Abstract
Idiopathic orbital inflammation (IOI) is a noninfectious inflammatory disease whose etiology remains unknown. Treatment is focused on reducing inflammation, which becomes challenging in nonresponding cases. We report the case of a 59-year-old woman with refractory IOI that showed a positive response to tocilizumab therapy. The patient was diagnosed with a unilateral sclerosing IOI for 9 years and showed a negative control with previous oral steroids, peribulbar steroid injections, radiotherapy, immunosuppressors, and intravenous rituximab. After the initiation of 8 mg/kg intravenous tocilizumab, a complete reduction of the pain and the orbital inflammation signs was observed and her condition remained stable for the following 6 years under a monthly dose of 4 mg/kg. In recalcitrant IOI cases, tocilizumab could be considered a possible treatment to reduce inflammatory signs and symptoms with positive long-term outcomes as in our case.
Keywords: Idiopathic orbital inflammation, Tocilizumab, Orbit, Inflammation, Eye
Introduction
Idiopathic orbital inflammation (IOI) or orbital pseudotumor is an orbital noninfectious inflammatory disease caused by a polymorphic lymphoid infiltration with varying degrees of fibrosis and without any local or systemic identifiable cause [1].
Treatment is based on reducing the underlying inflammation. Systemic corticosteroids followed by descendent oral steroids are the first-line therapy and a positive response is usually observed [1, 2]. However, many cases of nonresponders and recurrences are to be considered. In such cases, the use of radiotherapy, immunosuppressive agents (methotrexate, azathioprine, mycophenolate mofetil, cyclosporine A, cyclophosphamide), and biologic antibodies (rituximab, daclizumab, infliximab) has been reported [3]. Unfortunately, there are no other alternatives described when all these therapies fail to control the disease.
Tocilizumab is a humanized monoclonal antibody against interleukin-6 (IL-6) receptor that has been widely used in systemic and ocular inflammatory diseases with positive outcomes [4]. Despite showing good response in other inflammatory diseases, there is no evidence in the literature of positive responses to tocilizumab in cases of IOI [5]. To date, only one article mentions a negative response and persistence of the inflammation after 9 months under tocilizumab therapy, but no clinical nor radiological evidence is provided [6].
The aim of this case is to report the clinical and radiologic outcomes after 6 years of follow-up in a woman affected with severe IOI who showed no response to multiple therapies and was successfully treated with intravenous tocilizumab.
Case Report
A 59-year-old woman with a previous diagnosis 9 years before of IOI in her right orbit consulted our hospital in 2014 for disabling pain that affected her daily life activities. During the last 6 years, she had had several clinical manifestations including dacryoadenitis, episcleritis, myositis of the external rectus muscle, anterior uveitis, and perineuritis in her right eye (RE). Secondary to the compressive neuropathy, visual acuity was no light perception in her RE for the last years. A biopsy of the right tear gland and orbital fat tissue revealed scarce interstitial lymphoplasmacytic cells in the fat tissue and adjacent to the gland lobes, as well as some dense fibrotic tissue. A complete blood test was performed (including a complete blood count and biochemical profile, C-reactive protein, erythrocyte sedimentation rate, levels of IgG4, antineutrophil cytoplasmic antibodies, complement, angiotensin converting enzyme, and serologic profile) to rule out the presence of an underlying systemic inflammatory disease such as IgG4 disease, vasculitis, sarcoidosis, and other infectious diseases. At that moment she was under 375 mg/m2 of intravenous rituximab perfusions every week. She had been treated several times with corticosteroid boluses (500 mg of methylprednisolone daily for 3 days) and with oral and topical corticosteroids in descending protocols, but the responses were always short term. Due to the high recurrences, she had also received peribulbar injections of triamcinolone (1 mL Trigon© 40 mL/mg), 10 sessions of local radiotherapy, subcutaneous injections of methotrexate (10-15-20 mg per week), and intravenous perfusions of rituximab (3 cycles of Mabthera© 375 mg/m2 of body surface, once a week for 4 weeks). However, all these treatments failed to control the inflammatory activity in the long term. Secondary to the long steroid treatment, hypertension arose and was well controlled with oral antihypertensives.
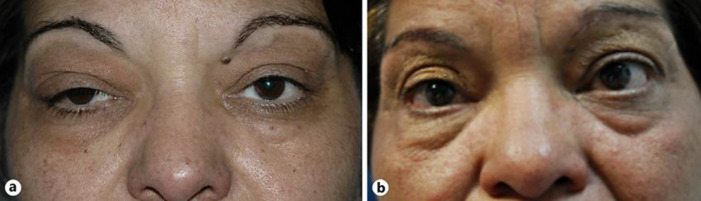
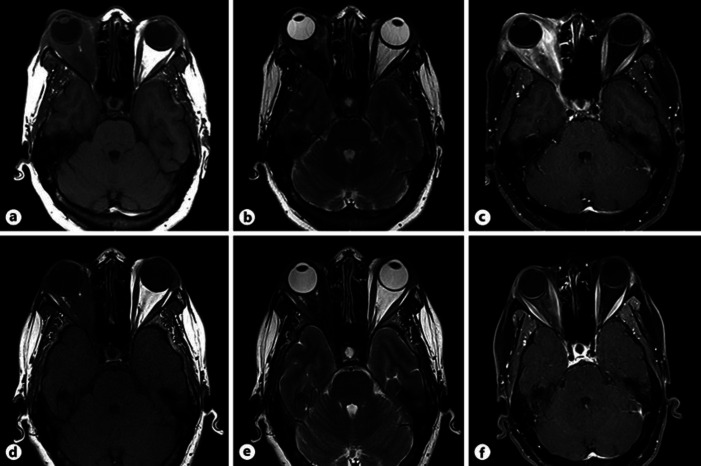
At the ophthalmological examination, the patient presented a diffuse right upper-lid edema with a thickening of the tear gland and a mild ptosis (Fig. 1). Visual acuity was no light perception in her RE and 1.0 in her left eye (LE). A relative afferent pupillary defect was observed in her RE. A binocular eye movement test, which was performed by asking the patient to follow the explorer's finger and employing a scoring system from 0 to −4 (from normal to a lack of muscle function, in 25% increments per grade), revealed a restriction of −3 in the RE in all positions, whereas the LE was preserved (grade 0). Proptosis of the RE was measured by the Hertel exophthalmometer (Oculus, Wetzlar, Germany), resulting in 22 mm in the RE and 20 mm in the LE (previous measurement a year before was 21 mm and 20 mm, respectively). The slit-lamp examination showed a mild hyperemia and chemosis in her RE. Intraocular pressure was within normal limits in both eyes. The fundoscopy of the RE showed a pale optic nerve secondary to previous compressive neuropathy with no other fundus alterations. Anterior and posterior pole examination was normal in the LE. Findings in the orbital MRI were compatible with sclerosant IOI and described an overall moderate radiologic worsening of the right orbit compared to the previous one a year ago. A 1-mm increase of the exophthalmos of the RE was measured and the intra- and extraconal orbital fat fibrosis presented a more intense inflammatory compound (Fig. 2a–c). Due to the clinical, histological, and radiological findings, sclerosing type of IOI was diagnosed [7].
Fig. 1.
a Acute stage of IOI presenting right upper lid edema and ptosis. Temporary mild hyperemia is observed. b Photograph 3 years after tocilizumab therapy. Eyelid edema is absent and the interpalpebral aperture is similar in both eyes.
Fig. 2.
a–c Multimodal images captured during the acute stage before treatment with tocilizumab in 2014. a Orbital T1-weighted axial MRI showing bilateral asymmetric exophthalmos with progression in the right eye in respect to the previous image (1 mm). Diffuse alteration of intra- and extraconal orbital fat pushing the posterior part of the globe can be observed. b T2-weighted axial MRI presenting hypointense signal of the orbital fat, translating a major fibrous compound compatible with sclerosant subtype of IOI. c T1-weighted contrast-enhanced MRI. High-contrast caption is objectivized. d–f Multimodal images taken 1 year after initiation of tocilizumab in 2015. d T1-weighted axial MRI, showing mild bilateral symmetric exophthalmos, with important reduction of the proptosis in the right eye. Intra- and extraconal fat presenting notorious improvement. e T2-weighted axial MRI presenting hypointense signal of the orbital fat due to the high fibrotic tissue but with remarkable improvement in comparison to the previous image. f T1-weighted contrast-enhanced MRI in which intravenous contrast capture is clearly reduced.
With this situation, together with the Rheumatology service, it was decided to initiate oral steroids to stop the acute inflammation and the addition of a new immunosuppressor. Treatment consisted of 60 mg of oral prednisone daily in a weekly descendent protocol and 8 mg/kg of intravenous tocilizumab (Roactembra©) every month. High doses of oral NSAIDs and analgesia were necessary to control the pain. No adverse events of this drugs were noticed. During the next 6 months, an important progressive clinical and symptomatic improvement was objectivized. The eyelid edema and the hyperemia were reduced completely and extraocular movements of the RE improved remarkably to −1 in the grading score. Proptosis was slightly reduced in the RE, with an exophthalmos of 21 mm in the RE and 20 mm in the LE, measured by the Hertel exophthalmometer. Orbital MRI showed a diffuse reduction of the intraorbital inflammation (Fig. 2d–f). Oral analgesia and NSAIDs were no longer needed as the pain ceased. Oral steroids were stopped after 6 months, and tocilizumab was continued in a maintenance dose of intravenous perfusions of 4–6 mg/kg every 6 weeks up to the present time. The patient is currently asymptomatic and only one relapse was observed when treatment was stopped for a dental implant 2 years ago. No side effects of tocilizumab have been identified so far. Routine blood tests are performed every 3–6 months in order to detect any disorder.
Discussion
To our knowledge, this is the first case in the literature to report a positive response to tocilizumab in the treatment of IOI and to describe the results of a 6-year follow-up.
Tocilizumab is a recombinant humanized monoclonal IgG1 antibody that binds to the soluble and membrane-bound IL-6 receptor. By blocking the IL-6 signal, it has shown efficacy in immuno-mediated diseases such as rheumatoid arthritis, juvenile idiopathic arthritis, Crohn's disease, Castleman disease, and more recently, giant cell arteritis [8]. With regard to ocular diseases, the efficacy of this drug has been widely studied in Grave's orbitopathy [9, 10]. Moreover, a recent review by Ruiz-Medrano et al. [11] describes the good response to tocilizumab in other ocular inflammatory diseases such as anterior uveitis associated with juvenile idiopathic arthritis, uveitis in association to Castleman disease, uveitis-related refractory macular edema, Birdshot chorioretinopathy, Behçet disease, and in retinal vasoproliferative tumors. Despite all these diseases showing a positive response to the treatment, there is no evidence of a favorable response in IOI. Only one article, published by Silpa-Archa et al. [6], reports the outcomes of tocilizumab in 10 cases of uveitis, 6 cases of scleritis, and 1 case of orbital pseudotumor refractory to corticosteroids and immunosuppressants. In that study, poor clinical and radiological response is mentioned in the case of the orbital pseudotumor, but specific clinical, radiologic, and response data are absent. Therefore, we consider that our case reports the first positive case with specific and detailed data on the effects of tocilizumab in a case of refractory IOI.
The mechanism of tocilizumab in IOI is still not well defined. Recent studies have demonstrated an elevation of cytokines such as IL-2, −8, −10, −12, gamma interferon, and tumor necrosis factor alpha in IOI biopsy specimens and describe a T-helper 1 leucocyte disorder in the pathogenesis of IOI [12], but the involvement of IL-6 is yet to be defined. Another study documented the strong expression of CD20 and CD25 in IOI specimens, suggesting a possible benefit of rituximab (anti-CD20 monoclonal antibody) and daclizumab (anti-CD25 monoclonal antibody) in this disease [13]. However, our patient's condition was not controlled enough with infliximab, and daclizumab was not available. The involvement of IL-6 in inflammatory cascades and the good response of tocilizumab in other inflammatory diseases previously mentioned could explain the positive response in cases of IOI, where orbital inflammation is the landmark.
Tocilizumab can be used alone or in association with methotrexate [14]. In our case, monotherapy was preferred due to the poor response shown previously to methotrexate. Side effects of this biologic agent have been well described in the literature. The most common adverse events are upper respiratory tract infections, followed by headache, skin and soft tissue infections, hepatic enzymes elevation, hypercholesterolemia, and neutropenia [15]. Hypersensitivity reactions and anaphylaxis are rare but severe side effects that have been related to the development of anti-tocilizumab antibodies [14]. Thus, routine blood-tests are necessary during the follow-up for the early diagnosis and treatment of these possible adverse events.
In our experience, tocilizumab presented positive long-term clinical and radiological outcomes in a case of sclerosant IOI that was refractory to corticosteroids and other immunosuppressive treatments such as methotrexate and rituximab, and therefore could be considered a therapeutic option in nonresponding patients. Follow-up should be carried out between Ophthalmology and Rheumatology services and should include complete ophthalmological examinations as well as routine blood analysis in order to detect any early alteration. However, results from an isolated case such as this should be taken cautiously. Further research and the performance of clinical trials in this area would provide more information about the efficacy and safety profile of tocilizumab in IOI.
Statement of Ethics
The patient gave her written informed consent to publish the case (including publication of images).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding sources to disclose.
Author Contributions
June ArtaechevarriaArtieda made substantial contribution to the design of the work, the acquisition and interpretation of data, and drafting the work. Ignacio TapiasElias made substantial contribution to the design of the work, the acquisition and interpretation of data, and revising the work critically for important intellectual content. Both authors agree to be accountable for all aspects of the work.
Acknowledgments
We thank Dr. Olga Sánchez-Pernaute, PhD, for substantial contribution in the management of the therapy with tocilizumab and supervision of the clinical evolution of the patient, and Dr. Jose Fortes, PhD, for substantial contribution in the analysis and description of the histopathological specimens.
References
- 1.Yuen SJ, Rubin PA. Idiopathic orbital inflammation: distribution, clinical features, and treatment outcome. Arch Ophthalmol. 2003 Apr;121((4)):491–9. doi: 10.1001/archopht.121.4.491. [DOI] [PubMed] [Google Scholar]
- 2.Swamy BN, McCluskey P, Nemet A, Crouch R, Martin P, Benger R, et al. Idiopathic orbital inflammatory syndrome: clinical features and treatment outcomes. Br J Ophthalmol. 2007 Dec;91((12)):1667–70. doi: 10.1136/bjo.2007.124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeşiltaş YS, Gündüz AK. Idiopathic orbital inflammation: review of literature and new advances. Middle East Afr J Ophthalmol. 2018 Apr-Jun;25((2)):71–80. doi: 10.4103/meajo.MEAJO_44_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol. 2008;181:151–60. doi: 10.1007/978-3-540-73259-4_7. [DOI] [PubMed] [Google Scholar]
- 5.American academy of Ophthalmology Nonspecific Orbital Inflammation (Idiopathic orbital inflammation, Orbital inflammatory syndrome, Orbital pseudotumor) [cited 2019 June 27>] Available from: https://eyewiki.aao.org/Nonspecific_Orbital_Inflammation_(Idiopathic_orbital_inflammation,_Orbital_inflammatory_syndrome,_Orbital_pseudotumor)
- 6.Silpa-Archa S, Oray M, Preble JM, Foster CS. Outcome of tocilizumab treatment in refractory ocular inflammatory diseases. Acta Ophthalmol. 2016 Sep;94((6)):e400–6. doi: 10.1111/aos.13015. [DOI] [PubMed] [Google Scholar]
- 7.Zakir R, Manners RM, Ellison D, Barker S, Crick M. Idiopathic sclerosing inflammation of the orbit: a new finding of calcification. Br J Ophthalmol. 2000 Nov;84((11)):1322–4. doi: 10.1136/bjo.84.11.1318f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubbert-Roth A, Furst DE, Nebesky JM, Jin A, Berber E. A review of recent advances using Tocilizumab in the treatment of Rheumatic Disease. Rheumatol Ther. 2018 Jun;5((1)):21–42. doi: 10.1007/s40744-018-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Moreiras JV, Gómez-Reino JJ, Maneiro JR, Pérez-Pampin E, Romo López A, Rodríguez Alvarez FM, et al. Tocilizumab in Graves Orbitopathy Study Group Efficacy of Tocilizumab in Patients With Moderate-to-Severe Corticosteroid-Resistant Graves Orbitopathy: A Randomized Clinical Trial. Am J Ophthalmol. 2018 Nov;195:181–90. doi: 10.1016/j.ajo.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Moreiras JV, Alvarez-López A, Gómez EC. Treatment of active corticosteroid-resistant graves' orbitopathy. Ophthal Plast Reconstr Surg. 2014 Mar-Apr;30((2)):162–7. doi: 10.1097/IOP.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Medrano J, Díaz-Valle D, Cuiña R, Gegúndez JA, Chhablani J, Majumder PD, et al. The role of tocilizumab in the treatment of inflammatory diseases of the eye and orbit: A useful alternative. J Fr Ophtalmol. 2018 Oct;41((8)):759–66. doi: 10.1016/j.jfo.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Wladis EJ, Iglesias BV, Gosselin EJ. Characterization of the molecular biologic milieu of idiopathic orbital inflammation. Ophthal Plast Reconstr Surg. 2011 Jul-Aug;27((4)):251–4. doi: 10.1097/IOP.0b013e31820768f7. [DOI] [PubMed] [Google Scholar]
- 13.Ho VH, Chevez-Barrios P, Jorgensen JL, Silkiss RZ, Esmaeli B. Receptor expression in orbital inflammatory syndromes and implications for targeted therapy. Tissue Antigens. 2007 Aug;70((2)):105–9. doi: 10.1111/j.1399-0039.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency Assessment report for RoActembra. EMA/742879/2018.
- 15.H. Venkiteshwaran A. Tocilizumab MAbs. 2009;1((5)):432–8. doi: 10.4161/mabs.1.5.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]