Introduction
For the last 18 years, the Breast Committee of the Arbeitsgemeinschaft Gynäkologische Onkologie (German Gynecological Oncology Group, AGO) has been preparing and updating evidence-based recommendations for the diagnosis and treatment of patients with early and metastatic breast cancer (MBC). The AGO Breast Committee consists of gynecological oncologists specialized in breast cancer and interdisciplinary members specialized in pathology, radiologic diagnostics, medical oncology, and radiation oncology. This update has been performed according to a documented rule-fixed algorithm, by thoroughly reviewing and scoring chapter by chapter the recent publications for their scientific validity (Oxford level of evidence [LoE], www.cebm.net) [1] (for all references, see the online supplementary material; for all online suppl. material, see www.karger.com/doi/10.1159508736) and clinical relevance (AGO grades of recommendation; Table 1). We herewith present the 2020 update; the full version of the updated slide set is available online as a PDF file in both English and German [2]. Moreover, a special version for patients is also available at www.ago-online.de.
Table 1.
AGO grades of recommendation
| ++ | This investigation or therapeutic intervention is highly beneficial for patients, can be recommended without restrictions, and should be performed |
| + | This investigation or therapeutic intervention is of limited benefit for patients and can be performed |
| +/− | This investigation or therapeutic intervention has not shown benefit for patients and may be performed only in individual cases; according to current knowledge a general recommendation cannot be given |
| − | This investigation or therapeutic intervention can be of disadvantage for patients and might not be performed |
| −− | This investigation or therapeutic intervention is of clear disadvantage for patients and should be avoided or omitted in any case |
Options for Primary Prevention and Lifestyle Factors
Individual risk factors can be classified into nonmodifiable and modifiable lifestyle factors. Currently, there is good evidence that changes in some modifiable risk factors could substantially decrease the individual breast cancer risk.
Relevant lifestyle factors such as obesity, alcohol consumption [LoE 2a/B/AGO+], physical inactivity, fiber-containing foods, smoking, and exposition to ionizing radiation are well known. A Mediterranean diet with consumption of extra virgin olive oil [LoE 2b/B/AGO+], nuts [LoE 2b/B/AGO+] (>10 g/day), and reduced consumption of fat [LoE 2a/B/AGO+] and red meat may decrease the incidence of breast cancer. For other factors such supplementation of vitamin D3, vegetarian or vegan diet, vegetables, fruits, or phytoestrogens there is yet no sufficient or contradictory data regarding the reduction of breast cancer incidence [3]. In contrast, physical exercise (metabolic equivalents to 3–5 h of moderate pace walking per week) has been demonstrated to be efficient in reducing breast cancer risk [LoE 2a/B/AGO++] [4].
Avoidance of hormone replacement therapy (especially estrogen/progestin combination regimens) in postmenopausal women may reduce breast cancer risk [LoE 1b/A/AGO+] [5], whereas oral contraceptives do not increase the risk of mortality from breast cancer [LoE 1a].
Breast Cancer Risk and Prevention
Germline testing for hereditary breast and ovarian cancer is becoming increasingly relevant not only for risk-adapted prevention measures but also for therapeutic decision making. With the increasing number of identified risk genes, the clinical benefit should always be measured by the effectiveness of preventive and therapeutic measures. A checklist facilitates the identification of individuals for whom genetic counseling and testing is an option. In addition, BRCA1/2 germline (gBRCA1/2) testing should be performed in all patients with advanced/metastatic HER2-negative breast cancer (irrespective of hormone receptor [HR] status), as they may receive poly(ADP-ribose)-polymerase (PARP) inhibitors in case of mutation detection (LoE 1b/B/+) [6, 7].
Women with gBRCA1/2 mutations should be offered nondirective counseling for primary preventive measures (e.g., risk-reducing bilateral salpingo-oophorectomy [RRSO] after completion of family planning, for women with BRCA1 mutations at age 35–40 years and for women with BRCA2 mutations at age 40–45 years) (LoE 2a/B/AGO++) or risk-reducing bilateral mastectomy (RRBM) (LoE 2a/B/AGO+). A recent study shows that RRSO does not reduce breast cancer risk for BRCA1 mutation carriers and shows only a potentially beneficial effect for BRCA2 mutation carriers (particularly after 5 years following RRSO) [8]. Moreover, RRBM was associated with lower mortality compared to imaging surveillance for BRCA1 mutation carriers. In BRCA2 mutation carriers, RRBM may lead to similar breast cancer-specific survival compared to surveillance (LoE 2b/B/AGO+) [9]. RRBM after diagnosis of ovarian cancer is not generally indicated and can be discussed depending on tumor stage, recurrence-free interval (> = 5 years) and age (<55 years) (LoE 4/C/AGO+/−). However, uni- or bilateral mastectomy is not indicated in the absence of clearly defined genetic risk factors (LoE 2a/B/AGO+). RRSO is associated with a significant reduction of the incidence of ovarian cancer and overall mortality (LoE 2a/B/++). Another reasonable preventive option is breast cancer surveillance. Follow-up results of the German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC) have recently been published for 4,573 patients based on 14,142 screening rounds with magnetic resonance imaging (MRI) [10]. The stage distribution in the cohort, with 84.5% of all observed primary breast cancers in the study being stage 0 or I, underlines the importance of implementing a multimodal screening program with annual MRI for women at high risk. The data demonstrate that high-risk screening with MRI was successfully implemented in the GC-HBOC with high sensitivity and specificity (LoE 2b/B/++).
Breast Cancer Diagnostics
Screening mammography (MG) is recommended for women aged 50–74 years (LoE 1a/A/++). For women aged 40–49 years, individual shared decision making is recommended (LoE 1a/B/+). For women aged >75 years, screening can be offered to women in good health with a life expectancy of 10 years or longer (LoE 4/C/+) [11].
Glandular tissue density is a known risk factor for breast cancer development and decreased MG sensitivity. Nevertheless, neither use of hand-held ultrasound (US) nor automated whole-breast US can be recommended as sole modality for screening (LoE 3a/C/AGO−) [11].
Using digital breast tomosynthesis (DBT) the recall and biopsy rates were low (LoE 2a/B/AGO+). Synthetic 2D image reconstruction of the 3D data set can significantly reduce radiation dose. Nevertheless, it is very important to use the complete data set for diagnosis and provide them for the subsequent treatment [12].
In a recent randomized controlled trial, MRI in the extremely dense breast screening group with negative MG showed a significantly reduced interval cancer rate at the cost of slightly increased false-positive cases (LoE 1b/B/+/−) [13].
For patients with breast symptoms, clinical examination (LoE 3b/B/++), MG (LoE 1b/A/AGO++), DBT (LoE 2b/B/AGO+) or contrast-enhanced MG (LoE 3a/B/AGO+/−), US (LoE 2b/B/AGO++), and minimal invasive biopsies (LoE 1c/A/AGO++) should be performed [14].
Pretherapeutic MRI after careful considerations can be helpful for some patients, e.g., those with a reduced sensitivity of MG and US, nipple involvement, lobular invasive cancer, suspicion of multilocular disease, and/or high risk (LoE 1b/B/AGO+). Second-look US is recommended in cases of MRI-only detected lesions. MRI-guided vacuum-assisted biopsy access must be available [15].
In patients with clinically and/or sonographically suspicious axillary lymph nodes, core needle biopsy (CNB) is recommended (LoE 2b/B/AGO++). If lymph node involvement is proven in a patient undergoing neoadjuvant therapy who is a potential candidate for targeted axillary dissection (TAD), a marker should be inserted in the lymph node after biopsy.
Staging is recommended in those patients being candidates for neoadjuvant or adjuvant chemotherapy and/or anti-HER2 treatment including thorax/abdomen CT scan and bone scans (LoE 2b/B/AGO+).
Pathology
Conventional histopathology including immunohistochemistry remains the gold standard pathological workup of breast cancer specimens. Histological tumor typing is performed according to WHO classification, 5th edition [16].
For pathological evaluation after neoadjuvant chemotherapy (NACT), intraoperative frozen sections should be avoided as tissue consumption may lower sensitivity of final analyses (LoE 5/D/AGO−). Immunohistochemistry may help to identify minimal tumor residues in lymph nodes (LoE 2b/B/AGO+/−). In sentinel lymph node excision (SLNE) after NACT, about 17% of cases with isolated tumor cells and 64% with micrometastases may have additional nodal metastases when axillary lymph node dissection (ALND) is performed [17].
Several gene alterations are currently therapeutically relevant: germline BRCA1/2 testing needs to be performed in all HER2-negative MBC (independent of family history) in order to assess eligibility for PARP inhibitor treatment (LoE 1b/A/AGO++). As the registration studies did not use somatic BRCA, tissue testing only constitutes a preliminary screening procedure until its relevance in MBC is elucidated (LoE 2b/B/AGO+/−). FDA approval of the PI3K inhibitor Alpelisib has been granted; EMA approval is expected for 2020. Thus, PIK3CA mutation testing (exons 7,9, 20) in the primary tumor, or preferentially metastases or plasma, is recommended in luminal MBC (LoE 1b/A/AGO+).
Mutations of the estrogen receptor (ER) gene ESR1 render ER constitutionally active and are linked to secondary endocrine resistance [96]. ESR1 mutations (exons 4,7, 8) are preferentially found after (adjuvant) aromatase inhibitor (AI) treatment and thus best evaluated in metastases or plasma. They may be a helpful MBC biomarker if AI monotherapy is considered. Whether this mechanism of resistance is also relevant for AI+ targeted therapy has not yet fully been evaluated.
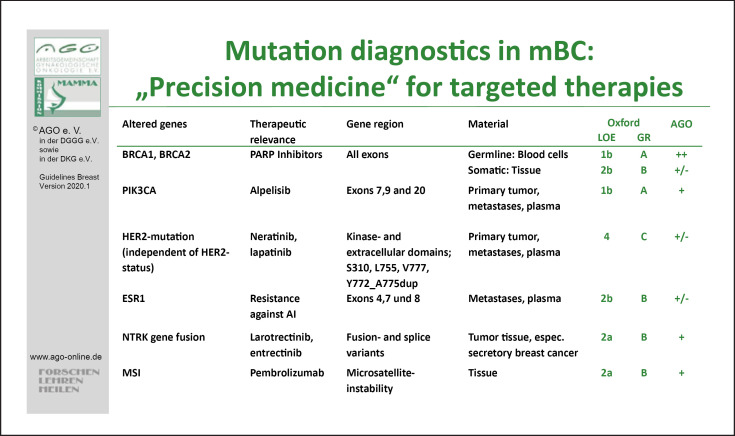
The following alterations are rare but therapeutically relevant and should be included in molecular panel diagnostics. HER2 mutations are found in luminal or HER2-positive tumors. They may be linked to resistance to endocrine- or antibody-based anti-HER2 therapy, whereas anti-HER2 tyrosine kinase inhibitor (TKI) therapy may still be effective [94]. Tumor agnostic regulatory approvals are available for neurotrophic receptor TKI and for pembrolizumab in MSI-high tumors. Neurotrophic receptor TKI gene fusion [97] is a very rare event in breast cancer; it is very frequent in the rare subtype of secretory breast cancer where it should be evaluated upon diagnosis (Fig. 1). Immunohistochemistry pre-screening can be performed before confirmatory molecular analysis.
Fig. 1.
Mutation diagnostics in MBC: “Precision medicine” for targeted therapies.
The ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT) Guidelines helps to further understand which molecular markers offer therapeutically relevant information in breast cancer [98].
Lesions of Uncertain Malignant Potential (B3)
Lesions of uncertain malignant potential (B3) are detected typically in core or vacuum-assisted biopsy in asymptomatic women. The risk associated with B3 lesions cannot be strictly categorized according to the type of lesion (atypical ductal hyperplasia [ADH], FEA, LIN, papilloma, radial scar) and additional clinical and pathological factors must be taken into consideration. The aim of further excision of B3 lesions is to detect more severe lesions (ductal carcinoma in situ, DCIS) but also to minimize the risk of progression of a lesion of low malignant potential to an in situ or invasive carcinoma.
ADH has a particularly high risk for breast cancer when combined with BIRADS IV/V and high breast tissue volume. ADH on core biopsy may represent inadequately sampled DCIS.
Open excision can be avoided after the diagnosis of classical LIN has been established on core biopsy if no discordant imaging, especially no focal lesion, is present [18]. In contrast, high-risk variants of lobular neoplasia, which include pleomorphic and florid lobular carcinoma in situ (LCIS) (pLCIS and fLCIS), are recommended for open biopsy, and preferably complete excision.
The diagnosis of solitary or multiple papillomas on core biopsy carries a risk of up to 30% (with atypia) for an invasive carcinoma or DCIS. However, upgrade rates are widely different in the literature and may be as low as 3.1% [19]. Conservative management is justified provided that the biopsy has been sufficiently representative and no discordance to imaging results was evident.
A radial scar may mimic carcinoma mammographically because of its stellate appearance. Radial sclerosing lesions are only rarely associated with atypia or DCIS. When radial scar is associated with atypia (such as FEA, ADH, or classical LIN), management can be similar to atypia alone [20].
Medical prevention (e.g., low-dose tamoxifen (Tam) (LoE 2b/B/AGO+/−) for lesions with uncertain biological behavior may be performed only in individual cases [21].
Prognostic and Predictive Factors
Most of the established prognostic markers, including estrogen (ER) and progesterone (PR) receptors (HER2 and Ki67), are provided by traditional pathology and overlap with parameters used for the prediction of therapeutic response. Commonly, they serve as surrogates for intrinsic subtypes such as luminal A and luminal B. In addition, uPA/PAI-1 provides strong prognostic information and allows prediction of the chemotherapy effect [22].
In particular, for differentiation of luminal A and B subtype implying the alternative choice between endocrine and chemo-endocrine therapy, there are no generally accepted immunohistochemical algorithms or cut-off values. Several RNA expression assays have been tested to enable a more accurate assignment of individual patients to prognostic groups. The different assays are not interchangeable and the level of concordance is only about 70% [23]. They should only be used in selected patients if all other criteria are inconclusive for therapeutic decision making.
Pathological complete response (pCR) provides a relevant prognostic parameter after neoadjuvant systemic therapy (NAST). Besides clinically determined parameters to predict the prospective success of NAST, such as young age and cT1/cT2 tumors, pathological parameters (G3 grade, pN0, HR negativity, triple negativity, and HER2 type) are predictive. Some histological types such as lobular breast cancer or metaplastic cancers show poor response to NAST.
In MBC, expression of PD-L1 by tumor-infiltrating lymphocytes (at least 1% of cells) either in the primary tumor or the metastasis itself is predictive for the response to checkpoint inhibitors [24]. DNA sequencing and detection of mutations yield a couple of novel predictive markers in MBC. BRCA1/2 mutations are predictive for PARP inhibitor efficacy. PIK3CA mutations indicate response to corresponding inhibitors, such as Alpelisib [25]. Besides amplification and overexpression, HER2 may be activated as an oncogene by point mutations, which render affected cells sensitive to inhibitors such as neratinib and lapatinib [26]. ESR1 is mutated in about 15–25% of metastatic luminal breast cancers which induces resistance against AI [27].
Ductal Carcinoma in situ
The diagnosis of DCIS increased by the implementation of screening MG and comprises approximately >20% of all newly diagnosed breast cancers. However, the removal of DCIS lesions has not been accompanied by a reduction in the incidence of invasive breast cancer [28]. DCIS is commonly diagnosed by MG, but up to 20% of DCIS remain mammographically occult. The use of additional breast MRI may be helpful to detect more DCIS or to describe the extent of a DCIS lesion and define surgical treatment. The sensitivity of breast MRI for the diagnosis of DCIS is lower (77–96%) than that for invasive breast cancer (90–100%). However, there is yet no evidence demonstrating an improvement of surgical results using MRI for patients with DCIS [29].
The biological characteristics of DCIS often predict recurrence and a type of subsequent invasive cancer. The breast cancer-specific mortality is associated with age at diagnosis, ethnicity, grade, size, and ER status [28]. Breast-conserving surgery (BCS) aims at the complete removal of the DCIS and represents the most favorable treatment in a majority of patients. Clear margins of at least 2 mm are associated with a reduced risk of ipsilateral DCIS or invasive tumor recurrence (ipsilateral breast tumor recurrence, IBTR) compared with positive margins defined as ink on DCIS. Factors known to impact rates of IBTR should be considered in determining the need for re-excision [30].
Both adjuvant endocrine treatment and radiation therapy (RT) reduce IBTR but do not affect mortality. RT after BCS has been shown to reduce both in situ and invasive recurrences (LoE 1a) [31]. Omitting radiotherapy implies a slightly elevated risk for local recurrence without an effect on overall survival (OS). Omission of adjuvant RT in low-risk patients such as those with tumors <2.5 cm, low and intermediate nuclear grade, and mammographically detected DCIS might be discussed. Retrospective evaluation of the ER status showed that Tam reduced any subsequent breast events by 42% in ER-positive DCIS [32]. AI might be used as an alternative option in postmenopausal women. However, OS is not improved by endocrine therapy. Newer data support an effectiveness of low-dose Tam (5 mg/day for 3 years) regarding event-free survival in premalignant lesions with lower side effects (DCIS, LCIS, ADH).
Hence, potential side effects of radiation and endocrine therapy must be weighed carefully when making treatment decisions.
Breast Cancer Surgery under Oncological Aspects
Oncoplastic methods led to the transition from radical surgical concepts towards BCS. “No ink on tumor” is the accepted standard for resection margins for patients who undergo primary BCS or surgery, provided that all suspicious lesions according to preoperative imaging are resected (LoE 2a/A/AGO++). Therefore, BCS is also an option for patients with multifocal and multicentric disease, when R0 resection is confirmed (LoE 2b/B/AGO+). SLNE is the standard of care staging procedure in cN0 patients with invasive disease (LoE 1b/A/AGO++). Suspicious lymph nodes should be assessed by CNB. The significance of axillary surgery for patients with 1–2 positive sentinel lymph nodes (SLNs) after primary surgery continues to decline. This is mainly due to the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial, confirmed by the 10-year follow-up [33] and the AMAROS trial [34] as well as confirming studies with no differences in locoregional control, disease-free survival (DFS), and OS.
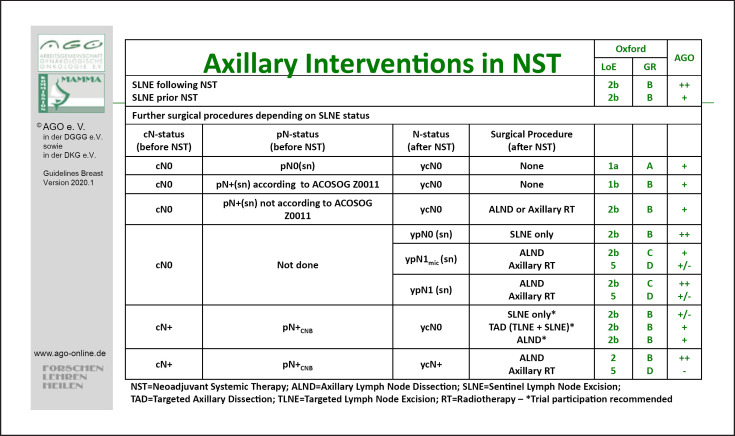
Following neoadjuvant therapy (Fig. 2), SLNE should be available and performed after NACT since it reduces the rate of ALND (ycN0 after cN+) and axillary remission provides additional prognostic information (LoE 1b/A/AGO++). SLNE before NACT remains an option if an impact on adjuvant treatment decisions is expected (LoE 1b/B/AGO+/−). In patients with a clinically negative axilla (cN0) before NACT and tumor-infiltrated SLN after NACT (including micrometastases), a full ALND should be performed (LoE2b/B/AGO+) [35].
Fig. 2.
Management of the axillary lymph nodes in the neoadjuvant chemotherapy concept.
For patients who presented initially with (CNB proven) positive axillary lymph nodes (pN+) and converted to ycN0 after NACT, the accuracy of SLNE is lower than in the adjuvant setting [36]. Since unselected axillary sampling is not indicated and ALND may be harmful, TAD offers an alternative in these patients (LoE 2b/B/AGO+). TAD implies the combination of SLNE and removal of the CNB-positive target lymph node (TLNE) marked with a clip, coil, seed, or tattoo. Caudle et al. [37] described a significant reduction of FNR from 10.1% with SLNE alone to 4.2% with TLNE alone, and 1.4% in case of combination of SLNE and TLNE (TAD). Of note, in 23% of the patients the TLN was not the SLN. The impact of TAD on DFS is still unclear and further studies are required. Collection of patient data in a joint registry is recommended.
Oncoplastic and Reconstructive Surgery
Oncoplastic surgery represents an essential component in the framework of an integrated treatment strategy for patients with breast carcinoma. It is defined as the use of plastic surgery techniques at the time of tumor removal, in order to achieve safe resection margins and an esthetic breast shape [38]. Oncoplastic surgery focuses on favorable scar positioning, adequate soft tissue shaping, the choice of a suitable reconstruction procedure (particularly when radiotherapy is indicated), and reconstruction of the contralateral breast in order to achieve symmetry. For breast reconstruction, patient-reported outcome measures should be systematically recorded for every oncoplastic breast surgery. For implant reconstruction, a pre- or retropectoral implant placement can be performed [39, 40]. Participation in studies to evaluate the procedures should be supported.
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) represents a rare malignant disease after implantation of a breast implant, which usually occurs with textured implants. It represents 3% of non-Hodgkin lymphomas and causes only 0.04–0.5% of all malignant breast diseases with an incidence of 0.6–1.2/100,000 women with breast implants. The median interval to diagnosis of BIA-ALCL is about 8 years. The symptoms include swelling and late seroma (60%), solid tumor (17%), or seroma and solid tumor (20%). All newly diagnosed seroma more than a year after breast implant placement should be clarified by US and cytology (LoE 3a/D/AGO++). The diagnosis is made by aspiration of seroma fluid (at least 50 mL) with subsequent cytology (flow cytology) with immunohistological examination of CD 30 (LoE 3a/D/AGO++). CNB in solid lesions should be performed, and lymphoma assessment of the resected tissue as well as histologic staging are indicated (LoE 3a/D/AGO++). BIA-ALCL must be reported to the regulatory authorities (in Germany: Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM). The treatment approach (implant removal and total capsulectomy plus removal of the tumor and suspicious lymph nodes) should be discussed with lymphoma specialists (LoE 3a/C/AGO++). Despite the accumulation of ALCL in textured implants, the European Society of Breast Cancer Specialists (EUSOMA) and AGO currently see no safety concerns in the use of this type of implant [41, 42].
Neoadjuvant Chemotherapy
If systemic chemotherapy in patients with early operable breast cancer is indicated, the AGO recommends NAST. For patients with increased risk of recurrence dose-dense chemotherapy schedules are recommended, including weekly taxane regimens (AGO+/AGO++). In patients with HER2-positive tumors NAST should contain anthracyclines, taxanes, and trastuzumab, in patients with N+ plus pertuzumab (AGO++), or an anthracycline-free regimen with carboplatin (AGO+). In triple-negative breast cancer (TNBC), dose-dense chemotherapy with anthracyclines including weekly taxanes is recommended. The addition of platinum is recommended, irrespective of BRCA mutation (AGO++).
PCR (ypT0/is ypN0) is associated with improved survival [43]. A recent Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta-analysis with a follow-up of 15 years showed equal DFS and OS compared to adjuvant therapy. The increased rate of local recurrence cannot be attributed to NAST, but to suboptimal locoregional management in patients who received NAST [44]. In first-generation NAST trials localization of the tumor and multidisciplinary management were not standardized. Modern regimens are considered to be more efficient (higher pCR) and better surgical and RT as well as pathology assessment have improved outcome after NAST. In subgroups where a pCR is strongly associated with improved survival (TNBC, HER2-positive, and luminal B-like), NAST (plus HER2-targeted therapy) should be the preferred therapeutic approach (AGO++). The addition of carboplatin in TNBC (regardless of gBRCA1/2 mutation status) was not only associated with an increased pCR rate in 2 neoadjuvant trials, but also resulted in a significant improvement in outcome with a DFS rate of 85.8% (with carboplatin) versus 76.1% without carboplatin (HR = 0.56; p = 0.0350) in GeparSixto and a clinically meaningful, albeit statistically not significant, improvement in DFS (absolute 5%) in the CALGB 40603 study [45, 46]. Based on the results of the GeparSepto trial, the AGO recommends nab-paclitaxel 125 mg twice weekly instead of paclitaxel for patients with TNBC (LoE 1b/B/AGO+) [47]. For patients with HER2-positive tumors, the addition of pertuzumab and trastuzumab to chemotherapy is highly recommended [48] (LoE 2b/B/AGO++). The indication for neoadjuvant therapy in inflammatory breast cancer remains unchanged. Delayed initiation of NAST for thorough diagnosis (imaging and/or molecular pathology) is not correlated with a negative outcome (LoE 2b/B) [49].
Post-Neoadjuvant Therapy Options: AGO Recommendations
HER2−/ER+
For patients with HR-positive tumors, endocrine therapy according to the menopausal status is standard of care.
TNBC
Given the positive results of the CREATE-X trial, further chemotherapy with capecitabine in patients with TNBC and no pCR is recommended (LoE 2b/B/AGO+) [50].
HER2+/Non-pCR
Based on the KATHERINE trial results, patients who have residual invasive tumor cells in the breast and or in the ipsilateral lymph nodes after optimal chemotherapy (anthracyclines and taxanes or taxanes and platin) plus anti-HER2 therapy (trastuzumab or trastuzumab and pertuzumab) should receive 14 cycles of T-DM1 after NAST [51].
HER2+/pCR
In patients with a pCR after optimal NAST the continuation of anti-HER2 therapy is recommended up to the completion of 1 year (LoE 2b/C/AGO+). In patients with a pCR and low risk of recurrence (node negative before and after NAST) a de-escalation to trastuzumab monotherapy is recommended (LoE 2a/C/AGO++). In patients with node-positive breast cancer prior to NAST responding with a pCR, dual HER2- blockade based on the data from the APHINITY trial (AGO+) is recommended.
Adjuvant Cytotoxic and Targeted Therapy
Adjuvant anthracycline- and taxane-based chemotherapy reduces breast cancer mortality by approximately one-third depending on the absolute risk [52].
Very recently, a patient-level meta-analysis of 37,298 women with early breast cancer from 26 randomized trials showed that increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling moderately improved 10-year risk of recurrence and death from breast cancer without increasing mortality from other causes [53]. Because of this strong evidence, dose-dense chemotherapy (including weekly regimens) (LoE 1a/A/AGO++) instead of conventionally dosed anthracycline- and taxane-based chemotherapy (q3w) (LoE 1/A/AGO+) should be the preferred treatment option. In patients with high risk of recurrence with 4 or more involved axillary lymph nodes, dose-dense and dose-intensified chemotherapy demonstrated superior survival compared with a conventional schedule [54]. Most strikingly, OS was improved with an absolute difference of 10% after 10 years of follow-up (LoE 1/A/AGO++).
A meta-analysis of individual patient data from 15,457 patients showed that only patients with TNBC and non-pCR after optimal NAST might benefit from the addition of capecitabine (LoE 1aa/A/AGO+). Thus, a general use in TNBC cannot be recommended (LoE 1aa/A/AGO−) [55].
In patients with reduced left ventricular ejection fraction, anthracycline-free therapy with docetaxel/cyclophosphamide (LoE 1b/B/AGO+) [56] might be an option, whilst other regimes like weekly paclitaxel (LoE 1b/B/AGO+/−) or CMF (LoE 1a/A/AGO+/−) [52] may not be sufficiently effective. In patients with TNBC tumors and negative lymph nodes, AGO recommends adjuvant chemotherapy for tumors of 5–10 mm, while for tumors <0.5 cm there is no indication for adjuvant chemotherapy (LoE 2b/B/AGO−) [57].
In patients with HER2-positive early breast cancer, anti-HER2 therapy with trastuzumab is highly recommended (LoE 1a/A/AGO++). Trastuzumab might be either combined with an anthracycline- and taxane-based chemotherapy (LoE 1a/A/AGO++) or an anthracycline-free regimen like carboplatin/docetaxel (LoE 1b/A/AGO+) [58]. In patients with HER2-positive node-negative early breast cancer with a maximum diameter of 2 cm, trastuzumab might be combined with weekly paclitaxel (LoE 2b/B/AGO+) [59]. Based on the updated follow-up of the APT trial, node-negative patients treated with this de-escalated regimen had an excellent 7-year invasive DFS of 93% [60]. The updated data from the APHINITY trial support adjuvant pertuzumab in addition to trastuzumab and chemotherapy only in node-positive patients with HER2-positive early breast cancer (LoE 1ba/B/AGO+) [61, 62]. At a median follow-up of 74.1 months, invasive DFS of node-positive patients was 87.9% for trastuzumab and pertuzumab versus 83.4% for trastuzumab. In the node-negative cohort, no additional clinical benefit was seen for the dual blockade (LoE 1ba/B/AGO+/−).
For extended adjuvant treatment, the TKI neratinib in combination with standard endocrine treatment for 12 months may be an option for HR-positive patients who have completed 1 year of trastuzumab-based therapy (LoE 1b/B/AGO+). The ExteNET trial [63] demonstrated that 1 year of neratinib significantly improved the 5-year invasive DFS (91.2 vs. 86.8%) in this cohort. However, none of these patients had received prior pertuzumab.
Adjuvant Endocrine Therapy
Endocrine therapy is indicated in all patients with HR-positive breast cancer (LoE 1/A/AGO++). A meta-analysis of the Good Behavior Game (GBG) of several neoadjuvant trials suggests that tumors with low HR expression (≥1–9%) are biologically similar to triple-negative carcinoma. Thus, omitting endocrine therapy may be an option in cases with very low expression of ER and PR (LoE 1/A/AGO++) [64]. In case of ER–/PR+ (≥10%), immunohistochemical reevaluation should be performed. False positivity for PR should be excluded [65]. Treatment duration of 5 years remains standard of care. Extended adjuvant treatment might be indicated in patients with increased risk of relapse.
Premenopausal Patients
An update of the SOFT and TEXT trials reported 8-year OS data recently and showed superiority of the addition of ovarian function suppression (OFS) to Tam or an AI in patients treated with additional chemotherapy. In SOFT the effect was best with OFS + Tam versus OFS + AI versus Tam alone [66]. In the ASTRRA trial, 5-year DFS and OS was significantly increased after 2 years of OFS + 5 years of Tam versus 5 years of Tam alone. All patients had (neo)adjuvant chemotherapy and menopausal status post-chemotherapy was evaluated for 2 years. In case of switch to a premenopausal status, patients could still be enrolled up to 2 years after chemotherapy [67]. Thus, the AGO recommendation changed to consider OFS in cases of resumption of ovarian function within 24 months. All statements from 2019 are still valid.
Postmenopausal Patients
The recommendations from 2019 have not been changed. However, for patients with lobular breast cancer a nonsteroidal AI should be preferred over a steroidal AI due to better OS [68] (LoE 2b/B/AGO+).
Extended Adjuvant Therapy in Premenopausal Women
Tam can be extended for up to 10 years (LoE 1a/A/AGO++). Extended adjuvant treatment with 5 years of Tam should also be offered to those patients with ovarian suppression and Tam or AI for their initial treatment (LoE 5/D/AGO+). If the patient is confirmed as being postmenopausal within the first 5 years, endocrine therapy can be continued after 5 years of Tam with 2.5–5 years of letrozole (LoE 1b/B/AGO+).
Extended Adjuvant Therapy in Postmenopausal Women
After 5 years of Tam, extended therapy with 5 years of Tam is still an option (LoE 1a/A/AGO+), but switching to an AI for 2–5 years should be preferred (LoE 1a/A/AGO++). If patients received an AI (upfront or switch), patients at higher risk should be offered 2–5 additional years of AI (LoE 1b/B/AGO+).
Adjuvant Radiotherapy
The guidelines on the use of radiotherapy (RT) were jointly developed by the AGO and the German Society of Radiation Oncology (DEGRO). Hypofractionated whole-breast RT is considered standard of care after BCS (LoE 1a/A/AGO++). The use of a tumor bed boost should be adapted to the local recurrence risk: premenopausal patients (LoE 1b/B/AGO++) or postmenopausal patients with additional risk factors (LoE 2b/B/AGO+). In elderly patients with low-risk early-stage breast cancer and a life expectancy <10 years, RT can be omitted, thus accepting an increased risk of local recurrence (LoE 1a/B/AGO+).
The statements regarding accelerated partial breast irradiation (APBI) have been updated. APBI should only be used in patients with low-risk early-stage breast cancer (>50 years, pT1 pN0 R0 G1–2, HR positive, nonlobular histology, no EIC). Intraoperative RT with 50-kV photons or electrons should only be used in patients >70 years (LoE 1b/A/AGO+). Postoperative APBI can be delivered with multicatheter brachytherapy or external beam RT. Publication of the NSABP B-39/RTOG 0413 [69] and the RAPID trial [70] as well as the presentation of the long-term results of the Florence trial [71] have significantly increased the evidence for the use of EBRT. Regimens using 15 × 2.67 Gy over 3 weeks [72] and 5 × 6 Gy over 2 weeks with intensity-modulated RT [73] showed low rates of local recurrence and improvements in acute and late toxicity over whole-breast RT (LoE 1b/A/AGO+). Due to the higher rates of late toxicity and adverse cosmesis in the RAPID trial [70], which employed 10 × 3.85 Gy in two daily fractions, this regimen should be used with caution (LoE 1b/A/AGO+/−). Interdisciplinary discussion of clip placement and target volume delineations between the involved breast surgeons and radiation oncologists during implementation of APBI is crucial.
Postmastectomy RT should be used in patients with pT3/4 and/or N2–3 (LoE 1a/A/AGO++) and in patients with pT1–2 pN1 and additional high-risk factors (LoE 1a/A/AGO+). Regional nodal irradiation (RNI) to the supra-/infraclavicular and internal mammary nodes (IMN) is recommended in patients with pN2–3 or when there is involvement of level 3 or the IMN. It should be strongly considered in patients with pN1 and additional risk factors (premenopausal patient and G2–3 or HR-negative as well as central/medial tumor location). RT to the IMN should not be applied to patients receiving trastuzumab or those with cardiac comorbidities (LoE 2b/A/AGO−). In patients with cN0 and 1–2 involved sentinel lymph nodes not meeting the Z0011 criteria, RNI according to the AMAROS trial can be applied (LoE 1b/B/AGO++). Normofractionated RT is advised when applying RNI (LoE 1a/A/AGO++).
Breast Cancer: Special Situations
While Tam remains the standard for endocrine treatment of male breast cancer, some evidence also exists for use of AI and fulvestrant. There is very limited data supporting the use of CDK-4/6 inhibitors among male patients with breast cancer [74]. However, the FDA has recently expanded the approved indications to include men. Based on this, AGO recommends the use of CDK-4/6 inhibitors for male patients with advanced breast cancer/MBC (LoE 2b/B/AGO+).
Surgical therapy of phyllodes tumor of the breast has been a matter of debate. Based on recent evidence [75], AGO has agreed to revise the minimal resection margins required for the disease subtype. For benign phyllodes tumors, simply complete resection is requested (LoE 2b/B/AGO++). For cases with borderline/malignant phyllodes tumors a resection margin of ≥1 mm is considered sufficient (LoE 2b/B/AGO++). However, local control might be improved if borderline/malignant tumors are resected with a resection margin of >10 mm (LoE 2b/B/AGO+) [76].
The indications for mastectomy after NACT remain unchanged: positive margins after repeated excisions (LoE 3b/C/AGO++), lack of feasibility of radiotherapy (LoE 5/D/AGO++), and presence of inflammatory breast cancer (with no more than clinical complete response, LoE 2b/C/AGO+/−).
Regarding BIA-ALCL, please refer to the section on Oncoplastic and Reconstructive Surgery.
Complementary Therapy and Survivorship
The integration of complementary interventions is still a challenge in the standard treatment of breast cancer. The two main reasons for this are:
lacking general definition of complementary medicine, and
only a few “conventional studies” exist providing clear evidence on the efficacy of complementary approaches and benefit-risk ratios.
In 2020, the AGO recommendations for “Complementary Therapy and Survivorship” did not change substantially compared to 2019. Recently published studies and review articles underline the effects of physical exercise (endurance training 3 times a week in combination with workout exercises 2 times a week) on quality of life, cardio-respiratory fitness, physical performance, sleep, pain, depression, lymphedema, and fatigue (LoE 1a/A/ AGO++) [77]. Evidence is growing that mind-body interventions, including cognitive behavioral therapies, relaxation techniques, and meditation, improve quality of life among breast cancer patients, and therefore clinical guidelines have begun to include recommendations. A systemic review and meta-analysis of 19 RCTs (n = 2,806) revealed evidence that mind-body interventions are efficacious for reducing fear of cancer recurrence, although further investigations are recommended to analyze the optimal integration of mind-body practices (LoE 1a/A/AGO+) [78].
Gynecological Issues in Breast Cancer Patients/Contraception
Treatment of Menopausal Symptoms
Classical replacement therapy to alleviate menopausal symptoms is contraindicated in breast cancer patients (LoE 1b/B/AGO−), while topical vaginal application of low-dose estriol may be used for urogenital symptoms (LoE 4/D/AGO+/−). Hot flushes may be treated with serotonin reuptake inhibitors (i.e., venlafaxine, LoE 1a/A/AGO+). A recent study found that a single dose of depomedroxyprogesterone acetate to prevent hot flushes had no negative effect on survival in ER-positive breast cancer patients [79]. Homeopathy had no effect on hot flushes in a large randomized trial compared with placebo in breast cancer survivors (LoE 1b/B/−) [80]. Sleep disturbances might be treated with melatonin (LoE2b/C/+).
Physical exercise has positive effects on menopausal symptoms and, to a lesser degree, on the sexuality of patients experiencing treatment-induced menopause (LoE 1a/A/AGO++) [81]. Mind-body medicine results in a moderate improvement in hot flushes scores, joint pain, fatigue, and sleep (LoE 1b/B/AGO+). Cognitive behavioral therapy is effective in alleviating treatment-induced menopausal symptoms (LoE 1b/B/++). There are contradictory data about the effect of acupuncture on hot flushes, depression, and sleep disturbances but it can be used to treat AI-induced joint pain (LoE 1b/B/+) [82].
Fertility Preservation
Fertility counseling on fertility preservation should be offered to all patients who wish to retain their fertility (LoE 4/C/AGO+). Application of GnRH analogs given 1–2 weeks prior to chemotherapy has shown an improved rate of recovery of ovarian function after 2 years (LoE 1a/B/AGO+) and might have a moderate effect on preservation of fertility (LoE 2a/B/AGO+/−).
Low AMH levels seem to be indicative of reduced ovarian reserve in chemotherapy-treated breast cancer patients (LoE 1b/B/+).
Contraception
Hormone-free contraceptive methods are the first choice for patients with breast cancer.
Sexual Health
Sexual complaints are common in breast cancer patients and should be assessed. Screening tools may help physicians to address sexual health issues (LoE 4/C/AGO+).
Nonhormonal lubricants and moisturizers are the primary treatment for vaginal dryness (LoE 1b/B/AGO+). Microablative fractionated laser or vaginal YAG/erbium laser may be an option for some patients to alleviate genital atrophy (LoE 2a/A/+/−) [83].
Follow-up of Breast Cancer
Recommendations for follow-up of breast cancer have remained unchanged for several years, including this year.
Still, the rationale in breast cancer follow-up is the early detection of curable breast cancer events (LoE 1a/B/AGO++). Early detection of symptomatic metastases is desirable (LoE 3b/C/AGO+); however, with regard to the early detection of asymptomatic metastases (LoE 1a/A/AGO−), data are inconsistent and, most importantly, do not suggest a survival benefit.
Beyond improvement of survival, additional issues like improvement of quality of life and physical performance and the reduction and early detection of treatment-related side effects are important concerns in this matter (LoE 2b/B/AGO+). In addition, re-evaluation of current adjuvant therapies and the assessment or improvement of treatment adherence is an essential part of follow-up care (LoE 2b/B/AGO++). It should thus be pointed out that every patient has the right to obtain a second opinion (LoE 2c/B/AGO++); genetic counseling should be offered if indicated, as should hormone replacement therapy, prophylactic surgery, and breast reconstruction (LoE 2c/C/AGO+). Lifestyle modifications and interventions with regard to comorbidities are further important aspects of follow-up.
Most importantly, follow-up examinations in asymptomatic patients in routine situations should not consist of tumor marker measurements and imaging methods. For the detection of curable events, physical and self-examination with MG and adjunctive US are recommended. Follow-up of male breast cancer should follow the same procedures as in female breast cancer (LoE 5/D/AGO+). There is no data that may support tailoring breast cancer follow-up according to breast cancer (molecular) subtype.
In case of increased risk such as age <50 years, HR negativity, and decreased diagnostic accessibility C/D in MG and US, MRI should be considered [84].
In this context, screening for secondary malignancies according to guidelines is meaningful. Patients and physicians should be aware of the increased risk of hematologic malignancies after chemotherapy and lung cancer after radiotherapy to the breast or chest wall. Further, a DXA scan at baseline and a repeated scan according to individual risk in women with premature ovarian failure or in women on AI therapy are recommended [85].
Health Literacy and Communication
The options for healthy people and patients in cancer prevention and therapy are constantly increasing. At the same time, a change has taken place in the health care system, which significantly strengthens the patients' right of self-determination and anchors the informed and shared decision making of patients and doctors in law. Instead, doctors should no longer make decisions on prevention and treatment concepts alone. They should involve healthy people and patients as “experts in their own affairs” in the process of preventing cancer and treating it as much as possible, and accompany them on the way to decisions for which they are responsible. This places new demands on counsellors, patients, and doctors. The main focus is on improving health literacy and shared decision making, which depends on successful doctor-patient communication.
Health Literacy
Despite media presence of specialist content, for the majority it is difficult to find out what is important and how to make the right decisions for a healthy life or for coping with illness (health literacy). According to a current survey from 2017, half of all Germans have insufficient or clearly limited health literacy. As a result, numerous initiatives and offers were launched to improve health literacy (Alliance for Health Literacy, National Action Plan Health Literacy). They focus on the special form of doctor-patient relationship and are based on an overarching set of values: respect for the right of self-determination of the individual, the principle of non-harm, care, and equality.
Communication
Good communication skills are a medical core competence and the basis for a trusting doctor-patient relationship. This in turn has an important influence on the understanding of the disease, cooperation in diagnosis, treatment, and rehabilitation, and thus on the success of treatment. “Talking medicine” is becoming increasingly important in the health care system (remuneration) and is offered across sectors as a part of training and continuing education programs for all health care professionals.
Shared Decision Making and Patient Decision Aids
Successful communication and the development of a trustful doctor-patient relationship is an important cornerstone for patient participation in the shared decision-making process. The open discussion of prevention options and treatment methods as well as the joint and equal decision with the doctor demonstrably lead to improved treatment results.
Locoregional Recurrence
The primary goal of early breast cancer treatment is the prevention of locoregional (LRR) and distant recurrences. In case of LRR, pre-therapeutic re-assessment of the tumor histology, immunohistochemistry, and re-staging are recommended (AGO++). PET-CTs should not be routinely included in re-staging procedures (LoE 2b/B/AGO−) [86]. Numerous risk factors are described which can be assigned to different groups: patient-related, tumor-related, and further factors (Fig. 1). Residual tumor disease after NAST is an important prognostic factor (LoE 2b) for postneoadjuvant treatment options [87]. An increasing number of nomograms and scores are under development for predicting individual risk.
Although mastectomy reduces the risk of LRR, several parameters affect the individual risk as shown in a recently published meta-analysis of 20 studies with 11,233 patients. In patients with pT1–2 pN0 after mastectomy, the pooled results indicated that young age (HR = 1.77; p = 0.001), positive lymphovascular invasion (L1/ V1) (HR = 2.23; p < 0.001), high histologic grade (HR = 1.66; p < 0.001), positive HER2 status (HR = 1.65; p = 0.027), premenopausal status (HR = 1.36; p = 0.015) and positive surgical margins (HR = 2.56; p = 0.014) are associated with a significant increased risk of LRR [88].
Independent risk factors for the development of a second recurrence are the omission of radiotherapy (OR = 4.6; p = 0.011) and systemic therapy (OR = 3.7; p = 0.015) at first recurrence and high tumor grade (OR = 3.1, p = 0.013) (LoE 3b/C) [89]. These data confirm the importance of adequate treatments in improving local control.
In patients with ER-positive LRR following complete resection (R0), endocrine therapy is considered standard (LoE 2b/B/AGO++). For patients with triple-negative (TN) tumors, chemotherapy (LoE 2b/B/AGO+), and in cases with human epidermal growth factor receptor (HER)2-positive disease, chemotherapy in combination with HER2-targeted therapy are the recommended options (LoE 5/D/AGO+).
In patients with positive surgical margins or unresectable tumors, systemic treatment should be adjusted analogous to distant recurrences (LoE 2b/B/AGO++).
After previous BCS and RT, re-BCS and brachytherapy can be considered (LoE 2b/B/AGO+). Several, even prospective, studies demonstrated comparable safety and effectiveness to mastectomy [90]. Concerning this, treatment of LRR should always be planned and performed in an interdisciplinary approach.
Prognostic and Predictive Factors
Most of the established prognostic markers, including ER and PR HER2 and Ki67, are provided by traditional pathology and overlap with parameters used for the prediction of therapy response.
In MBC, expression of programmed cell death 1 ligand (PD-L1) by tumor-infiltrating leukocytes either in the primary tumor or the metastasis itself is predictive for the response to check point inhibitors, such as atezolizumab [91]. For the prediction of atezolizumab efficacy in TN MBC, immune cell PD-L1 positivity seems essential (punch biopsies, resection specimens). At least 1% cytoplasmic staining of the leukocyte stromal infiltrate (lymphocytes, macrophages, plasma cells, granulocytes outside of abscesses) is considered positive. Tumor staining should not be assessed. In IMPASSION 130, Ventana antibody SP142 with positive control (tonsil) was used [91]. Other antibodies are probably equivalent and different cutoffs may apply. Participation in the National Pathology Society QA Program is obligatory and reference pathology is needed if a center is not yet qualified.
A couple of novel predictive markers in MBC are yielded by DNA sequencing and detection of mutations. BRCA1/2 mutations are predictive for PARP inhibitor efficacy [92]. Phosphatidylinositol-4,5-bisphosphonate 3-kinase catalytic subunit alpha (PIK3CA) mutations indicate response to corresponding inhibitors, such as alpelisib [93]. Besides amplification and overexpression, HER2 may be activated as an oncogene by point mutations, which render affected cells sensitive to inhibitors such as neratinib and lapatinib [94]. ESR1 is mutated in about 15–25% of metastasized luminal breast cancers, which induces autocrine ligand independence and consequently generates resistance against deprivation of estrogen supply by AI [95].
Endocrine and Targeted Therapy in MBC
In women with HR-positive, HER2-negative MBC, endocrine-based therapy should be considered first choice, irrespective of menopausal status. Premenopausal women rendered postmenopausal by either GnRH analogs or other means of OFS should then be treated like postmenopausal women.
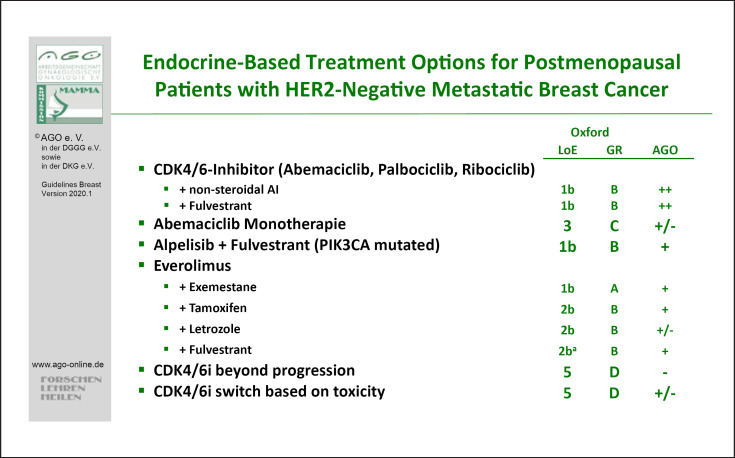
The majority of patients are candidates for a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor combination therapy. The evidence concerning palbociclib, ribociclib, and abemaciclib has been completed with regard to a variety of patient populations according to therapy line, menopausal status, and endocrine combination partners. Those combination therapies are rated with LoE 1b/B/AGO++ for postmenopausal patients (Fig. 3), and for premenopausal patients with LoE 1b/B/AGO++ and 2b/B/AGO++, respectively. All three drugs have been thoroughly investigated in first and further therapy lines in endocrine-sensitive and endocrine-resistant MBC and demonstrated a homogeneous improvement of progression-free survival (PFS) with hazard ratios between 0.42 and 0.58 [summarized in 99, 100]. Thus, no subgroup could be identified either by clinical markers or by biomarkers that does not benefit from using a CDK4/6 inhibitor in addition to endocrine therapy. An OS was shown in premenopausal patients in the MONALEESA-7 [101] study and in postmenopausal patients who had participated in the MONALEESA-3 [102] and MONARCH-2 studies [103]. Due to the efficacy of the CDK4/6 inhibitors, endocrine monotherapy with fulvestrant, AI, or Tam was downgraded to AGO+.
Fig. 3.
Endocrine-based treatment options for postmenopausal patients with HER2-negative MBC.
Patients with HR-positive breast cancer carrying a germline BRCA mutation might be candidates for PARP inhibitors. Both confirmatory studies OlympiAD with olaparib (LoE 1b/A/AGO++) [104] and EMBRACA with talazoparib (LoE 1b/B/AGO+) [105] included about 50% HR-positive breast cancers and showed a better PFS compared to standard of care monochemotherapies. The final OS analysis of the OlympiAD study showed a significant OS benefit for olaparib in patients without prior metastatic treatment. However, this analysis had a low patient number [106].
In PIK3Ca-mutated patients, the PI3Kα-specific inhibitor alpelisib has shown a significant improvement in combination with fulvestrant when compared to fulvestrant alone after progress on an AI (LoE 1b/b/+) [93]. Therefore, another treatment option to overcome endocrine resistance exists, most likely to be effective after CDK4/6 inhibition.
Chemotherapy with or without Targeted Drugs in MBC
While on treatment for MBC, reevaluation of quality of life, signs and symptoms, and general health status is important (A/AGO++). Monochemotherapy is the treatment of choice in slow progressing disease or if secondary resistance to endocrine therapy arises (LoE 1b/A/AGO++). In case of disease progression after taxanes or anthracyclines eribulin (LoE 1b), capecitabine (LoE 2b) or vinorelbine (LoE 2b) are recommended (AGO++). A recent post hoc analysis of the 301 trial in HER2-negative MBC revealed a significant survival benefit for eribulin over capecitabine (HR = 0.77; 95% CI 0.62–0.97) [107]. In contrast, combination chemotherapy is recommended in case of need of urgent remission or visceral crisis according the ABC-4 definition [108].
In MBC, treatment selection is based on ER and/or PR and HER2 status either from the primary tumor or from the metastatic site (AGO++). In TNBC patients with newly diagnosed MBC, the evaluation of the PD-L1 status on immune cells is recommended [93]. In TNBC patients with PD-L1 immune cell-positive status, the combination of nab-paclitaxel and the PD-L1 inhibitor atezolizumab is a new option in first-line therapy of MBC (LoE 1b/B/AGO+).
Next to the recommendation for platinum salts in TNBC (LoE 1b/B/AGO+), PARP inhibitors improved PFS in two trials (OlympiAD, EMBRACA) compared to any chemotherapy as “doctors' best choice” in HER2-negative MBC with germline BRCA (gBRCA) mutation [104, 105]. Thus, olaparib (LoE 1b/B/AGO++) or talazoparib (LoE 1b/B/AGO+) are new treatment options in this setting.
In HER2-positive MBC, taxane-based chemotherapy plus dual blockade of the HER2 receptor by trastuzumab and pertuzumab is recommended as first-line combination. After progression, T-DM1 is recommended for second-line therapy (LoE 1b/A/AGO++).
Bone Metastasis
In breast cancer, over 65–70% of patients with advanced disease develop skeletal metastasis [109]. Bisphosphonates and denosumab have been successfully used to reduce hypercalcemia (LoE 1a/A/AGO++), skeletal events/complications (LoE 1a/A/AGO++), and bone pain (LoE 1a/A/AGO++), and increase the bone pain-free survival (bisphosphonates: LoE 1a/A/AGO++; denosumab: LoE 1b/A/AGO++) [110]. The approved schedule for denosumab (120 mg s.c.) is every 3–4 weeks (LoE 1b/A/AGO++), whereas the de-escalation treatment of zoledronic acid every 12 weeks (q12w) i.v. seems to be superior to standard treatment (q4w) in terms of safety, efficacy, and costs (LoE 1a/A/AGO++) [111]. Severe side effects must be considered, and as prevention of osteonecrosis of the jaw the ASORS evaluation should be performed [112].
CNS Metastases
Metastases to the CNS in breast cancer are of high relevance since the incidence has increased to more than 30% in high-risk groups such as HER2-positive or triple-negative MBC patients [113]. Despite this high incidence, evidence of breast cancer-specific treatment approaches is very limited. Therefore, the AGO breast group encourages centers to participate in the German Brain Metastases in Breast Cancer (BMBC) Registry (BMBC) [114]. Local therapy is the first treatment of choice. Stereotactic radiotherapy (radiosurgery or fractionated stereotactic radiotherapy) or surgery in case of limited numbers (1–3, in some studies also up to 4) of cerebral foci (LoE 2b/B/AGO++) are recommended. Whole-brain irradiation therapy (WBRT) (LoE 2a/B/AGO+) is an additional treatment option if surgery or stereotactic radiotherapy are not feasible. In general, outcome is not improved by surgery compared with radiotherapy. Indications for surgery are histological verification or need for immediate decompression. Discordance between primary tumor and brain metastases is shown for ER in 16%, for PR in 25.2%, and for HER2 neu in 10.4% of cases, respectively [115].
After surgery, radiotherapy of the resection area is recommended (LoE 1b/B/AGO++) [116]. Additional WBRT does not improve OS despite better brain control. Decline in cognitive function was described to be more frequent with the addition of WBRT to stereotactic radiotherapy. Initial treatment with stereotactic radiotherapy and close monitoring is recommended to better preserve cognitive function in patients with newly diagnosed brain metastases (LoE 2b/B/AGO++) [117] and should even be discussed in patients with multiple brain metastases in which WBRT is still standard of care. New radiation techniques can protect the hippocampal region and improve preservation of memory (LoE 2b/C/AGO+/−) [118]. In the case of local recurrence in the brain, re-irradiation can be discussed (LoE 4/C/AGO+/−).
Systemic therapy in patients with brain metastases in addition to local therapy should be performed as for other metastatic sites. Systemic therapy might be continued if the patient has the first diagnosis of brain metastases with stable extracranial disease (LoE 2c/C/AGO+).
In patients with HER2-positive disease, HER2-directed therapy should be continued if remission of extracranial disease is achieved. TKI (e.g., ONT-380) and new chemotherapeutic options are being investigated in clinical trials. For patients with poor prognosis and reduced performance status best supportive care is an option (LoE 5/B/AGO+).
Specific Sites of Metastases
Management of primary stage IV breast cancer should focus primarily on systemic therapy (LoE 2a/B/AGO++). Surgical treatment should be considered on an individual basis as it does not seem to influence OS [119]. Only in women with limited metastatic disease and a good response to systemic treatment should surgical procedures at the primary site as well as at the metastatic sites be considered (LoE 2b/C/AGO+) [120, 121, 122].
If surgery of the primary tumor is performed in the metastatic setting, local excision or mastectomy should be done, resulting in free margins [123]. Axillary surgery is only indicated for bulky disease (LoE 5/D/AGO+/−). Local radiotherapy of the primary tumor can be performed after local surgical treatment according to the indications of the adjuvant setting (LoE 3a/C/AGO+).
Systemic treatment of metastatic disease is the therapy of choice. Before treatment, metastases should be confirmed by histology including evaluation of the HR and HER2 status. If surgery for distant metastases is considered, good overall health, oligometastatic disease, and a long-time interval between primary treatment and the occurrence of metastases are favorable factors regarding an improved outcome. Resection of liver metastases may be considered if R0 resection is feasible, if no extrahepatic metastases are present, and if the tumor biology shows an HR-positive breast cancer responding well to former systemic therapy with a long disease-free interval and ≤3 metastases (LoE 3a/B/AGO+/−) [124, 125]. Other procedures like regional radiotherapy, stereotactic body radiosurgery with volumetric intensity-modulated arc therapy, thermoablation, or chemoembolization are also possible options in individual cases (LoE 3b/C/AGO+/−) [126, 127].
For patients with lung metastases, the LoE for a curative approach is low, but some patients might benefit from an R0 resection of metastases followed by appropriate systemic treatment (LoE/B/AGO+) [128, 129].
Malignant pleural effusion should be treated in symptomatic cases. To control malignant pleural effusion, thoracoscopy with talcum pleurodesis (LoE 1a/B/AGO+) or povidone-iodine (20 mL of a 10% solution) (LoE 1b/B/AGO+), i.e., video-assisted thoracoscopy (LoE 1b/B/AGO++) or continuous pleural drainage with indwelling pleural catheters (LoE/B/AGO++) are options of choice. More rarely, other sclerosing agents are used (bleomycin, doxycycline, and mitoxantrone) [130]. Local antibody therapy with catumaxomab in the management of ascitis remains not recommended [131].
In symptomatic patients with malignant pericardial effusion and cardiac tamponade, drainage and pericardial fenestration are probably the treatment options of choice (LoE 3b/B/AGO++). For individual patients, video-assisted thoracoscopy or US-guided puncture with instillation of bleomycin, cisplatinum, mitomycin C, mitoxantrone, or bevacizumab may be an alternative. A retrospective analysis suggests benefit from the combination of systemic treatment and pericardial drainage (LoE 4/C/AGO++) [132].
In oligometastatic cases and single metastatic lesions, a surgical approach has to be considered as part of the whole treatment strategy.
Breast Cancer: Supportive Care and Side Effect Management
Optimal side effect management and supportive care is a major contributor to the overall risk/benefit balance associated with oncological therapies. This section of the AGO recommendations details aspects that are particularly relevant for the treatment of breast cancer patients and is based on the most recent version of the S3 guidelines [133] as well as other international guidelines such as ESMO wherever available.
Chemotherapy can lead to reactivation of hepatitis B in carriers [134]. Before the start of chemotherapy, screening for hepatitis B (HBsAG, anti-HBC) should therefore be performed in all patients (LoE 2c/AGO+). If one of the tests is positive, HBV DNA needs to be determined. In case of HBV DNA detection, virustatic therapy needs to be initiated (AGO++).
The essential drug management for antiemetic therapy has been revised (https://www.mascc.org/antiemetic-guideline). For patients in the acute and as well in the delayed emetic high-risk group, olanzapine on days 1–4 may be offered, particularly if nausea is a concern. As sedation and weight gain are side effects a dose reduction, 10 mg/day to 5 mg/day are a valid option [135, 136].
Chemotherapy-induced peripheral neuropathy is a common toxicity following taxane or subsequent T-DM1 therapy with an incidence of up to 50% grade 1–2 and up to 20% grade 3–4. Thus, besides continuing measures for neuropathy prevention such as tight surgical gloves and compression stockings (LoE 2b/B/AGO+), cooling gloves and stockings (LoE 2b/B/AGO+/−) and tactile stimulation (LoE 5/D/AGO+) are very important. While drug-based treatment options are limited (AGO+/−) non-drug-based therapy might be an option (AGO+) with functional treatment (LoE 2a/C), physiotherapy (LoE 5/D), and acupuncture (LoE2b/B).
Detailed and practical management information for new drugs such as CDK 4/6 inhibitors or immunotherapy can be found in the respective package inserts which are regularly updated.
Palliative Care
It is well accepted that MBC in an early phase is incurable but treatable. However, the late “palliative” phase has to be differentiated, as the focus is set on an end of life care. Early introduction of palliative care concurrent with active treatment is important to improve symptoms and quality of life. Furthermore, discussions about patient preferences at the end of life should begin early in the course of metastatic disease [109, 137].
It is very important to point out that with recent therapeutic progress with innovative and effective compounds the patient goals are differing in each phase. Meanwhile, we are in the position to prolong PFS without increasing toxicity, and the very recent results of studies with CDK4/6 inhibitors, checkpoint inhibitors, and PARP inhibitors presented an OS benefit. With such compounds the targeted and more individual treatment strategies take center stage. Therefore, patients are no longer satisfied to be treated with a palliative approach instead of being treated with a curable and life-prolonging approach. Thus, patient-reported outcome data are crucial to estimate treatment success and course.
Disclosure Statement
Prof. Dr. med. Nina Ditsch: MSD, Roche, AstraZeneca, TEVA, Mentor, and MCI Healthcare.
Prof. Dr. med. Michael Untch: Presentations, travel grants, all paid to his institution from: Abbvie, Amgen GmbH München, AstraZeneca, BMS, Celgene GmbH München, Daiji Sankyo, Eisai GmbH München, Janssen Cilag, Johnsen & Johnsen, Lilly Deutschland, Lilly Int., MSD Merck, Mundipharma, Myriad Genetics GmbH Zürich, Odonate, Pfizer GmbH Berlin, PUMA Biotechnology, Riemser, Roche Pharma AG, Grenzach Wyhlen, Sanofi Aventis Deutschland GmbH, Sividon Diagnostics Köln, TEVA Pharmaceuticals Ind. Ltd., and Berlin Pharmaceutical Industry.
Prof. Dr. med. Cornelia Kolberg-Liedtke: Consulting: Phaon Scientific, Novartis, Pfizer, Pfizer, Novartis, SurgVision, Carl Zeiss Meditec, Amgen, and Onkowissen; presentations: Roche, Novartis, Pfizer, Lilly, Pfizer, Novartis, Roche, Genomic Health, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, TEVA, Theraclion Janssen-Cilag, GSK, LIV Pharma, and Theramex; travel grants: Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, and Daiichi Sankyo.
Prof. Dr. med. Christian Jackisch: Roche, AstraZeneca, and Novartis.
Dr. David Krug: Merck Sharp & Dome.
Prof. Dr. med. Michael Friedrich: Roche, Pfizer, AstraZeneca, and Novartis.
Prof. Dr.med. Wolfgang Janni: Research grants and/or honoraria from: Sanofi-Aventis, Novartis, Roche, Pfizer, AstraZeneca, Chugai, GSK, Eisai, Cellgene, Lilly, Janssen, and Menarini.
Prof. Dr. med. Volkmar Müller: Speaker honoraria from Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, Pfizer, MSD, Novartis, Roche, Teva, and Seattle Genetics; consultancy honoraria from Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, Tesaro, and Nektar; institutional research support from Novartis, Roche, Seattle Genetics, and Genentech.
Prof. Dr. med. Ute-Susann Albert: Lectures and/or consulting: Medexo GmbH, Institut für Versicherungsmedizin (IMV), and Pfizer.
PD Dr. Malgorzata Banys-Paluchowski: Pfizer, Roche, Novartis, and Eli Lilly.
Dr. med. Ingo Bauerfeind: Aurikamed and J. Eickeler.
Prof. Dr. med. Jens-Uwe Blohmer: Honoraria: Amgen, AstraZeneca, Genomic Health Recipient, MSD Oncology, Myriad Genetics, Novartis, Pfizer, Roche, and Sonoscape; travel, accommodations, expenses: Pfizer and Roche.
Prof. Dr. med. Wilfried Budach: No conflicts of interest.
Prof. Dr. med. Peter Dall: Advisory boards: Olympus, Roche, Novartis, and Tesaro; congress support: Roche; lecture fees: Amgen and Roche.
Prof. Dr. med. Ingo Diel: No conflicts of interest regarding this manuscript.
PD Dr. med. Eva Maria Fallenberg: GE-Healthcare, Siemens, ECR, EUSOBI, ESOR, KCR, and DFG.
Prof. Dr. med. Peter A. Fasching: Grants from Novartis, Biontech, and Cepheid; personal fees from Novartis, Roche, Pfizer, Celgene, Daiichi-Sankyo, AstraZeneca, Merck Sharp & Dohme, Myelo Therapeutics, Macrogenics, Eisai, Puma, and Lilly.
Prof. Dr. med. Tanja Fehm: AstraZeneca, Celegene, Pfizer, Novartis, Roche, Teva, and Daichii Sankyo.
Prof. Dr. med. Bernd Gerber: No conflicts of interest.
PD Dr. med. Oleg Gluz: Honoraria for lectures and/or consulting: Celgene, Roche, Genomic Health, Amgen, Pfizer, Novartis, Lilly, Nanostring, Eisai, and MSD; travel grants: Celgene, Roche, and Daiichi Sankyo.
Prof. Dr. Volker Hanf: No conflicts of interest regarding this manuscript.
Prof. Dr. med. Nadia Harbeck: Honoraria for lectures and/or consulting: Agendia, Amgen, AstraZeneca, BMS, Celgene, Daiichi-Sankyo, Genomic Health, Lilly, MSD, Novartis, Odonate, Pierre Fabre, Pfizer, Roche, Sandoz/Hexal, and Seattle Genetics; Minority shareholder: Westdeutsche Studiengruppe (WSG).
Prof. Dr. med. Jörg Heil: No conflicts of interest.
Prof. Dr. med. Jens Huober: Research grants: Celgene, Novartis, and Hexal; lecture honoraria: Lilly, Novartis, Roche, Pfizer, AstraZeneca, MSD, Celgene, Eisai, and Abbvie; consulting: Lilly, Novartis, Roche, Pfizer, Hexal, AstraZeneca, MSD, Celgene, and Abbvie; travel expenses: Roche, Pfizer, Novartis, Celgene, and Daiichi.
Herr Prof. Dr. med. habil. Hans H. Kreipe: Reimbursement for attending symposia: Ventana; other expenses (advisory boards, lectures): AMGEN, AstraZeneca, Genomic Health, Lilly, and Roche Pharma.
Prof. Dr.med. Thorsten Kühn: Celgene, Roche, and Pfizer.
Prof. Dr. med. Sherko Kümmel: Roche, Genentech, Novartis, AstraZeneca, Amgen, Celegene, SOMATEX, Daiichi Sankyo, Puma Biotechnologx, pfm medical, Pfizer, and MSD Oncology.
Prof. Dr. med. Sibylle Loibl: Abbvie, Amgen, AstraZeneca, Celgene, Novartis, Pfizer, Roche, Seattle Genetics, PriME/Medscape, Chugai, Teva, Vifor, Daiichi-Sankyo, Lilly, Samsung, Eirgenix, BMS, Puma, MSD, and Immunomedics.
Prof. Dr. med. Diana Lüftner: Advisory boards/oral presentations: Amgen, AstraZeneca, Celegene, Lilly, Loreal, MSD, Mundipharma, Mylan, Novartis, Pfizer, Roche, Teva, and Tesaro.
Prof. Dr. med. Michael Lux: Lilly, Pfizer, Roche, MSD, Hexal, Novartis, AstraZeneca, Eisai, Medac, and Genomic Health for advisory boards, lectures, and travel support.
Prof. Dr. med. Nicolai Maass: No conflicts of interest.
Prof. Dr. med. Volker Moebus: Amgen, Celegene, Roche, Myelotherapeutics, Roche, and Amgen.
Prof. Dr. med. Christoph Mundhenke: Not specified.
Prof. Dr. Tjoung-Won Park-Simon: Advisory role or expert testimony and lecture honoraria: Roche, AstraZeneca, Tesaro, Pfizer, Daichii, Lilly, and MSD; participation in clinical trials: Roche, AstraZeneca, Tesaro, Pfizer Daichii, Lilly, MSD, Novartis, and Seattle Genetics; other financial relationships, e.g., travel: Roche, AstraZeneca, Tesaro, Pfizer, Lilly, MSD, and Novartis.
Prof. Dr. med. Toralf Reimer: grants from German Cancer Aid and Else Kroener-Fresenius-Stiftung; personal fees from Pfizer, Roche, Novartis, and AstraZeneca, outside the submitted work.
PD. Dr. med. Kerstin Rhiem: AstraZeneca, Tesaro, Pfizer, and Roche.
Prof. Dr. med. Achim Rody: Roche, Pfizer, Novartis, Celgen, Novartis, Genomic Health/Exact Sciences, AstraZeneca, Eisai, MSD, Hexal, and Amgen.
Prof. Dr. med. Markus Schmidt: Honoraria: AstraZeneca, Novartis, Pfizer, and Roche; consulting or advisory role: AMGEN, AstraZeneca, BioNTech, Eisai, Lilly, Myelo Therapeutics, Novartis, Pantarhei Bioscience, Pfizer, and Roche; travel, accommodations, expenses: BioNTech, Pantarhei Bioscience, Pfizer, and Roche; patents, royalties, other intellectual property: Patent issued EP2951317.
Prof. Dr. med. Andreas Schneeweiss: Grants: Celgene, Roche, Abbvie, and Molecular Partner; personal fees: Roche, AstraZeneca, Celgene, Pfizer, Novartis, MSD, Tesaro, and Lilly.
Prof. Dr. med. Christine Solbach: Travel, accommodations: Roche, Novartis, AstraZeneca, Amgen, Celgene, Hexal, Daiichi Sankyo, Dialog Service GmbH, Lilly, Pfizer, MSD Oncology, ESAI, Gedon Richter, Mylan, and Tesaro.
Prof. Dr. med. Erich-Franz Solomayer: Roche, AstraZeneca, Pfizer Roche, Amgen, Celgen, Tesaro, AstraZeneca, Pfizer, Storz, Erbe, Gedeon Richter, Eisai, Medac, MSD, Vifor, Teva, and Ethikon.
Prof. Dr. med. Elmar Stickeler: Consulting: Novartis, Pfizer, AstraZeneca, Roche, and Tesoro; honoraria: Novartis, Pfizer, AstraZeneca, and Roche.
Prof. Dr. med. Christoph Thomssen: Advisory boards, lectures: Amgen, AstraZeneca, Celgen, Daiichi-Sankyo, Eisai, Lilly, MSD, Mundipharma, Medapharm, Novartis, Pfizer, Pierre-Fabre, Roche, Tesaro, and Vifor.
PD Dr. Isabell Witzel: Not specified.
Prof. Dr. med. Achim Wöckel: Amgen, AstraZeneca, Aurikamed, Celgene, Eisai, Lilly, Novartis, Pfizer, Roche, and Tesaro.
PD. Dr. med. Marc Thill: Advisory board: Amgen, AstraZeneca, Celgene, ClearCut, Clovis, Daiichi Sankyo, Exact Sciences, Lilly, MSD, Neodynamics, Novartis, Pfizer, pfm Medical, Pierre-Fabre, Roche, and Tesaro; manuscript support: Amgen, Celgene, and Roche; travel expenses: Amgen, Art Tempi, AstraZeneca, Celgene, Clovis, Connect Medica, Daiichi Sankyo, Exact Sciences, Hexal, I-Med-Institute, Lilly, MCI, MSD, Novartis, Pfizer, pfm Medical, Roche, and Tesaro; congress costs: Amgen, AstraZeneca, Celgene, Daiichi Sanyko, Hexal, Novartis, Pfizer, and Roche; lectures: Amgen, Art Tempi, AstraZeneca, Celgene, Clovis, Connect Medica, Exact Sciences, Hexal, I-Med-Institute, Lilly, MCI, MSD, Novartis, OnkoLive, Pfizer, pfm Medical, and Roche; trial funding: Exact Sciences.
Supplementary Material
Supplementary data
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data