, such as postural hypotension and incontinence, central nervous system dysfunction, such as megaloblastic madness, myelopathy, subacute combined degeneration of spinal cord, optic atrophy, loss of taste, and glossitis, and hematological abnormalities, such as macrocytic red cells and hypersegmented neutrophils [3]. Severe pancytopenia and hemolysis occur in some patients with severe B12 deficiency in addition to megaloblastic anemia. The reticulocyte count in such cases is usually suppressed, suggesting bone marrow dysfunction.
Case Report
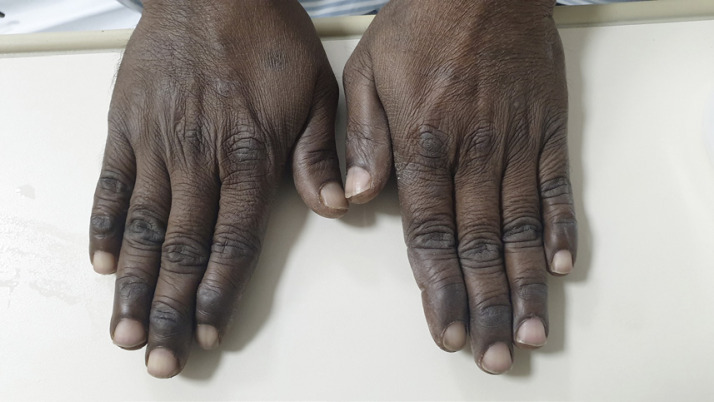
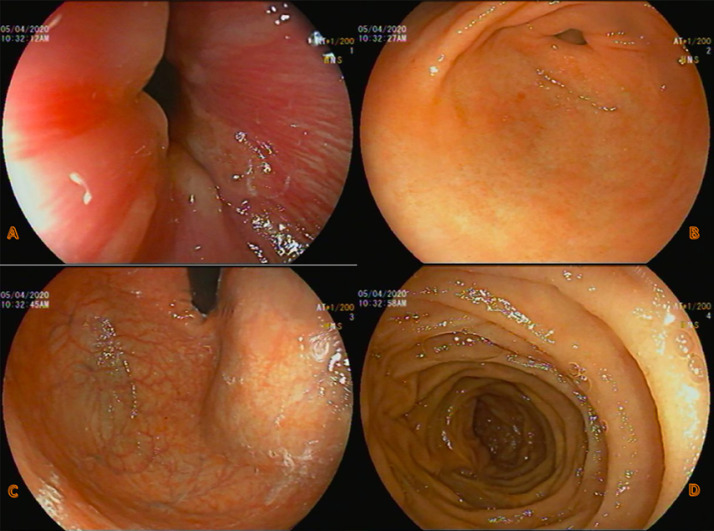
A 48-year-old gentleman came to the emergency department with complaints of dizziness and generalized weakness for 3 days. Dizziness was explained by him as a feeling of lightheadedness when standing up. There was no syncope or vertigo. His stool was dark and tar-like for 1 week. He denied any chest or abdominal pain, nausea, or vomiting. The review of systems was negative. His past was not significant for any medical or surgical conditions. He was a chronic tobacco chewer for 20 years but never smoked or used alcohol. He was a lacto-ovo-vegetarian and was working as a steel fitter in construction sites. Family history was negative for any easy bruising, bleeding, or clotting disorders. General examination showed tachycardia with a heart rate of 104, normal blood pressure and saturation. He was afebrile. There was scleral icterus, conjunctival pallor, and hyperpigmentation of the knuckles of both hands (Fig. 1). Oral hygiene was poor with tobacco staining of teeth. Chest and abdomen were normal without organomegaly. Neurological exam showed diminished reflexes in all 4 limbs. He was walking with a wide-based gait. Per-rectal examination was positive for melena. Urine dipstick was negative for hematuria. Complete blood counts on admission showed pancytopenia and macrocytic anemia with high red cell distribution width. There was significant neutropenia (Table 1). His PT-INR was elevated (16, 1.4). Peripheral smear showed features of pancytopenia, macrocytosis with hypersegmented neutrophils, few spherocytes and schistocytes. Hemolysis workup showed indirect hyperbilirubinemia, high lactate dehydrogenase (LDH), low haptoglobin, normal reticulocyte count, and positive direct antiglobulin (DAT) (Table 2). Serum iron studies and thyroid functions were normal, but vitamin B12 level was remarkably low (<37). Folate level was normal (35.12 nmol/L). Anti-intrinsic factor (IF) antibodies were positive with a level of 17.0 U/mL, and anti-parietal cell antibodies were positive at a titer of 1:160. Autoimmune tests including ANA, anti-DNA, and rheumatoid factor were negative. As a part of pancytopenia evaluation, a pan CT scan was done which was unremarkable. Upper gastrointestinal endoscopy was normal except for generalized gastritis (Fig. 2). He was started on intramuscular vitamin B12 injection of 1,000 μg once weekly. His blood cell counts started showing an upward trend on day 4 after starting the treatment. He was discharged asymptomatic with outpatient appointments for weekly B12 injections after 10 days of hospital stay. The complete and differential cell counts and vitamin B12 levels on discharge are shown in Table 1
Fig. 1.
Hyperpigmentation of the knuckles of both hands.
Table 1.
Complete differential cell counts and vitamin B12 levels at the time of admission and discharge
| Patient's values |
Normal range | ||
|---|---|---|---|
| on admission | on discharge | ||
| WBC, ×103/µL | 1.4 | 3.0 | 4.0–10.0 |
| RBC, ×106/µL | 1.9 | 3.0 | 4.5–5.5 |
| Hemoglobin, g/dL | 6.5 | 9.8 | 13.0–17.0 |
| Hematocrit, % | 19.5 | 29.0 | 40.0–50.0 |
| MCV, fL | 102.6 | 95.7 | 83.0–101.0 |
| MCH, pg | 34.2 | 32.3 | 27.0–32.0 |
| MCHC, g/dL | 33.3 | 33.8 | 31.5–34.5 |
| RDW-CV, % | 21.8 | 20.3 | 11.6−l4.5 |
| Absolute neutrophil count (ANC), ×103/µL | 0.4 | 1.0 | 2.0–7.0 |
| Lymphocytes, ×103/µL | 0.91 | 1.45 | 1.00–3.00 |
| Monocytes, ×103/µL | 0.09 | 0.53 | 0.20–1.00 |
| Eosinophils, ×103/µL | 0.0 | 0.0 | 0.0–0.5 |
| Basophils, ×103/µL | 0.00 | 0.02 | 0.02–0.10 |
| Platelets, ×103/µL | 58 | 66 | 150–400 |
| Vitamin B12, pmol/L | <37 | 369 | 133–675 |
Table 2.
Results of hemolysis workup
| Patient's values | Normal range | |
|---|---|---|
| Bilirubin total, µmol/L | 27.7 | 3.4–20.5 |
| Bilirubin indirect, mmol/L | 21.6 | 0.0–3.0 |
| LDH, U/L | 1,117 | 125–220 |
| Haptoglobin, mg/dL | <10 | 30–200 |
| Reticulocyte count, | 1.5 | 0.5–2.5 |
| DAT | positive | |
| DAT AHG | W+ | |
| DAT C3D | negative | |
| Elution result | negative | |
| DAT CTR | negative |
Fig. 2.
Upper gastrointestinal endoscopy images which were normal except for generalized gastritis. A Gastroesophageal junction. B Body (greater curvature). C Pylorus. D D1 duodenum.
Discussion/Conclusion
Vitamin B12, also known as cobalamin, cannot be synthesized by human cells and is typically derived from animal sources, including meat, liver, dairy products, and eggs. The recommended daily intake of vitamin B12 is 2.4 μg, and the total body store is 2–5 mg, the bulk of which is in the liver. Hence, B12 deficiency develops only when its dietary supplementation or absorption is affected for more than 2 years [4]. Risk factors for vitamin B12 deficiency are decreased ileal absorption (Crohn's disease, ileal resection, tapeworm infection), decreased IF (atrophic gastritis, pernicious anemia [PA], postgastrectomy syndrome), transcobalamin II deficiency, inadequate intake (vegans, exclusively breastfed infants of vegan mothers), alcohol abuse, age >75 years, and prolonged medication use (H2 antihistamine or proton pump inhibitor >1 year, metformin >4 months). Screening is recommended in those with 1 or more of these risk factors [5]. PA refers to vitamin B12 deficiency caused by autoantibodies that interfere with its absorption by targeting IF, gastric parietal cells, or both. The sensitivity of IF autoantibodies is relatively low (70%), but their specificity is high; if present, anti-IF autoantibodies are considered confirmatory for a diagnosis of PA. Many patients with PA have autoantibodies to parietal cells; however, anti-parietal cell antibodies are also seen in some patients with gastritis and are not diagnostic of PA [4]. Pancytopenia is a rare hematological manifestation of vitamin B12 deficiency. Commonest are macrocytosis (54%), hypersegmented neutrophils (32%), leukopenia (14%), and thrombocytopenia (10%). Pancytopenia was seen in 5% of patients and hemolytic anemia in 1.5% [6]. Hemolysis in B12 deficiency may be intramedullary or extramedullary. Intramedullary hemolysis is more common and is due to destruction of megaloblastic cells by bone marrow macrophages. Extramedullary hemolysis results from impaired deformability of the red blood cell membrane leading to their fragmentation in capillaries. This is known as pseudo-thrombotic microangiopathy. Hyper-homocysteinemia in B12 deficiency causes endothelial dysfunction leading to extramedullary hemolysis [7]. PA can be associated with autoimmune hemolytic anemia diagnosed with a positive Coombs test, as in our patient. In such cases, if anemia does not respond to vitamin B12 supplementation, corticosteroid therapy or therapeutic splenectomy is indicated. Forshaw and Harwood [8] studied the association of a positive antiglobulin reaction with megaloblastic erythropoiesis. Thirty-two patients with megaloblastic anemia were investigated, and the direct antiglobulin test was positive in 10. There was no correlation between the result of the test and the degree of anemia, and no difference in the incidence of positive results between PA and other causes. The antiglobulin test became negative in 9 patients after they had received treatment with vitamin B12 for 1–3 weeks. Recognition of vitamin B12 deficiency as a cause of pancytopenia and DAT-positive autoimmune hemolytic anemia can help in avoiding unwanted investigations and aid in early diagnosis and treatment.
Statement of Ethics
The case was approved by Hamad Medical Corporation Medical Research Center. Written informed consent was given by the patient to publish his case information and details.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The publication of this article was funded by the Qatar National Library.
Author Contributions
Sreethish Sasi: manuscript writing, manuscript editing, and patient management. He will act as a study guarantor. Mohamed A. Yassin: concept, manuscript review, and manuscript editing. All authors have approved the final version of the manuscript.
Acknowledgment
The authors would like to acknowledge the Internal Medicine Residency Program of Hamad Medical Corporation for scientific support.
References
- 1.Weinzierl EP, Arber DA. Bone marrow evaluation in new-onset pancytopenia. Hum Pathol. 2013 Jan 1;44((6)):1154–64. doi: 10.1016/j.humpath.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Kohla S, Ali E, Amer A, Yousif T, Yassin MA. A rare case of severe copper deficiency in an infant with exclusive breast feeding mimicking myelodysplastic syndrome. Case Rep Oncol. 2020;13((1)):62–8. doi: 10.1159/000505483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kollipara VK, Brine PL, Gemmel D, Ingnam S. A case of asymptomatic pancytopenia with clinical features of hemolysis as a presentation of pernicious anemia. J Community Hosp Intern Med Perspect. 2016;6((4)):32493. doi: 10.3402/jchimp.v6.32493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood. 2017 Nov;129((19)):2603–11. doi: 10.1182/blood-2016-10-569186. [DOI] [PubMed] [Google Scholar]
- 5.Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. 2017 Sep 15;96((6)):384–9. [PubMed] [Google Scholar]
- 6.Andrès E, Affenberger S, Zimmer J, Vinzio S, Grosu D, Pistol G, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. 2006 Feb;28((1)):50–6. doi: 10.1111/j.1365-2257.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 7.Acharya U, Gau JT, Horvath W, Ventura P, Hsueh CT, Carlsen W. Hemolysis and hyperhomocysteinemia caused by cobalamin deficiency: three case reports and review of the literature. J Hematol Oncol. 2008 Dec;1:26. doi: 10.1186/1756-8722-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forshaw J, Harwood L. The direct antiglobulin (Coombs) test in megaloblastic anaemia. J Clin Pathol. 1965 Jan;18((1)):119–20. doi: 10.1136/jcp.18.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]