Abstract
Ethnopharmacological relevance
Multiple plant species were used traditionally in southern Africa to treat bacterial respiratory diseases. This review summarises this usage and highlights plant species that are yet to be verified for these activities.
Aim of the study
This manuscript reviews the traditional usage of southern African plant species to treat bacterial respiratory diseases with the aim of highlighting gaps in the literature and focusing future studies.
Materials and methods
An extensive review of ethnobotanical books, reviews and primary scientific studies was undertaken to identify southern African plants which are used in traditional southern African medicine to treat bacterial respiratory diseases. We also searched for southern African plants whose inhibitory activity against bacterial respiratory pathogens has been conmfirmed, to highlight gaps in the literature and focus future studies.
Results
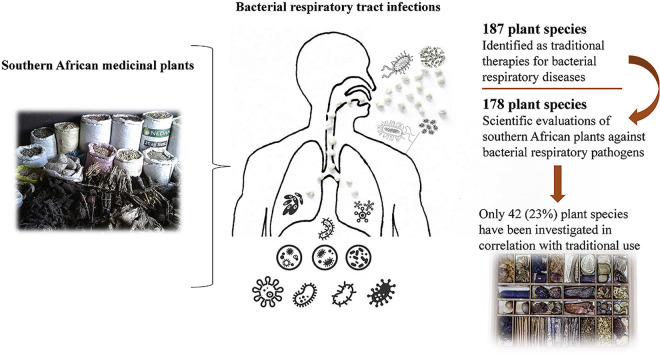
One hundred and eighty-seven southern African plant species are recorded as traditional therapies for bacterial respiratory infections. Scientific evaluations of 178 plant species were recorded, although only 42 of these were selected for screening on the basis of their ethnobotanical uses. Therefore, the potential of 146 species used teraditionally to treat bacterial respiratory diseases are yet to be verified.
Conclusions
The inhibitory properties of southern African medicinal plants against bacterial respiratory pathogens is relatively poorly explored and the antibacterial activity of most plant species remains to be verified.
Keywords: Southern African plants, Tuberculosis, Diphtheria, Pertussis, Whooping cough, Pneumonia, Traditional medicine
Graphical abstract
1. Introduction
Four diseases account for the majority of bacterial respiratory infections globally. Of these, tuberculosis (caused by Mycobacterium tuberculosis) has the greatest burden and is classified as one of the top ten causes of death globally (Floyd et al., 2018). This disease is highly contagious and is readily spread via airborne transmission. Indeed, the World Health Organisation (WHO) estimates that more than 10 million people fell ill with tuberculosis in 2018, with 1.5 million people dying from the disease (WHO, 2019a). Of the people contracting M. tuberculosis infections, only 10% develop the active form of the disease and fall ill (Houben and Dodd, 2016). Therefore, it is estimated that a pool of 100 million new potentially infective people contracted M. tuberculosis infections in 2018. Bacterial pneumonia is also a considerable cause of mortality and morbidity. Indeed, it is classed as the second highest cause of mortality of any communicable disease (after tuberculosis), with more than 800,000 deaths estimated in 2017 (WHO, 2018). Diphtheria and pertussis caused similarly high mortality rates prior to the widespread introduction of effective vaccination (Holý et al., 2017). Vaccines have been particularly effective and the rates of infection and mortality have decreased dramatically. For example, the number of reported cases of diphtheria decreased from >1 million cases in 1980 to approximately 4500 in 2018 (WHO, 2019b; Holý et al., 2017). Similar trends for the incidence of pertussis have been reported although it still causes a considerable health burden, with 150,000 new cases and 90,000 deaths reported in 2018 (WHO, 2019c; (Holý et al., 2017). This review concentrates on the use of southern African plants to treat these four diseases due to their relevance to southern African health. Whilst other bacteria such as Legionella pneumophila (Legionaires disease) may also cause respiratory diseases, they make relatively minor contributions to southern African health (Muchesa et al., 2018) and thus are not a focus of this review.
1.1. Tuberculosis
Tuberculosis (TB) is the most serious of the bacterial respiratory infections globally (Floyd et al., 2018). It is classified by the World Health Organisation (WHO) as one of the top ten causes of death globally, and the leading cause of death by a single pathogen, ranking substantially ahead of human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) (WHO, 2019a). TB has a high infection rate due to its air-borne route of transmission. When an infected person coughs or sneezes (or even talks), small droplets of saliva containing the bacterium are released and dispersed into the air. If other individuals breathe in these droplets, they may also become infected. The spread from person to person is rapid and there is a high infection rate. Indeed, the WHO estimates that approximately a quarter of the world's population is infected with M. tuberculosis at any given time and thus at risk of developing TB (WHO, 2019a; Floyd et al., 2018). These high rates of infection also increase the risks of infection to non-infected members of the population.
Tuberculosis generally affects the lungs, although it can also infect other parts of the body. Most infections are asymptomatic and are known as latent TB. People with latent TB do not suffer from the disease's symptoms, nor do they generally spread the disease (Furin et al., 2019). However, they have the potential to spread the bacterium if the disease progresses and therefore they constitute a substantial potential disease reservoir. However, active TB infections are substantially more frequent in immune-compromised individuals, such as people with HIV/AIDS (Pawlowski et al., 2012). When active TB develops, there is a high mortality rate, with approximately 50% of afflicted individuals dying unless they receive timely and effective medical treatment (Floyd et al., 2018).
A number of symptoms are evident in people with TB: chronic coughing with blood containing sputum, fever, night sweats and rapid weight loss (which is responsible for the historical name ‘consumption’) (Furin et al., 2019). Medical intervention for TB most frequently involves vaccination and >90% of children are vaccinated globally. The efficacy of the vaccine is low, decreasing the risk of acquiring a M. tuberculosis by only 20%. However, once an infection occurs, the vaccination decreases the chances that the infection will develop from a latent to active form of TB by 60%. If active TB develops, treatment with antibiotics for 6–9 months is often effective in curing the disease and blocking the transmission from the infected person to others (Nguyen, 2016). However, limited classes of antibiotics are effective against M. tuberculosis as the cell wall blocks cell entry for most antibiotic classes. A combination of rifampicin, isoniazid, pyrazinamide and ethambutol is generally used to increase the efficacy of the treatment (Furin et al., 2019). Of considerable concern, there are increasing reports of M. tuberculosis strains with resistance to these antibiotics, rendering these combinations of little use (Nguyen, 2016). Effective new antibiotic therapies are urgently required to treat these resistant strains.
1.2. Diphtheria
Diphtheria is another bacterial infection that can cause substantial mortality. Indeed, 5–10% of infections result in death, although the mortality rate can be as high as 20% in children less than 5 years of age and in adults over 40 years old (WHO, 2019b; Holý et al., 2017). Outbreaks are rare in developed countries due to medical advances, although they are more common in developing populations. Diphtheria primarily affects the upper respiratory tract, causing symptoms that range from mild to severe (Truelove et al., 2019). The disease is caused by the bacterium Cornyebacterium diphtheria and is transmitted in a similar way to TB: from person to person via air-borne pathways. Once a person breathes in the bacterium, disease progression is rapid, with symptoms usually evident within 2–5 days after exposure. The symptoms may include a sore throat, fever, chills, fatigue, cyanosis, coughs, headaches, difficulty swallowing and swollen lymph nodes, resulting in swelling of the neck. Grey or white pseudo-membrane patches may also develop in the throat of infected people, restricting the airways and causing a ‘barking’ cough similar to that seen for croup. In severe cases, myocarditis, nerve inflammation, renal disease and decreased blood clotting (due to low platelet levels) may also occur.
The widespread usage of an effective vaccine has substantially reduced the incidence of diphtheria globally (WHO, 2019b; Holý et al., 2017). This vaccine is now routinely given to children (in conjunction with whooping cough and tetanus vaccines) as a three or four dose regimen. Immunity is not life-long and repeated vaccinations are recommended every ten years after the initial vaccination. The diphtheria vaccination is generally quite effective and has greatly reduced the incidence of the disease since its widespread introduction. Indeed, the WHO estimates that approximately 4500 cases of diphtheria are now reported each year, down from >1 million cases a year prior to 1980 (WHO, 2019b; Holý et al., 2017). When C. diphtheriae infections occur (generally in non-vaccinated people in developing countries), antibiotic therapy may be effective in curing the disease and blocking its further spread. Metronidazole, erythromycin, penicillin-G, rifamin or clindamycin are most frequently used to treat diphtheria (Truelove et al., 2019). However, multi-antibiotic resistant C. diphtheriae strains are increasingly being reported (Floros et al., 2018; Mohankumar et al., 2018) and new antibiotic therapies are required.
1.3. Pertussis (whooping cough)
Pertussis (commonly known as whooping cough) is a highly contagious bacterial disease that infects large numbers of people annually despite the availability of an effective vaccine (Holý et al., 2017). Indeed, the WHO estimated that over 150,000 new cases were reported in 2018, with nearly 90,000 deaths (WHO, 2019c). However, not all cases are reported, particularly in developing countries, and it is likely that the WHO estimate substantially understates the prevalence of this disease. Indeed, other studies have estimated that there were 24.1 million pertussis cases and over 160,000 deaths of children under five years of age in 2014 (Yeung et al., 2017). Whilst these incidence rates remain unacceptably high, the introduction of a pertussis vaccine in the 1940's has resulted in dramatic reductions in regions that have introduced pertussis vaccination programs (Holý et al., 2017). For example, before the introduction of the vaccine, the incidence of pertussis in the United States of America was estimated to be approximately 180,000 annually (CDC, 2019). Following vaccination, the incidence in that country fell dramatically to an estimated 1000 new cases per year in 1976. Since that time, the incidence has risen again to nearly 19,000 in 2017.
Pertussis is caused by the bacterium Bordetella pertussis (Holý et al., 2017). It is an airborne disease and is spread in a similar manner to TB and diphtheria. Once a person is infected with the bacterium, it generally takes 6–20 days for the symptoms to become evident. Initially the symptoms are similar to a common cold, with a runny nose, fever and mild cough being common. These rapidly progress to the characteristic severe coughing fits, followed by a sudden inhalation, producing the ‘whooping’ sound that gives the disease the common name whooping cough. The disease is protracted, with the symptoms often lasting up to 10 weeks. The coughing can be so severe that it can cause subconjunctivial haemorrhages, rib fractures, hernias, urinary incontinence and vertebral artery dissection (WHO, 2019c).
Vaccination is the main form of pertussis control and is approximately 70–85% effective, dependent on the B. pertussis strain (Holý et al., 2017). However, recent genetic shifts in the bacterium have rendered some strains less susceptible to the vaccine (Mooi et al., 2014). Furthermore, immunity conferred by vaccination is not life-long and has been estimated to only last 4–12 years (Wendelboe et al., 2005). If a person contracts pertussis, macrolide antibiotics including erythromycin, clarithromycin or azithromycin are generally effective. However, macrolide resistant B. pertussis strains have been reported (Liu et al., 2018; Lönnqvist et al., 2018) rendering these antibiotics of little use. Effective new therapies are urgently required.
1.4. Bacterial pneumonia
Bacterial pneumonia is characterised by lung inflammation due to bacterial infections (Brooks, 2020). It is not a single disease and can be caused by multiple bacterial species including Haemophilus influenzae, Klebsiella pnuemoniae, Moraxella catarrhalis, Pseudomonas aeruginosa, Staphyloccus aureus and Streptococcus pneumoniae. Bacterial pneumonia is a significant medical burden and causes considerable loss of life annually. Indeed, lower respiratory infections (of which bacterial pneumonia is the major mortality causing disease) are one of the highest causes of death of all communicable diseases (Brooks, 2020; WHO, 2018). The severity of bacterial pneumonia varies widely from mild to life-threatening, or even death. The severity is dependent on a number of factors including the bacterial species and strain causing the infection, the age of the infected person (children and older people tend to suffer more severe symptoms), the immunological status of the infected person, and their general health. The symptoms are generally the same irrespective of the bacterial species/strain or the age of the infected person and include chest pains, shortness of breath, frequent coughing which produces yellow or green coloured mucus, fever, lethargy and chills. In severe cases, infected individuals may develop complications including respiratory failure, sepsis, lung abscesses or empyema (accumulation of pus in the pleural cavity surrounding the lungs).
Infection with these bacteria is most frequently via similar transmission pathways as TB, diphtheria and pertussis (i.e. air-borne transmission), and is classified as community acquired pneumonia (Brooks, 2020). This accounts for the vast majority of bacterial pneumonia cases. However, hospital-acquired bacterial pneumonia (HCAP) is also relatively common and occurs when a sick patient (who has a compromised immune system due to an existing medical condition) contracts an infection whilst in hospital. Ventilator-acquired pneumonia (VAP) may also occur when contaminated equipment is used to ventilate a patient. However, whilst HCAP and VAP are significant issues, air-borne transmission is the major route of transmission.
In contrast to the other bacterial respiratory diseases already discussed, there are few effective vaccines to prevent bacterial pneumoniae, although vaccines are available against Pneumonococcal spp. (Brooks, 2020). Treatment for bacterial pneumonia is generally reactive and is reliant on the use of antibiotics to kill the infective bacteria. The specific antibiotic(s) used are dependent on the infective bacterium. Antibiotic therapy is generally effective against most infective strains, although resistance of bacterial pneumonia strains to antibiotics is becoming relatively common and antibiotic therapies are increasingly failing (Peyrani et al., 2019). Of particular concern, extremely resistant strains of Klebsiella pneumoniae have been reported in China and Greece (Cheesman et al., 2017). Both of these strains were resistant to nearly all frontline antibiotics. The same study reported that shortly after those resistant strains were isolated, another K. pneumoniae strain that was resistant to all classes of antibiotics was detected in the United States of America. This is particularly concerning as medical science has no effective treatment against that strain and new therapies are urgently required. Similarly, methicillin resistant Staphyloccus aureus (MRSA) and extended spectrum β-lactamase resistant (ESBL) strains of some bacterial causes of pneumonia are now relatively common (Cheesman et al., 2017). The development of new therapies that are effective against these antibiotic-resistant species is urgently required.
2. An overview of bacterial respiratory diseases in South Africa
The incidence of TB is particularly high in southern Africa (Nanoo et al., 2015). Indeed, the WHO issues an annual global TB report on the 30 highest TB burden countries based on the number of cases and the severity of diseases burden (WHO, 2019a). Six southern African countries (Lesotho, Mozambique, Namibia, South Africa, Zambia, Zimbabwe) are included in that list. South Africa has the highest overall number of cases (301,000 total infections), which corresponds to the higher population numbers in South Africa compared to the other southern African countries. The TB rate as a percentage of population is approximately 5% for South Africa, which is similar to the rates in Mozambique and Namibia, and lower than the rates in Lesotho. Interestingly, the rates in Zambia (3.5%) and Zimbabwe (2%) were substantially lower and may correlate to the higher average temperatures (particularly in winter) in those countries. Alternatively, the lower rates may be due to less effective and incomplete reporting of these diseases in those countries. Due to the higher temperatures, it is likely that people in those countries spend less time in groups indoors, thus decreasing pathogen transmission. The southern African infection rates are substantially higher than the global average of 1%, demonstrating the health burden that TB has in southern Africa.
Notably, the WHO statistics only report the cases of active TB. As only approximately 10% of M. tuberculosis infections cause the active form of the disease, the actual infection rate may be as high as 50% of the population, providing a vast reservoir of bacteria for potent transmission of TB. Other studies have reported higher incidence of M. tuberculosis infection in specific populations. Screening studies in an adult population (<30 years old) in a mining community detected M. tuberculosis infections in 89% of the population (Hanifa et al., 2009). A similar study screened adolescent school students (12–18 years old) in rural regions of the Western Cape province of South Africa, within 100 km of Cape Town, and reported nearly 60% of the students had latent TB infections (Mahomed et al., 2011). Both of these studies screened specific groups and these statistics do not necessarily represent the overall prevalence of latent M. tuberculosis infections in the entire southern Africa region and the prevalence in urban regions of southern Africa may be substantially different.
The Hanifa et al. study (2009) highlights the prevalence of TB on the mining industry. The conditions under which miners may work constitute ideal conditions for the transmission of M. tuberculosis. Miners often work in enclosed spaces underground for extended periods. If a miner has TB, airborne transmission is highly likely under those conditions. Furthermore, miners often share equipment, which may further facilitate the spread of the bacterium. High density lower socio-economic urban communities also have higher incidences of M. tuberculosis infections than other regions of southern Africa. High density living provides ideal conditions for airborne transmission, thereby increasing the likelihood of person to person transfer. Of concern, antibiotic resistant M. tuberculosis strains are highly prevalent in southern Africa, with >90% of new infections reported to be resistant to several frontline antibiotics (WHO, 2019a). The high prevalence of resistant M. tuberculosis strains contributes to the overall burden of the disease in the region. Not only is it more difficult to treat the disease in infected people, but this also allows for further transmission of the bacterium.
The relatively low level of childhood vaccination uptake in several of the southern African countries also contributes to the levels of TB in the region. The WHO report (2019a) estimates that only 59% of children below five years of age have been immunised against TB in South Africa. This contrasts dramatically with the worldwide vaccination rates where it has been estimated that more than 90% of children below the age of five years have been vaccinated for TB. The low vaccination uptake in South Africa is surprising given the incidence of TB in the region and subsidisation of TB vaccination programs by the South African government. Vaccination is relatively cheap and is generally readily available in most areas of the country. We were unable to find a further breakdown of the vaccination statistics on a geographical and ethnic basis, but it is likely that low levels of uptake in isolated and rural communities skew the statistics for the entire country. Isolated and rural communities often have limited access to clinical care, and when medical care is available, rural populations are often poor and may be unable to afford westernised health care. Instead, rural communities are often reliant on traditional healers. However, encouraging TB vaccination programs may be effective in reducing the infection levels in southern Africa, even amongst non-vaccinated people, by providing ‘herd immunity’, thereby reducing transmissibility.
Not surprisingly, the incidence of M. tuberculosis infections is also higher in health care workers than in the general population, due to their levels of exposure to respiratory pathogens. Indeed, one study estimated that the risk of contracting TB is approximately 2.5 times higher for medical professionals than the general population in several countries with similar socio-economic profiles as southern Africa (Joshi et al., 2006). Furthermore, that study demonstrated that specific health care sectors have substantially increased risks of contracting TB. In particular, the risks to workers in emergency departments, TB treatment facilities, clinical laboratories, and internal medicine departments were particularly high rates of infection. Within those departments, paramedics, nurses, patient attendants, ward attendants and radiology technicians had substantially increased risks of contracting a M. tuberculosis infection. Whilst that study examined the incidence of TB in the health care sector in other countries, it is likely that similar trends occur in southern Africa.
Immuno-compromised people also have higher rates of infection than non-immunocompromised people. Indeed, the WHO report on TB in the southern African countries (WHO, 2019a) estimated that 59% of individuals diagnosed with TB in 2018 in South Africa also had HIV/AIDS. Of further concern, people with HIV/AIDS had a substantially worse prognosis than the general population, with approximately twice the mortality rate. Whilst the WHO report did not break the data down on the basis of age, it is likely that similar trends would occur in children and in the elderly. Both of these groups have lower immuno-competence than healthy adults. Thus, they are likely to have higher incidences of TB, and higher rates of mortality once they contract a M. tuberculosis infection. However, we were unable to find statistics to support this and further studies are needed for confirmation.
The other bacterial respiratory diseases generally follow similar trends to other countries with similar socio-economic profiles. As with other regions of the world, widespread diphtheria and pertussis vaccination programs in children have substantially decreased the incidence of those diseases in southern Africa. Indeed, between January 2008 and March 2015, only four cases of diphtheria were reported in South Africa. An outbreak of diphtheria occurred in South Africa in March 2015, with fifteen confirmed cases in rural Kwa-Zulu Natal, of which four died (Mahomed et al., 2015). All but four of the infected people were either not vaccinated, or their vaccinations were out of date. The outbreak was rapidly contained and the incidence rates have remained low since. Similarly low rates of infection occurred throughout other southern African countries across the same period.
Pertussis is far more common than diphtheria in southern Africa. It is difficult to find incidence statistics for individual countries in southern Africa as the WHO provides figures for the African global region instead. According to the WHO, 14 million cases of pertussis were reported from a population of approximately one billion people, which equates to an infection rate of 1.4% of the population. However, vaccination programs are widespread in South Africa and have a far greater take up rate in southern Africa than in central, eastern and western Africa (WHO, 2019c), so it is likely that the incidence in South Africa (and other southern African countries) is substantially less than this. Pertussis is substantially more common in children than in adults and is one of the most common diseases in children under five years of age. It also has higher incidences in immuno-compromised people than in the general population. A recent study screened children hospitalised for respiratory illnesses in South Africa and reported that pertussis was the cause of approximately 7% of the cases of respiratory illness (Muloiwa et al., 2016). The rate was significantly higher in HIV positive children (15.8%) and in HIV exposed but negative children (10.9%) than in HIV unexposed children (5.4%). Notably, there have been marked recent increases in the incidence of pertussis in the WHO African world region. Indeed, the number of reported B. pertussis infections in that region has increased from approximately 1.5 million to over 14 million between 2016 and 2018 (WHO, 2019d). Although specific figures for South Africa are not available from the WHO, it is likely that it has similar trends for those of the rest of the Africa region. It is likely that decreased rates of pertussis vaccination uptake in recent years may contribute to this trend. Indeed, a recent report by the Centre for Communicable Diseases (2018) reported that pertussis vaccination rates in South Africa had decreased to 66% of the population in 2016, allowing for the resurgence of the disease in the region.
Bacterial pneumonia is common in both children and adults in southern Africa and it is the most common cause of hospitalisation in South Africa. Indeed, approximately 12 million children were hospitalised and 1.2 million children died from bacterial pneumonia in 2010 in South Africa (Dept of Paediatrics and Child Health, South Africa, 2019). The same study also reported that bacterial pneumonia is the second most common cause of death in South African adults. The disease is substantially more common in immune-compromised individuals (both children and adults).
Pertussis and bacterial pneumonia transmission trends are similar to TB. High density urban living allows for efficient transmission of these diseases, therefore the incidence is higher under those conditions. Unfortunately, we were unable to locate occupation specific statistics as reported for TB and further research is required in that area. However, it is likely that similar trends occur (i.e. higher rates in occupations that require workers to work together in confined spaces such as mining; high rates in health care sector professionals through greater contact with infected people). However, these trends have not been reported for pertussis and bacterial pneumonia and further studies are required to confirm this.
3. Materials and methods
3.1. Search strategy
Our study aimed to identify southern African plants used traditionally to treat bacterial respiratory diseases in humans. A systematic search was undertaken using a variety ethnobotanical books (Smith, 1888; Watt and Breyer-Brandwijk, 1962; Van Hutchings et al., 1996; Von Koenen, 2001; Ngwenya et al., 2003; Wyk et al., 2009) and ethnobotanical reviews (Hulley and Van Wyk, 2017; De Beer and Van Wyk, 2011; Nortje and Van Wyk, 2011; Philander, 2011; Van Wyk, 2008). Ethnobotanical research articles published prior to June 2020 were also searched via Google-Scholar, Science-Direct, PubMed and Scopus using the following terms as filters, and were searched both alone and as combinations: “South African”, “medicinal plant”, “traditional medicine”, “ethnobotany”, “respiratory infection” “tuberculosis”, “pneumonia”, “bacterial pneumonia”, “pertussis”, “diphtheria”, “whooping cough”. All terms were searched alone and as combinations.
Each plant species identified by this initial search were subjected to a further literature review to establish the extent (if any) of the scientific research into the efficacy of that species. Specific criteria to filter studies included the terms ethnomedicine, southern African medicinal plants and other key words related to bacterial respiratory infections and the specific pathogens.
3.2. Eligibility criteria
A screening of publication titles was initially performed, and eligible publications were selected. The abstracts of were then read to ensure that the selected publications met the eligibility criteria. Full text manuscripts were retrieved for all publications that met the eligibility requirements and these were further studied.
3.2.1. Inclusion criteria
To meet the eligibility criteria for this study, a publication had to meet the following inclusion criteria:
-
•
Only English language publications published prior to June 2020 were used in the preparation of this review.
-
•
This study is non-biased and does not have taxonomic preference (although several of the studies we review targeted specific families or genera).
-
•
For the ethnobotanical studies, the plant species must be stated to be used against the specific bacterial respiratory diseases examined in this study, rather against generic symptoms.
-
•
For the screening studies, preparations prepared from plant extracts must have been screened against at least one of the pathogens responsible for the bacterial respiratory diseases. Alternatively, studies that tested the plant preparations against human or animal models with the bacterial respiratory diseases were also included in this study.
-
•
For introduced species to be included in this report, they must either be naturalised or widely cultivated, and there must be documented evidence that they are commonly used by at least one southern African ethnic group to treat viral respiratory disease.
3.2.2. Exclusion criteria
Studies with the following criteria were excluded from this study:
-
•
Studies where the species identity was in doubt. By necessity, several relatively old publications were searched (e.g. Smith, 1888; Watt and Breyer-Brandwijk, 1962). Where possible, the species names were confirmed or updated using the Plant List website (http://www.theplantlist.org/). Where a species name could not be definitively verified, that species was omitted from this study.
-
•
Only plant species definitively described as being used to treat specific bacterial respiratory diseases are included in this study. Due to symptom similarity with numerous diseases, when a plant was described as being used to treat symptoms consistent with bacterial respiratory diseases without specifying the diseases they are used to treat were excluded from this study.
-
•
Whilst plant species that are not native to southern Africa are included in this study, introduced plant species were excluded unless there is evidence of their usage in at least one southern African traditional healing system and their widespread cultivation in southern Africa.
3.3. Data collection
Ethnopharmacological studies from southern Africa that are linked with the treatment of bacterial respiratory disease were collected and examined in this study. Additionally, studies testing the activity of the southern African medicinal plants against the bacterial respiratory pathogens, or against infected human or animal models, were examined, irrespective of the study origin. The following data was collected:
-
•
Genus, species and family name for each species examined in the individual publications. All species names were standardised using the Plant List website (http://www.theplantlist.org/).
-
•
Ethnic grouping that traditionally used the plant species medicinally. Where possible, the common and ethnic names were also collected.
-
•
The plant part used and the method of preparation were collected (where available).
-
•
For screening studies, the bacterial pathogen species and (where possible) the strain were listed and the MIC values (where available) are included.
-
•
For animal and human trial studies, the animal model (where appropriate), route of administration, doses and toxicity data (where available) was noted.
All data was managed using Excel® software.
4. Results
4.1. South African medicinal plants used traditionally to treat bacterial respiratory diseases
One hundred and eighty-seven southern African plants which are used in at least one southern African traditional healing system to treat bacterial respiratory infections were identified following an extensive literature search (Table 1 ). As indicated in our review of southern African plants to treat viral respiratory infections (Cock and Van Vuuren, part one), several pathogenic respiratory diseases exhibit generic symptoms that are common with other bacterial diseases, as well as viral respiratory diseases. For example, the early stage symptoms of TB are often similar to those of influenza or severe acute respiratory syndrome (SARS). Similar symptoms are also evident for numerous other non-respiratory diseases including bubonic plague, Lyme disease, malaria, measles, rabies, and the early phases of AIDS. As ethnobotanical texts may report the usage of plant species to treat the symptoms of a disease, the specific disease treated is often not definitive. For this study, we have only included plant species that have specifically been reported for the treatment of bacterial respiratory infections in humans. Where the disease pathogen targeted by a plant is ambiguous, we have excluded that species from this study. Thus, it is likely that this list underestimates the number of plant species used to treat bacterial respiratory disease.
Table 1.
South African plants used traditionally to treat bacterial respiratory illnesses.
| Plant species | Family | Common name(s) | Plant part used | Used for | References |
|---|---|---|---|---|---|
| Abrus precatorius subsp. africanus Verdc. | Fabaceae | Bead vine, coral bead plant, coral bean, crabs eye, licorice vine, love bean, lucky bean creeper, prayer beads, weather vine (English), umkhokha (Zulu) | Leaves, roots | Used to treat TB and whooping cough. Preparation and application not specified. | Madikizela et al. (2013) |
| Abutilon angulatum (Guill. & Perr.) Mast. | Malvaceae | Unknown | Root | Used to treat bacterial pneumonia. Preparation and application are not specified. | Von Koenen (2001) |
| Acacia eriloba E.Mey. | Fabaceae | Camel thorn, giraffe thorn (English), kameeldoringboom (Afrikaans) | Leaves | Leaf infusions are drunk to treat bacterial pneumonia. | Von Koenen (2001) |
| Acacia nilotica (L.) Delile | Fabaceae | Redheart, scented thorn (English), lekkerreulpeul (Afrikaans) | Root | Used to treat TB. Preparation and application are not specified. | Watt and Breyer-Brandwijk (1962) |
| Acacia xanthophloea Benth. | Fabaceae | Fever tree (English) | Bark | Used to treat TB. Preparation and application are not specified. | McGaw et al. (2008) |
| Aclepias crispa P.J.Bergius | Apocynaceae | witvergeet, kalmoes (Afrikaans) | Not specified | Used to treat bacterial pneumonia. Preparation and application are not specified. | Hulley and Van Wyk, 2017 |
| Acorus calamus L. | Acoraceae | Sweet flag (English), makkalmoes (Afrikaans), ikalamuzi (Zulu) | Root | Volatile compounds produced from the root are used to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Adenia fruticosa Burtt Davy | Passifloraceae | Green-stem (English) | Not specified | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Ajuga ophrydis Burch. ex Benth. | Lamiaceae | Senyarrla (Southern Sotho) | Roots | Used to treat TB. Preparation and application not specified. | Kose et al. (2015) |
| Allium cepa L. | Amaryllidaceae | Onion (English) | Bulb | Consumed to treat whooping cough. | Watt and Breyer-Brandwijk (1962) |
| Allium sativum L. | Amaryllidaceae | Garlic (English) | Bulb | Consumed to treat whooping cough and TB. | Watt and Breyer-Brandwijk (1962) |
| Aloe arborescens Mill. | Xanthorrhoeaceae | Krantz aloe (English), kransaalwyn (Afrikaans), ikalene (Xhosa), inkalane, umhlabana (Zulu) | Leaves | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Aloe ferox Mill. | Xanthorrhoeaceae | Cape aloe (English), Bitteraalwyn, Winkelaalwyn (Afrikaans), iKhala (Xhosa), iNhlaba (Zulu) | Leaves | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Aloe maculata All. | Xanthorrhoeaceae | Soap aloe, zebra aloe (English) | Leaves | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Aloe noblis Haw. | Xanthorrhoeaceae | Golden toothed aloe | Leaves | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Aloe plicatilis (L.) Mill. | Xanthorrhoeaceae | Fan aloe, Franschhoek aloe (English), waaieraalwyn, Franschoekaalwyn, bergaalwyn, tongaalwyn (Afrikaans) | Leaves | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Anginon difforme (L.) B.L.Burtt. | Apiaceae | Wildeanys (Afrikaans) | Leaves | Leave infusions were drunk by the Nama to treat TB. | Nortjie and Van Wyk (2015) |
| Aptosimum depressum Burch. ex Benth. | Scrophulariaceae | Unknown | Not specified | An infusion was used as a gargle to treat diphtheria. | Watt and Breyer-Brandwijk (1962) |
| Artemisia afra Jacq. ex Willd. | Asteraceae | African wormwood (English), als, alsem, wildeals (Afrikaans), lengana (Sotho, Tswana), umhlonyane (Xhosa, Zulu) | Leaves | The leaves are boiled and the steam inhaled to treat whooping cough and diphtheria. The resultant infusion can also be drunk for the same purposes. | Von Koenen, 2001; McGaw et al., 2008; |
| Aspalathus cordata (L.) R.Dahlgren | Fabaceae | Unknown | Leaves | An infusion is drunk to treat whooping cough. | Watt and Breyer-Brandwijk (1962) |
| Aspalathus linearis (Burm.f.) R.Dahlgren | Fabaceae | Red bush, bush tea (English), Rooibos (Afrikaans) | Leaves | An infusion is drunk to treat TB and whooping cough. | Watt and Breyer-Brandwijk (1962) |
| Asparagus africanus Lam. | Asparagaceae | Bush asparagus (English) | Root | Root infusions are consumed several times per day to treat TB. | Watt and Breyer-Brandwijk, 1962; Madikizela et al., 2013; Hulley and Van Wyk, 2017 |
| Asparagus capensis L. | Asparagaceae | Katdoring (Afrikaans) | Root | Root infusions are consumed several times per day to treat TB. | Watt and Breyer-Brandwijk, 1962; Philander, 2011; Hulley and Van Wyk, 2017 |
| Asparagus densiflorus (Kunth) Jessop | Asparagaceae | Katdoring (Afrikaans) | Root | Root infusions are consumed to treat TB. | Hulley and Van Wyk, 2017 |
| Asparagus falcatus L. | Asparagaceae | Unknown | Leaves and roots | Root infusions are consumed to treat TB. | Pallant and Steenkamp, 2008; Madikizela et al., 2013; |
| Asparagus linearis (Brum.f.) R.Dahlgren | Asparagaceae | T'nuance, katdoring (Afrikaans) | Roots | Root infusions are consumed to treat TB. | Van Wyk (2008) |
| Asparagus retrofractus L. | Asparagaceae | Ming fern (English) | Root | Root infusions are consumed to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Asparagus setaceus (Kunth) Jessop | Asparagaceae | Asparagus fern, climbing fern, lace fern (English) | Root | Root infusions are consumed several times per day to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Asparagus striatus (L.f.) Thunb. | Asparagaceae | Bergappel, bergappeltjie, bobbejaanappel (Afrikaans) | Root | Root infusions are consumed several times per day to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Asparagus suaveolens (Burch.) Baker | Asparagaceae | Bushveld Asparagus, wild aspoaragus (English), katdoring (Afrikaans), mvane (Xhosa) | Root | Root infusions are consumed to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Astridia velutina (L.Bolus) Dinter | Aizoaceae | Unknown | Sap | used to treat diphtheria. Preparation and application not specified. | Von Koenen (2001) |
| Buddleja saligna Willd. | Scrophulariaceae | False olive (English), witolien (Afrikaans), lelothwane (Southern Sotho), ungqeba (Xhosa), igqeba-elimhlope (Zulu) | Leaves, stems | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Callilepis laureola DC | Asteraceae | Oxe-eye daisy (English), Wile margriet (Afrikaans), amafuthomhlaba, ihlamvu, impila (Zulu) | Root | Preparation and application methods are not specified. | Watt and Breyer-Brandwijk (1962) |
| Capparis tomentosa Lam. | Capparaceae | Wooly caper-bush (English), wollerige kapperbos, wag-‘n-bietjie (Afrikaans), inkunzi-ebomvu, iqwaningi, umqoqolo, ukhokhwana, umabusane (Zulu), imfishlo, intshihlo, intsihlo, umpasimani (Xhosa) | Bark, Roots | The bark is burned and the smoke is inhaled to treat TB. The Venda also drank a root decoction for the same purpose. | Watt and Breyer-Brandwijk, 1962; Pallant and Steenkamp, 2008 |
| Carissa edulis (Forssk.) Vahl. | Apocynaceae | Simple-spined num-num, climbing num-num, small num-num (English), enkeldoringnoemnoem, ranknoemnoem, kleinnoemnoem (Afrikaans), mothokolo (North Sotho), murungulu (Venda) | Root, leaves | A root decoction is used by the Venda to treat TB. Leaf juice was gargled to treat diphtheria | Pallant and Steenkamp, 2008; Van Wyk, 2008 |
| Carpobrotus acinaciformis (L.) L.Bolus | Aizoaceae | Eland's sourfig (English), elandssuurvy (Afrikaans) | Leaves | The boiled fruit is consumed to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Carpobrotus edulis (L.) N.E.Br. | Aizoaceae | Sour fig, Cape fig, Hottentot's fig (English), vyerank, ghaukum, ghoenavy, hotnotsvye, Kaapvy, perdevy, rankvy (Afrikaans), ikhambi-lamabulawo, umgongozi (Zulu) | Leaves | A leaf decoction is consumed to treat diphtheria. Leaf juice is consumed to treat TB. | al.Watt and Breyer-Brandwijk, 1962; Van Wyk et al., 2009; Philander, 2011; Nortjie and Van Wyk, 2015 |
| Cassine aethiopica Thunb. | Celastraceae | Saffron wood, forest saffron (English), saffraan, bossafraan (Afrikaans) | Leaves | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Cephalaria pungens Szabó | Caprifoliaceae | Unknown | Roots | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Chaetachme aristata Planch. | Ulmaceae | Thorny elm (English), basterwitpeer (Afrikaans) | Leaves | Used to treat TB. Preparation and application not specified. | Dzoyem et al. (2016) |
| Chamaecrista mimosoides (L.) Greene | Fabaceae | Boesmantee (Afrikaans) | Not specified | Used to treat bacterial pneumonia. Preparation and application are not specified. | Von Koenen (2001) |
| Chenopodium ambrosioides L. | Chenopodiaceae | Wormsalt (English), sinkingbossie (Afrikaans) | Not specified | Used to treat bacterial pneumonia. Preparation and application are not specified. | al.; Von Koenen, 2001; McGaw et al., 2008 |
| Chironia baccifera L. | Gentianaceae | Bitterbos, skilparbos (Afrikaans) | Not specified | Infusions are used to treat TB and pneumonia. | Hulley and Van Wyk, 2017 |
| Chrysanthemum frutescens L. | Asteraceae | Paris daisy (English) | Roots | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Chrysanthemum segetum L., | Asteraceae | Corn marigold (English) | Leaves | Decoctions are drunk to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Cinnamomum camphora (L.) J.Presl. | Lauraceae | Camphor tree (English), kanferboom (Afrikaans), uroselina (Zulu) | Leaves. Essential oil (distilled from the wood) | The leaves are smoked by the Southern Sotho to treat TB. The bark is used to treat bacterial pneumonia, Preparation and application not specified. | Philander, 2011; Van Wyk et al., 2009; Watt and Breyer-Brandwijk, 1962 |
| Cissampelos capensis L.f. | Menispermaceae | Dawidjies, fynblaarklimop (Afrikaans) | Leaves | Infusions are drunk to treat TB. | Hulley and Van Wyk, 2017 |
| Cliffortia odorata L.f. | Rosaceae | Wildewingerd (Afrikaans) | Not specified | An infusion is drunk to treat diphtheria. | Watt and Breyer-Brandwijk (1962) |
| Combretum molle R.Br. ex G.Don. | Combretaceae | Velvet bush willow (English), fluweelboswilg, basterrooibos (Afrikaans), mokgwethe (Sotho), mugwiti (Venda), umBondwe-omhlope (Zulu) | Bark | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Combretum platypetalum Welw. ex M.A. Lawson | Combretaceae | Unknown | Root | Root decoctions are drunk to treat bacterial pneumonia | Von Koenen (2001) |
| Croton pseudopulchellus Pax | Euphorbiaceae | Small lavender fever-berry (English), kleinlaventelkoorsbessie, sandkoorsbessie (Afrikaans), uHubeshane (Zulu) | Leaves | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Cryptocarya latifolia latifolia Sond. | Lauriaceae | Bastard stinkwood, broad-leaved aurel, broad-leaved quince (English), baster-stinkhout, basterswartstinkhout, breëblaarkweper, pondo-kweper (Afrikaans),umgxaleba, umgxobothi (Xhosa), umhlangwenya, umkhondweni, umdlangwenya (Zulu), | Bark | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Cyclopia genistoides (L.) Vent. | Fabaceae | Honeybush tea (English), heuningbos (Afrikaans) | Leaves | infusions are drunk as an expectorant in people with TB. | Watt and Breyer-Brandwijk (1962) |
| Dahlia pinnata Cav. | Asteraceae | Garden dahlia (English) | Flowers | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Datura metel L. | Solanaceae | Thorn apple, angel's trumpet (English) | Root | Dried roots are smoked to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Dicoma capensis Less. | Asteraceae | Karmadik, baarbos, sandsalie (Afrikaans) | Leaves | Decoctions are drunk to treat TB. | Nortjie and Van Wyk (2015) |
| Dichrostachys cinerea (L.) Wight & Arn. | Fabaceae | Kalahari Christmas tree, sickle bush, bell mimosa, Chinese lantern tree (English) | Leaves and roots | Leaves and roots are burned and the smoke inhaled to treat TB and bacterial pneumonia. | Watt and Breyer-Brandwijk, 1962; Von Koenen, 2001; |
| Diplorhynchus condylocarpon (Müll.Arg.) Pichon | Apocynaceae | Wild rubber, horn-pod tree (English), horingpeulbos, melkbos (Afrikaans), muthowa (Venda) | Not specified | Used to treat TB. Preparation and application method not specified. | Watt and Breyer-Brandwijk (1962) |
| Dodonaea viscosa (L.) Jacq. | Sapindaceae | Sand olive (English), sandolien, ysterbos (Afrikaans), mutata-vhana (Venda) | Leaves, twigs | A decoction is drunk to treat TB and diphtheria. Also useful for the treatment of bacterial pneumonia. | al.Watt and Breyer-Brandwijk, 1962; McGaw et al., 2008; Hulley and Van Wyk, 2017 |
| Drosera capensis L. | Droseraceae | Cape sundew (English), sondouw (Afrikaans) | Leaves | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Ekebergia capensis Sparm. | Meliaceae | Cape ash, dogplum (English), essenhout (Afrikaans), mmidibidi (Sotho) | Leaves bark, roots | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Elytropappus rhinocerotis (L.f) Less. | Asteraceae | Rhinoceros bush (English) | Unspecified | Used to treat TB. Preparation and application methods are not specified. | Hulley and vanWyk, 2017 |
| Empleurum unicapsulare (L.f.) Skeels | Rutaceae | Bergboegoe (Afrikaans) | Unspecified | Used to treat bacterial pneumonia. Preparation and application methods are not specified. | Hulley and vanWyk, 2017 |
| Erigeron canadensis L. | Asteraceae | Horseweed, coltstail, marestail, butterweed (English) | Leaf | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Eriocephalus africanus L. | Asteraceae | Kapokbos, skaapkaroo (Afrikaans) | Not specified | Infusions are used to treat TB. | Hulley and vanWyk, 2017 |
| Erythrina humeana Spreng. | Fabaceae | Umsinsana (Zulu) | Not specified | Used to treat TB. Preparation and application not specified. | Corrigan et al. (2011) |
| Eucalyptus globulus Labill. | Myrtaceae | Southern blue gum, Tasmanian blue gum (English) | Leaves | The leaves are boiled and the vapour is inhaled to treat TB and diphtheria. | Van Wyk, 2008; Watt and Breyer-Brandwijk, 1962 |
| Eucela natalensis A. DC. | Ebenaceae | Natal guarri, Natal ebony, large-leaved guarri (English), Natalghwarrie, berggwarrie, swartbasboom (Afrikaans), umTshekisani, umKhasa (Xhosa), iDungamuzi, iChitamuzi, umZimane, umTshikisane, inKunzane, inKunzi-emnyama, umHlalanyamazane, umAnyathi (Zulu) | Roots | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Euphorbia heterophylla L. | Euphorbiaceae | Japanese poinsettia, desert poinsettia, painted spurge, milkweed (English) | Leaves and flowers | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Euphorbia neriifolia L. | Euphorbiaceae | Milk hedge, milk bush, oleander spurge, oleander-leaved euphorbia (English), melkbos (Afrikaans) | Stem latex | Used to treat whooping cough. Preparation and application method not specified. | Watt and Breyer-Brandwijk (1962) |
| Felicia filifolia (Vent.) Burtt Davy | Asteraceae | Steenbokbossie, vaderlandsrapuisbos (Afrikaans) | Not specified | Used for the treatment of TB. Preparation and application not specified. | Hulley and Van Wyk, 2017 |
| Ficus carica L. | Moraceae | Common fig (English) | Leaves and roots | A decoction of the roots and leaves is drunk to treat diphtheria. | Watt and Breyer-Brandwijk, 1962; Hulley and Van Wyk, 2017 |
| Ficus sur Forssk | Moraceae | Cape fig, broom cluster fig (English) | Root and bark | The Zulu drink a decoction of the root and bark to treat TB. | al.Watt and Breyer-Brandwijk, 1962; Pallant and Steenkamp, 2008; Madikizela et al., 2013 |
| Ficus sycomorus L. | Moraceae | Sycamore fig, common cluster fig, mulberry fig (English), trosvy, geelrriviervy, geelstamvy, gewone trosvy, wildevyeboom, sycomorusvy (Afrikaans), mogo, mogoboya, mohlole (Sotho), muhuvhoya, muhuyu, muhuyu-lukuse, mutole, muvhuyu-vhutwa (Venda), mogoboya, umkhiwane isikhukhuboya, umncongo, umkhiwane (Zulu) | Fruit | A fruit infusion is drunk by the Venda to treat TB. | Pallant and Steenkamp (2008) |
| Galenia africana L. | Aizoaceae | Yellow bush (English), brakkraalbossie, geelbos, kraalbos, muisbos, muisgeelbossie, perdebos (Afrikaans), iqina (Xhosa) | Leaves | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Garcinia polyantha Oliv. | Clusiaceae | Unknown | Bark | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Ginkgo biloba L. | Ginkgoaceae | Ginkgo | Leaves | Decoctions and infusions are drunk to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Glycyrrhiza glabra glabra L. | Fabaceae | Liquorice, licorice (English) | Rhizome | Root infusions are used to treat TB. | Watt and Breyer-Brandwijk, 1962; Van Wyk et al., 2009 |
| Gomphocarpus fruticosus (L.) W.T.Aiton | Apocynaceae | Milkweed (English), melkbos, tontelbos (Afrikaans), lebegana, lereke-la-ntja (Sotho), modimolo (Southern Sotho), umsinga-lwesalukazi (Zulu) | Leaves | Used to treat TB. Preparation and application method not specified. | Watt and Breyer-Brandwijk, 1962; Van Wyk et al., 2009; |
| Gunnera perpensa L. | Gunneraceae | Wild rhubarb, river pumpkin (English), wilde ramenas, ravierpampoen (Afrikaans), qobo (Sotho), rambola-vhadzimu (Venda), iphuzi, ighobo (Xhosa), ugobhe (Zulu) | Roots | Used to treat TB. Preparation and application method not specified. | McGaw et al. (2008) |
| Gymnosporia buxifolia (L.) Szyszył. | Celestraceae | lemoendoring, wondedoring, pendoringbos (Afrikaans) | Not specified | Used to treat TB. Preparation and application not specified. | Hulley and Van Wyk, 2017 |
| Helianthus tuberosus L. | Asteraceae | Jerusalem artichoke (English) | Root/tuber | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Helichrysum crispum (L.) D.Don. | Asteraceae | Hottentot's Bedding (English), Hotnotskooigoed, Hottentotskooigoed, Hottentotskruie, Kooigoed (Afrikaans) | Leaves, whole plant | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Helichrysum imbricatum (L.) Less. | Asteraceae | Gold-and-silver (English) | Not specified | Used as a remedy for whooping cough. Preparation and application not specified. | Watt and Breyer-Brandwijk, 1962; McGaw et al., 2008; |
| Helichrysum krausii Sch. Bip. | Asteraceae | Straw everlasting (English), sewejaartjie (Afrikaans), isipheshane, isiqoqo (Zulu) | Flowers and seeds | Dried flowers and seeds are smoked to treat TB. | Watt and Breyer-Brandwijk, 1962; McGaw et al., 2008; |
| Helichrysum melanacme DC. | Asteraceae | Hotnotskooigoed (Afrikaans) | Leaves, whole plant | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Helichrysum nudifolium (L.) Less. | Asteraceae | Everlastings (English), hottentotsteebossie, kooigoed (Afrikaans), isicwe, indlebe zebhokwe, undleni (Xhosa), icholocholo, imphepho (Zulu) | Leaves, whole plant | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Helichrysum odoratissimum (L.) Sweet | Asteraceae | Everlantings (English), kooigoed (Afrikaans) imphepho (Zulu) | Leaves, whole plant | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Helichrysum vestitum (L.) Willd. | Asteraceae | Cape snow (English) | Not specified | Used to treat diphtheria. Preparation. Application not specified. | ; Watt and Breyer-Brandwijk, 1962; McGaw et al.., 2008 |
| Helipterum eximium (L.) DC. | Asteraceae | Unknown | Not specified | Used to treat diphtheria. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Helipterum speciosissimum (L.) DC. | Asteraceae | Unknown | Not specified | Used to treat diphtheria. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Helipterum variegatum DC. | Asteraceae | Unknown | Not specified | Used to treat diphtheria. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Hermannia salviifolia L.f. | Malvaceae | Katjiedrieblaar (Afrikaans) | Not specified | Used to treat bacterial pneumonia. Preparation and application not specified. | Hulley and Van Wyk, 2017 |
| Hoodia gordonii (Masson) Sweet ex Decne. | Apocynaceae | Hoodia, ghaap, kakimas (Afrikaans) | Fleshy stems | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Hoodia pilifera subsp. annulata (N.E. Br.) Bruyns | Apocynaceae | Unknown | Fleshy stems | Used to treat TB. Preparation and application method not specified. | Watt and Breyer-Brandwijk (1962) |
| Hypericum perforatum L. | Hypericaceae | St John's wort (English) | Roots, leaves and flowers | Decoctions and infusions are drunk to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Indigofera tinctoria L. | Fabaceae | True indigo (English) | Juice | consumed to treat whooping cough. | Watt and Breyer-Brandwijk, 1962; Madikizela et al., 2013; |
| Jatropha zeyheri Sond. | Euphorbiaceae | Verfbol (Afrikaans), sefapabadia (Sotho), ugodide (Zulu) | Unspecified | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Lactuca sativa L. | Asteraceae | Lettuce (English) | Whole plant | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Lannea edulis (Sond.) Engl. | Anacardiaceae | Wild grape (English), wildedruif (Afrikaans), muporotso (Venda) | Root | Decoctions and infusions of the root bark is drunk to treat whooping cough. | Van Wyk et al. (2009) |
| Leonotis leonoris (L.) R.Br. | Lamiaceae | Wild dagga (English), wildedagga, duiwelstabak (Afrikaans), mvovo (Xhosa), uyshwala-bezinyoni (Zulu) | Leaves and stems | A tincture is drunk to treat TB and whooping cough. | Watt and Breyer-Brandwijk, 1962; Pallant and Steenkamp, 2008; |
| Leucaena leucocephala (Lam.) de Wit | Fabaceae | Wild tamarind, wild lead tree (English) | Bark leaves, seeds | Used to treat TB. Preparation and application not specified. | Dzoyem et al. (2016) |
| Lessertia frutescens (L.) Goldblatt & J.C. Manning | Fabaceae | Keurtjie, beeskeurtiebos, kankerbos (Afrikaans) | Not specified | An infusion is used to treat TB. | Hulley and Van Wyk, 2017 |
| Leyssera gnaphalioides L. | Asteraceae | Skilpadteebossie, hongertee, duinetee, teringteebos Afrikaans) | Leaf | An infusion is drunk to treat TB. | Watt and Breyer-Brandwijk (1962); McGaw et al. (2008) |
| Malva neglecta Wallr. | Malvaceae | Low mallow (English) | Roots | Decoctions and infusions are drunk to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Malva paviflora L. | Malvaceae | Marshmallow, cheeseweed mallow, little mallow, small flower mallow (English) | Roots | Decoctions and infusions are drunk to treat TB. | Von Koenen (2001) |
| Matricaria chamomilla L. | Asteraceae | German chamomile, wild chamomile, scented mayweed (English) | Not specified | Used to treat diphtheria and TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Maytenus heterophylla (Eckl. & Zeyh.) N.Robson | Celestraceae | Gewone pendoring (Afrikaans) | Leaves | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Melilotus alba Ledeb. | Fabaceae | White sweet clover (English) | Whole plant | Decoctions and infusions are drunk to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Melolobium candicans (E.Mey.) Eckl. & Zeyh. | Fabaceae | Wild dagga (English), wildedagga (Afrikaans) | Leaves and stems | A decoction of the leaves and stems is drunk to treat TB. | De Beer and Van Wyk (2011) |
| Mentha longifolia (L.) L. | Lamiaceae | Wild mint (English), kruisement, balderjan (Afrikaans), koena-ya-thabo (Sotho), inixina, inzinziniba (Xhosa), ufuthana, lomhlanga (Zulu) | Leaves, roots and stems | Used to treat TB, whooping cough and diphtheria. Preparation and application method not specified. | Watt and Breyer-Brandwijk (1962) |
| Mesembryanthemum tortuosum L. | Aizoaceae | Koegoed, kanna (Afrikaans) | Not specified | Used to treat TB. Preparation and application not specified. | Hulley and Van Wyk2017 |
| Montinia caryophyllacea Thunb. | Montiniaceae | Pepper-bush, wild clove bush (English), bergklapper, peperbos (Afrikaans) | Leaves | The dried pulverised leaves are used as a snuff to treat TB. | Von Koenen (2001) |
| Mundulea sericea (Willd.) A.Chev. | Fabaceae | Cork bush, silver bush, (English), kurkbos, olifantshout, visboontjie, visgif, mangaanbos (Afrikaans), mosetla-thlou (Sotho), mukunda-ndou (Venda), umsindandlovu (Zulu) | Bark and roots | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Nasturtium officinale R.Br. | Brassicaceae | Watercress (English) | Whole plant | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Nicotiana glauca Graham | Solanaceae | Mustard tree, tree tobacco (English), tabakboom, wildetabak, volstruisgifboom (Afrikaans), mohlafotha (Sotho) | Leaves | Powdered leaves are used as a snuff to treat TB. | Von Koenen (2001) |
| Nidorella anomala Steetz | Asteraceae | Unknown | Whole plant | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Nidorella auriculata DC. | Asteraceae | Unknown | Whole plant | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Olea europea L. | Olacaceae | Wild olive (English) olienhout (Afrikaans), mohlware (Sotho), umnquma (Zulu, Xhosa), mutlhwari (Venda) | Leaves | A leaf decoction is used as a gargle to treat diphtheria. | Watt and Breyer-Brandwijk (1962); Von Koenen (2001); |
| Oncosiphon suffruticosum (L.) Källersjö | Asteraceae | Stinkkruid, wirmkruid (Afrikaans) | Whole plant | An infusion is drunk to treat bacterial pneumonia. | Van Wyk et al. (2009) |
| Opuntia ficus-indica (L.) Mill. | Cactaceae | Indian pear (English), turksvy (Afrikaans) | Leaves | Leaf infusions are drunk to treat whooping cough. | Von Koenen (2001) |
| Opuntia vulgaris Mill. | Cactaceae | Prickly pear (English) | Leaves | A leaf infusion is consumed to treat whooping cough. | Watt and Breyer-Brandwijk (1962) |
| Pegolettia baccharidifolia Less. | Asteraceae | Ghwarrieson, heuningdou (Afrikaans) | Not specified | Used to treat bacterial pneumonia. Preparation and application not specified. | Hulley and Van Wyk2017 |
| Pelargonium graveolens L'Hér. | Geraniaceae | Rose geranium (English), wildemalva (Afrikaans) | Leaves | Leaves are steamed and vapours are inhaled to treat TB. | Van Wyk et al. (2009) |
| Pelargonium myrrhifolium (L.) L'Hér | Geraniaceae | Unknown | Tuber | Used to treat TB. Preparation and application not specified. | Van Wyk (2008) |
| Pelargonium ramosissimum Willd. | Geraniaceae | Dassieboegoe (Afrikaans) | Tuber | Used to treat TB. Preparation and application not specified. | Van Wyk (2008) |
| Pelargonium rentiforme Curtis | Geraniaceae | Kidney-leaved pelargonium (English), rooirabas (Afrikaans), iyeza lesikhali, ikubalo, umsongelo (Xhosa) | Tuber | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Pelargonium sidoides DC. | Geraniaceae | Black pelargonium (English), kalwerbossie, rabassam (Afrikaans), ikubalo, iyeza lesikhali (Xhosa), khoara-e-nyenyane (Southern Sotho) | Tuber | Used to treat TB and pneumonia. Preparation and application not specified. | McGaw et al. (2008); Hulley and Van Wyk2017 |
| Pelargonium triste (L.) L'Hér | Geraniaceae | Kaneelbol, rooirabas (Afrikaans) | Tuber | Used to treat TB and pneumonia. Preparation and application not specified. | Philander (2011) |
| Pentanisia prunelloides (Klotzsch) Walp. | Rubiaceae | Wild verbena (English), sooibrandbossie (Afrikaans), setimamollo (Sotho), icimamlilo (Zulu) | Roots | The Xhosa drink a root infusion to treat TB. | Watt and Breyer-Brandwijk (1962); Van Wyk et al. (2009); Philander (2011); Madikizela et al. (2013) |
| Pentzia incana (Thunb.) Kuntze | Asteraceae | Skaapkaroobos, ankerkaroo, kleinskaapkaroobos (Afrikaans) | Not specified | Used to treat bacterial pneumonia. Preparation and application not specified. | Hulley and Van Wyk2017 |
| Pharnaceum lineare L. f. | Molluginaceae | Droëdaskruie (Afrikaans) | Not specified | An infusion is consumed to treat TB. | Watt and Breyer-Brandwijk (1962); Van Wyk (2008); |
| Polycarpaea corymbosa (L.) Lam. | Caryophyllaceae | Old man's cap (English) | Not specified | Used to treat TB. Treatment and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Polygala fruticosa P.J. Bergius | Polygonaceae | Butterfly bush, heart-leaf polygala (English), ertjieblom (Afrikaans), ulopesi, ulapesi, umabalabala (Xhosa), ithethe (Zulu) | Roots | Root decoctions are used by the Zulu to treat TB. | Watt and Breyer-Brandwijk (1962); Madikizela et al. (2013); |
| Polygala myrtifolia L. | Polygonaceae | September bush (English), septemberbossie, augustusbossie, blouertjie, langelede (Afrikaans), ulopesi, ulapesi, umabalabala (Xhosa), uchwasha (Zulu) | Aerial parts | Used to treat TB. Treatment and application not specified. | McGaw et al. (2008) |
| Polygonum aviculare L. | Polygonaceae | Knotgrass, bird weed, pig weed (English) | Not specified | Used to treat TB. Treatment and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Polysiphonia virgata (C. Agardh.) Sprengel | Rhodomeleaceae | Red algae (English) | Not specified | Used to treat TB. Treatment and application not specified. | McGaw et al. (2008) |
| Protea nitida Mill. | Protaceae | Waboom (Afrikaans) | Not specified | Used to treat TB and pneumonia. Treatment and application not specified. | Hulley and Van Wyk2017 |
| Prunus africana (Hook.f.) Kalkman | Rosaceae | Red stinkwood, African almond (English), rooistinkhout, Afrika-amandel, Wilde-kersieboom (Afrikaans), inyazangoma-elimnyama, inkokhokho, ngubozinyeweni, umdumezulu (Zulu); uMkakase, inyazangoma, itywina-elikhul, Umkhakhase (Xhosa), mogohloro (Sotho), mulala-maanga (Venda) | Bark | Used to treat TB. Treatment and application not specified. | McGaw et al. (2008) |
| Prunus cerasus L. | Rosaceae | Sour cherry, tart cherry, dwarf cherry (English) | Leaf | Used to treat TB. Treatment and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Prunus persica (L.) Batsch | Rosaceae | Peach tree (English) | Leaves | Used to treat whopping cough. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Pteronia camphorata (L.) L. | Asteraceae | Wakkerbos, koorsbos (Afrikaans) | Leaves and twigs | Leaf and twig infusions are drunk to treat TB. | Nortjie and Van Wyk (2015) |
| Quercus spp. | Fagaceae | Oak (English) | Bark | The bark is boiled and the fumes inhaled to treat diphtheria | Watt and Breyer-Brandwijk (1962) |
| Rapanea melanophloeos (L.) Mez | Primulaceae | Cape beech (English); boekenhout, beukehout (Afrikaans), isiCalabi, umaPhipha, iKhubalwane, isiQalaba sehlati (Zulu), isiQwane sehlati (Xhosa) | Leaves and twigs | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Rhynchosia caribaea (Jacq.) DC. | Fabaceae | Unknown | Roots | A root extract is consumed to treat bacterial pneumonia. | Von Koenen (2001) |
| Rhamnus prinoides L'Hér | Rhamnaceae | Mofifi (Southern Sotho) | Branches | Used to treat bacterial pneumonia. Preparation and application not specified. | Kose et al. (2015) |
| Ricinus communis L. | Euphorbiaceae | Castor bean, castor oil plant (English) | Leaves | Used to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Rumex crispus L. | Polygonaceae | Yellow dock (English) | Whole plant | Used to treat TB. Preparation and application methods are not specified. | McGaw et al. (2008) |
| Salvia africana-lutea L. | Lamiaceae | Bloebloomsalie (Afrikaans) | Not specified | A tincture is drunk to treat whooping cough. | Watt and Breyer-Brandwijk (1962) |
| Salvia chamdaeagnea Berg. | Lamiaceae | Bloublomsalie (Afrikaans) | Leaves and flowers | Used to treat whooping cough. Preparation and application method not specified. | Watt and Breyer-Brandwijk (1962) |
| Salvia microphylla Kunth | Lamiaceae | Rooisalie, rooiblomsalie (Afrikaans) | Not specified | Infusions are drunk to treat bacterial pneumonia. | Hulley and Van Wyk2017 |
| Schinus molle L. | Anacardiaceae | Peruvian pepper (English), peperboom (Afrikaans) | Not specified | Used to treat bacterial pneumonia. Preparation and application not specified. | Hulley and Van Wyk2017 |
| Searsia lancea (L.f.) F.A. Barkley | Anacardiaceae | Makkaree, kareeboom (Afrikaans) | Not specified | Infusions are drunk to treat bacterial pneumonia. | Hulley and Van Wyk2017 |
| Securidaca longpedunculata Fresen. | Polygalaceae | Violet tree (English), krinkhout, rooipeultjie, seepbasboom (Afrikaans), mpesu (Venda), iphuphuma (Zulu) | Roots | A decoction is consumed by the Venda to treat TB. | Pallant and Steenkamp (2008) |
| Senecio serratuloides DC. | Asteraceae | Two-day cure (English), ichazampukane, insukumbili, umaphozisa umkhuthelo (Zulu) | Aerial parts | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Solanum nigrum L. | Solanaceae | Black nightshade (English) | Leaves | Fresh leaves are consumed to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Solanum retrofractum Vahl | Solanaceae | Nastergal, nasgal (Afrikaans) | Not specified | Used to treat TB. Preparation and application not specified. | Hulley and Van Wyk2017 |
| Spergula arvensis L. | Caryophyllaceae | Corn spurry (English) | Whole plant | An essential oil used to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Strophanthus grandiflorus (N.E.Br.) Gilg | Apocynaceae | Unknown | Whole plant | An alcohol extract is consumed to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Sutherlandia frutescens (L.) R.Br. | Fabaceae | Cancer bush (English), kankerbos (Afrikaans), ‘musa-pelo, motlepelo (Sotho), insiswa, unwele (Xhosa, Zulu) | Leaves | A decoction is used to treat TB. | Nortjie and Van Wyk (2015) |
| Syzygium cordatum Hochst. ex Krauss | Myrtaceae | Waterberry (English), waterbessie, waterboom (Afrikaans), undoni (Zulu), umswi, umjomi (Xhosa), mawthoo (Southern Sotho), motlho (Northern Sotho), mutu (Venda) | Leaves | Used by the Zulu to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962); Hutchings et al., (1996); Corrigan et al. (2011); |
| Syzygium gerrardii (Harv. ex Hook.f.) Burtt Davy | Myrtaceae | Unknown | Leaves | Used by the Zulu to treat TB. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962); McGaw et al. (2008) |
| Tabernaemontana elegans Stapf | Apocynaceae | Toad tree (English), laeveldse paddaboom (Afrikaans), umKhahlwana, umKhadu (Zulu) | Leaves, roots | Used to treat TB. Preparation and application not specified. | Pallant and Steenkamp (2008); Dzoyem et al. (2016) |
| Taraxacum officinale (L.) Weber ex F.H.Wigg | Asteraceae | Dandelion (English) | Flowers, leaves, roots, whole plant | Used to treat TB. Extracts were consumed orally to treat tuberculosis. | Sharifi-Rad et al. (2018); Smith (1895); Watt and Breyer-Brandwijk (1962) |
| Terminalia phanerophlebia Engl. & Diels | Combretaceae | Lebombo cluster-leaf (English), lebombotrosblaar (Afrikaans), amaNgwe-amnyama, amaNgwe-omphofu (Zulu) | Roots | Used to treat TB. Preparation and application not specified. | Madikizela et al. (2013) |
| Terminalia sericea Burch. ex DC. | Combretaceae | Silver cluster leaf (English), vaalboom (Afrikaans), mususu (Venda) | Roots and leaves | Decoctions and infusions are consumed to treat bacterial pneumonia. | McGaw et al. (2008); Van Wyk et al. (2009); York et al. (2011); |
| Tetradenia riparia (Hochst.) Codd | Lamiaceae | Misty plume bush, ginger bush (English), gemerbos, watersalie (Afrikaans), iboza, ibozane (Zulu) | Leaves, roots | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Thesium hystrix A.W. Hill | Santalaceae | Kleinswartstorm (Afrikaans) | Root | Large volumes of a root decoction are drunk to treat TB. | Smith (1895),Watt and Breyer-Brandwijk (1962); Van Wyk et al. (2009) |
| Thymus serpyllum L. | Lamiaceae | Breckland thyme, wild thyme, creeping thyme, elfin thyme (English) | Leaves and flowers | Used to treat whooping cough. Preparation and application methods are not specified. | Smith (1895); Watt and Breyer-Brandwijk (1962); |
| Thymus vulgaris L. | Lamiaceae | German thyme, common thyme (English) | Leaves | Leaf essential oil is used to treat whooping cough. Application methods is not specified. | Smith (1895); Watt and Breyer-Brandwijk (1962); McGaw et al. (2008); |
| Trachyandra laxa (N.E.Br.) Oberm. | Xanthorrhoeaceae | Unknown | Roots | Used to treat whooping cough. Preparation and application are not specified. | Von Koenen (2001) |
| Trema orientalis (L.) Blume | Cannabaceae | Pigeon wood (English), hophout (Afrikaans) | Leaves and fruit | Infusions of the leaves and fruit are drunk to treat bacterial pneumonia. | Von Koenen (2001) |
| Trichilia emetica Vahl | Meliaceae | Ixolo, umathunzini, umkhula (Zulu) | Leaves | Decoctions are used to treat pneumonia and whooping cough. | York et al. (2011) |
| Trifolium pratense L. | Fabaceae | Red clover (English) | Flowers | Infusions are drunk to treat TB and whooping cough. | Smith (1895); Watt and Breyer-Brandwijk (1962); |
| Tulbaghia alliacea L.f. | Amaryllidaceae | Wild garlic, woodland garlic (English), wildeknoflok (Afrikaans), molela (Southern Sotho), ishaladilezinyoka, umwelela (Zulu) | Bulbs | Used to treat TB. Preparation and application not specified. | Smith (1895); Watt and Breyer-Brandwijk (1962); Van Wyk (2008) |
| Tulbaghia maritima Vosa | Amaryllidaceae | Unknown | Bulbs | Used to treat TB. Preparation and application not specified. | Smith (1895); Watt and Breyer-Brandwijk (1962) |
| Tulbaghia violacea Harv. | Amaryllidaceae | Wild garlic (English), wilde knoffel (Afrikaans), isihaqa (Zulu) | Bulbs | Decoctions are drunk to treat TB. | Philander (2011); Smith (1895); Watt and Breyer-Brandwijk (1962); Van Wyk et al. (2009) |
| Urtica urens L. | Urticaceae | Annual nettle, burning nettle, sting nettle bush, dwarf stinging nettle (English), brandnekel (Afrikaans) | Bark | Bark infusions are drunk to treat TB, pneumonia and whooping cough. | Watt and Breyer-Brandwijk (1962); Van Wyk (2008); Hulley and Van Wyk2017 |
| Viscum capense L. f. | Santalaceae | Cape mistletoe (English), lidjiestee, voelent (Afrikaans) | Whole plant | Used to treat TB. Preparation and application not specified. | Philander (2011) |
| Vitis vinifera L. | Vitaceae | Common grape (English) | Fruit | A syrup prepared by boiling the fruit juice is used in the Transvaal to treat diphtheria. | Watt and Breyer-Brandwijk (1962) |
| Warburgia salutaris (G.Bertol.) Chiov. | Canellaceae | Pepper-bark tree (English), peperbasboom (Afrikaans), mulanga, manaka (Venda), isibhaha (Zulu) | Bark | Used to treat TB. Preparation and application not specified. | McGaw et al. (2008) |
| Withania somnifera (L.) Dunal | Solanaceae | Indian ginseng, poison gooseberry, winter cherry (English), bitterappelliefie, koorshout (Afrikaans), ubuvuma (Xhosa), ubuvimbha (Zulu) | Root | Alcohol root extracts are drunk to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Zanthoxylum capensis (Thunb.) Harv. | Rutaceae | Knobwood (English) | Bark | The Zulu use a root bark decoction to treat TB. | Watt and Breyer-Brandwijk (1962) |
| Ziziphus mucronata Willd. | Rhamnaceae | Buffalo thorn (English), blinkblaar-wag-'n-bietjie (Afrikaans), umphafa, umlahlankosi, isilahla (Zulu), umphafa (Xhosa), mutshetshete (Venda), mokgalô, moonaona (Sotho) | Leaves, bark and roots | A decoction of the leaves, roots and bark is drunk to treat TB. | McGaw et al. (2008); Suliman (2010) |
The relatively high number of plant species used to treat bacterial respiratory diseases may relate to the seriousness and relative prevalence of these infections. Indeed, the vast majority of the plants recorded for use against bacterial respiratory infections were used against bacterial pneumonia (139 species) or TB (81 species). Both of these diseases are relatively common in southern Africa and produce relatively high mortality rates. It is therefore perhaps not surprising that high numbers of plant species were identified for the treatment of these diseases.
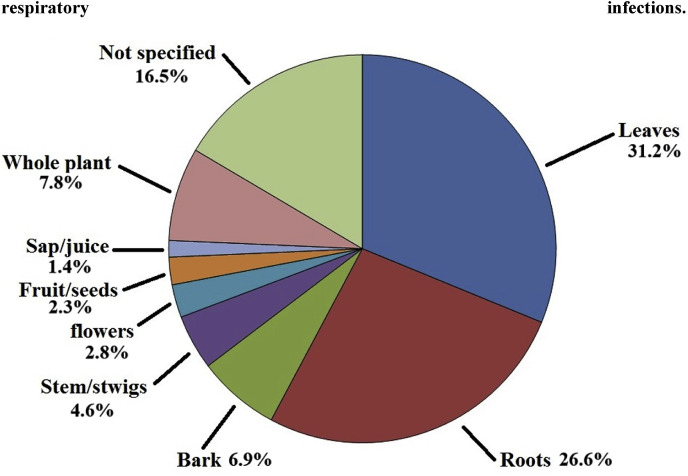
Many of the ethnobotanical books, reviews and primary studies used in this review did not specify the preparation of the traditional medicine or how it was used and further studies are required to clarify this. However, many of the recent ethnobotanical surveys did report these details, further emphasising the importance of updated ethnobotanical information. From those studies, a further trend was also evident: decoctions and infusions were most widely used in the treatment of bacterial respiratory infections, with 64 plant-based medicines reported to be used in these ways. Previous studies have also reported that decoctions and infusions are the most common methods for treating most pathogenic diseases (Afolayan et al., 2014; Asong et al., 2019; Cock et al., 2018; Cock et al., 2019; De Beer and Van Wyk, 2011; Hulley and Van Wyk, 2017; Nortje and Van Wyk, 2011; Philander, 2011). Tinctures were prepared and consumed for a further four species, volatiles targeted from three species via inhalation, and a syrup was prepared and consumed from the fruit of various species. This contrasts dramatically with the preparation and usage of plant species to treat viral respiratory diseases, where inhalation was the main method of administration (unpublished results). The use of southern African plants to treat viral respiratory plants will be the basis of another manuscript in preparation.
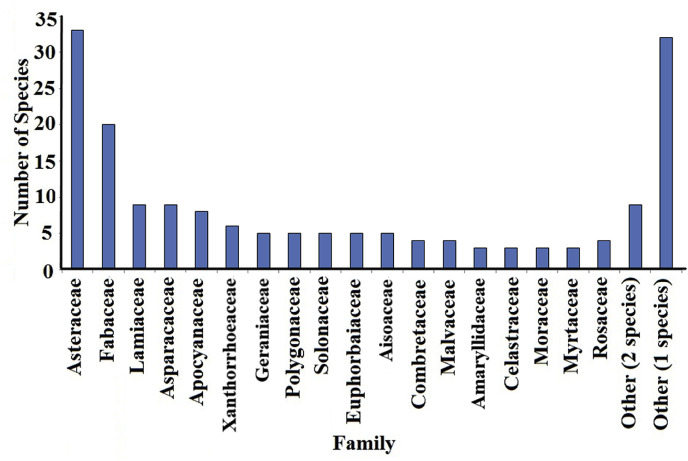
A wide variety of families of southern African plant species including Apiaceae, Asparagaceae, Asphodelaceae, Apocynaceae, Asteraceae, Brassicaceae, Celastraceae, Combretaceae, Euphorbiaceae, Fabaceae, Lamiaceae, Lauraceae, Malvaceae, Moraceae, Myrtaceae, Polygonaceae, Rosaceae and Solanaceae (Fig. 1 ) were traditionally used to treat bacterial respiratory diseases. Although the bioactivity of several of these species has already been screened against bacterial respiratory pathogens via in vitro testing (Table 2 ), most species are yet to be screened against respiratory bacterial pathogens. Asteraceae (33 species) and Fabaceae (20 species) were commonly used traditionally to treat bacterial respiratory diseases (Fig. 1). Lamiaceae (9 species), Asparagaceae (9 species), Apocynaceae (8 species), Xanthorrhoeaceae (6 species), Aisoaceae, Geraniaceae, Euphorbiaceae, Polygonaceae and Solanaceae were also well represented with 5 species each. Four members of Combretaceae, and Malvaceae were also identified, as well as three members each of Amaryllidaceae, Celastraceae, Moraceae, Myrtaceae and Rosaceae. Two or less species of fourty-one other families were also identified as being traditionally used to treat bacterial respiratory diseases.
Fig. 1.
The number of southern African plant species per family related to southern African medicinal plants for the treatment of bacterial respiratory infections. Others refers to the number of other genuses (not named individually) that are represented by the indicated number of species.
Table 2.
Scientific evaluations of the inhibitory activity of South African plants against bacterial respiratory pathogens.
| Plant species | Family | Common name(s) | Extract tested | Test micro-organism | Activity | References |
|---|---|---|---|---|---|---|
| Acacia nilotica (L.) Delile | Fabaceae | Scented-pod acacia (English), lekkerruikpeul (Afrikaans), mogohlo (Sotho), umNqawe (Zulu) | Leaf, bark and root extracts | K. pneumoniae | MIC = 780 μg/mL | Eldeen et al. (2005) |
| Acacia sieberiana DC. | Fabaceae | Paperbark thorn (English), papierbasdoring (Afrikaans), mphoka (Northern Sotho), musaunga (Venda), umKhamba (Zulu) | Leaf extracts | K. pneumoniae | MIC = 800–2000 μg/mL | Suliman (2010); Eldeen et al. (2005) |
| Acacia xanthophloea Benth. | Fabaceae | Fever tree (English) | Bark extracts | M. tuberculosis | 500 μg/mL | Lall and Meyer (1999) |
| Leaf, bark and root extracts | K. pneumoniae | 800 μg/mL | Eldeen et al. (2005) | |||
| Acanthospermum glabratum (DC.) Wild | Asteraceae | Unknown | Whole plant extracts | M. smegmatis (model for TB) | MIC = 670 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 3300 μg/mL | |||||
| Agathosma betulina (P.J.Bergius) Pillans | Rutaceae | Buchu (English), boegoe, rondeblaarboegoe (Afrikaans) | Leaf extracts | K. pneumoniae | MIC = 1876 μg/mL | Cock and Van Vuuren (2015a) |
| Aloe barberae Dyer | Xanthorrhoeaceae | Tree aloe (English) | Leaf extracts | K. pneumoniae | MIC = 780 μg/mL | Ndhlala et al. (2009) |
| Aloe marlothii A.Berger | Xanthorrhoeaceae | Mountain aloe, flat-flowered aloe (English), bergalwyn (Afrikaans), inhlaba, umhlaba (Zulu) | Leaf extracts | M. smegmatis (model for tuberculosis) | MIC = 3330 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 2670 μg/mL | |||||
| Abrus precatorius subsp. africanus Verdc. | Fabaceae | Bead vine, coral bead plant, coral bean, crabs eye, licorice vine, red bead vine (English), umkhokha (Zulu) | Leaf and seed extracts | M. arum (model for TB) | MIC = 780 μg/mL (leaf) | Madikizela et al. (2013) |
| K. pneumoniae | MIC = 1560 μg/mL (seed) | |||||
| Androstachys johnsonii Prain | Picrodendraceae | Ironwood (English), musimbiri (Venda) | Root bark and leaf extracts | K. pneumoniae | MIC = 3000 μg/mL | Samie et al. (2005) |
| P. aeruginosa | MIC = 620 μg/mL | |||||
| Artemisia afra Jacq. ex Willd. | Asteraceae | African wormwood (English), als, alsem, wildeals (Afrikaans), lengana (Sotho, Tswana), umhlonyane (Xhosa, Zulu) | Leaf extracts | M. tuberculosis | Inactive | Mativandlela et al. (2008) |
| M. smegmatis (model for TB) | MIC = 1563 μg/mL | |||||
| M. arum (model for TB) | MIC = 1560 μg/mL | Buwa and Afolayan (2009) | ||||
| K. pneumoniae | MIC = 1560 μg/mL | |||||
| Asparagus africanus Lam. | Asparagaceae | Bush asparagus (English) | Leaf extracts | M. arum (model for TB) | MIC = 390 μg/mL | Madikizela et al. (2013) |
| K. pneumoniae | MIC = 1560 μg/mL | |||||
| Asparagus falcatus L. | Asparagaceae | Unknown | Leaf extracts | H. influenzae | MIC>1000 μg/mL | Pallant and Steenkamp (2008) |
| M. arum (model for TB) | MIC = 390 μg/mL | Madikizela et al. (2013) | ||||
| K. pneumoniae | MIC = 3130 μg/mL | |||||
| Ballota africana (L.) Benth. | Lamiaceae | kattekruid, kattekruie (Afrikaans) | Leaf extracts | K. pneumoniae | MIC = 379 μg/mL | Cock and Van Vuuren (2015a) |
| Bauhinia petersiana Bolle | Fabaceae | Large white bauhinia (English), mubondo, mumwando, mun'ando or mupondo (Shona) | Leaf and root extracts | M. tuberculosis | MIC = 312 μg/mL | Dzoyem et al. (2016) |
| Berchemia discolor (Klotzsch) Hemsl. | Rhamnaceae | Bird plum, brown ivory (English), voëlpruim (Afrikaans), mogokgomo (Sepedi), nmumu, ubalatsheni-omkhulu, umadlozane, umhlungulo, uvuku (Zulu) | Bark acetone extract | M. tuberculosis | MIC = 10.5–12.5 μg/mL | Green et al. (2010) |
| Brachylaena discolor DC | Asteraceae | Coast silver oak (English), kusvaalbos (Afrikaans), phahla (Zulu), mphahla (Sotho), umPhahla (Xhosa) | Leaf extracts | M. smegmatis (model for TB) | MIC = 900 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 4000 μg/mL | |||||
| Bridelia cathartica Bertol. | Phyllanthaceae | Blue sweetberry, knobby bridelia (English) | Leaf extracts | M. smegmatis (model for TB) | MIC = 670 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 4000 μg/mL | |||||
| Bridelia micrantha (Hochst.) Baill. | Phyllanthaceae | Munzere (Venda) | Root, bark and seed extracts | K. pneumoniae | MIC = 6000 μg/mL | Samie et al. (2005) |
| P. aeruginosa | MIC = 6000 μg/mL | |||||
| Bark acetone extract | M. tuberculosis | MIC = 25 μg/mL | Green et al. (2010) | |||
| Brunsvigia grandiflora Lindl. | Amaryllidaceae | Giant candellabria (English), reusekandelaarblom (Afrikaans) | Bulb extracts | M. arum (model for TB) | MIC = 3130 μg/mL | Madikizela et al. (2013) |
| K. pneumoniae | MIC = 3130 μg/mL | |||||
| Calpurnia aurea (Aiton) Benth. | Fabaceae | Calpurnia, wild laburnum, Natal laburnum, Cape laburnum (English), geelkeurboom, geelkeur, Natalse geelkeur (Afrikaans), inDloli, umSitshana (Xhosa), umKhiphampethu, inSiphane-enkulu, umHlahlambedu, umLalandlovana (Zulu) | Leaf extracts | K. pneumoniae | MIC = 80 μg/mL | Elisha et al. (2017) |
| Carpobrotus edulis (L.) N.E.Br. | Aizoaceae | Sour fig, Cape fig, Hottentot's fig (English), vyerank, ghaukum, ghoenavy, hotnotsvye, Kaapvy, perdevy, rankvy (Afrikaans), ikhambi-lamabulawo, umgongozi (Zulu) | Leaf extracts | M. arum (model for TB) | MIC = 3125 μg/mL | Buwa and Afolayan (2009) |
| K. pneumoniae | MIC = 320–1040 μg/mL | |||||
| Cassia fistula L. | Fabaceae | Purging cassia, holden shower tree (English) | Flower extract | K. pneumoniae | Inactive | Duraipandiyan and Ignacimuthu (2007); Buwa and Afolayan (2009),Cock and Van Vuuren (2015a) |
| P. aeruginosa | Up to 13 mm zones of inhibition in disc diffusion assay. MIC not determined. | |||||
| Cassia petersiana (Bolle) Lock | Fabaceae | Dwarf Cassia (English), apiespeul (Afrikaans), Munembenembe (venda), Uhwabile, Umnembenembe (Zulu) | Bark acetone extract | M. tuberculosis | MIC = 50 μg/mL | Green et al. (2010) |
| Cassine papillosa (Hochst.) Kuntze | Celastraceae | Unknown | Bark extracts | M. tuberculosis | MIC = 1000 μg/mL | Lall and Meyer (1999) |
| Chaetachme aristata Planch. | Ulmaceae | Thorny-Elm (English), basterwitpeer (Afrikaans) | Leaf extracts | M. tuberculosis | MIC = 39 μg/mL | Dzoyem et al. (2016) |
| Chamaecrista mimosoides (L.) Greene | Fabaceae | Fishbone dwarf cassia (English), boesmanstee (Afrikaans), imbubu, umbonisela (Zulu), umnyana, unobothungwana (Xhosa) | Leaf and root extracts | K. pneumoniae | MIC = 2000 μg/mL (roots) | Suliman (2010) |
| Cheilanthes viridis (Forssk.) Sw | Pteridaceae | Ikhambi, lesilonda (Zulu) | Leaf and stem extracts | P. aeruginosa | MIC = 460 μg/mL (leaves) | Kelmanson et al. (2000) |
| Chenopodium ambrosioides L. | Chenopodiaceae | Wormsalt (English), sinkingbossie (Afrikaans) | Aerial parts extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Leaf and root extracts | K. pneumoniae | MIC = 1500 μg/mL (leaves) | Lall and Meyer (1999) | |||
| Citrus limon (L.) Osbeck | Rutaceae | Lemon (English) | Leaf extracts | M. smegmatis (model for TB) | MIC = 2000 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 2670 μg/mL | |||||
| Clausena anisata (Willd.) Hook.f. ex Benth. | Rutaceae | Unknown | Leaf extracts | M. smegmatis (model for TB) | MIC = 1670 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 1670 μg/mL | |||||
| Clematis brachiata Thunb. | Ranunculaceae | Ihlonzo leziduli, inhlongo, umdloza, umfufuna (Zulu) | Leaf and stem extracts | M. smegmatis (model for TB) | MIC = 2000 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 2000 μg/mL | |||||
| Clematis oweniae Harv. | Ranunculaceae | Bridal wreath, traveller's joy, old man's beard (English), klimop, lemoenbloeisels (Afrikaans), ihlonzo leziduli, Inhlabanhlanzi (Zulu), ityolo (Xhosa), morarana-oa-mafehlo (Southern Sotho) | Leaf extracts | K. pneumoniae | MIC = 2000 μg/mL | Suliman (2010) |
| Clerodendrum glabrum E.Mey. | Lamiaceae | Tinderwood (English), tontelhout (Afrikaans), moswaapeba (Sotho), munukha-tshilongwe (Venda), umqwaqwanam (Xhosa), umqoqonga (Zulu) | Leaf, bark and root extracts | M. tuberculosis | MIC = 156 μg/mL | Dzoyem et al. (2016) |
| Leaf extracts | K. pneumoniae | MIC = 4000 μg/mL | Suliman (2010) | |||
| Combretum apiculatum Sond. | Combretaceae | Red bush willow (English), rooiboswilg (Afrikaans), umbondwe (Zulu), mohwelere (Pedi), muvuvha (Venda) | Leaf extracts | P. aeruginosa | MIC = 1600 μg/mL | Eloff (1999) |
| Combretum bracteosum (Hochst.) Engl. & Diels | Combretaceae | Hiccup nut (English), hikklimop (Afrikaans), uQotha (Xhosa) | Leaf extracts | P. aeruginosa | MIC = 1600 μg/mL | Eloff (1999) |
| Combretum caffrum (Eckl. & Zeyh.) Kuntze | Combretaceae | Eastern Cape bushwillow (English) | Leaf extracts | P. aeruginosa | MIC = 6000 μg/mL | Eloff (1999) |
| Combretum celastroides Welw. ex M.A. Lawson | Combretaceae | Trailing bushwillow (English) | Leaf extracts | P. aeruginosa | MIC = 3000 μg/mL | Eloff (1999) |
| Combretum collinum Fresen. | Combretaceae | Weeping bushwillow, bicoloured bushwillow (English), vaiërende boswilg (Afrikaans) | Leaf extracts | K. pneumoniae | MIC = 315 μg/mL | Cock and Van Vuuren (2015b) |
| P. aeruginosa | MIC = 555–1600 μg/mL | Eloff (1999); Cock and Van Vuuren (2015b); | ||||
| Combretum edwardsii Exell. | Combretaceae | Forest climbing bushwillow (English) | Leaf extracts | P. aeruginosa | MIC = 3000 μg/mL | Eloff (1999) |
| Combretum erythrophyllum (Burch.) Sond. | Combretaceae | River bushwillow (English), riviervaderlandswilg, rooiblaar, rooiblad (Afrikaans), umbondwe, umdubu-wehlandze, umhlalavane (Zulu), umdubu (Xhosa), miavana, modubo (Southern Sotho), modibo (Northern Sotho), mugavhi, mugwiti, muvuvhu (Venda) | Leaf extracts | K. pneumoniae | MIC = 430 μg/mL | Eloff (1999); Cock and Van Vuuren (2015b) |
| P. aeruginosa | MIC = 500–3000 μg/mL | |||||
| Combretum hereroense Shinz | Combretaceae | Russet bushwillow, mouse-eared combretum (English), kierieklapper (Afrikaans), mokabi (Sotho), mugavhi (Venda), umhlalavane (Zulu) | Leaf extracts | P. aeruginosa | MIC = 1600 μg/mL | Eloff (1999) |
| Combretum imberbe Wawra | Combretaceae | Leadwood (English), hardekool (Afrikaans), motswiri (Pedi), ummono (Sotho), umbondwe omnyama (Zulu), muhiri (Venda) | Leaf extracts | P. aeruginosa | MIC = 3000 μg/mL | Eloff (1999) |
| Combretum kraussii Hochst. | Combretaceae | Forest bushwillow (English), bosvaderlandswilg (Afrikaans), uhwabhu, umdubu-wehlathi (Zulu), ulandile (Xhosa), modlubu (Sotho), muvuvhu, muvuvhu-wannda, muvuvhu-wa-thavhani (Venda) | Leaf extracts | P. aeruginosa | MIC = 1600 μg/mL | Eloff (1999) |
| Leaf, bark and root extracts | K. pneumoniae | MIC = 3125 μg/mL | Eldeen et al. (2005) | |||
| Combretum microphyllum Klotzsch | Combretaceae | Flame creeper (English) | Leaf extracts | K. pneumoniae | MIC = 205 μg/mL | Cock and Van Vuuren (2015b) |
| P. aeruginosa | MIC = 1650 μg/mL | Eloff (1999); Cock and Van Vuuren (2015b) | ||||
| Combretum mkuzense J.D.Carr & Retief | Combretaceae | Maputaland bushwillow (English) | Leaf extracts | P. aeruginosa | MIC = 1600 μg/mL | Eloff (1999) |
| Combretum moggii Exell | Combretaceae | Rock bushwillow (English), muvuvhatavha (Venda) | Leaf extracts | P. aeruginosa | MIC = 3000 μg/mL | Eloff (1999) |
| Combretum molle R.Br. ex G.Don | Combretaceae | Umbondo, umbondwe (Zulu) | Leaf extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| M. smegmatis (model for TB) | MIC = 1330 μg/mL | Eloff (1999); York et al. (2011); Cock and Van Vuuren (2015b) | ||||
| K. pneumoniae | MIC = 550–670 μg/mL | |||||
| P. aeruginosa | MIC = 800–1870 μg/mL | |||||
| Combretum mossambicense (Klotzsch) Engl. | Combretaceae | Knobbly creeper (English), knoppiesklimop (Afrikaans) | Leaf extracts | P. aeruginosa | MIC = 800 μg/mL | Eloff (1999) |
| Combretum nelsonii Dummer | Combretaceae | Waterberg bushwillow (English) | Leaf extracts | P. aeruginosa | MIC = 3000 μg/mL | |
| Combretum paniculatum Vent. | Combretaceae | Burning bush, forest flame creeper (English) | Leaf extracts | P. aeruginosa | MIC = 1600 μg/mL | |
| Combretum woodii Dummer | Combretaceae | Large-leaved forest bushwillow (English), grootblaarvaderlandswilg (Afrikaans), iWaphu (Zulu) | Leaf extracts | P. aeruginosa | MIC = 800 μg/mL | |
| Combretum padoides Engl. & Diels. | Combretaceae | Thicket bushwillow (English) | Leaf extracts | P. aeruginosa | MIC = 800 μg/mL | |
| Combretum zeyheri Sond. | Combretaceae | Large-fruited bushwillow, Zeyher's bushwillow (English), raasblaar, fluisterboom (Afrikaans), moduba-tshipi (Pedi), umbondwe wasembudwini (Zulu), mufhatelathundu (Venda) | Leaf extracts | P. aeruginosa | MIC = 800 μg/mL | |
| Cremaspora triflora (Thonn.) K.Schum. | Rubiaceae | Unknown | Leaf extracts | K. pneumoniae | MIC = 80 μg/mL | Elisha et al. (2017) |
| Croton megalobotrys Müll.Arg. | Euphorbiaceae | Feverberry (English), koorsbessie, grootkoorsbessie (Afrikaans), motsibi (Sotho), muruthu (Venda) | Leaf extracts | P. aeruginosa | MIC = 313 μg/mL | Selowa et al. (2010) |
| Croton pseudopulchellus Pax | Euphorbiaceae | Small lavender fever-berry (English), kleinlaventelkoorsbessie, sandkoorsbessie (Afrikaans), uHubeshane (Zulu) | Aerial parts extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Croton silvaticus Hochstetter ex Krauss | Euphorbiaceae | Forest fever berry (English) | Leaf extracts | P. aeruginosa | MIC = 1250 μg/mL | Selowa et al. (2010) |
| Conyza scabrida DC. | Asteraceae | Oven bush (English), bakbesembossie, bakbos, oondbos (Afrikaans), | Leaf extracts | M. tuberculosis | MIC = 300 μg/mL | Seaman (2005) |
| K. pneumoniae | MIC = 2000 μg/mL | |||||
| Cyperus articulatus L. | Cyperaceae | Jointed flat sedge (English) | Root extracts | M. smegmatis (model for TB) | Inactive | York et al. (2011) |
| K. pneumoniae | MIC = 2670 μg/mL | |||||
| Cryptocarya latifolia Sond. | Lauraceae | Bastard stinkwood, broad-leaved laurel (English), baster-stinkhout, breëblaarkweper, breëblaar-kweper, pondo-kweper (Afrikaans), umdlangwenya, umthungwa (Zulu), umgxaleba, umgxoboth, umncatyana (Xhosa) | Bark extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Datura stramonium L. | Solanaceae | Jimsome weed, Devoil's snare (English) | Leaf extracts | M. tuberculosis | MIC = 300 μg/mL | Seaman (2005) |
| K. pneumoniae | MIC = 4000 μg/mL | |||||
| Dioscorea dregeana (Kunth) T.Durand & Schinz | Discoreaceae | Ilabatheka (Zulu) | Tuber extracts | P. aeruginosa | MIC = 390 μg/mL | Kelmanson et al. (2000) |
| Dioscorea sylvatica Eckl. | Discoreaceae | Ilabatheka (Zulu) | Root and tuber extracts | P. aeruginosa | Inactive | Kelmanson et al. (2000) |
| Dioscorea sylvatica Eckl. | Discoreaceae | Forest elephant's foot (English) | Tuber extract | M. tuberculosis | MIC = 1000 μg/mL | Seaman (2005) |
| K. pneumoniae | MIC = 4000 μg/mL | |||||
| Dodonaea viscosa subsp. angustifolia (L.f.) J.G.West | Sapindaceae | Sand olive (English) | Leaf extracts | M. tuberculosis | MIC = 5000 μg/mL | Mativandlela et al. (2008) |
| M. smegmatis (model for TB) | MIC = 3125 μg/mL | |||||
| Drosera capensis L. | Droseraceae | Cape sundew (English), sondouw (Afrikaans) | Leaf extracts | M. tuberculosis | Inactive | Mativandlela et al. (2008) |
| M. smegmatis (model for TB) | MIC = 3125 μg/mL | |||||
| Diospyros mespiliformis Hochst. ex A.DC. | Ebenaceae | African ebony, jackal-berry (Eng.); jakkalsbessie (Afr.); Musuma (Tshivenda); Mgula (Tsonga) | Leaf hexane extract | M. tuberculosis | MIC = 100 μg/mL | Green et al. (2010) |
| Ekebergia capensis Sparrm. | Meliaceae | Cape ash, dogplum (English), essenhout (Afrikaans), mmidibidi (Sotho) | Bark extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Leaf extracts | M. smegmatis (model for TB) | Inactive | York et al. (2011) | |||
| K. pneumoniae | MIC = 1330 μg/mL | |||||
| Elaeodendron croceum (Thunb.) DC | Celastraceae | Forest saffron (English) | Leaf extracts | K. pneumoniae | MIC = 310 μg/mL | Elisha et al. (2017) |
| Eriocephalus africanus L. | Asteraceae | Wild rosemary (English), wilderoosmaryn, kapokbos (Afrikaans) | Leaf extracts | M. tuberculosis | MIC = 500 μg/mL | Seaman (2005) |
| K. pneumoniae | MIC = 500 μg/mL | |||||
| Erythrina caffra Thunb. | Fabaceae | Coast coral tree (English), kuskoraalboom (Afrikaans), umsinsi (Zulu), umsintsi (Xhosa) | Root extracts | M. smegmatis (model for TB) | Inactive | York et al. (2011) |
| K. pneumoniae | MIC = 8000 μg/mL | |||||
| Eucalyptus grandis W. Hill | Myrtaceae | Red gum, flooded gum (English) | Leaf extracts | M. smegmatis (model for TB) | MIC = 2000 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 670 μg/mL | |||||
| Euclea natalensis A.DC. | Ebenaceae | Natal guarri, Natal ebony, large-leaved guarri (English), Natalghwarrie, berggwarrie, swartbasboom (Afrikaans), umTshekisani, umKhasa (Xhosa), iDungamuzi, iChitamuzi, umZimane, umTshikisane, inKunzane, inKunzi-emnyama, umHlalanyamazane, umAnyathi (Zulu) | Root extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Euphorbia tirucalli L. | Euphorbiaceae | Pencil plant, rubber-hedge euphorbia (English), kraalmelkbos (Afrikaans) | Stem extracts | M. smegmatis (model for TB) | Inactive | York et al. (2011) |
| K. pneumoniae | MIC = 2670 μg/mL | |||||
| Faidherbia albida (Delile) A.Chev. | Fabaceae | Ana tree (English), anaboom (Afrikaans), mogabo (Pedi), umHlalankwazi (Zulu), muhoto (Venda) | Leaf and bark extracts | K. pneumoniae | MIC = 6269 μg/mL | Eldeen et al. (2005) |
| Ficus sur Forssk | Moraceae | Cape fig, broom cluster fig (English) | Bark and root extracts | M. arum (model for TB) | MIC = 3130 μg/mL (bark and roots) | Madikizela et al. (2013) |
| K. pneumoniae | MIC = 780–1560 μg/mL (roots) | Madikizela et al. (2013); Eldeen et al. (2005) | ||||
| Flueggea virosa (Roxb. ex Willd.) Royle | Phyllanthaceae | Snowberry tree, white-berry bush (English), witbessiebos (Afrikaans), isibangamhlota sehlati, umyaweyane (Zulu) | Leaf, root, bark and fruit extracts | M. tuberculosis | MIC = 312 μg/mL | Dzoyem et al. (2016) |
| Galenia africana L. | Aizoaceae | Yellow bush (English), brakkraalbossie, geelbos, kraalbos, muisbos, muisgeelbossie, perdebos (Afrikaans), iqina (Xhosa) | Bark extracts | M. tuberculosis | MIC = 1200 μg/mL (bark and roots) | Mativandlela et al. (2008) |
| M. smegmatis (model for TB) | MIC = 780 μg/mL (roots) | |||||
| Gerrardina foliosa Oliv. | Gerrardinaceae | Krantz-berry (English), Umaluleka (Zulu), Umlulama (Zulu) | Aqueous bark extracts | M. tuberculosis | MIC = 310 μg/mL | Madikizela, and McGaw (2018) |
| Gunnera perpensa L. | Gunneraceae | Wild rhubarb, river pumpkin (English), wilde ramenas, ravierpampoen (Afrikaans), qobo (Sotho), rambola-vhadzimu (Venda), iphuzi, ighobo (Xhosa), ugobhe (Zulu) | Root extracts | M. tuberculosis | Inactive | Lall and Meyer (1999) |
| Helichrysum kraussii Sch.Bip. | Asteraceae | Straw everlasting (English), sewejaartjie (Afrikaans), isipheshane, isiqoqo (Zulu) | Leaf and stem extracts | M. smegmatis (model for TB) | MIC = 2000 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 1330 μg/mL | |||||
| Helichrysum melanacme DC. | Asteraceae | Hotnotskooigoed (Afrikaans) | Whole plant extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Helichrysum odoratissimum Less. | Asteraceae | Everlantings (English), kooigoed (Afrikaans) imphepho (Zulu) | Whole plant extracts | M. tuberculosis | MIC = 300–500 μg/mL | Meyer, 1999; Seaman (2005); Lall and |
| Whole plant extracts | K. pneumoniae | MIC = 2000 μg/mL | Seaman (2005) | |||
| Heteromorpha arborescens (Spreng.) Cham. & Schltdl. | Apiaceae | Parsley tree (English), wildepietersielie (Afrikaans) | Leaf extracts | K. pneumoniae | MIC = 160 μg/mL | Elisha et al. (2017) |
| Heteromorpha trifoliata (H.L.Wendl.) Eckl. & Zeyh. | Apiaceae | Acetone, ethanol and aqueous leaf extracts | M. tuberculosis | MIC = 80–5000 μg/mL | Madikizela, and McGaw (2018) | |
| Heteropogon contortus (L.) P.Beauv. ex Roem. & Schult. | Poaceae | Tanglehead, spear grass (English), pylgras, assegaaigras (Afrikaans), isitupe (Zulu), selokana, seloka (Southern Sotho) | Leaf and stem extracts | P. aeruginosa | Inactive | Kelmanson et al. (2000) |
| Heteropyxis natalensis Harv. | Myrtaceae | Lavender tree, natal lavender (English), laventelboom (Afrikaans), inkunzi (Zulu), mudedede (Venda), inkhuzwa (Zulu). | Leaf bark and root extracts | M. tuberculosis | MIC = 312 μg/mL | Dzoyem et al. (2016) |
| Hexalobus monopetalus (A.Rich.) Engl. & Diels. | Annonaceae | Baboons' breakfast, Shakama plum (English), muhodzongwa, mukorongwa, mukwingiziri, munyani, mupodzongo, mupodzongwa, musakama (Shona) | Leaf, stem, root, bark and fruit extracts | M. tuberculosis | MIC = 156 μg/mL | Dzoyem et al. (2016) |
| Hypericum roeperianum Schimp.ex A. Rich. | Hypericaceae | Unknown | Leaf extracts | K. pneumoniae | MIC = 160 μg/mL | Elisha et al. (2017) |
| Hypoxis spp. | Hypoxidaceae | Yellow stars, star lily, african potato (English), sterretjie, Afrika-patat (Afrikaans), inkomfe, ilabatheka (Zulu) | Root extracts | M. smegmatis (model for TB) | MIC = 5330 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 1670 μg/mL | |||||
| Hypoxis colchicifolia Baker | Bulb acetone and ethanol extracts | M. tuberculosis | MIC = 160–630 μg/mL | Madikizela, and McGaw (2018) | ||
| Bark, acetone, aqueous and ethanol extracts | MIC = 1250–2500 μg/mL | |||||
| Indigofera arrecta A.Rich. | Fabaceae | African indigo (English) | Leaf and root extracts | M. arum (model for tuberculosis) | MIC = 780 μg/mL (leafs) | Madikizela et al. (2013) |
| K. pneumoniae | MIC = 390 μg/mL (roots) | |||||
| Kigelia africana (Lam.) Benth. | Bignoniaceae | Sausage tree (English), worsboom (Afrikaans), umVunguta, umFongothi (Zulu), muvevha (Venda) | Leaf and fruit extracts | K. pneumoniae | MIC = 663 μg/mL | Arkhipov et al. (2014); Cock and Van Vuuren (2015a); |
| Krauseola mosambicina Pax & Hoffm. | Caryophyllaceae | Unknown | Leaf and stem extracts | M. smegmatis (model for TB) | MIC = 1000 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 3000 μg/mL | |||||
| Lantana rugosa Thunb. | Verbenaceae | Bird's beer, bird's brandy (English), voëlbrandewyn, wildesalie (Afrikaans), utyani-bentaka, utywala bentaka (Xhosa), impema, ubukhwebezane, ubungungundwane, uguguvama (Zulu), mabele-mabutsoa-pele, molutoane (Southern Sotho). | Leaves and stems | K. pneumoniae | MIC = 3000 μg/mL | Suliman (2010) |
| Leonotis intermedia Lindl. | Lamiaceae | Minaret-flower (English), klipdagga (Afrikaans), fincane, isihlungu sedobo (Xhosa), joala-ba-li-nonyana, moseneke (Southern Sotho) | Leaf and stem extracts | M. arum (model for TB) | MIC = 195 μg/mL (leaves) | Madikizela et al. (2013) |
| K. pneumoniae | MIC = 3130 μg/mL (leaves and stems) | |||||
| Leucaena leucocephala (Lam.) De Wit | Fabaceae | Wild tamarind, white lead tree, lead tree, koa haole, ekoa, horse tamarind, jumbie bean, white popinac (English) | Leaf, bark and seed extracts | M. tuberculosis | MIC>2500 μg/mL | Dzoyem et al. (2016) |
| Leucas martinicensis (Jacq.) R.Br. | Lamiaceae | Unknown | Leaf and flower extracts | K. pneumoniae | MIC = 2000 μg/mL | Suliman (2010) |
| Lippia javanica (Burm.f.) Spreng | Verbenaceae | Fever tea, lemon bush (English), koorbossie, beukesbossie, lemoenbossie (Afrikaans), inzinzinba (Xhosa), umsuzwane, umswazi (Zulu) | Leaf, twig and root extracts | M. tuberculosis | MIC>2500 μg/mL | Dzoyem et al. (2016) |
| M. smegmatis (model for TB) | MIC = 1000 μg/mL | York et al. (2011); Cock and Van Vuuren (2015a); | ||||
| K. pneumoniae | MIC = 538 μg/mL | |||||
| Maesa lanceolata Forssk. | Primulaceae | False assegai (English), valsassegaai (Afrikaans), umalunguzalazikhakhona, inhlavubele, umaguqu, isidenda, ubhoqobhoqo (Zulu), intendekwane (Xhosa), muunguri (Venda) | Leaf extracts | K. pneumoniae | MIC = 630 μg/mL | Elisha et al. (2017) |
| Maytenus senegalensis (Lam.) Excell | Celastraceae | Confetti spike-thorn, confetti tree (English), rooidoring, rooi-pendoring (Afrikaans), isihlangu, Isihlangwane (Zulu), sephatwa (Sotho), tshibavhe, tshiphandwa (Venda) | Aerial parts extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Morus mesozygia Stapf | Moraceae | African mulberry, black mulberry (English) | Leaf extracts | K. pneumoniae | MIC = 80 μg/mL | Elisha et al. (2017) |
| Nidorella anomala Steetz | Asteraceae | Unknown | Whole plant extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Nidorella auriculata DC. | Asteraceae | Unknown | Whole plant extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Ozoroa obovata (Oliv) R.Fern. & A.Fern. | Anacardaceae | Broad-leaved resin tree (English), Breë blaarharpuisboom (Afrikaans) | Leaf and bark extracts | M. smegmatis (model for TB) | MIC = 300–2000 μg/mL | Seaman (2005); York et al. (2011); |
| K. pneumoniae | MIC = 1000–1700 μg/mL | |||||
| Parinari capensis Harv. | Chrysobalanaceae | Dwarf mobola-plum, sand apple (English), bosappel, gruisappeltjie, sandappeltjie, witappeltjie (Afrikaans), mobolo-oa-fatsi (Southern Sotho) | Root extracts | M. smegmatis (model for TB) | MIC = 2000 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 1500 μg/mL | |||||
| Peucedanum caffrum Phil | Apiaceae | Unknown | Root extracts | K. pneumoniae | MIC = 6000 μg/mL | Suliman (2010) |
| Pentanisia prunelloides (Klotzsch) Walp. | Rubiaceae | Wild verbena (English), sooibrandbossie (Afrikaans), setimamollo (Sotho), icimamlilo (Zulu) | Leaf and root extracts | M. arum (model for TB) | MIC = 390 μg/mL (leaves) | Madikizela et al. (2013) |
| K. pneumoniae | MIC = 390 μg/mL (roots) | |||||
| Pelargonium betulinum (L.) L'Hér. ex Aiton | Geraniaceae | Camphor-scented pelargonium, birch-leaved pelargonium (English), kanferblaar, maagpynbossie, suurbos (Afrikaans) | Aerial parts extracts | K. pneumoniae | MIC = 1000 μg/mL | Lalli et al. (2008) |
| Pelargonium citronellum J.J.A. Van der Walt | Geraniaceae | Citronella pelargonium, lemon-scented pelargonium (English) | Aerial parts extracts | K. pneumoniae | MIC = 3000 μg/mL | Lalli et al. (2008) |
| Pelargonium cordifolium Curtis | Geraniaceae | Unknown | Aerial parts extracts | K. pneumoniae | MIC = 1500 μg/mL | Lalli et al. (2008) |
| Pelargonium crispum (P.J.Bergius) L'Hér. | Geraniaceae | Lemon-scented pelargonium, crisped-leaf pelargonium (English) | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium cucullatum (L.) L'Hér. | Geraniaceae | Hooded-leaf pelargonium, (English), wildemalva (Afrikaans) | Aerial parts extracts | K. pneumoniae | MIC = 1500 μg/mL | Lalli et al. (2008) |
| Pelargonium fasciculaceum E.M. Marais | Geraniaceae | Unknown | Leaf extracts | K. pneumoniae | MIC = 374 μg/mL | Cock and Van Vuuren (2015a) |
| Pelargonium glutinosum (Jacq.) L'Hér. | Geraniaceae | balm-scented pelargonium, sticky-leaf pelargonium (English) | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium graveolens L'Hér. | Geraniaceae | Rose-scented pelargonium (English), wildemalva (Afrikaans) | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium greytonense J.J.A.Van der Walt | Geraniaceae | Greyton pelargonium (English) | Aerial parts extracts | K. pneumoniae | MIC = 3200 μg/mL | Lalli et al. (2008) |
| Pelargonium hermanniifolium Jacq. | Geraniaceae | Unknown | Aerial parts extracts | K. pneumoniae | MIC = 1500 μg/mL | Lalli et al. (2008) |
| Pelargonium hispidum Willd. | Geraniaceae | Hispid pelargonium (English), grofharig (Afrikaans) | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium panduriforme Eckl. & Zeyh. | Geraniaceae | Balsam-scented geranium, fiddle leaf geranium (English) | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium papilionaceum (L.) L'Hér. ex Aiton | Geraniaceae | Butterfly pelargonium (English), rambossie (Afrikaans) | Aerial parts extracts | K. pneumoniae | MIC = 4000 μg/mL | Lalli et al. (2008) |
| Pelargonium pseudoglutinosum R.Knuth | Geraniaceae | Unknown | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium quercifolium (L.f) L'Hér. | Geraniaceae | Oak-leaf pelargonium (English), muishondbos (Afrikaans) | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium radens H.E. Moore | Geraniaceae | Rasp-leaved, multifid-leaved pelargonium (English) | Aerial parts extracts | K. pneumoniae | MIC = 2500 μg/mL | Lalli et al. (2008) |
| Pelargonium scabroide R. Knuth | Geraniaceae | Unknown | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium scabrum (L) L'Hér. | Geraniaceae | Rough-leaved pelargonium, three-pointed pelargonium (English), hoenderbos (Afrikaans) | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium sublignosum R. Knuth | Geraniaceae | Unknown | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium tomentosum Jacq. | Geraniaceae | Peppermint-scented pelargonium (English) | Aerial parts extracts | K. pneumoniae | MIC = 2000 μg/mL | Lalli et al. (2008) |
| Pelargonium vitifolium (L.) L'Hér. | Geraniaceae | Vine-leaved pelargonium, balm-scented pelargonium (English) | Aerial parts extracts | K. pneumoniae | MIC = 4000 μg/mL | Lalli et al. (2008) |
| Phymaspermum acerosum (DC.) Källersjö | Asteraceae | Geelblombos (Afrikaans), isibhaha-segceke, umhlonishwa (Zulu) | Acetone, ethanol and aqueous leaf extracts | M. tuberculosis | MIC = 80–630 μg/mL | Madikizela, and McGaw (2018) |
| Acetone, ethanol and aqueous root extracts | MIC = 40–310 μg/mL | |||||
| Piper capense L.f. | Piperaceae | Wild pepper (English) | Root acetone extract | M. tuberculosis | MIC = −100 μg/mL | Green et al. (2010) |
| Pittosporum viridiflorum Sims | Pittosporaceae | Cheesewood, white cape beech (English), kasuur, witboekenhout (Afrikaans), umVusamvu, Umkhwenkwe, Umphushane (Zulu), kgalagangwe (Sotho), mosetlela (Southern Sotho), mulondwane, mutanzwakhamelo (Venda), umgqwengqwe (Xhosa) | Leaf extracts | K. pneumoniae | MIC = 160 μg/mL | Elisha et al. (2017) |
| M. tuberculosis | MIC = −310-2500 μg/mL | Madikizela, and McGaw (2018) | ||||
| Plectranthus neochilus Schltr. | Lamiaceae | Smelly spur flower, lobster flower (English), rotstuinsalie (Afrikaans) | Leaf extracts | M. smegmatis (model for TB) | MIC = 1000 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 1330 μg/mL | |||||
| Podocarpus elongatus (Aiton) L'Hér. ex Pers. | Podocarpaceae | Breede River yellowwood (English), breeriviergeelhout, westelike geelhout (Afrikaans) | Leaf and stem extracts | K. pneumoniae | MIC = 1040 μg/mL | Abdillahi et al. (2008) |
| Podocarpus falcatus (Thunb.) R.Br. ex Mirb. | Podocarpaceae | Outeniqua yellowwood (English), Outeniekwageelhout (Afrikaans), mogôbagôba (Sotho), umsonti (Zulu) | Leaf and stem extracts | K. pneumoniae | MIC = 650 μg/mL | Abdillahi et al. (2008) |
| Podocarpus henkelii Stapf ex Dallim. & B.D.Jacks. | Podocarpaceae | Henkel's yellowwood (English), Henkel-se geelhout (Afrikaans), umSonti (Zulu) | Leaf and stem extracts | K. pneumoniae | MIC = 390 μg/mL | Abdillahi et al. (2008) |
| Podocarpus latifolius (Thunb.) R.Br. ex Mirb. | Podocarpaceae | Real yellowwood (English), opregte geelhout (Afrikaans), umkhomba (Xhosa), mogobagoba (Sotho), muhovho-hovho (Venda), umsonti (Zulu) | Leaf and stem extracts | K. pneumoniae | MIC = 650 μg/mL | Abdillahi et al. (2008) |
| Polygala fruticosa P.J. Bergius | Polygalaceae | Butterfly bush, heart-leaf polygala (English), ertjieblom (Afrikaans), ulopesi, ulapesi, umabalabala (Xhosa), ithethe (Zulu) | Leaf and root extracts | M. arum (model for TB) | MIC = 1560 μg/mL (leaves) | Madikizela et al. (2013) |
| K. pneumoniae | MIC = 3130 μg/mL (roots) | |||||
| Polygala myrtifolia L. | Polygalaceae | September bush (English), septemberbossie, augustusbossie, blouertjie, langelede (Afrikaans), ulopesi, ulapesi, umabalabala (Xhosa), uchwasha (Zulu) | Aerial parts extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Pouzolzia mixta Solms | Urticacaceae | Soap nettle (English), muthanzwa (Venda) | Leaf, root and stem extracts | K. pneumoniae | MIC = 6000 μg/mL | Samie et al. (2005) |
| Protorhus longifolia (Bernh.) Engl. | Anacardiaceae | Red beech, purple currant, red Cape beech (English), rooiboekenhout, rooimelkhout (Afrikaans), ikhubalo, isifuce (isiXhosa), umkomiso, uzintlwa (Zulu) | Leaf acetone and ethanol extracts | M. tuberculosis | MIC = −160-1250 μg/mL | Madikizela, and McGaw (2018) |
| Pterocelastrus echinatus N.E. Br. | Celastraceae | Hedgehog-tree, white candlewood (English), Ibholo (Xhosa), Inqayi-elibomvu (Zulu), Mutongola (Venda), Wit-kershout (Afrikaans) | Bark acetone and ethanol extracts | M. tuberculosis | MIC = −160-630 μg/mL | Madikizela, and McGaw (2018) |
| Leaf acetone, aqueous and ethanol extracts | MIC = −160-630 μg/mL | |||||
| Prunus africana (Hook.f.) Kalkman | Rosaceae | Red stinkwood, African almond (English), rooistinkhout, Afrika-amandel (Afrikaans), inyazangoma-elimnyama, inkokhokho, ngubozinyeweni, umdumezulu (Zulu), uMkakase, inyazangoma, Itywina-elikhul, Umdumizulu (Xhosa), mogohloro (Sotho), mulala-maanga (Venda) | Leaf extracts | M. arum (model for TB) | Inactive | Madikizela et al. (2013) |
| P. aeruginosa | MIC = 1500 μg/mL | Samie et al. (2005) | ||||
| K. pneumoniae | MIC = 1500–6000 μg/mL | Eldeen et al. (2005); Samie (2005) | ||||
| Psidium guajava L. | Myrtaceae | Common guava, yellow guabva (English) | Leaf extracts | M. smegmatis (model for TB) | MIC = 2000 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 670 μg/mL | |||||
| Ptaeroxylon obliquum (Thunb.) Radlk. | Rutaceae | Sneezewood (English), nieshout (Afrikaans), umThathi (Xhosa) | Leaf extracts | K. pneumoniae | MIC = 1977 μg/mL | Cock and Van Vuuren (2015a) |
| Rapanea melanophloeos (L.) Mez. | Primulaceae | Cape beech, rapanea (English), boekenhout, kaapse, boekenhout, rooiboekenhout, swartbas (Afrikaans), ikhubalwane, inhluthe, isicalabi, isiqalaba-sehlathi, umaphipha-khubalo, umhluti-wentaba, uvukwabafile (Zulu) | Leaf, bark and root extracts | M. tuberculosis | MIC = 150–500 μg/mL | Lall and Meyer (1999); Dzoyem et al. (2016) |
| Rhamnus prinoides L'Hér. | Rhamnaceae | Camdeboo, dogwood, glossy-leaf, shiny leaf, stinkwood (English), alinkbaar, hondepishout, kamdeboo-stinkhout, seerkeelboom (Afrikaans), ulenyenye, umgilindi, umhlinye, umnyenye (Zulu) | Leaf, bark, root, fruit and seed extracts | M. tuberculosis | MIC = 625 μg/mL | Dzoyem et al. (2016) |
| Rhoicissus tridentata (L.f.) Wild & R.B.Drumm. | Vitaceae | Bitter grape (English), Murumbulashedo (Venda) | Root, tuber and fruit extracts | K. pneumoniae | MIC = 3000 μg/mL | Samie et al. (2005) |
| P. aeruginosa | MIC = 3000 μg/mL | |||||
| Leaf acetone extract | M. tuberculosis | MIC = 50 μg/mL | Green et al. (2010) | |||
| Rhus dentate Thunb. | Anacardiaceae | Nana-berry (English), Mubikasadza (Shona) | Bark acetone extract | M. tuberculosis | MIC = 50 μg/mL | Green et al. (2010) |
| Rumex crispus L. | Polygonaceae | Yellow dock (English) | Aerial parts extracts | M. tuberculosis | Inactive | Lall and Meyer (1999) |
| Salix mucronate Thunb. | Salicaceae | Cape willow (English), Safsaf-wilger, kaapse wilger, wildewilgerboom (Afrikaans), munengeledzi (Venda), mogokare (Sotho), umngcunube (Xhosa), umnyezane, umzekana (Zulu) | Leaf, bark and root extracts | K. pneumoniae | MIC = 6250 μg/mL | Eldeen et al. (2005) |
| Sansevieria hyacinthoides (L.) Druce | Asparagaceae | Mother-in-law's tongue, piles root, bowstring hemp (English), skoonma-se-tong, aambeiwortel, haasoor (Afrikaans), kai, ghaiwortel (Khoi), isikholokotho (Xhosa, Zulu), isikwendle, isitokotoko (Zulu) | Leaf extracts | M. smegmatis (model for TB) | Inactive | York et al. (2011) |
| K. pneumoniae | MIC = 4000 μg/mL | |||||
| Scadoxus puniceus (L.) Friis & Nordal | Amaryllidaceae | Paintbrush lily, snake lily (English), rooikwas (Afrikaans), isisphompho, umgola (Zulu) | Leaf extracts | M. smegmatis (model for TB) | MIC = 2670 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 3000 μg/mL | |||||
| Schkuhria pinnata (Lam.) Kuntze ex Thell. | Compositae | Dwarf marigold (English) | Leaf extracts | K. pneumoniae | MIC = 3000 μg/mL | Suliman (2010) |
| P. aeruginosa | MIC = 270 μg/mL | Kudumela et al., 2018 | ||||
| M. smegmatis | MIC = 270 μg/mL | Masiphephethu (2019); Masoko and Masiphephethu (2019). | ||||
| Schotia brachypetala Sond. | Fabaceae | Weeping boer-bean, tree fuchsia, African walnut (English), huilboerboon (Afrikaans) umfofofo (Xhosa), uvovovo (Zulu), molope (Northern Sotho), mutanswa (Venda), nwavilombe (Tsonga); umutwa (Tswana), uvovovo (Swati) | Bark acetone extracts | M. tuberculosis | MIC = 50 μg/mL | Green et al. (2010) |
| Sclerocarya birrea (A. Rich.) Hochst. | Anacardaceae | Marula (English), morula (Northern Sotho) | Bark extracts | M. smegmatis (model for TB) | MIC = 1330 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 670 μg/mL | |||||
| Securidaca longipedunculata Fresen. | Polygonaceae | Violet tree (English), krinkhout, rooipeultjie, seepbasboom (Afrikaans), mpesu (Venda), iphuphuma (Zulu) | Roots | Haemophilus influenzae | Inactive | Pallant and Steenkamp (2008) |
| Senecio deltoideus Less. | Asteraceae | Canary creeper (English), undenze (Xhosa) | Leaf extracts | Mycobacterium smegmatis (model for TB) | MIC = 1330 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 1330 μg/mL | |||||
| Senecio serratuloides DC. | Asteraceae | Two-day cure (English), ichazampukane, insukumbili, umaphozisa umkhuthelo (Zulu) | Aerial parts extracts | M. tuberculosis | MIC = 5000 μg/mL | Lall and Meyer (1999) |
| Leaf extracts | M. smegmatis (model for TB) | MIC = 1330 μg/mL | York et al. (2011) | |||
| K. pneumoniae | MIC = 1330 μg/mL | |||||
| Siphonochilus aethiopicus (Schweinf.) B.L. Burtt | Zingiberaceae | Natal ginger, wild ginger (English), wildegemmer (Afrikaans), indungulo, isiphephetho (Zulu) | Root extracts | M. tuberculosis | MIC = 300 μg/mL | Seaman (2005) |
| K. pneumoniae | MIC = 4000 μg/mL | |||||
| Syzygium cordatum Hochst. ex Krauss | Myrtaceae | Waterberry (English), waterbessie, waterboom (Afrikaans), undoni (Zulu), umswi, umjomi (Xhosa), mawthoo (Southern Sotho), motlho (Northern Sotho), mutu (Venda) | Bark extracts | H. influenzae | MIC = 1000 μg/mL | Pallant and Steenkamp (2008) |
| M. tuberculosis | MIC = 300 μgmL | Seaman (2005) | ||||
| M. smegmatis (model for TB) | MIC = 1330 μg/mL | York et al. (2011) | ||||
| P. aeruginosa | MIC = 310 μg/mL | Samie et al. (2005) | ||||
| K. pneumoniae | MIC = 300–2000 μg/mL | Cock and Van Vuuren (2015a); York et al. (2011); Samie et al. (2005); Seaman (2005) | ||||
| Tabernaemontana elegans Stapf | Apocynaceae | Toad tree (English),laeveldse paddaboom (Afrikaans), umKhahlwana, umKhadu (Zulu) | Leaves, roots, rhizome, sap | M. tuberculosis | MIC = 1250 μg/mL | Dzoyem et al. (2016) |
| H. influenzae | Inactive | Pallant and Steenkamp (2008) | ||||
| Terminalia phanerophlebia Engl. & Diels | Combretaceae | Lebombo cluster-leaf (English), lebombotrosblaar (Afrikaans), amaNgwe-amnyama, amaNgwe-omphofu (Zulu) | Leaf, root and twig extracts | M. arum (model for TB) | MIC = 195 μg/mL (leaves and twigs) | Madikizela et al. (2013) |
| K. pneumoniae | MIC = 195 μg/mL (twigs) | |||||
| Terminalia pruinoides M.A.Lawson | Combretaceae | Purple-pod terminalia (English), deurmekaar, sterkbos (Afrikaans) | Leaf extracts | K. pneumoniae | MIC = 432 μg/mL | Cock and Van Vuuren (2015a); Cock and Van Vuuren (2015b); Eloff (1999) |
| P. aeruginosa | MIC = 360–1600 μg/mL | |||||
| Terminalia sericea Burch. ex DC. | Combretaceae | Silver cluster leaf (English), vaalboom (Afrikaans), mususu (Venda) | Bark, root and leaf extracts | M. smegmatis (model for TB) | MIC = 670 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 250–670 μg/mL | York et al. (2011); Suliman (2010); Cock and Van Vuuren (2015a) | ||||
| P. aeruginosa | MIC = 6000 μg/mL | Eloff (1999) | ||||
| Bark acetone extract | M. tuberculosis | MIC = 25 μg/mL | Green et al. (2010) | |||
| Tetradenia riparia (Hochst.) Codd | Lamiaceae | Misty plume bush, ginger bush (English), gemerbos, watersalie (Afrikaans), iboza, ibozane (Zulu) | Root extracts | M. tuberculosis | MIC = 300 μg/mL | Seaman (2005) |
| Leaf extracts | M. smegmatis (model for TB) | MIC = 1000 μg/mL | York et al. (2011) | |||
| K. pneumoniae | MIC = 1750 μg/mL | |||||
| Thymus vulgaris L. | Lamiaceae | German thyme, common thyme (English) | Aerial parts extracts | M. tuberculosis | MIC = 500 μg/mL | Lall and Meyer (1999) |
| Trema orientalis (L.) Blume | Cannabaceae | Pigeonwood (English), hophout (Afrikaans), ifamu, iphubane, isakasaka, isikhwelamfene, sakasaka, ubathini, umbengele, umbhangabhanga, umdindwa, umsekeseke, umvangazi (Zulu) | Leaves, stems, roots, bark, fruit, twigs and seeds | M. tuberculosis | MIC = 312 μg/mL | Dzoyem et al. (2016) |
| Trichilia dregeana Sond. | Meliaceae | Forest mahogany, Cape mahogany, red ash (English), rooiessenhout, bosrooiessenhout, basteressenhout (Afrikaans), umKhuhlu, uMathunzini (Zulu), umKhuhlu (Xhosa), mmaba (Sotho), mutuhu, mutshikili (Venda) | Leaf, bark and root extracts | K. pneumoniae | MIC = 6250 μg/mL | Eldeen et al. (2005) |
| Trichilia emetica Vahl | Meliaceae | Natal mahogany (English), rooiessenhout (Afrikaans), mamba (Sotho), umathunzini (Zulu), umkhuhlu (Xhosa), mutuhu (Venda) | Leaf extracts | M. smegmatis (model for TB) | MIC = 2670 μg/mL | York et al. (2011) |
| K. pneumoniae | MIC = 1670 μg/mL | |||||
| Tulbaghia violacea Harv. | Amaryllidaceae | Wild garlic (English), wilde knoffel (Afrikaans), isihaqa (Zulu) | Root and leaf extracts | M. arum (model for TB) | MIC = 780 μg/mL | Buwa and Afolayan (2009) |
| K. pneumoniae | MIC = 277 μg/mL | Cock and Van Vuuren (2015a) | ||||
| Vernonia colorata (Willd.) Drake | Asteraceae | English bitter leaf, bitters tree (English) | Leaf, stems and root extracts | P. aeruginosa | MIC = 520 μg/mL (leaves) | Kelmanson et al. (2000) |
| Vitex rehmannii Gürke | Lamiaceae | Pipe-stem fingerleaf (English), umluthu (Zulu), munyongatshifumbu (Venda) | Leaf extracts | K. pneumoniae | MIC = 2000 μg/mL | Suliman (2010) |
| Warburgia salutaris (G.Bertol.) Chiov. | Canellaceae | Pepper-bark tree (English), peperbasboom (Afrikaans), mulanga, manaka (Venda), isibhaha (Zulu) | Bark and leaf extracts | M. tuberculosis | MIC = 156 μg/mL | Dzoyem et al. (2016) |
| K. pneumoniae | MIC = 624 μg/mL | Cock and Van Vuuren (2015a) | ||||
| Leaf acetone extracts | M. tuberculosis | MIC = 25 μg/mL | Green et al. (2010) | |||
| Xerophyta retinervis Baker | Velloziaceae | Black-stick lily (English), besembos, bobbejaanstert (Afrikaans), isigqumana, isiphmba (Zulu) | Bark extracts | M. tuberculosis | MIC = 300 μg/mL | Seaman (2005) |
| K. pneumoniae | MIC = 500 μg/mL | |||||
| Ximenia caffra Sond. | Olacaceae | Sour plum (English), mutshili (Venda) | Leaf and root extracts | K. pneumoniae | MIC = 3000 μg/mL | Samie et al. (2005) |
| P. aeruginosa | MIC = 6000 μg/mL | Samie et al. (2005) | ||||
| Zanthoxylum davyi Waterm. | Rutaceae | Forest knobwood (English) | Root and bark extracts | K. pneumoniae | MIC = 2000 μg/mL (bark) | Suliman (2010) |
| Zornia milneana Mohlenbr. | Fabaceae | Lukandulula (Venda) | Whole plant extracts | K. pneumoniae | MIC = 3000 μg/mL | Samie et al. (2005) |
| P. aeruginosa | MIC = 3000 μg/mL | |||||
| Ziziphus mucronata Willd. | Rhamnaceae | Buffalo thorn (English), blinkblaar-wag-'n-bietjie (Afrikaans), umphafa, umlahlankosi, isilahla (Zulu), umphafa (Xhosa), mutshetshete (Venda), mokgalô, moonaona (Sotho) | Bark extracts | M. smegmatis (model for TB) | Inactive | Mativandlela et al. (2008) |
| Leaf, bark and root extracts | M. tuberculosis | MIC = 156 μg/mL | Dzoyem et al. (2016) | |||
| K. pneumoniae | MIC = 4000 μg/mL | Suliman (2010) | ||||
| Bark extract | M. tuberculosis | MIC = 50 μg/mL | Green et al. (2010) |
Where multiple solvent extractions and/or multiple plant parts were tested for a plant species, the MIC values reported here were for the most active extract (as judged by MIC value). Plant species indicated in bold are species documented ethnobotanically (Table 1).
Several trends regarding the plant part used traditionally were also apparent. The use of leaves was particularly common for the treatment of bacterial respiratory infections, with the use of leaves recorded for 68 southern African plant species (Fig. 2 ). Interestingly, leaves are also the most frequently used plant part for the treatment of many other pathogenic diseases (Afolayan et al., 2014; Asong et al., 2019; Cock et al., 2018; Cock et al., 2019; De Beer and Van Wyk, 2011; Hulley and Van Wyk, 2017; Philander, 2011). The roots of 58 plant species were also used to treat several bacterial respiratory diseases, whilst bark (15 species) and stems and twigs (10 species) were also relatively widely used. In contrast flowers, fruit and seeds were only rarely used to treat bacterial respiratory disease. In contrast, the whole plant of some species was used therapeutically in southern African traditional medicine. Complete ethnobotanical records were not available for 36 plant species and we were therefore not able to determine the part used traditionally to treat bacterial respiratory diseases.
Fig. 2.
Frequency of use of different southern African plant parts to treat bacterial respiratory infections.
4.2. Scientific studies into the growth inhibitory activity of South African plants against bacterial respiratory pathogens
Whilst the ethnobotanical usage of many southern African plants to treat bacterial respiratory infections are yet to be scientifically evaluated, 189 southern African plant species have been screened for the ability to inhibit the growth of bacterial respiratory infections (Table 2). Interestingly, only 42 species (indicated in bold within Table 2) (of the 186 species that are used in traditional medicine to treat bacterial respiratory diseases have been tested against one or more bacterial respiratory pathogens. This equates to approximately only 23% of the species traditionally used to treat bacterial respiratory diseases. The remaining 77% are yet to be evaluated against any of the respiratory pathogens. Furthermore, the majority of the plants species that have been screened have only been tested against a single bacterial respiratory pathogen and further studies are required to test them against other bacterial species and strains.
The vast majority of plants that have been tested against pneumonia-causing bacterial pathogens have been screened against K. pneumoniae and P. aeruginosa. Indeed, 139 plant species were tested against one or both of these pathogens. Eighty-nine plant species were also screened against M. tuberculosis, or other Mycobacterium spp. (M. arum and M. smegmatis) as TB model organisms. Furthermore, many of these species were screened against both the TB and pneumonia-causing bacteria. The high numbers of plants screened against these pathogens may be due to the serious consequences of contracting TB or pneumonia, both of which may result in mortality.
The high numbers of plants screened against the pneumonia-causing bacteria may also result from these bacteria also being implicated in several other illnesses. Often, when plant extracts are screened against these bacteria, the testing was targeting those other diseases. For example, several of the southern African plants screened against K. pneumoniae (Agathosma betulina (Berg.) Pillans, Ballota africana Benth., Carpobrotus edulis (L.) N.E. Br, Combretum collinum Fresen., Combretum erythrophyllum (Burch.) Sond., Combretum microphyllum Klotzsch, Combretum molle R.Br., Kigellia africana (Lam.) Benth., Lippia javanica (Burm F.) Spreng, Pelargonium fasiculata Colvill ex Sweet, Ptaeroxylon obliquum (Thunb.) Radlk., Syzygiuym cordatum (Hochst.), Terminalia pruinoides Lawson, Terminalia Sericea Burch. ex DC., Tulbaghia violacea Harv., Warburgia salutaris (Bertol.f.) Chiov.) were tested in studies focused on the trigger mechanisms of the autoimmune disease ankylosing spondylitis (Arkhipov et al., 2014; Cock and Van Vuuren, 2015; Cock and Van Vuuren, 2014). Despite the different focus, these studies also evaluate the use of the plants for the treatment of bacterial pneumonia.
Due to the important pathogenesis of TB in Southern Africa, a relatively large number of southern African plant species have already been screened for anti-M. tuberculosis activity (Green et al., 2010; Dzoyem et al., 2016; Madikizela and McGaw, 2018). However, the majority of plants identified as being used to treat TB are yet to be tested, and substantially more work is required in this field.
Other bacterial pathogens associated with respiratory diseases have been poorly studied with respect to validation and southern African medicinal plant use. Indeed, only four plants species were screened against Haemophilus influenzae (Asparagus falcatus (L.) Oberm., S. longpedunculata, S. cordatum, T. elgans) and we were unable to find any studies that screened southern African plants against C. diptheriae or B. pertussis (the causes of diphtheria and whooping cough respectively). These diseases all cause considerable distress. However, effective vaccines against C. diptheriae or B. pertussis are available, and their use is now routine. Therefore, cures for these diseases are considered of lower priority, possibly accounting for the lack of screening against these pathogens. However, these vaccines are only effective as preventatives and are of little use if a pathogen infection occurs. Furthermore, neither vaccination nor prior exposure to C. diptheriae or B. pertussis confer lifelong immunity and a vaccinated individual may still contract the disease several years after initial vaccination. Therefore, regular ‘booster’ vaccinations are required to prevent these diseases. Cures are still required to overcome the illness once infection with these bacteria occurs, and treatments for these diseases should not be neglected.
A critical analysis of the plant species studied in Table 2 demonstrates that 75% do not demonstrate noteworthy activities. It has been recommended (Van Vuuren and Holl, 2017), that efficacies ≤ 160 μg/mL should only be considered as noteworthy. One does, however, need to take cognisance that each study may not have been tested against all known respiratory pathogens and as such, the plant species while not demonstrating efficacy against a particular tested bacterial strain, may prove to be more active against other bacterial strains not included in the pathogen set. Some plant species demonstrating noteworthy efficacy against M. tuberculosis include B. discolour (10.5–12.5 μg/mL), B. micrantha (25 μg/mL), C. petersiana (50 μg/mL), D. mespiliformis (100 μg/mL) (Green et al., 2010), as well as C. aristate (39 μg/mL), C. glabrum (156 μg/mL) and H. monopetalus (156 μg/mL) (Dzoyem et al., 2016). Other plant species also demonstrating noteworthy activity against M. tuberculosis were P. acerosum, P. capense, P. longifolia, P. echinatus, R. melanophloeos. R. tridentate, R. dentate, S. brachypetala, T. sericea, W. salutaris and Z. mucronata (Green et al., 2010; Dzoyem et al., 2016; Madikizela, and McGaw, 2018). Other plant species such as C. aurea and C. triflora demonstrated noteworthy efficacy against K. pneumoniae at 80 μg/mL (Elisha et al., 2017). Other plant species demonstrating noteworthy activity against K. pneumoniae include Heteromorpha spp., H. roeperianum, H. colchicifolia, M. mesozygia, P. viridiflorum (Elisha et al., 2017; Madikizela, and McGaw, 2018). What is even more interesting is the fact that only four plant species (C. aristata T. sericea, W. salutaris and Z. mucronate) were affiliated to traditional use (Table 1). Furthermore, only two bacterial species (M. tuberculosis and K. pneumoniae) correlated with noteworthy activity.
All the investigations that screened southern African plants against bacterial respiratory diseases used in vitro methods and none of the plant preparationshave been evaluated in vivo. This is may be due to ethical concerns and the understandable global trend to replace in vivo test models (where possible) with in vitro testing. However, whilst the in vitro properties may be promising and indicate further evaluation is required, many other pharmacodynamic and pharmacokinetic factors also affect the safety and efficacy of all chemotherapies. Where promising activity is detected, fuerther in vivo studies are required to evaluate the suitability of these plants for therapeutic use.
There are no records of ethnobotanical use to treat bacterial respiratory diseases for the other majority plant species that have been screened against bacterial respiratory pathogens. Indeed, 212 of the 254 plant species that have been already screened (approximately 83%) were selected for study for reason other than ethnobotanical usage. Those plant species were selected based on alternative criteria, including the investigators bias for a specific genus/family. For example, 21 Combretum species and three Terminalia species have been screened against bacterial respiratory pathogens (Cock and Van Vuuren, 2015a; Cock and Van Vuuren, 2015b; Madikizela et al., 2013; York et al., 2011; Eldeen et al., 2005; Eloff, 1999; Lall and Meyer, 1999) despite only two Combretum species and two Terminalia species being traditionally used for that purpose. The selection of the Combretum and Terminalia species correlates with the research background of specific researchers where interest in the plant family Combretaceae, was the focus. Plant species from the family Combretaceae from other regions of the world have demonstrated similar interesting activities (Cock, 2015; Lima et al., 2012; Eloff, 1999) and therefore these species are good candidates for testing.
5. Discussion
Traditional herbal medicines are promising sources of potential new antibacterial chemotherapeutics. In many cases, their use has been documented for hundreds (or even thousands of years), aiding in species selection for study. Southern African traditional plant use has particularly good potential as there are high biodiversity levels and relatively good records of plant use. Through extensive review of the available ethnobotanical literature, we identified 187 plant species that are used in one or more southern African healing systems to treat either TB, diphtheria, pertussis or bacterial pneumonia. Surprisingly given the ease of transmission and severity of these diseases, only 42 of the identified species have been screened against bacterial causes of these diseases. All of those species were reported to be effective against one or more bacterial respiratory pathogens. However, the other 145 species identified as traditional treatments for respiratory diseases of bacterial origin remain to be screened.
Even when a plant species has been screened against a bacterial respiratory pathogen, they were usually only tested against one or two pathogens. The plants have been most extensively tested against TB and pneumonia causing pathogens, because of the ease of transmission and relatively high mortality rates caused by these diseases. However, with a few notable exceptions, those studies screen against bacterial strains that are also susceptible to other conventional antibiotics. Given the high incidence of multiple drug resistant strains of M. tuberculosis (~92% in southern Africa; WHO, 2019a) and of some pneumonia causing bacteria, testing against resistant strains of these bacteria is important to develop new therapies. Ideally, any new therapies targeting those pathogens should function via different mechanisms to the existing drugs so that they may also be effective in resistant bacterial strains. Thus, where a plant-based therapy has demonstrated efficacy towards one or more bacterial respiratory pathogens, follow-up studies should also test the preparations against resistant and neglected strains.
In comparison to TB and bacterial pneumonia, studies screening plant extracts against C. diphtheriae and B. pertussis are lacking. Indeed, we were unable to find any studies screening southern African plants against these bacteria. To an extent, this is understandable, particularly for C. diphtheriae. Highly efficient vaccines are available against this pathogen and the rates of new diphtheria infections in particular have decreased dramatically and there are now only approximately 4500 new infections reported annually worldwide (WHO, 2019b). However, when an individual is infected with C. diptheriae, there is a 5–10% mortality rate. The incidence of pertussis has also declined substantially since the introduction of widespread vaccination programs, with the levels of infection decreased to 150,000 new cases in 2018, with nearly 90,000 deaths (WHO, 2019c). These rates are still concerning and studies seeking new cures for these diseases should not be ignored, particularly given the rapid emergence of antibiotic resistant strains of C. diphtheriae and B. pertussis in recent years.
Notably, all of the screening studies have only used in vitro models to screen the traditional medicines. We were unable to find any studies that screened the plants in in vivo systems. In vitro modelling is best regarded as an initial step in the developing of new drugs from natural sources. Activity in an in vitro system may not necessarily translate to activity in vivo. A large number of bioavailability issues will influence the activity of a plant preparation in vivo. Where promising activity is detected in vitro, the plant preparations should be re-screened using an appropriate in vivo model organism. Furthermore, pharmacodynamics and pharmacokinetic parameters should be examined so that appropriate doses and treatment regimens can be modelled.
Of the plants that have been screened for inhibitory activity against the bacterial respiratory pathogens, few were also tested for toxicity. These plants have often been used therapeutically for hundreds of years and it is possible that researchers assume that they are safe and have therefore not focused on the toxicological aspects of the traditional medicine. Many of these plants have been screened for toxicity in other studies (Fennell et al., 2004; Steenkamp and Gouws, 2006). However, the focus of those studies was solely on evaluation of toxicity of the extracts, or on investigating the safety of the extracts in conjunction with different bioactivities. Toxicological screening studies should be performed in parallel with therapeutic screening studies to allow the safety index to also be reported. Differences in the methods used to process the plants, as well as differences between collection locations, seasons of collection etc. may result in substantial differences between phytochemical profiles of the different preparations. This may significantly alter their toxicity profiles of the different preparations. It may therefore not be valid to assume that the toxicity profile reported in one study also indicates that bioactive extracts in another study will also be safe. Instead, toxicity evaluations and bioactive screening studies should be included in the same report. Furthermore, to allow for comparisons between different studies, toxicity evaluations should ideally incorporate more than one toxicity model.
6. Conclusions
In conclusion, despite ethnobotanical records of a substantial number of southern African plant species used to treat bacterial respiratory diseases, many are yet to be tested for inhibitory activity against any repiratory bacterial pathogen and substantially more work is need in this field. Indeed, one One hundred and eighty-seven southern African plant species are recorded as traditional therapies for bacterial respiratory infections. Scientific evaluations of 178 plant species were recorded. Of the species evaluated for therapeutic properties, only 42 species were selected for screening on the basis of their ethnobotanical uses. Therefore, the potential of 146 species used teraditionally to treat bacterial respiratory diseases are yet to be verified. Furthermore, the species that have been tested against some bacterial respiratory pathogens generally have only been tested against one a few bacteria respiratory pathogens and testing against other bacteria is required. Furthermore, most plant species have only been tested against antibiotic-sensitive pathogen strains. Given the rapid development of antibiotic resistance, the southern African plant species identified in our study should also be screened against antibiotic resistant bacterial strains.
Whilst substantial effort is required to screen southern African plants against respiratory bacterial pathogens, a directed approach is recommended. Plant preparations should initially be screened against the pathogens for which they were traditionally used. Furthermore, testing against the pathogens that cause the more serious respiratory diseases (tuberculosis, bacterial pneumonia) is recommended to decrease the burden caused by these diseases. Diphtheria and pertussis are less of a concern than tuberculosis and pneumonia due to the development of effective vaccines against those pathogens. However, they also should not be neglected as the bacteria responsible for these diseases are increasingly evading the vaccines and are concurrently developing resistance to clinical antibiotics. It is hoped that this review will highlight promising species for testing, which may aid in the development of new therapies against bacterial respiratory diseases.
Acknowledgements
The authors are grateful to the Environmental Futures Research Institute, Griffith University, Australia and the Department of Pharmacy and Pharmacology, University of the Witwatersrand, South Africa and by for the support provided to undertake these studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jep.2020.113204.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdillahi H.S., Stafford G.I., Finnie J.F., Nan Staden J. Antimicrobial activity of South African Podocarpus species. J. Ethnopharmacol. 2008;119:191–194. doi: 10.1016/j.jep.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Afolayan A.J., Grierson D.S., Mbeng W.O. Ethnobotanical survey of medicinal plants used in the management of skin disorders amongst the Xhosa communities of Amathole District, Eastern Cape, South Africa. J. Ethnopharmacol. 2014;153:220–232. doi: 10.1016/j.jep.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Arkhipov A., Sirdaarta J., Rayan P., McDonnell P.A., Cock I.E. An examination of the antibacterial, antifungal, anti-Giardial and anticancer properties of Kigellia africana fruit extracts. Pharmacogn. Commun. 2014;4(3):62–76. [Google Scholar]
- Asong J.A., Ndhlovu P.T., Khosana N.S., Aremu A.O., Otang-Mbeng W. Medicinal plants used for skin-related among the batswanas in ngaka district municipality, South Africa. South Afr. J. Bot. 2019 doi: 10.1016/j.sajb.2019.05.002. [DOI] [Google Scholar]
- Brooks W.A. Hunter's Tropical Medicine and Emerging Infectious Diseases. tenth ed. Elsevier; Amsterdakm, Netherlands: 2020. Bacterial pneumonia; pp. 446–453. [Google Scholar]
- Buwa L.V., Afolayan A.J. Antimicrobial activity of some medicinal plants used for the treatment of tuberculosis in the Eastern Cape Province, South Africa. Afr. J. Biotechnol. 2009;8(23):6683–6687. [Google Scholar]
- CDC Pertussis cases year by year (1922-2015) 2019. https://www.cdc.gov/pertussis/surv-reporting/cases-by-year.html
- Centre for Communicable Diseases Pertussis: NCID Recommendations for diagnosis, management and public health response. 2018. http://www.nicd.ac.za/wp-content/uploads/2017/03/Guidelines_pertussis_v2_21-December-2018_Final.pdf
- Cheesman M.J., Ilanko A., Blonk B., Cock I.E. Developing new antimicrobial therapies: are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharm. Rev. 2017;11:57–72. doi: 10.4103/phrev.phrev_21_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock I.E. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae) Inflammopharmacology. 2015;23(5):203–229. doi: 10.1007/s10787-015-0246-z. [DOI] [PubMed] [Google Scholar]
- Cock I.E., Selesho I., Van Vuuren S.F. A review of the traditional use of South African medicinal plants for the treatment of malaria. J. Ethnopharmacol. 2019 doi: 10.1016/j.jep.2019.112176. [DOI] [PubMed] [Google Scholar]
- Cock I.E., Selesho M.I., Van Vuuren S.F. A review of the traditional use of southern African medicinal plants for the treatment of selected parasite infections affecting humans. J. Ethnopharmacol. 2018;220:250–264. doi: 10.1016/j.jep.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Cock I.E., Van Vuuren S.F. The potential of South African plants with anti-Klebsiella activity for the treatment and prevention of ankylosing spondylitis. Inflammopharmacology. 2015;23(1):21–35. doi: 10.1007/s10787-014-0222-z. [DOI] [PubMed] [Google Scholar]
- Cock I.E., Van Vuuren S.F. A comparison of the antimicrobial activity and toxicity of six Combretum and two Terminalia species from southern Africa. Phcog. Mag. 2015;11(41):208–218. doi: 10.4103/0973-1296.149740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock, I.E., Van Vuuren, S.F., (in press). A review of the traditional use of southern African medicinal plants for the treatment of selected parasite infections affecting humans. J. Ethnopharmacol. [DOI] [PubMed]
- Corrigan B.M., Van Wyk B.-E., Gledenhuys C.J., Jardine J.M. Ethnobotanical plant uses in the KwaNibela penninsula, st lucia, South Africa. South Afr. J. Bot. 2011;77:346–359. [Google Scholar]
- De Beer J.J.J., Van Wyk B.-E. An ethnobotanical survey of the agter-hantam, northern Cape province, South Africa. South Afr. J. Bot. 2011;77:741–754. [Google Scholar]
- Dept of Paediatrics and Child Health, South Africa National syndromic surveillance for pneumonia in South Africa. 2019. http://www.paediatrics.uct.ac.za/national-syndromic-surveillance-pneumonia-south-africa
- Duraipandiyan V., Ignacimuthu S. Antibacterial and antifungal activity of Cassia fistula L.: an ethnomedicinal plant. J. Ethnopharmacol. 2007;112:590–594. doi: 10.1016/j.jep.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Dzoyem J.P., Aro A.O., McGaw L.J., Eloff J.N. Antimycobacterial activity against different pathogens and selectivity index of fourteen medicinal plants used in South Africa to treat tuberculosis and respiratory ailments. South Afr. J. Bot. 2016;102:70–74. [Google Scholar]
- Elisha I.L., Jambalang A.R., Botha F.S., Buys E.M., McGaw L.J., Eloff J.N. Potency and selectivity indices of acetone extracts of nine selected South African trees against six opportunistic Enterobacteriaceae isolates from commercial chicken eggs. BMC Compl. Alternative Med. 2017;17:90. doi: 10.1186/s12906-017-1597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldeen I.M.S., Elgorashi E.E., Van Staden J. Antibacterial, anti-inflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. J. Ethnopharmacol. 2005;102:457–464. doi: 10.1016/j.jep.2005.08.049. [DOI] [PubMed] [Google Scholar]
- Eloff J.N. Antibacterial activity of 27 southern African members of the Combretaceae. South Afr. J. Sci. 1999;95:148–152. [Google Scholar]
- Fennell C.W., Lindsey K.L., McGaw L.J., Sparg S.G., Stafford G.I., Elgorashi E.E., Grace O.M., Van Staden J. Assessing African medicinal plants for efficacy and safety: pharmacological screening and toxicology. J. Ethnopharmacol. 2004;94:205–217. doi: 10.1016/j.jep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Floros P., Gunaratne D.A., Chang A., Coman W.B. A rare case of toxigenic diphtheria tonsillitis resistant to penicillin causing sepsis and death. Australian J. Otolaryngol. 2018;1 [Google Scholar]
- Floyd K., Glaziou P., Zumla A., Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Resp. Med. 2018;6(4):299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- Furin J., Cox H., Pai M. Tuberculosis. Lancet. 2019;393:1642–1656. doi: 10.1016/S0140-6736(19)30308-3. [DOI] [PubMed] [Google Scholar]
- Green E., Samie A., Obi C.L., Bessong P.O., Ndip R.N. Inhibitory properties of selected South African medicinal plants against Mycobacterium tuberculosis. J. Ethnopharmacol. 2010;130:151–157. doi: 10.1016/j.jep.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Hanifa Y., Grant A.D., Lewis J., Corbett E.L., Fielding K., Churchyard G. Prevalence of latent tuberculosis infection among gold miners in South Africa. Int. J. Tubercul. Lung Dis. 2009;13(1):39–46. [PubMed] [Google Scholar]
- Holý O., Vlčková J., Janoušková L., Matoušková I. Prevalence of diphtheria, tetanus and pertussis in the world. Klinická Mikrobiol. a Infekcní Lékarství. 2017;23(1):10–16. [PubMed] [Google Scholar]
- Houben R.M., Dodd P.J. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10) doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulley, I.M., Van Wyk, B.-E. Quantitative medicinal ethnobotany of Kannaland (western Little Karoo, South Africa): non-homogeneity amongst villages. South Afr. J. Bot. 122, 225-265.
- Hutchings A., Scott A.H., Lewis G., Cunningham B. first ed. University of Natal Press; Pietermaritzburg: 1996. Zulu Medicinal Plants: an Inventory. [Google Scholar]
- Joshi R., Reingold A.L., Menzies D., Pai M. Tuberculosis among health-care workers in low-and middle-income countries: a systematic review. PLoS Med. 2006;3(12):e494. doi: 10.1371/journal.pmed.0030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelmanson J.E., Jäger A.K., Van Staden J. Zulu medicinal plants with antibacterial activity. J. Ethnopharmacol. 2000;69:241–246. doi: 10.1016/s0378-8741(99)00147-6. [DOI] [PubMed] [Google Scholar]
- Kose L.S., Moteetee A., Van Vuuren S. Ethnobotanical survey of medicinal plants used in the Maseru district of Lesotho. J. Ethnopharmacol. 2015;170:184–200. doi: 10.1016/j.jep.2015.04.047. [DOI] [PubMed] [Google Scholar]
- Lall N., Meyer J.J.M. In vitro inhibition of drug-resistant and drug-sensitive strains of Mycobacterium tuberculosis by ethnobotanically selected South African plants. J. Ethnopharmacol. 1999;66:347–354. doi: 10.1016/s0378-8741(98)00185-8. [DOI] [PubMed] [Google Scholar]
- Lalli J.Y.Y., Van Zyl R.L., Van Vuuren S.F., Viljoen A.M. In vitro biological activities of South African Pelargonium (Geraniaceae) species. South Afr. J. Bot. 2008;74:153–157. [Google Scholar]
- Lima G.R., de Sales I.R., Caldas Filho M.R., de Jesus N.Z., Falcão H.D., Barbosa-Filho J.M., Cabral A.G., Souto A.L., Tavares J.F., Batista L.M. Bioactivities of the genus Combretum (Combretaceae): a review. Molecules. 2012;17(8) doi: 10.3390/molecules17089142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang Z., Zhang J., Li F., Luan Y., Li H., Li Y., He Q. Pertussis outbreak in a primary school in China: infection and transmission of the macrolide-resistant Bordetella pertussis. Pediatr. Infect. Dis. J. 2018;37(6):e145–e148. doi: 10.1097/INF.0000000000001814. [DOI] [PubMed] [Google Scholar]
- Lönnqvist E., Barkoff A.M., Mertsola J., He Q. Antimicrobial susceptibility testing of Finnish Bordetella pertussis isolates collected during 2006–2017. J. Global Antimicrob. Resist. 2018;14:12–16. doi: 10.1016/j.jgar.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Madikizela B., Ndhlala A.R., Finnie J.F., Van Staden J. EBCAM 2013; 2013. In Vitro Antimicrobial Activity of Extracts from Plants Used Traditionally in South Africa to Treat Tuberculosis and Related Symptoms. Article ID 840719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madikizela B., McGaw L.J. Scientific rationale for traditional use of plants to treat tuberculosis in the eastern region of the OR Tambo district, South Africa. J. Ethnopharmacol. 2018;224:250–260. doi: 10.1016/j.jep.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Mahomed S., Archary M., Mutevedzi P., Mahabeer Y., Govender P., Ntshoe G., Kuhn W., Thomas J., Olowolagba A., Blumberg L., McCarthy K. An isolated outbreak of diphtheria in South Africa, 2015. Epidemiol. Infect. 2015;145(10):2100–2108. doi: 10.1017/S0950268817000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahomed H., Hawkridge T., Verver S., Geiter L., Hatherill M., Abrahams D.A., Ehrlich R., Hanekom W.A., Hussey G.D., Satvi Adolescent Study Team Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int. J. Tubercul. Lung Dis. 2011;215(3):331–336. [PubMed] [Google Scholar]
- Masiphephethu M.V. Doctoral dissertation, University of Limpopo; 2019. Isolation and Characterisation of Antimycobacterial Compounds from Schkuhria Pinnata (lam.) Duntse Ex Thell against Mycobacterium Smegmatis. [Google Scholar]
- Masoko P., Masiphephethu M.V. Phytochemical investigation, antioxidant and antimycobacterial activities of Schkuhria pinnata (Lam) Thell extracts against Mycobacterium smegmatis. J. Evid.-Based Integr. Med. 2019;24 doi: 10.1177/2515690X19866104. 2515690X19866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mativandlela S.P.N., Meyer J.J.M., Hussein A.A., Houghton P.J., Hamilton C.J., Lall N. Activity against Mycobacterium smegmatis and M. tuberculosis by extract of South African medicinal plants. Pythother. Res. 2008;22:841–845. doi: 10.1002/ptr.2378. [DOI] [PubMed] [Google Scholar]
- McGaw L.J., Lall N., Meyer J.J.M., Eloff J.N. The potential of South African plants against Mycobacterium infections. J. Ethnopharmacol. 2008;119:482–500. doi: 10.1016/j.jep.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Mohankumar S.K., Paschapur S., Mailare S. Epidemiology of diphtheria and antimicrobial resistance among diphtheria cases, Bijapur District, Karnataka, India, 2012–2015. Open Forum Infectious Diseases. 2018;5(Suppl. 1):S243. (Oxford University Press) [Google Scholar]
- Mooi F.R., Van Der Maas N.A., De Melker H.E. Pertussis resurgence: waning immunity and pathogen adaptation - two sides of the same coin. Epidemiol. Infect. 2014;142(4):685–694. doi: 10.1017/S0950268813000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchesa P., Leifels M., Jurzik L., Barnard T.G., Bartie C. Detection of amoeba-associated Legionella pneumophila in hospital water networks of Johannesburg. S. Afr. J. Infect. Dis. 2018;33(3):72–75. [Google Scholar]
- Muloiwa R., Dube F.S., Nicol M.P., Zar H.J., Hussey G.D. Incidence and diagnosis of pertussis in South African children hospitalized with lower respiratory tract infection. Pediatr. Infect. Dis. J. 2016;35(6):611–616. doi: 10.1097/INF.0000000000001132. [DOI] [PubMed] [Google Scholar]
- Nanoo A., Izu A., Ismail N.A., Ihekweazu C., Abubakar I., Mametja D., Madhi S.A. Nationwide and regional incidence of microbiologically confirmed pulmonary tuberculosis in Soiuth Africa, 2004-12: a time series analysis. Lancet Infect. Dis. 2015;15(9):1066–1076. doi: 10.1016/S1473-3099(15)00147-4. [DOI] [PubMed] [Google Scholar]
- Ndhlala A.R., Amoo S.O., Stafford G.I., Finnie J.F., Van Staden J. Antimicrobial, anti-inflammatory and mutagenic investigation of South African tree Aloe (Aloe barberae) J. Ethnopharmacol. 2009;124:404–408. doi: 10.1016/j.jep.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Nguyen L. Antibiotic resistance mechanisms in M. tuberculosis: an update. Arch. Toxicol. 2016;90(7):1585–1604. doi: 10.1007/s00204-016-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwenya M.A., Koopman A., Williams R. National Botanical Institute; Durban, South Africa: 2003. Zulu Botanical Knowledge. [Google Scholar]
- Nortje J.M., Van Wyk B.-E. Medicinal plants of the kamiesberg, namaqualand, South Africa. J. Ethnopharmacol. 2015;171:205–222. doi: 10.1016/j.jep.2015.04.049. [DOI] [PubMed] [Google Scholar]
- Pallant C.A., Steenkamp V. In vitro bioactivity of Venda medicinal plants used in the treatment of respiratory conditions. Hum. Exp. Toxicol. 2008;27:859–866. doi: 10.1177/0960327108099526. [DOI] [PubMed] [Google Scholar]
- Pawlowski A., Jansson M., Sköld M., Rottenberg M.E., Källenius G. Tuberculosis and HIV co-infection. PLoS Pathog. 2012;8(2) doi: 10.1371/journal.ppat.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrani P., Mandell L., Torres A., Tillotson G.S. The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expet Rev. Respir. Med. 2019;13(2):139–152. doi: 10.1080/17476348.2019.1562339. [DOI] [PubMed] [Google Scholar]
- Philander L.A. An ethnobotany of Western Cape Rasta bush medicine. J. Ethnopharmacol. 2011;138:578–594. doi: 10.1016/j.jep.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Samie A., Obi C.L., Bessong P.O., Namrita L. Activity profiles of fourteen selected medicinal plants from Rural Venda communities in South Africa against fifteen clinical bacterial species. Afr. J. Biotechnol. 2005;4(12):1443–1451. [Google Scholar]
- Seaman T. Witwatersrand University; 2005. The Antimicrobial and Antimycobacterial Activity of Plants Used for the Treatment of Respiratory Ailments in Southern Africa and the Isolation of Anacardic Acid from Ozoroa Paniculosa. MSC thesis. [Google Scholar]
- Selowa S.C., Shai L.J., Masoko P., Mokgotho M.P., Magano S.R. Antibacterial activity of extracts of three Croton species collected in Mpumalanga region in South Africa. Afr. J. Trad. CAM. 2010;7(2):98–103. doi: 10.4314/ajtcam.v7i2.50861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad M., Roberts T.H., Matthews K.R., Bezerra C.F., Morais‐Braga M.F., Coutinho H.D., Sharopov F., Salehi B., Yousaf Z., Sharifi‐Rad M., del Mar Contreras M. Ethnobotany of the genus Taraxacum—phytochemicals and antimicrobial activity. Phytother Res. 2018;32(11):2131–2145. doi: 10.1002/ptr.6157. [DOI] [PubMed] [Google Scholar]
- Smith A. second ed. Lovedale Institution Press; South Africa: 1895. A Contribution to South African Materia Medica. [Google Scholar]
- Steenkamp V., Gouws M.C. Cytotoxicity of six South African medicinal plant extracts used in the treatment of cancer. South Afr. J. Bot. 2006;72:630–633. [Google Scholar]
- Suliman A. University of Witwatersrand; 2010. The Antimicrobial Activity and Chemical Profile of Traditional Medicinal Plants Indigenous to Southern Africa Used to Treat Respiratory Tract Infections. Masters of Science thesis. [Google Scholar]
- Truelove S.A., Keegan L.T., Moss W.J., Chaisson L.H., Macher E., Azman A.S., Lessler J. Clinical and epidemiological aspects of diphtheria: a systematic review and pooled analysis. Clin. Infect. Dis. 2019 doi: 10.1093/cid/ciz808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vuuren S., Holl D. Antimicrobial natural product research: a review from a South African perspective for the years 2009-2016. J. Ethnopharmacol. 2017;208:236–252. doi: 10.1016/j.jep.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Van Wyk B.-E., Van Oudtshoorn B., Gericke N. second ed. Briza Publications; Pretoria, South Africa: 2009. Medicinal Plants of South Africa. [Google Scholar]
- Van Wyk B.-E. A review of Khoi-San and Cape Dutch medicinal ethnobotany. J. Ethnopharmacol. 2008;119:331–341. doi: 10.1016/j.jep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Von Koenen E. fourth ed. Klaus Hess Publishers; Windhoek, Namibia: 2001. Medicinal, Poisonous and Edible Plants in Namibia. [Google Scholar]
- Watt J.M., Breyer-Brandwijk M.G. second ed. Livingstone; Edinburg and London: 1962. The Medicinal and Poisonous Plants of Southern and Eastern Africa. [Google Scholar]
- Wendelboe A.M., Van Rie A., Salmaso S., Englund J.A. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 2005;24(5 Suppl. l):S58–S61. doi: 10.1097/01.inf.0000160914.59160.41. [DOI] [PubMed] [Google Scholar]
- WHO TB statistics South Africa – national, incidence, provincial. 2019. https://tbfacts.org/tb-statistics-south-africa/#:~:targetText=TB%20incidence&targetText=Out%20of%20the%20estimated%20322,109%2C799)%20are%20on%20ARV%20therapy
- WHO Immunisation, vaccines and biologicals: Diphtheria. 2019. https://www.who.int/immunization/diseases/diphtheria/en/
- WHO Immunisation, vaccines and biologicals: Pertussis. 2019. https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/pertussis/en/
- WHO Global and regional immunisation profile. African region. 2019. https://www.who.int/immunization/monitoring_surveillance/data/gs_afrprofile.pdf?ua=1
- WHO The top ten causes of death. 2018. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Yeung K.H., Duclos P., Nelson E.A., Hutubessy R.C. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect. Dis. 2017 Sep 1;17(9):974–980. doi: 10.1016/S1473-3099(17)30390-0. [DOI] [PubMed] [Google Scholar]
- York T., de Wet H., Van Vuuren S.F. Plants used for treating respiratory infections in rural Maputaland, KwaZulu-Natal, South Africa. J. Ethnopharmacol. 2011;135:696–710. doi: 10.1016/j.jep.2011.03.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.