Abstract
The use of immune checkpoint inhibitors has dramatically improved the chance of surviving malignant melanomas; however, the effect comes at the cost of toxicities that are difficult to predict. Immune-mediated hepatitis is the most common form of liver toxicity, but fatal outcome is uncommon. We report the case of a 70-year-old female with metastatic malignant melanoma who developed severe liver toxicity characterized by bile duct injury and cholestasis. The condition progressed despite potent immunosuppressive treatment, plasmapheresis, and intensive supportive care; and the patient died while still having tumor response.
Keywords: Immune checkpoint inhibitors, Vanishing bile duct syndrome, Malignant melanoma
Introduction
The use of immune checkpoint inhibitors (ICPIs) has dramatically improved the chance of surviving malignant melanomas. Pembrolizumab is a monoclonal antibody that selectively inhibits programmed cell death-1 (PD1) activity at the tumor site, thus being able to restore anti-tumor immune response. In metastatic disease, pembrolizumab significantly improves overall survival, with 55% of all treated patients still being alive 24 months after treatment start.
Liver toxicity is a rare complication of pembrolizumab, with 2% of all patients included in the Checkpoint 006 study experiencing grade 3–4 injury [1]. A recent meta-analysis suggests that the risk of hepatotoxicity related to ICPIs largely depends on the type of cancer treated, dosing schedule, and the regimen used, with the combination of ipilimumab and nivolumab posing the highest risk [2].
The pathophysiological mechanisms of immune-related adverse events (irAEs) are poorly understood. Immune-mediated hepatitis is believed to be mediated by an immune-related T-cell activation, but it differs in several respects from both drug-induced and idiopathic autoimmune hepatitis [3]. Details in the reporting of hepatotoxicity vary widely across publications, with some studies registering isolated elevations of various liver tests, for example, alanine aminotransferase (ALT), aspartate aminotransferase, alkaline phosphatase (ALP), γ-glutamyl transpeptidase, or bilirubin, while others use general terms such as “liver toxicity” or “hepatitis.”
Mortality due to liver toxicity is a rare event in prospective studies, but in a retrospective analysis of individual safety data from the World Health Organization database VigiLyze, approximately 22% of all irAE-related deaths in patients on anti-PD1/PD-L1 monotherapy were caused by liver injury [4].
The general algorithm for managing irAEs is based on treatment with high-dose steroids with prompt conversion to other immunosuppressants in case of treatment failure [5]. A thorough investigation to exclude other causes of liver dysfunction should be performed before or simultaneously with immunosuppressive treatments, and this should include virology tests, radiological assessment, and liver biopsy. Liver-specific autoimmune antibody tests are not always elevated in case of irAEs.
Case Report
A 70-year-old Caucasian female with no prior history of cancer was examined in December 2017, after the discovery of a growing lump in her left axilla. Her past medical history included hypertension, paroxysmal tachycardia, and gout. Her medication was lisinopril dehydrate, verapamil colchicine, and allopurinol. A biopsy of the axillary mass revealed lymph node metastasis from a malignant melanoma, BRAF wild type. A CT scan showed several small lung nodules and enlarged lymph nodes on the left side of her neck, giving suspicion of disseminated malignancy. No primary tumor was identified at skin checkup.
At assessment in our outpatient clinic, she was in good clinical condition (ECOG 1) and her blood tests revealed activated inflammatory parameters (CRP 103 mg/L, leukocytes 11.8 × 109/L, granulocytes 8 × 109/L), normal liver function (ALT, γ-glutamyl transpeptidase, bilirubin, and ALP), and normal kidney function (creatinine). LDH was moderately elevated at 353 U/L.
She was offered treatment with pembrolizumab 2 mg/kg every 3 weeks and received the regimen as scheduled. Reimaging after 5 cycles of treatment showed good partial response and her CRP and LDH had normalized. At that time point, she was experiencing itching and skin rash corresponding to grade 2 toxicity [6] that were successfully managed with topical corticosteroid as well as hypothyroidism that was corrected with levothyroxine.
During treatment, her laboratory results including liver tests were followed every 3 weeks and were normal until symptom onset.
After the twelfth infusion with pembrolizumab, she was admitted to the hospital in poor clinical condition; she had developed jaundice and suffered from painful joints and inspiratory chest pain. Her blood examinations showed: CRP 19 mg/L, hemoglobin 15 g/dL, leukocytes 15.1 × 109/L, Na 132 mmol/L, creatinine 137 μmol/L, ALT 217 U/L, ALP 417 U/L, LD 369 U/L, bilirubin 216 μmol/L, and S-glucose 22 mmol/L.
The tentative diagnosis upon admission was irAEs affecting the liver, kidney, pancreas, joints, and possibly lungs and/or pleura. The patient received intravenous treatment with methyl prednisolone 125 mg daily and insulin along with adequate supportive care according to international guidelines [5]. Imaging with liver ultrasound and CT of the chest and abdomen excluded tumor progression or other organ-related causes. Serum virology test (hepatitis B and C, HBV, cytomegalovirus) were normal. Magnetic resonance cholangiopancreatography was not considered necessary based on previous radiology findings.
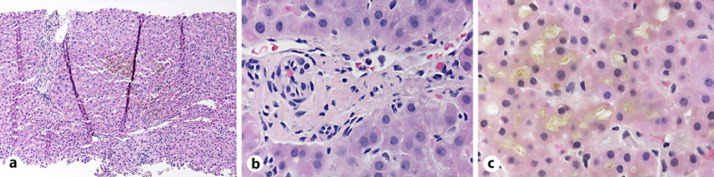
Three days after admission, her symptoms improved; and creatinine was normalized, while ALT, ALP, and bilirubin levels continued to rise (Fig. 1). Given the lack of improvement in her liver tests, mycophenolate 1 g twice daily was added to her corticosteroid treatment. A liver biopsy was obtained and showed portal fibrosis and inflammation dominated by lymphocytes, with scattered histiocytes and a few plasma cells and only focal interface activity. The most striking finding was the lack of identifiable bile ducts in the majority of the portal tracts, as well as the absence of ductular proliferation. In the lobules, there was extensive intracellular and intracanalicular cholestasis, mild lymphocytic inflammation, and mild microvesicular steatosis (Fig. 2).
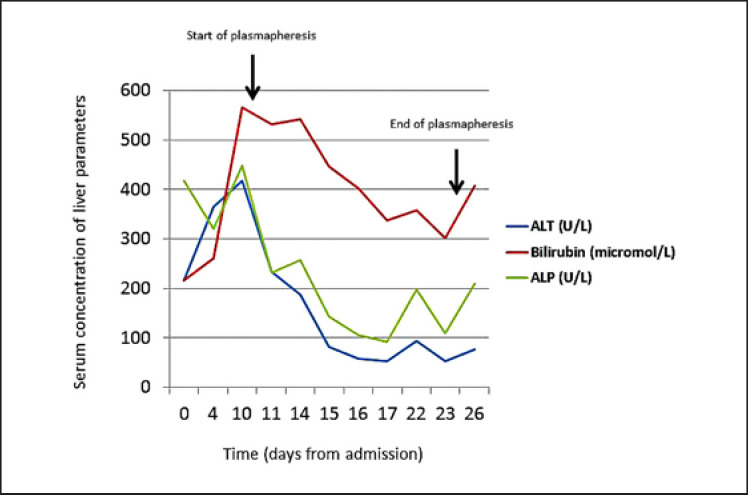
Fig. 1.
Graph showing the changes in liver tests over time. Start and stop of plasmapheresis is depicted on the graph.
Fig. 2.
Photomicrographs showing liver biopsy findings, including a mild portal and lobular inflammation, b small portal tract with bile duct loss, and c marked intracanalicular and intracellular cholestasis.
Ten days after admission and despite high-dose corticosteroids and mycophenolate, her liver tests continued to worsen, and plasmapheresis was instituted along with doubling of the mycophenolate dose. She received 8 cycles in total of plasmapheresis that were given daily in the early phase. All her liver tests improved slightly, but we observed a rapid deterioration when plasmapheresis was delayed (e.g., during weekends), thus indicating a short-lived effect of this treatment modality. Her clinical condition eventually worsened with no signs of reversible cause, and she died on day 26 after symptom onset.
Discussion
We present here the case of an atypical form of liver toxicity associated with ICPIs therapy, histologically characterized by severe ductopenia and cholestasis. According to previous reports, the predominant histopathological findings in liver injury associated with ICPIs, including pembrolizumab, include hepatitis with lobular inflammation and some degree of portal and/or periportal inflammation, sometimes with bile duct injury [3, 7, 8]. The biopsy findings in the present case are suggestive of a rare form of liver injury known as vanishing bile duct syndrome (VBDS). This type of injury may be caused by an infection, autoimmune disease, ischemia, and drugs, or it may occur as a paraneoplastic event. This condition is characterized by a progressive destruction of intrahepatic bile ducts resulting in severe cholestasis. The prognosis is largely dependent on the underlying cause and whether epithelial regeneration occurs.
A retrospective register-based analysis [9] showed a mortality rate of 20% among patients with drug-related VBDS, with the extent of ductopenia being the most important predictive factor for outcome. Doherty et al. [10] reported a series of 3 cases of severe intrahepatic biliary injury that occurred under treatment with ICPIs, one of which presented as VBDS after a single infusion of pembrolizumab. The pathogenesis of this type of ICPI-related biliary injury is unknown, but the lack of autoimmune hepatitis-like features, and the apparently poor effect of immunosuppressive treatment, suggests involvement of mechanisms distinct from those of immune-mediated hepatitis.
Liver toxicity due to ICPIs is treated with high-dose corticosteroids (1–2 mg prednisolone/kg/day). In the event of treatment failure, mycophenolate should be added. The TNF-alpha inhibitor infliximab, which is commonly used to treat gastrointestinal immunological side effects, is not recommended due to concerns regarding added liver toxicity. Other immunosuppressive alternatives are tacrolimus or anti-thymocyte globulin. Our patient showed no treatment response to either corticosteroids or mycophenolate. Due to the rapid aggravation of her liver function, plasmapheresis was started with the intention of reducing exposure to pathogenic autoantibodies. We observed a slight but only temporary improvement in her liver parameters with this treatment modality (Fig. 1).
The median time from onset of symptoms to death from irAEs due to ICPIs (all toxicities analyzed together) is 40 days in the retrospective analysis of Wang et al. [4]. In the other reported case of VBDS due to pembrolizumab, toxicity occurred after the first infusion [10]. In the case of our patient, liver injury became manifest almost 10 months after treatment initiation. We also noticed several other toxicities occurring prior to or concurrently with liver toxicity (dermatitis, hypothyroidism, arthritis, diabetes, and possibly pneumonitis/pleuritis).
Clinical features, time to onset and severity of liver injury associated with ICPIs vary widely and make prediction and management of these conditions challenging. To our knowledge, this is the second reported case of VBDS associated with treatment with ICPIs, and the first case with fatal outcome. With the dramatic increase in the use of checkpoint inhibitors across cancer types, efforts should be made to improve the understanding of toxicities and to optimize their treatment, especially in the context of complex, severe irAEs. The identification of cases with severe biliary ductal injury may be particularly important, as these may require other treatment regimens than patients with immune-mediated hepatitis.
Statement of Ethics
Publication of these data was approved post mortem by the patient's closest family and by the Data Security Office at Vestre Viken HF, Norway.
Disclosure Statement
T.T., T.L., and H.M.R. have nothing to declare. A.C.P. has received honorarium from Brystol-Myers Squibb Norway for scientific lectures.
Funding Sources
None.
Author Contributions
All authors contributed to the treatment and clinical evaluation of this patient as well as to the preparation of this manuscript.
References
- 1.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017 Oct 21;390((10105)):1853–62. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Lie P, Guo M, He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis of published data. Int J Cancer. 2017 Sep 1;141((5)):1018–28. doi: 10.1002/ijc.30678. [DOI] [PubMed] [Google Scholar]
- 3.Jennings JJ, Mandaliya R, Nakshabandi A, Lewis JH. Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol. 2019 Mar;15((3)):231–44. doi: 10.1080/17425255.2019.1574744. [DOI] [PubMed] [Google Scholar]
- 4.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018 Dec 1;4((12)):1721–8. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul 1;28((Suppl l_4)):iv119–iv42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE) 2017. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- 7.Aivazian K, Long GV, Sinclair EC, Kench JG, McKenzie CA. Histopathology of pembrolizumab-induced hepatitis: a case report. Pathology. 2017 Dec;49((7)):789–2. doi: 10.1016/j.pathol.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 8.De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018 Jun;68((6)):1181–90. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017 Apr;65((4)):1267–7. doi: 10.1002/hep.28967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV, et al. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO open. 2017;2((4)):e000268. doi: 10.1136/esmoopen-2017-000268. [DOI] [PMC free article] [PubMed] [Google Scholar]