Abstract
Pathogenic microorganisms are sensed by the inflammasome, resulting in the release of the pro-immune and proinflammatory cytokine interleukin-1β (IL-1β). In humans, the paired <underline>s</underline>ialic acid-binding Ig-like lectin receptors Siglec-5 (inhibitory) and Siglec-14 (activating) have been shown to have reciprocal roles in regulating macrophage immune responses, but their interaction with IL-1β signaling and the inflammasome has not been characterized. Here we show that in response to known inflammasome activators (ATP, nigericin) or the sialic acid-expressing human bacterial pathogen group B Streptococcus (GBS), the presence of Siglec-14 enhances, whereas Siglec-5 reduces, inflammasome activation and macrophage IL-1β release. Human THP-1 macrophages stably transfected with Siglec-14 exhibited increased caspase-1 activation, IL-1β release and pyroptosis after GBS infection, in a manner blocked by a specific inhibitor of nucleotide-binding domain leucine-rich repeat protein 3 (NLRP3), a protein involved in inflammasome assembly. Another leading pathogen, Streptococcus pneumoniae, lacks sialic acid but rather prominently expresses a sialidase, which cleaves sialic acid from macrophages, eliminating cis- interactions with the lectin receptor, thus attenuating Siglec-14 induced IL-1β secretion. Vimentin, a cytoskeletal protein released during macrophage inflammatory activation is known to induce the inflammasome. We found that vimentin has increased interaction with Siglec-14 compared to Siglec-5, and this interaction heightened IL-1β production by Siglec-14-expressing cells. Siglec-14 is absent from some humans because of a SIGLEC5/14 fusion polymorphism, and we found increased IL-1β expression in primary macrophages from SIGLEC14+/+ individuals compared to those with the SIGLEC14–/+ and SIGLEC14–/– genotypes. Collectively, our results identify a new immunoregulatory role of Siglec-14 as a positive regulator of NLRP3 inflammasome activation.
Keywords: Siglec, Interleukin-1β, Caspase-1, Inflammasome, Macrophages, Innate immunity, Vimentin
Introduction
Pathogenic and beneficial microbes establish intimate interactions with host immune cells to manipulate immune responses and resist immune clearance [1, 2]. To detect and respond to microbial invasion, host cells express a range of pattern recognition receptors that recognize specific pathogen-associated molecular patterns to alert the broader immune system [1]. Several pathogen-associated molecular patterns are glycoconjugates expressed on the microbial cell surface or secreted into the environment [3]. Conversely, immune cells recognize common glycan “self-associated molecular patterns” ubiquitous in the host (e.g., sialic acid, hyaluronic acid) with inhibitory receptor signaling to dampen immune responses during homeostatic conditions [4]. Consequently, some microbes have evolved molecular mimicry of host glycan structures to subvert these immunoregulatory pathways and promote their survival within the host [5, 6].
CD33-related sialic acid-binding immunoglobulin-like lectins (Siglecs) are a family of lectins having characteristic amino acid sequences for recognition of sialic acid-containing glycans on immune cells [7]. Siglecs play important roles in regulating immune responses, in host-pathogen interactions, and in tumorigenesis [8, 9]. To date, 15 human Siglecs have been identified and sequenced, 10 of which are inhibitory receptors containing immunoreceptor tyrosine-based inhibition motif (ITIM) or ITIM-like domain(s) in their intracellular signaling domains [7, 10]. Upon engagement of sialic acid-containing ligands, the cytoplasmic tyrosine phosphatases SHP1/2 are recruited to the ITIM domain to deliver inhibitory signal(s) that modulate and counteract immune responses [7, 8, 9]. Some microbes, for example, the prominent human neonatal pathogen group B Streptococcus(GBS), manipulate host immune cells through sialic acid mimicry by engaging inhibitory Siglecs to promote bacterial immune evasion [5, 11, 12]. Perhaps in response to the selective pressures exerted by such pathogen subversion, some Siglecs with activating signaling potential have evolved, for example, human Siglecs-14 and 16, in which the ITIM/ITIM-like intracellular domains are replaced with an immunoreceptor tyrosine-based activation motif [13] that recruits the activating adapter protein DAP12 [14, 15].
The human SIGLEC14 (activating) and SIGLEC5 (inhibitory) genes are located near one another on chromosome 19q13.41 and possess partial gene conversions leading Siglec-14 to share high sequence similarity and glycan-binding preference with Siglec-5 [14]. Interestingly, in some human populations, SIGLEC14 and SIGLEC5 are undergoing unequal crossover within these highly homologous regions to generate a new fusion gene product, SIGLEC14/5. This fusion gene is almost identical to SIGLEC5 encoding the inhibitor ITIM/ITIM-like domains, but is expressed under control of the SIGLEC14 promoter, resulting in the functional deletion of SIGLEC14 [16]. In the human population, individuals either express intact SIGLEC5 and SIGLEC14 (denoted here Sig14+/+), have one of their alleles replaced by SIGLEC14/5 fusion (heterozygous Sig14+/−), or have both alleles replaced by SIGLEC14/5 (homozygous Sig14−/−) [16].
GBS and other sialic acid-expressing bacterial pathogens (e.g., Neisseria meningitidis, Campylobacter jejuni, Haemophilus influenzae) can engage various human Siglecs (e.g., Siglec-1, −5, −9) on leukocytes to impact phagocytosis, microbicidal activities and inflammatory responses [5, 17, 18, 19, 20]. In addition to sialylated ligands, certain strains of GBS can engage paired receptors Siglec-14 and −5 through a non-sialic acid-dependent ligand, the β-protein [21, 22]. By β-protein-mediated engagement of inhibitory Siglec-5, GBS can suppress immune responses in neutrophils and monocytes [21], and this suppression can be counteracted by activating Siglec-14 [22]. Recently, heat shock protein 70 (HSP70), which can be secreted from cells during inflammatory responses, was identified as a host-derived sialic acid-independent ligand of Siglec-5 and −14. Whereas HSP70 binding to Siglec-5 delivered anti-inflammatory signals, engagement of HSP70 with Siglec-14 delivered counter-regulatory proinflammatory signals [23].
A major current paradigm in the innate immune system response to invading pathogens or cellular damage involves the function of inflammasomes – intracellular oligomeric assemblies containing a nucleotide-binding domain leucine-rich repeat protein (NLRP), the adaptor molecule apoptosis-associated speck-like protein containing a CARD domain (ASC), and the protease caspase-1 that work together to promote the maturation of the critical pro-immune and proinflammatory cytokine interleukin-1β (IL-1β) as well as IL-18 [24, 25]. To date, the interaction between immunoregulatory Siglecs and IL-1β/inflammasome signaling has not been characterized. In this work, we identified an important role of Siglec-14 in modulating NLRP3-inflammasome activation, in a manner influenced by sialic acid-dependent and non-sialic acid-dependent interactions with pathogens and host ligands.
Materials and Methods
Bacterial Strains and Mutants
Wild-type (WT) GBS serotype Ia strain A909 (GBS-WT) and its isogenic β-hemolysin/cytolysin (β-h/c) knockout mutant (GBSΔcylE) [26], Pseudomonas aeruginosa strain PAK (PA-WT) and its corresponding flagellin structural gene fliC knockout mutant (PAΔfliC) [27], Bacillus anthracis Sterne 7702 (BA-WT) and its isogenic lethal factor knockout mutant (BAΔLF) [28], and Streptococcus pneumoniaestrain D39 (SPN-WT) and its corresponding neuraminidase (sialidase) knockout mutant (SPNΔNanA) [29] have been described previously.
Antibodies and Immunological Reagents
Immunoprecipitation was performed with anti-Siglec5/14 antibody (MAB10721, R&D Systems, Minneapolis, MN, USA). Immunoblot analyses were performed with antibodies recognizing IL-1β and NLRP3 (12703 and 13158, Cell Signaling, Danvers, MA, USA), ASC (676502, Biolegend, San Diego, CA, USA), pro-caspase-1 + p10 (ab179515, Abcam, Cambridge, MA, USA), and β-actin (GTX109639, GeneTex, Irvine, CA, USA).
Cell Culture and Differentiation
For GBS exposure experiments, the human monocytic cell line THP1 expressing-Siglec-5 (THP1-Sig5), Siglec-14 (THP1-Sig14) and empty vector (THP1-EV) cells [16] were stimulated and differentiated with 50 ng/mL of phorbol 12-myristate 13-acetate in RPMI 1,640 medium containing 10% heat-inactivated fetal bovine serum (HI-FBS) + 0.05 mM 2-β-mercaptoethanol for 72 h. To test the effect of different inflammasome ligands, THP1-EV and Siglec cells were differentiated in the presence of phorbol 12-myristate 13-acetate and 10 µg/mL LPS (Sigma) for 18 h. Cells were stimulated for 2 h with 0.5 µg/mL Flagellin (AdipoGen) or 10 µg/mL Poly(dA:dT) (InvivoGen) resuspended in TransFast transfection reagent (Promega). Primary human monocyte-derived macrophages (MDM) from 5 to 7 different donors per SIGLEC14/15 genotype were used in this study. The mononuclear cell fraction was isolated from whole blood using PolymorphprepTM solution (Axis-Shield, Olso, Norway) as previously described [21]. Cells were then washed and cultured in RPMI 1640 + 10% HI-FBS. After 2–3 h of incubation, the culture was mildly washed and cells in suspension were removed. Attached MDMs were treated with 50 ng/mL of GM-CSF in complete RPMI 1640 medium for 7 days prior to study.
Cytokine Analysis and Cytotoxicity Measurement
Cytokine levels in cell culture supernatants were quantified using DuoSet IL-1β, IL-6, and tumor necrosis factor-α ELISA kits (R&D Systems, Minneapolis, MN, USA). Caspase-1 activity levels in culture supernatants were assayed using the Caspase-1 colorimetric kit (BioVision, Milpitas, CA, USA). Cytotoxicity was assessed by measuring lactate dehydrogenase (LDH) release from the cells using a CytoTox 96 Nonradioactive Cytotoxicity assay (Promega, Madison, WI, USA).
Detection of Cell Surface Siglec-5/14
Surface Siglec-5/14 staining was performed by seeding and differentiating 2 × 106 THP-1 cells in triplicate overnight. Cells were dislodged by incubation in cold 10 mM EDTA/phosphate-buffered saline (PBS) and gentle scraping using a rubber policeman, washed once with 1% heat-inactivated FBS (HI-FBS)/PBS, then resuspended in 200 µL of APC-conjugated anti-human CD170/Siglec-5 (352006, Clone 1A5, Biolegend) or APC-conjugated mouse IgG1, κ isotype control (400122, Clone: MOPC-21, Biolegend) mixture using 1 µg of antibody per 200 µL final volume of 1% HI-FBS/PBS. Staining was performed for 1 h at 4°C, followed by 2 washes and a final resuspension in 1% HI-FBS/PBS. Surface expression of Siglec-5/14 was measured using flow cytometry (FACSCalibur, BD Biosciences) and analyzed using FlowJo version 10.2 software (FlowJo LLC, Ashland, OR, USA). The mean fluorescence intensity was calculated on cells gated as THP-1 cells.
Siglec-5/14 Protein and mRNA Analysis
Soluble Siglec-5/14 (sSiglec-5/14) levels in cell supernatants were quantified using a Human Siglec-5/Siglec-14 DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA). Quantitative polymerase chain reaction (qPCR) analysis of Siglec mRNA expression was performed by extracting RNA from 2 × 106 cells using the RNeasy Plus Mini Kit (Qiagen) and DNase I treatment (Invitrogen). cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad), and qPCR was performed using the KAPA SYBR FAST qPCR Kit (Bio-Rad). Human GAPDH was used as a housekeeping gene control. qPCR primer sets were as follows: GAPDH Fwd: 5′-ACAACTTTGGTATCGTGGAAGG-3′, GAPDH Rev: 5′-GCCATCACGCCACAGTTTC-3′; Siglec-14 Fwd: 5′-CCTCTTCCTGCATATGTGTAACT-3′, Siglec-14 Rev: 5′-CCTCCACACCTGGTATAGTAGA-3′; Siglec-5 Fwd: 5′-CCCTCCCTTGGAAGAACAAA-3′, Siglec-5 Rev: 5′-TTAGGCTCCCTCGACTTCA-3′.
Human Macrophage Infection with GBS
GBS was propagated in Todd-Hewitt broth (Oxoid, Basingstoke, UK) at 37°C without shaking, washed and resuspended in RMPI 1640, and added to 4 × 104 macrophages at the indicated multiplicity of infection (MOI). Bacteria and macrophages were spun together at 1,600 rpm for 5 min to initiate the assay, gentamicin (100 µg/mL) was added 1 h after infection to suppress bacterial overgrowth, and bacteria and macrophages were co-incubated for an additional 16 h. When 2 × 106 THP-1 cells were needed for larger-scale experiments, GBS bacteria were similarly added at an MOI = 10 bacteria/cell. P. aeruginosa strains were cultured in Luria-Bertani broth overnight at 37°C with shaking, diluted 1:200 (v/v), and grown for 2 h. B. anthracis strains were grown in brain heart infusion broth for 12 h at 37°C with shaking, diluted 1:200 (v/v), and grown for 4 h. SPN strains were grown overnight on blood agar plates at 37°C and 5% CO2. A colony was then used to inoculate Todd-Hewitt broth supplemented with 0.5% yeast extract [30] and grown for 6 h.
Vimentin Binding Analysis
High-protein binding plates (Costar) were coated with 10 µg/mL of sialidase-treated Siglec-Fc proteins and allowed to bind overnight. Plates were washed 3 times with PBS + 0.05% Tween-20, blocked with 2% bovine serum albumin (BSA)/PBS for 1 h, washed 3 more times with PBS + 0.05% Tween-20, and then recombinant vimentin (Peprotech) resuspended in 2% BSA/PBS was added at varying concentrations and allowed to bind for 2 h. Afterwards, the plate was washed 3 times with PBS + 0.05% Tween-20. Vimentin binding was detected with a 1:500 dilution of anti-vimentin-HRP conjugated antibody (sc-6260 HRP, V9, Santa Cruz Biotechnology) allowed to bind for 2 h. The plate was washed 3 times with PBS + 0.05% Tween-20, and then developed with TMB substrate reagent for 20 min. Afterwards, the reaction was stopped with 2N H2SO4
SIGLEC5/14 Genotypic and Analysis of Cytokine Secretion
Genomic DNA was extracted from mononuclear cells of healthy donors using the DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD, USA). Primers used for specific amplification of SIGLEC14, SIGLEC5, and SIGLEC14/5 fusion genes and PCR conditions were described previously [16]. Cell culture supernatants were harvested for ELISA to determine the amount of IL-1β and IL-6 released using ELISA kits.
Protein Isolation and Immunoblot Analysis
Cell lysates of the 3 cell lines were prepared and separated by SDS-PAGE, transferred to PVDF membranes (EMD Millipore), and probed with anti-Capsase-1, IL-1β, ASC, and NLRP3 antibodies.
Statistical Analysis
Statistical significance was analyzed by using the paired t test for THP-1 cell experiments or an unpaired ttest for human sample experiments. Data are expressed as mean ± SD. Significance of p values was classified as follows: * p< 0.05, ** p< 0.005, *** p< 0.001.
Results
Expression of Siglec-14 on Macrophages Increases IL-1β Secretion through NLRP3 Inflammasome Activation
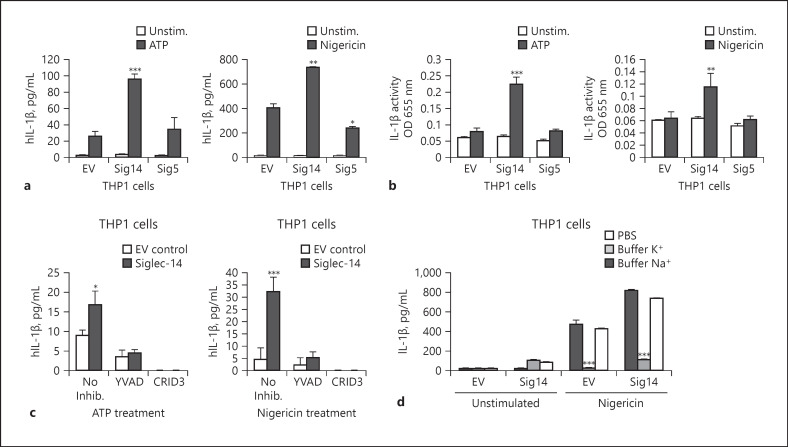
The paired receptors Siglec-14 and −5 modulate innate immune responses in human monocytes and neutrophils through interaction with microbial or host-expressed sialic acid or binding proteins [20, 21, 22, 23, 31]. However, the effect of Siglec-14/5 regulation on inflammasome activation triggered by bacterial infection [25] remains unexplored. To test this hypothesis, THP-1 cells (a human monocyte-like cell line) were stably transfected with Siglec-14 (THP1-Sig14), Siglec-5 (THP1-Sig5) or EV control (THP1-EV) [16] and cell surface expression of Siglec-14 and −5 confirmed by flow cytometry FACS (online suppl. Fig. S1a; for all online suppl. material, see www.karger.com/10.1159/000504323). When the macrophage lines were stimulated with the known inflammasome activators adenosine 5′-triphosphate (ATP) or nigericin, ELISA detected significantly increased IL-1β secretion from the THP-Sig14 macrophages compared to the EV control or THP1-Sig5 cell lines (Fig. 1a). Since Siglec-14 can interact with toll-like receptors 2 and lead to increased cytokine production [32], we also tested for the differential release of other cytokines. However, tumor necrosis factor-α or IL-6 cytokine release was not detected after THP1-Sig14 or THP1-Sig5 cell lines were stimulated with ATP or nigericin (online suppl. Fig. S1b). Therefore, under our experimental conditions we do not observe a general priming effect due to Siglec-14 or −5 exogenous expression; rather, we observed a specific effect on modulation of IL-1β cytokine production by THP1-Sig14 and THP1-Sig5 cells upon stimulation with NLRP3 inflammasome activators.
Fig. 1.
Expression of Siglec-14 on macrophages increases IL-1β secretion through NLRP3 inflammasome activation. a THP1-EV, THP1-Sig14 and THP1-Sig5 cells were treated with 25 nM of phorbol-12-myristate-13-acetate for 48 h and stimulated with 5 mM of ATP or 10 μM of nigericin for 30 min then incubated for additional 2 h; secreted IL-1β in supernatants was determined by ELISA. b The amount of secreted mature IL-1β in the supernatants of a were determined using IL-1β sensor cells (Invivogen) and optical density (OD) measurements at 655 nm. c Cells were treated with caspase-1 inhibitor Ac-YVAD-CMK (YVAD), NLRP3 inhibitor CRID3 sodium salt (CRID3) or mock control for 1 h and stimulated with ATP or nigericin for an additional 2 h followed by ELISA to determine the secreted IL-1β level in supernatants. d Cells were treated with PBS, K+ buffer or Na+ buffer for 2 h and exposed to nigericin or buffer control for an additional 2 h. Culture supernatants were used to determine secreted IL-1β levels by ELISA. Error bars represent the SD of data means from 3 independent experiments. Significance of p values was classified as follows: * p < 0.05, ** p< 0.005, *** p < 0.001. EV, empty vector; IL-1β, interleukin-1β; unstim., unstimulated; inhib, inhibition.
As the soluble form of Siglec-14 can be released by myeloid cells, and recombinant Siglec-14 can compete with membrane-localized Siglec-14 to suppress cytokine release [32]. Therefore, differences in Siglec surface expression could potentially lead to altered release of sSiglecs and effects on IL-1β production. We investigated how ATP or nigericin treatment affected the surface expression of Siglec-5/14 on the THP-1 Siglec overexpressing cells and THP-1 cells. Due to the high similarity between the extracellular regions of Siglec-5 and −14, the anti-Siglec-5 antibody utilized recognizes both Siglec-5 and −14 [14]. A small but statistically significant decrease in surface Siglec-5/14 expression was detected upon THP1-Sig14 and THP1-Sig5 treatment with ATP and upon EV and THP1-Sig14 cell line treatment with nigericin (online suppl. Fig. S2a). Neither ATP nor nigericin affected the levels of surface Siglec-5/14 on THP-1 cells (online suppl. Fig. S2b). Using a Siglec-5/14 ELISA, sSiglec-5/14 was not detected in cell supernatants collected after THP-1 or THP-1 Siglec cell stimulation with ATP or nigericin (online suppl. Fig. S2c). Together, these results indicate that the differential IL-1β production observed in the THP-1 and THP1-Sig14 and -Sig5 cells in response to ATP and nigericin was not affected by differences in release of sSiglec-5/14.
To further explore the interaction between Siglec-14 and NLRP3 inflammasome activation, a bioactive IL-1β reporter assay coupled to IL-1 receptor 1 activation was utilized. We confirmed that the IL-1β detected in the culture supernatant of the THP1-Sig14 macrophages after ATP or nigericin stimulation was in the form of the mature, signaling-competent cytokine (Fig. 1b). Enhanced IL-1β release seen in THP1-Sig14 macrophages in response to ATP or nigericin was mediated through NLRP3 inflammasome activation, since specific inhibitors of either caspase-1 (Ac-YVAD) or NLRP3 (CRID3 sodium salt) blocked the stimulatory effect of Siglec-14 on release of the cytokine (Fig. 1c). Furthermore, potassium efflux is required for NLRP3 inflammasome activation [33]. Replacement of the culture medium with high potassium buffer to collapse the required efflux gradient blocked the effect of Siglec-14 enhancement of NLRP3-inflammasome activation and IL-1β release (Fig. 1d). To test whether Siglec-14 had a specific effect to activate NLRP3 inflammasomes or a broader role in activating multiple inflammasome complexes, we tested different inflammasome inducers. Treatment of cells with LPS+ dsDNA poly(dA:dT) or with LPS+ flagellin (FLiC), which activate AIM2 and NLRC4 inflammasomes, respectively, did not increase bioactive IL-1β production by Siglec-14 cells (online suppl. Fig. S3). Together these results indicate that Siglec-14 promotes a proinflammatory effect via specific activation of NLRP3 inflammasomes.
Siglec-14 Boosts the Macrophage NLRP3 Inflammasome Response to GBS
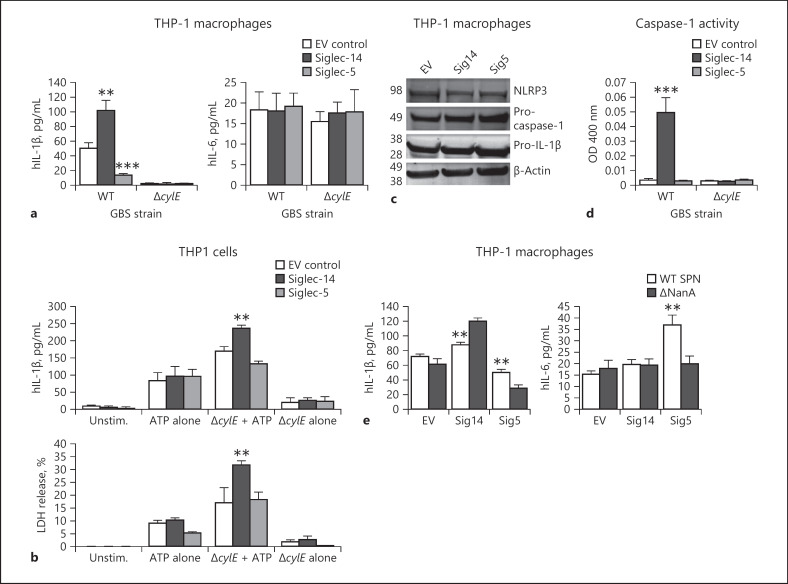
The important Gram-positive human bacterial pathogen GBS produces a pore-forming β-h/c protein that damages macrophage cell membranes [34] and induces NLRP3-inflammasome activation [35]. Siglec-14 and −5 modulate neutrophil and monocyte phagocytic and bactericidal responses to GBS [21, 22], but their linkage to inflammasome/IL-1β signaling had not been investigated. WT and β-h/c-deficient (ΔcylE) GBS were used to challenge THP1-EV, THP1-Sig14 and THP1-Sig5 macrophages at MOI = 10 bacteria:host cell, followed by measurement of IL-1β secretion. In a β-h/c-dependent manner, THP-1 macrophage release of IL-1β in response to GBS infection was increased by the presence of activating Siglec-14 and decreased by the presence of inhibitory Siglec-5 (Fig. 2a); no effect was observed for either Siglec on GBS-induced macrophage release of the inflammasome-independent proinflammatory cytokine IL-6 (Fig. 2a). Commonly, inflammasome-associated cell death or “pyroptosis” is quantified by release of LDH [25, 36], however in the case of GBS the pore-forming cytolytic activity of the β-h/c toxin itself rapidly induces LDH release [34], confounding simple interpretation. Therefore, we used ATP as a supplemental inflammasome stimulus together with the noncytolytic GBS ΔcylE mutant, and we found that expression of Siglec-14 resulted in increased macrophage IL-1β secretion (inflammasome activation) and LDH release (pyroptosis) compared to the EV or Siglec-5-expressing THP-1 macrophage lines (Fig. 2b).
Fig. 2.
Siglec-14 boosts the macrophage NLRP3 inflammasome response to GBS.a THP1-EV, THP1-Sig14 and THP1-Sig5 cells were treated with GBS WT and ΔcylE mutant at MOI = 10 bacteria/cell and incubated for 16 h; IL-1β and IL-6 release in supernatants were measured by ELISA. b THP1-EV, THP1-Sig14, and THP1-Sig5 cells were treated with the non-β-protein-expressing GBS ΔcylE mutant A9O9 for 1 h, then exposed to ATP or buffer control for 30 min. IL-1β release in supernatants was assessed by ELISA. LDH release was also assessed. c Western immunoblot analysis of NLRP3, pro-caspase-1, pro-IL-1β, and β-actin (loading control) expression in THP1-EV, THP1‑Sig14 and THP1-Sig5 cells. d Caspase-1 activity in supernatants of GBS-infected THP1-EV, THP1-Sig14, and THP1-Sig5 cells was assessed by colorimetric assay and optical density (OD) measurements at 400 nm. e THP1-EV, THP1-Sig14, and THP1-Sig5 cells were treated with SPNWTand sialidase deficient ΔNanA mutant at MOI = 10 bacteria/cell and incubated for 16 h. IL-1β and IL-6 release in the supernatant was assessed. Error bars represent the SD of data means from 3 independent experiments. ** p < 0.005, *** p < 0.001. IL-1β, interleukin-1β; GBS, group B Streptococcus; WT, wild-type; LDH, lactate dehydrogenase; EV, empty vector; NLRP3, nucleotide-binding domain leucine-rich repeat protein 3; unstim., unstimulated; SPN, Streptococcus pneumoniae
We previously observed that differential expression of Siglec-14 can affect Siglec-5 and Siglec-14 expression in neutrophils after LPS stimulation [22]. Thus, we investigated if exposure of THP-1 cells to GBS WT and to the GBS ΔcylEmutant affected Siglec expression. A small but significant decrease in the levels of surface Siglec-5/14 was observed on THP-1 cells exposed to GBS WT and to the ΔcylEmutant compared to media control (online suppl. Fig. S4a). At the mRNA level, exposure to GBS WT or the ΔcylEmutant did not affect Siglec-14 expression levels (online suppl. Fig. S4b). However, there was a statistically significant decrease in Siglec-5 expression upon GBS WT exposure compared to media controls (online suppl. Fig. S4c). This finding is similar to our previous report that Siglec-5 expression is decreased in neutrophils isolated from Sig14+/+ individuals when stimulated with LPS [22]. Future work is needed to determine whether there is a potential loss of Siglec-14 expressing cells due to induction of pyroptosis by GBS, leading to lysis and decreased detection of Siglec-14 expressing cells, or whether the decreased mRNA expression of Siglec-5 is causing the observed reduction of surface Siglec-5/14. Despite the differences in surface Siglec-5/14 expression, no differences in the release of sSiglec-5/14 were detected between untreated THP-1 cells versus those exposed to GBS WT or the GBS ΔcylEmutant. Therefore, release of sSiglec-5 or 14 is not predicted to differentially affect the IL-1β response observed after THP-1 exposure to GBS bacteria.
Microbial inflammasome activation is understood to require 2 signals: first, induction of transcription and translation of pro-IL-1β through NF-κB-dependent pattern-recognition systems such as toll-like receptors or dectin-1/2 receptors; second, inflammasome assembly for cleavage of pro-IL-1β into the active form in response to activators such as ATP and pore-forming toxins [37, 38]. In response to GBS WT infection, pro-IL-1β transcription was not elevated, but rather modestly reduced, in THP1-Sig14 and THP1-Sig5 macrophages compared to THP1-EV control cells (online suppl. Fig. S4e), and protein levels of NLRP3, pro-caspase-1 and pro-IL-1β were similar in the 3 cell lines (Fig. 2c). However, Siglec-14 expression triggered a marked increase in caspase-1 activity in the GBS-infected THP-1 cells (Fig. 2d). Together, these results show that Siglec-14 increases caspase-1 activation in response to GBS infection of macrophages, working at the level of signal two rather than signal one.
Siglec-14 and −5 exhibit similar glycan binding preference for sialic acid [14]. Comparing binding by WT and sialic-acid binding mutant (R119A) Fc chimeras of Siglec-5 and Siglec-14, we determined in both cases the presence of sialic acid-dependent and sialic acid-independent binding to cis-ligands expressed on THP-1 macrophages (online suppl. Fig. S5a). Another leading human streptococcal pathogen, SPN, expresses a potent sialidase (NanA) that removes sialic acid from cell surfaces. Compared to isogenic ΔNanA mutant bacteria, infection with WT SPN causing desialylation elicited less secretion of IL-1β from THP1-Sig14 macrophages. Conversely, WT SPN triggered increased secretion of IL-1β from THP1-Sig5 macrophages compared to infection with the ΔNanA mutant strain (Fig. 2e). A previous study demonstrated SPN sialidase expression reversed Siglec-5-mediated inhibition of NF-κB signaling and proinflammatory cytokine release [39]. We confirmed that in the case of NF-κB related proinflammatory cytokine IL-6, THP1-Sig5 macrophages showed increased secretion of IL-6 in WT vs. ΔNanA SPN infection; however, no effect was seen on IL-6 release from THP1-Sig14 cells. These results show Siglec-14 regulation of inflammasome activation is sialic acid-dependent, but the influence of Siglec-14 on the NF-κB signaling pathway may not be as strong as that of the inhibitory Siglec-5 in macrophages.
Vimentin can Engage Siglec-14 to Increase Inflammasome Activation
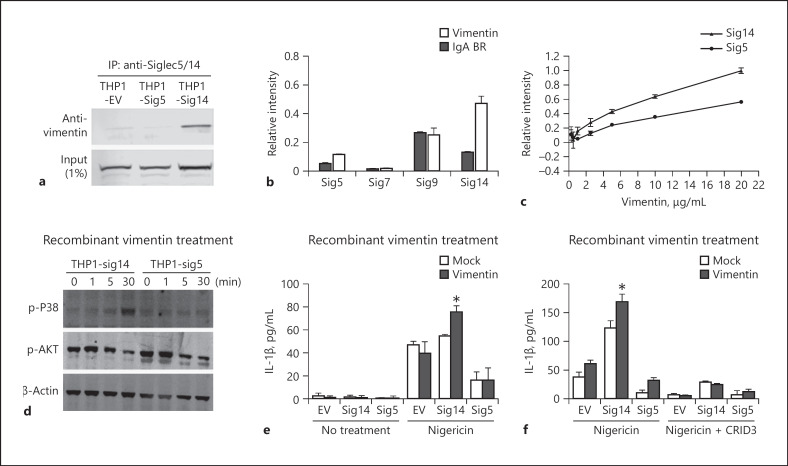
Our previous studies found that not only did bacterial GBS β-protein functionally engage Siglec-14 and −5 [22], but also HSP70, a non-sialic acid containing intracellular chaperone protein that can be released from monocytes, interacted with Siglec-14 and −5 to modulate monocyte immune responses [23]. To investigate the ability of additional ligands to bind Siglec-14 and −5 in macrophages, cell extracts of THP1-Sig14, THP1-Sig5 and THP1-EV macrophages were collected and immunoprecipitated using an anti-Siglec-5/14 antibody. Interestingly, vimentin, a cytoskeleton protein which can be secreted from activated macrophages [40], was pulled down in THP1-Sig14 immunoprecipitations, and this interaction was specific to Siglec-14, as vimentin was not found to interact with Siglec-5 or pulled down in the THP1-EV control (Fig. 3a). Indeed, Siglec-14-Fc chimera bound much more strongly to recombinant vimentin than Fc chimeras of Siglec-5, −7, or −9 (Fig. 3b). A dose-response study testing vimentin binding by Siglec-14-Fc vs. Siglec-5-Fc also detected increased Siglec-14-vimentin binding compared to Siglec-5 (Fig. 3c). Vimentin treatment also enhanced p38 MAPK phosphorylation in THP1-Sig14 macrophages compared to THP1-Sig5 cells; in contrast, AKT phosphorylation was not differentially regulated with expression of the activating versus inhibitory Siglec (Fig. 3d). Importantly, vimentin was previously shown to be released by macrophages and regulate NLRP3-inflammasome activation [41]. Thus, we hypothesized that Siglec-14 could engage soluble vimentin as a key factor to induce NLRP3 inflammasome activation. To test this hypothesis, recombinant vimentin was added to THP1-Sig14, THP1-Sig5, and THP1-EV macrophages exposed to the classical NLRP3 activator, nigericin. We found increased IL-1β secretion in vimentin-treated THP1-Sig14 macrophages compared to the others (Fig. 3e) and confirmed the increased IL-1β secretion was NLRP3 dependent by use of the specific NLRP3 inhibitor, CRID3 (Fig. 3f). Together, our results show that vimentin has a strong binding preference for Siglec-14 and is a host ligand that increases NLRP3 inflammasome activation through Siglec-14 signaling.
Fig. 3.
Vimentin can engage Siglec-14 to increase inflammasome activation. a Cell lysates of THP1-EV, THP1-Sig14 and THP1-Sig5 cells were immunoprecipitated with anti-Siglec-5/14 antibody and the interaction of vimentin assessed by western blotting. b Direct binding of Siglec5-Fc (Sig5), Siglec7-Fc (Sig7), Siglec9-Fc (Sig9) and Siglec14-Fc (Sig14) to recombinant vimentin as measured by ELISA; GBS β-protein immunoglobulin A binding region (IgA BR) was used as a negative control. c Direct binding of Siglec-14 and −5 to the various concentrations of recombinant vimentin measured by ELISA. d Cells were treated with 10 μg/mL of recombinant vimentin for the indicated time points, then phosphorylation of p38 mitogen-activated protein kinase P38 (p-P38) and AKT (p-AKT) protein levels were assessed by western blotting. e Cells were treated +/– recombinant vimentin for 2 h, then exposed to nigericin or buffer control for 30 min. Released IL-1β was assessed by ELISA. f Cells were treated with or without recombinant vimentin +/– NLRP3 inhibitor CRID3 sodium salt (CRID3) for 1 h, then exposed to nigericin or buffer control for an additional 2 h. IL-1β release was assessed by ELISA. Error bars represent the SD of data means from 3 independent experiments. * p < 0.05. IL-1β, interleukin-1β; EV, empty vector.
The Human SIGLEC14/5 Gene Polymorphism Influences Inflammasome Activation in Response to GBS
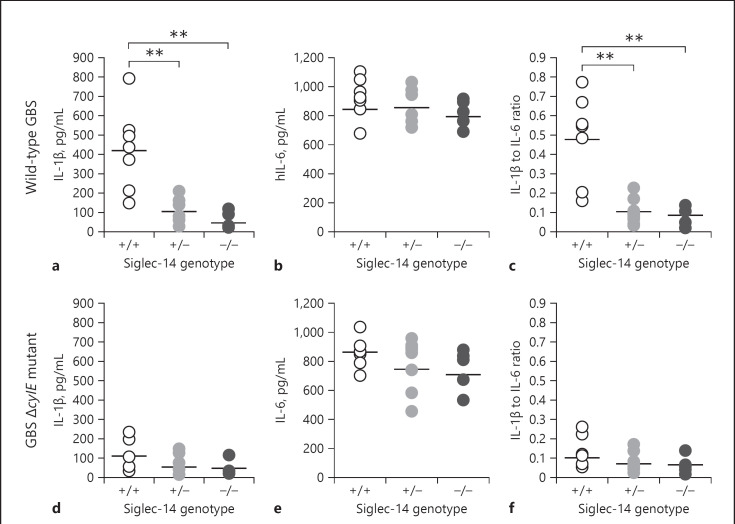
Based on our THP-1 macrophage cell line experiments, we hypothesized that the SIGLEC14/5 gene polymorphism could influence the magnitude of inflammasome activation. Genotyping was performed to identify SIGLEC14, SIGLEC5 and the SIGLEC14/SIGLEC5 fusion allele (functional SIGLEC5 gene driven under the SIGLEC14 promoter) [16] in different healthy donors (online suppl. Fig. S6a). In this study, the homozygous genotype carrying both normal SIGLEC14 allelesare referred as Sig14+/+; the heterozygous genotype carrying one SIGLEC14 allele and one SIGLEC14/SIGLEC5 fusion allele are referred to as Sig14+/−; and the homozygous genotype carrying both SIGLEC14/SIGLEC5 fusion alleles are referred to as Sig14−/−. Primary human MDM from 5 to 7 donors of each of the different genotypes were infected with GBS WT at MOI = 10 bacteria/host cell; GBS ΔcylE mutant bacteria served as negative (non-inflammasome inducing) control in this study. Primary macrophages from Sig14+/+ donors exhibited higher IL-1β secretion compared to primary macrophages from Sig14+/− or Sig14−/− donors in response to WT GBS infection (online suppl. Fig. S6b), whereas no significant differences in IL-6 secretion were observed across all genotypes (Fig. 4b). To normalize cytokine expression levels across the 3 genotypes, we divided the secretion level of IL-1β by that of IL-6, confirming Sig14+/+ donors yielded a higher IL-1β/IL-6 ratio than the others (Fig. 4c). In contrast, the response to GBS ΔcylE mutant infection did not reveal differences in IL-1β, IL-6, or IL-1β/IL-6 ratio among the 3 genotypes (Fig. 4d–f).
Fig. 4.
The human SIGLEC14/5 gene polymorphism influences inflammasome activation in response to GBS. Human MDM of the indicated genotypes were infected with GBS WT or the GBS ΔcylE mutant at MOI = 10 bacteria/cell with measurement of IL-1β (a, d) and IL-6 (b, e) release at 16 h by ELISA. The ratio of IL-1β to IL-6 was calculated (c, f). Data are from 5 to 7 different donors per SIGLEC-14/15 genotype. The lines indicate the means ± SEM of each group. ** p < 0.005. WT, wild-type; IL-1β, interleukin-1β; GBS, group B Streptococcus
Discussion
In this study, we described an immunoregulatory mechanism of the paired human Siglec-14 and −5 receptors in regulating NLRP3 inflammasome activation. Siglec-14-expressing THP1 cells exhibited increased secretion of mature IL-1β through NLRP3 inflammasome activation after treatment with ATP or nigericin and in response to GBS infection. Bacterial engagement with Siglec-14 (trans-interaction) and sialic acid-Siglec-14 interaction on the macrophage cell surface (cis-interaction) were both evident in modulation of Siglec-14 signaling to enhance inflammasome activation. We also identified vimentin as a new ligand of Siglec-14, which after its secretion from activated macrophages [40], can potentially functionally interact with Siglec-14 to boost inflammasome activation.
The innate immune response can be manipulated by bacterial glycosylation and by interaction of bacterial toxins and proteins with immunoregulatory receptors [1]. The virulence of GBS is associated with its surface capsular polysaccharide, which contain a terminal α2,3-linked Neu5Ac in all the repeating polysaccharide units of the 10 (Ia, 1b, II–IX) GBS serotypes. In particular, GBS serotype Ia and Ib contain terminal Neu5Acα2,3-lactosamine and interact with multiple human CD33-related Siglecs [42]. Through sialic acid mimicry, GBS can manipulate immune responses by engaging inhibitory Siglecs, such as Siglec-5 [21], Siglec-9 [5], and murine Siglec-E [11]. In addition to sialic acid mimicry, the GBS β-protein mediates an interaction with Siglec-14 and −5 [21, 22] that can influence innate immune phenotypes such as phagocytosis and IL-8 secretion. Our results extend the spectrum of innate immune phenotypes modulated by Siglec-5/14, since Siglec-14 enhances and Siglec-5 inhibits inflammasome activation in response to GBS infection. Additionally, we investigated how vimentin, a cytoskeleton protein known to mediate inside-out signaling in macrophages, can interact with Siglec-14 to stimulate inflammasome activation.
The expression of activating Siglec-14 with inhibitory paired receptor Siglec-5 controls a balance in inflammatory responses to pathogenic infection. However, activating Siglec-14 could represent a double-edged sword, which may promote inflammatory responses for pathogen clearance but also risk harmful excess inflammation, perhaps aggravated by pathogen manipulation. Exacerbations of chronic obstructive pulmonary disease (COPD) can be triggered by bacterial airway infection and is a significant cause of human mortality. The severity of COPD is associated with expression of Siglec-14 [31]; and moreover, the level of serum IL-1β correlated with disease severity in these patients [43]. Enhanced inflammasome activation through Siglec-14 signaling may thus represent a key mechanistic link between bacterial infection and excessive inflammatory responses, such as in COPD and our studies with GBS. However, further studies are necessary to ascertain that the differential inflammatory response to GBS is solely due to the Sig14+/+ genotype and not a co-segregating polymorphism.
In summary, we demonstrate a new role for Siglecs in modulating inflammasome activation in host-pathogen interactions and provide a mechanism for excess inflammation in individuals with a SIGLEC14 genotype. These results may inspire the examination of how other members of the Siglec family and other immunomodulatory lectin receptors influence inflammasome activation in response to pathogen infection and during inflammatory disease states.
Statement of Ethics
Human blood from healthy adult volunteers was collected with informed consent under a protocol approved by the University of California, San Diego Human Research Protection Program.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
Studies were supported by NIH/NHLBI Program of Excellence in Glycosciences (P01 HL107150 to V.N. and A.V.) and by NIH/NHLBI grant HL125352 (to V.N.). A.M.R. was supported by the UC President's Postdoctoral Fellowship and the San Diego IRACDA Fellowship (NIH/NIGMS K12 GM068524).
Author Contributions
C.-M.T., A.M.R. and V.N. conceived the study. C.-M.T., A.M.R., S.R.A, J.J.F., G.H. and J.Z.L conducted the experiments. C.-M.T., A.M.R., A.V, and V.N. provided key insights regarding reagents, the experimental design, and data interpretation. C.-M.T., A.M.R and V.N. wrote the paper, with all authors providing critical input and edits.
Supplementary Material
Supplementary data
References
- 1.Diacovich L, Gorvel JP. Bacterial manipulation of innate immunity to promote infection. Nat Rev Microbiol. 2010 Feb;8((2)):117–28. doi: 10.1038/nrmicro2295. [DOI] [PubMed] [Google Scholar]
- 2.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010 Mar;8((3)):171–84. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 3.Tytgat HL, Lebeer S. The sweet tooth of bacteria: common themes in bacterial glycoconjugates. Microbiol Mol Biol Rev. 2014 Sep;78((3)):372–417. doi: 10.1128/MMBR.00007-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology. 2011 Sep;21((9)):1121–4. doi: 10.1093/glycob/cwr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009 Apr;113((14)):3333–6. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secundino I, Lizcano A, Roupé KM, Wang X, Cole JN, Olson J, et al. Host and pathogen hyaluronan signal through human siglec-9 to suppress neutrophil activation. J Mol Med (Berl) 2016 Feb;94((2)):219–33. doi: 10.1007/s00109-015-1341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007 Apr;7((4)):255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 8.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012 Apr;1253((1)):16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014 Oct;14((10)):653–66. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30((1)):357–92. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang YC, Olson J, Beasley FC, Tung C, Zhang J, Crocker PR, et al. Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PLoS Pathog. 2014 Jan;10((1)):e1003846. doi: 10.1371/journal.ppat.1003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang YC, Nizet V. The interplay between Siglecs and sialylated pathogens. Glycobiology. 2014 Sep;24((9)):818–25. doi: 10.1093/glycob/cwu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, et al. A dietary fiber-deprived gut microbiota eegrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016 Nov;167((5)):1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angata T, Hayakawa T, Yamanaka M, Varki A, Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 2006 Oct;20((12)):1964–73. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 15.Cao H, Lakner U, de Bono B, Traherne JA, Trowsdale J, Barrow AD. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol. 2008 Aug;38((8)):2303–15. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka M, Kato Y, Angata T, Narimatsu H. Deletion polymorphism of SIGLEC14 and its functional implications. Glycobiology. 2009 Aug;19((8)):841–6. doi: 10.1093/glycob/cwp052. [DOI] [PubMed] [Google Scholar]
- 17.Jones C, Virji M, Crocker PR. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol Microbiol. 2003 Sep;49((5)):1213–25. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson HN, Mills DC, Jones H, Milioris E, Copland A, Dorrell N, et al. Pseudaminic acid on Campylobacter jejuni flagella modulates dendritic cell IL-10 expression via Siglec-10 receptor: a novel flagellin-host interaction. J Infect Dis. 2014 Nov;210((9)):1487–98. doi: 10.1093/infdis/jiu287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaas M, Oetke C, Lewis LE, Erwig LP, Heikema AP, Easton A, et al. Sialoadhesin promotes rapid proinflammatory and type I IFN responses to a sialylated pathogen, Campylobacter jejuni. J Immunol. 2012 Sep;189((5)):2414–22. doi: 10.4049/jimmunol.1200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angata T, Ishii T, Gao C, Ohtsubo K, Kitazume S, Gemma A, et al. Association of serum interleukin-27 with the exacerbation of chronic obstructive pulmonary disease. Physiol Rep. 2014 Jul;2((7)):e12069. doi: 10.14814/phy2.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlin AF, Chang YC, Areschoug T, Lindahl G, Hurtado-Ziola N, King CC, et al. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J Exp Med. 2009 Aug;206((8)):1691–9. doi: 10.1084/jem.20090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali SR, Fong JJ, Carlin AF, Busch TD, Linden R, Angata T, et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med. 2014 Jun;211((6)):1231–42. doi: 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fong JJ, Sreedhara K, Deng L, Varki NM, Angata T, Liu Q, et al. Immunomodulatory activity of extracellular Hsp70 mediated via paired receptors Siglec-5 and Siglec-14. EMBO J. 2015 Nov;34((22)):2775–88. doi: 10.15252/embj.201591407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storek KM, Monack DM. Bacterial recognition pathways that lead to inflammasome activation. Immunol Rev. 2015 May;265((1)):112–29. doi: 10.1111/imr.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015 May;25((5)):308–15. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol Microbiol. 2001 Jan;39((2)):236–47. doi: 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 27.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, et al. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998 Jan;66((1)):43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janes BK, Stibitz S. Routine markerless gene replacement in Bacillus anthracis. Infect Immun. 2006 Mar;74((3)):1949–53. doi: 10.1128/IAI.74.3.1949-1953.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winter AJ, Comis SD, Osborne MP, Tarlow MJ, Stephen J, Andrew PW, et al. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect Immun. 1997 Nov;65((11)):4411–8. doi: 10.1128/iai.65.11.4411-4418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slater SJ, Slater TF, Bailey JM. Discipline-based education research : a scientist's guide. New York: W.H Freeman; 2010. [Google Scholar]
- 31.Angata T, Ishii T, Motegi T, Oka R, Taylor RE, Soto PC, et al. Loss of Siglec-14 reduces the risk of chronic obstructive pulmonary disease exacerbation. Cell Mol Life Sci. 2013 Sep;70((17)):3199–210. doi: 10.1007/s00018-013-1311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang PJ, Low PY, Wang I, Hsu SD, Angata T. Soluble Siglec-14 glycan-recognition protein is generated by alternative splicing and suppresses myeloid inflammatory responses. J Biol Chem. 2018 Dec;293((51)):19645–58. doi: 10.1074/jbc.RA118.005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013 Jun;38((6)):1142–53. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, Tissi L, et al. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci USA. 2004 Oct;101((40)):14491–6. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa A, Gupta R, Signorino G, Malara A, Cardile F, Biondo C, et al. Activation of the NLRP3 inflammasome by group B streptococci. J Immunol. 2012 Feb;188((4)):1953–60. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009 Feb;7((2)):99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013 Jun;13((6)):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012 Mar;13((4)):333–42. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang YC, Uchiyama S, Varki A, Nizet V. Leukocyte inflammatory responses provoked by pneumococcal sialidase. MBio. 2012 Jan;3((1)):3. doi: 10.1128/mBio.00220-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003 Jan;5((1)):59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales-Nebreda L, et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun. 2015 Mar;6((1)):6574. doi: 10.1038/ncomms7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlin AF, Lewis AL, Varki A, Nizet V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol. 2007 Feb;189((4)):1231–7. doi: 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammada DR, Elgazzara AG, Essawya TS, Abd El Sameieb SA. Evaluation of serum interleukin-1 beta as an inflammatory marker in COPD patients. Egypt J Chest Dis Tuberc. 2015;64((2)):347–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data